Introduction
Childhood asthma, a heterogeneous condition with multiple phenotypes,1–4 originates from complex interactions between host genetics, environmental exposures (such as allergens and pollutants), and infectious agents. Traditionally, most research on the role of infectious agents in asthma inception has been focused on respiratory viruses, as these have been shown, in multiple studies, to be related to asthma onset and exacerbations.5–7 However, the recent advancements in microbiome research methodology have yielded valuable data on the role of respiratory bacteria in asthma. Emerging data suggest that certain compositions of the respiratory bacterial microbiome, during periods of health and/or disease in early life, are important determinants of asthma development and asthma exacerbations. In this manuscript, which is based on a Pro/Con seminar at the 2017 American Academy of Allergy, Asthma, and Immunology Annual Meeting, the debaters defend the roles of respiratory bacteria and viruses in childhood asthma and conclude with an integrated statement on this topic.
The Pro Position (Dr. Avraham Beigelman)
As described in the Con section of this manuscript, viral acute respiratory infections (ARIs) in infancy are significant determinants of childhood asthma inception and exacerbations.5–7 However, most children who experience early-life viral ARIs do not develop asthma. Moreover, respiratory viruses, mainly human rhinovirus (HRV), are commonly present in the upper airway of asymptomatic children and often do not cause exacerbations. Therefore, the obvious conclusion from these observations is that, in many occasions, respiratory viruses on their own are not sufficient to either promote asthma or to cause exacerbations.
Herby, I present evidence to support the hypothesis that bacteria are likely the “essential co-factor” that determines asthma inception and exacerbations. This hypothesis is supported by the following lines of evidence:
Airway “pathogenic” bacteria may induce episodic wheeze and childhood asthma. Bacteria may serve as the “first hit” on the pathway to asthma or may be the “second hit” as part of bacteria-virus interactions. These interactions, during early-life viral lower respiratory tract infections (LRTIs), may determine LRTI severity and the progression from the initial viral LRTI to subsequent episodes of recurrent wheeze (RW) and asthma.
In children with episodic wheeze and asthma, airway “pathogenic” bacteria are significant determinants of exacerbations.
Finally, as airway “pathogenic” bacteria are related to the inception and exacerbations of childhood asthma, anti-bacterial therapy may be utilized as a prevention and/or treatment modality.
Airway Bacteria and Bacteria-Virus Interactions are Important Determinants of Childhood Asthma
Airway bacteria as the “first hit” leading to childhood asthma
Certain airway bacteria may serve as the “first hit” leading to childhood asthma. It has been shown that asymptomatic, one-month old infants who had upper airway colonization with either S. pneumoniae, M. catarrhalis, and/or H. influenzae had almost 5 times higher odds to have asthma at the age of 5 years, compared to non-colonized infants.8 This observation is supported by a subsequent report from the same group, which provides mechanistic rationale to explain the role of airway bacteria in childhood asthma inception. In that subsequent study, children who eventually developed asthma had an aberrant early-life immune response evident by increased IL-5, IL-13, IL-17, and IL-10 production in peripheral blood mononuclear cells obtained at the age of 6 months. The authors proposed the following mechanistic pathway: aberrant immune response to “pathogenic” bacteria in the upper respiratory tract may predispose these children to persistent airway colonization with bacteria, which in-turn may result in T helper type 2 (pro-allergic) chronic airway inflammation and eventually asthma.9
Airway bacteria as the “second hit” (bacteria-virus interactions) leading to childhood asthma
It’s well known that early-life viral ARIs are associated with asthma inception.5–7 However, many children who experience viral ARIs do not develop asthma. Hence, the airway bacteria may be the “missing factor” (or the “second hit”) interacting with respiratory viruses, and the sum of these interactions may lead to episodic wheeze and asthma. This paradigm could be illustrated in a model of post-respiratory syncytial virus (RSV) RW. RSV ARIs are very common in early life; however, most of the RSV-infected children do not develop RW. The severity of the initial RSV ARI has been shown to be directly related to the likelihood of developing RW, as 43% of the children who had a prolonged hospitalization due to RSV ARI developed RW compared to only 12% of the children who did not have an RSV medical encounter.10 Yet, even among the children in the highest risk (the most severely ill children), more than half did not develop RW, suggesting that other factors, such as the composition of the airway microbiome, may determine the future development of post-RSV RW.
Another recent report provides information regarding the interactions between bacterial and viral airway microbiomes during the first year of life and their contribution to asthma inception.11 This report showed that the healthy upper airway microbiome during the first year of life was dominated by 3 main genera: Staphylococcus, Corynebacterium, and Alloiococcus; while samples taken during viral ARIs were dominated by Streptococcus, Moraxella, and Haemophilus. Bacteria, by themselves, were found to be an important determinant of asthma development as it was shown that asymptomatic high-abundance colonization with Streptococcus before the age of 2 months was a predictor of current wheeze at age of 5 years. Additional findings from that study support the “second hit” theory, in which certain compositions of the airway microbiome interact with respiratory viruses to determine the progressions to LRTI-related symptoms and RW:
The presence of Streptococcus, Moraxella, and Haemophilus during RSV ARI was associated with progression from upper respiratory tract infection to LRTI, which is a known risk factor for asthma development.
The presence of Moraxella during RSV LRTI was associated with a more severe infection (febrile episode), while febrile LRTI by itself (especially very early in life) was found to be a predictor of current wheeze at age of 5 years.
The evidence discussed above support the role of airway bacteria as the “first and/or second hit” leading to asthma. However, it should be acknowledged that the contribution of the airway bacteria to asthma inception should be viewed in the broader context of the effects of the environmental microbiomes on the immune system, as exposure to a wide range of microbes has found to be protective against asthma development.12
Airway Bacteria are Determinants of Childhood Asthma Exacerbations
A report from the COPSAC2000 birth cohort highlights the role of airway bacteria as determinants of asthma exacerbations among preschool children. The investigators sampled the upper airway of children during well and sick visits throughout the first 3 years of life to investigate the roles of virus and bacteria as triggers for wheezing episodes. It was found that bacteria, either alone or together with a virus, were identified in 86% of wheezing episodes. Importantly, the duration of the wheezing episodes did not differ between episodes dominated with virus or bacteria. The authors suggested that the commonly used term “viral wheeze” is inappropriate as it undervalues the role of bacteria in the pathophysiology of these wheezing episodes.13
Similar data supporting the role of bacteria in asthma exacerbations was reported in a cohort of older school-aged children who had weekly upper airway sampling during the fall season.14 The presence of HRV in upper airway samples was associated with an increased risk of detecting “pathogenic” bacteria (S. pneumoniae, H. influenzae, or M. catarrhalis) either within the same sample or within the following-week sample. However, bacteria detection did not increase the likelihood to detect HRV. Bacteria detection during HRV ARI was associated with the highest likelihood of asthma exacerbations, suggesting the role of bacteria as the “second hit” leading to exacerbation among these school age children.
Antibiotic Therapy (Macrolides) May Be Beneficial to Prevent Post-RSV RW
As the presence of certain airway bacteria during RSV LRTI determines illness severity and potentially the progression to RW and asthma,11, 15 the next apparent question is whether antibacterial therapy during RSV LRTI may modify the outcome of post-RSV RW. We recently completed a proof-of-concept, double-blinded, randomized trial in 40 infants hospitalized with RSV LRTI investigating the utility of adding 2 weeks of azithromycin therapy to routine bronchiolitis care. Infants who received azithromycin were less likely to develop RW and had fewer days with respiratory symptoms over the subsequent year.16 We chose azithromycin as the study intervention due to its anti-inflammatory properties demonstrated in other inflammatory airway diseases,17 and based on our previous findings in a murine model of viral bronchiolitis.18 Anti-inflammatory effects were detected in our study, as azithromycin reduced levels of upper airway IL-8, a major neutrophil chemoattractant.16 We also performed microbiome studies that showed that at the end of study treatment, Moraxella abundance was significantly lower in upper airway samples taken from children treated with azithromycin. Importantly, lower Moraxella abundance at the end of study treatment was associated with lower odds of post-RSV RW. This observation suggests that Moraxella may have a role in the pathogenesis of post-RSV RW, and that some of the potential beneficial effects of azithromycin to modify the outcome of post-RSV RW are mediated by anti-microbial effects and are not solely related to its direct anti-inflammatory effects. Overall, this proof-of-concept clinical trial and its microbiome studies identify airway bacteria during severe RSV LRTI as significant determinants of post-RSV RW and as a potential target for prevention. Nevertheless, this intervention should not be incorporated yet into the clinical practice, as a large confirmatory clinical trial is ongoing.19
Antibiotic Therapy (Macrolides) is Beneficial to Prevent and Treat Acute Episodic Wheeze Among Preschool Children
As airway bacteria may be significant determinants of exacerbations among preschool children with episodic wheeze, anti-bacterial therapy may provide benefits to prevent and/or to treat exacerbations. These potential approaches are supported by the results of the following 2 clinical trials in children: A recent clinical trial investigated whether early administration of azithromycin, started prior to the onset of severe LRTI-related symptoms and continued for 5 days, would prevent the progression to severe LRTI episodes that would trigger oral corticosteroids use.20 The results of that study revealed that early initiation of azithromycin reduced the risk of progressing to severe LRTI by 36% —an effect that is comparable to that of inhaled corticosteroids. Induction of azithromycin-resistant organisms was infrequent in that study. Another recent trial among preschool children with recurrent asthma-like symptoms (i.e., troublesome lung symptoms) investigated the utility of a 3-day course of azithromycin to treat respiratory symptoms that lasted at least 3 days.21 Compared to placebo, azithromycin therapy resulted in a 63% reduction in the duration of respiratory symptoms after initiation of therapy. The intervention was significantly more effective when the therapy was initiated earlier during the course of the illness and in episodes positive for H. influenzae. The investigators did not evaluate the effect of azithromycin on the induction of antibiotic resistance. An additional recent trial investigated the utility of azithromycin therapy in 222 pre-school children (87% with history of prior wheeze) presenting to the emergency department with acute wheeze. The results showed no difference in time to resolution of respiratory symptoms (p = 0.28) or in any of the secondary outcomes. However, no firm conclusion could be made from these results as the investigators recruited only ~50% of the target sample size, resulting in an underpowered study.22 Two additional clinical trials that investigated the utility of azithromycin therapy in asthmatic adults have yielded conflicting results.23, 24
Taken together, the results of the pediatric clinical trials suggest that azithromycin therapy may be utilized to prevent and/or to treat acute exacerbations among preschool children with severe episodic wheeze. More information is needed on the effect of azithromycin therapy on the development of antibiotic-resistant pathogens and whether non-macrolide antibiotics may also be beneficial. Nevertheless, these clinical trials highlight the important role of bacteria in the pathogenesis of severe exacerbations. Also, it must be stressed that macrolide use should be considered only during specific scenarios, such as described above, where there is sufficient evidence supporting its efficacy, because antibiotic use has the potential limitation of targeting commensal bacteria that may actually protect from asthma development,25 and these microbiome alterations may predispose to asthma development. This concept is supported by the results of a recent meta-analysis that detected a significant association (odds ratio [OR]: 2.18, 95% confidence interval [CI]: 1.04–4.60) between antibiotic use in the first year of life and the diagnosis of asthma at the age of 9–10 years in 2 large (n=9,393) population-based cohorts in Europe.26 Although these results may serve as an alarming sign for excessive antibiotic use, causation could not be proved from these epidemiological studies. It may be that antibiotic use is just a surrogate marker for viral ARIs, which by themselves may cause asthma. Nevertheless, more accurate approaches to target airway “pathogenic” bacteria (e.g., vaccines) are likely to be more desirable strategies for asthma prevention.
Potential Utilization of Bacterial Lysates for Childhood Asthma Prevention and Treatment
Bacterial lysates are products that may have immunostimulatory and/or immunomodulatory properties. One of the most investigated products is the OM-85 BV (Broncho-Vaxom®), a lyophilized extract of 21 bacterial lysates, which has been used for many years in Europe to prevent respiratory illnesses. A meta-analysis, which included 851 children, supported its use concluding that Broncho-Vaxom® was significantly effective in preventing recurrent viral ARIs in children.27 An additional clinical trial in preschool children with RW evaluated the effect of Broncho-Vaxom® for the prevention of episodic wheeze. Participants were treated for 10 days a month for 3 consecutive months and were followed for additional 9 months. The results showed that Broncho-Vaxom® treatment reduced the number and the duration of viral ARI-induced wheezing episodes, with effects that lasted beyond the treatment period.28 Based on these previous reports, the “Oral Bacterial Extract for the Prevention of Wheezing Lower Respiratory Tract Illness” (ORBEX) study, a primary prevention trial, is being currently conducted.29 Toddlers at high risk to develop asthma, based on family history of asthma and/or personal history of eczema, are being randomized to receive Broncho-Vaxom® or placebo for 10 days monthly for 2 consecutive years, and are being monitored for the development of wheezing LRTIs during a third observation year while off therapy.
Summary of the Pro Position
Undoubtedly, respiratory viruses are important in childhood asthma pathogenesis. However, and as supported by the data presented in this section, respiratory viruses on their own may not be sufficient determinants of asthma inception and/or exacerbations. Recent accumulating data have shown that airway bacteria, either alone or as part of bacteria-virus interactions, have a major role in the inception of asthma and are important determinants of acute asthma exacerbations (Figure 1). Finally, targeting airway bacteria by macrolides may provide benefits to prevent post-RSV RW and has been shown to be beneficial in the prevention and treatment of acute episodic wheeze among preschool children.
Figure 1.
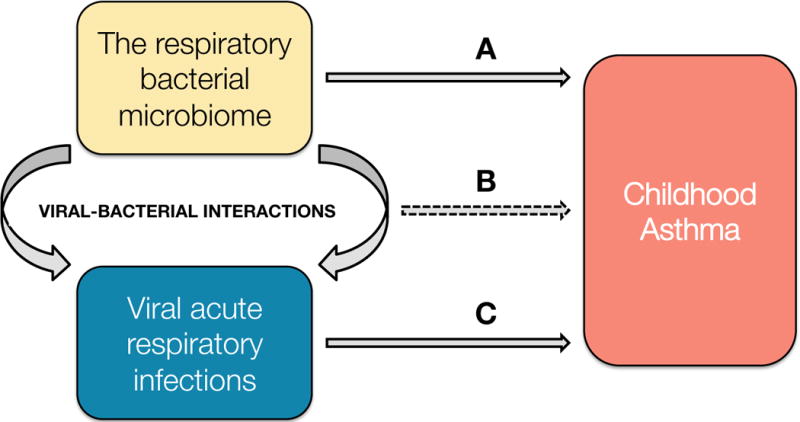
Potential effect of the respiratory bacterial microbiome (Pro position) (A), viral-bacterial interactions (Pro and Con position) (B), and acute respiratory viral infections (Con position) (C) in early life as causal agents of childhood asthma.
The Con Position (Drs. Christian Rosas-Salazar and Tina V. Hartert)
The link between respiratory viruses and the development of childhood asthma has long been recognized, with numerous epidemiological, laboratory, and semi-experimental studies demonstrating the importance of RSV and HRV ARIs in infancy —a critical period of lung and immune development— in the inception of this disease.5 In contrast, the role of respiratory bacteria in childhood asthma onset is less clear, as the available evidence is fairly limited and still accumulating.
The Role of Respiratory Bacterial Communities on Childhood Asthma Development
To date, the vast majority of studies examining the effect of the respiratory bacterial communities on childhood respiratory outcomes have focused on the interactions between upper airway bacteria and common respiratory viruses.11, 15, 30–35 In this context, we have shown marked differences in the taxonomic composition, richness, and/or abundance of certain taxa of the nasopharyngeal bacterial microbiome between healthy infants and infants with RSV ARI,30 as well as between infants with RSV ARI and HRV ARI.15 Unlike infants with asymptomatic HRV colonization, those with symptomatic HRV ARIs also appear to have alterations in the nasopharyngeal microbial diversity and bacterial density when compared to healthy infants with no respiratory virus detected.36 Other groups have also shown different nasopharyngeal microbial patterns in young children with severe LRTI due to isolated HRV infection when compared to those with severe LRTI due to HRV/RSV co-infection.31 In other studies, certain nasopharyngeal bacterial patterns in early childhood have been associated with a higher viral ARI severity of and/or a more pro-inflammatory immune response.31–34
On the other hand, most of the studies examining the role of the respiratory bacterial microbiome in asthma have been conducted in adults. In one of the few studies using next-generation sequencing conducted in children, Teo et al showed that a higher nasopharyngeal abundance of Streptococcus (i.e., more than 20%) in the first 2 months of life increased the odds of current wheeze at age 5 years, but not at age 10 years, in children born to atopic parents by ~4-fold (OR = 3.8, 95%CI = 1.3–12).11 In another recent study, Depner et al found a direct association between the nasal abundance of Moraxella and the odds of parental report of asthma in school-aged children, particularly in those not living in farms.37 In line with these findings, a prior study using conventional culture techniques showed that neonates born to asthmatic mothers with hypopharyngeal colonization with S. pneumoniae, M. catarrhalis, and/or H. influenzae had ~5-fold higher odds of asthma at age 5 years (OR = 4.6, 95% CI = 2.2 to 9.6).8 Interestingly, we have previously shown that infants with RSV ARI, who are at a particular high risk of developing childhood asthma, have higher nasopharyngeal abundances of Streptococcus, Moraxella, and Haemophilus, which suggests a compositional shift from the predominant nasopharyngeal bacterial genera in infancy (such as Corynebacterium, Dolosigranulum, and Staphylococcus) to genera that increase the risk of childhood asthma during early-life RSV ARI.30 Our observational studies also demonstrate a strong dose-response relationship between antibiotic courses during pregnancy and infancy on subsequent asthma risk.38 While this study cannot determine that antibiotics act through alterations in the microbiome, antibiotics have certainly been demonstrated to markedly alter the microbiome. Taken together, these observational studies do suggest that the early-life respiratory bacterial microbiome has an important role in the origins of childhood asthma; however, these are all association studies of the most common genera of the upper airways in children. Importantly, these studies assume that the most abundant bacteria are the most important, and thus preclude making any conclusions in regards to a potential causal bacteria or causal relationship. In a recent study we have shown that the nasopharyngeal genus associated with the greatest reduction in the risk of post-RSV RW is actually Lactobacillus, a generally low abundant bacteria in the airway, yet a bacteria decreased by the most commonly used antibiotics in the pediatric population.25 Other studies have shown that the protective effect of growing up in a traditional farm environment on childhood asthma development is most likely related to being exposed to house dust containing higher abundances of certain Listeria and Bacillus species, which are also uncommon in the upper airways.39 Thus, we must be cautious about targeting certain bacteria without regard to alterations in the entire respiratory bacterial microbiome and of particular genera that may be highly protective, despite being in low abundance.
Evidence of a Causal Role of Respiratory Viruses in the Inception of Childhood Asthma
Whereas the number of studies examining the association of the early-life respiratory bacterial microbiome and the development of childhood asthma is still very limited, the number of studies demonstrating the importance of early-life viral ARIs, particularly those due to RSV or HRV, in the inception of this disease is extensive.5 Taking into consideration the widely used Hill’s criteria of causality,40 the current body of research does support a causal role of these respiratory viruses —but not of respiratory bacteria—in childhood asthma onset. This is exemplified in Table 1, which shows that while only 3 out of the original Hill’s criteria are met for respiratory bacteria, at least 7 are met for respiratory viruses, in particular for RSV, as briefly discussed in the following paragraphs.
Table 1.
Summary of the available evidence in support of or against a causal relationship between respiratory bacteria or viruses and the inception of childhood asthma according to the Hill’s criteria.40
| Hill’s criteria for causality | Evidence for respiratory bacteria* | Evidence for respiratory viruses* |
|---|---|---|
| The effect size is large (strength of the association) | +/− | + |
| The results of studies are reproducible (consistency) | +/− | + |
| The exposure occurs prior to the cause (temporality) | + | + |
| There is a dose-response effect (biological gradient) | □ | + |
| There is coherence between observational, laboratory, and experimental findings (coherence) | □ | + |
| There is a potential biological mechanism between cause and effect (biological plausibility) | + | + |
| There are similar factors known to cause the disease (analogy) | + | + |
| There is experimental evidence in humans (experiment) | +/− | +/− |
| There is no other likely explanation (specificity) | − | − |
+ = There is evidence in support of a causal relationship; – = there is evidence against a causal relationship; +/– = the evidence is limited or inconclusive; □ = there is no evidence available.
Strength of the association: The effect size of the association between early-life RSV ARI and childhood asthma is large when compared to other pre- or post-natal risk factors of this disease. In a meta-analysis of 15 observational studies conducted up to 2012, Regnier et al showed that a hospitalization for RSV ARI in the first few years of life was associated with a ~4-fold increased odds (OR = 3.8, 95% CI = 3.2–4.6) of developing childhood asthma up to age 11 years.41 The individual studies included in this meta-analysis had odds ratios that ranged from 1.4 to 17.7. Not all infants with RSV ARI develop childhood asthma, but this does not mean that RSV is not a causal agent. For instance, not all individuals who smoke develop lung cancer, but as it is the case with RSV, this does not negate the fact that smoking is a well-established causal agent for pulmonary malignancies.
Consistency: Of the 15 observational studies that met pre-specified criteria in the aforementioned meta-analysis,41 all had a point estimate indicating a detrimental effect of a hospitalization for RSV ARI in the first few years of life on childhood asthma (i.e., an odds ratio above one), in spite of the different populations, sample sizes, and analytical approaches.
Temporality: Several longitudinal studies have shown that RSV ARIs precede childhood asthma onset. In a prospective cohort of otherwise healthy children followed until early adulthood in Sweden, children hospitalized for RSV ARI in their first year of life had increased odds of childhood asthma at ages 3, 7, 13, and 18 years.42–45 Likewise, children who are born just prior to the RSV circulating season (November to March) have higher odds of childhood asthma up to age 5 years when compared to those born during other periods.46
Biological gradient: We have previously shown a dose-response relationship between the severity of infant bronchiolitis and the risk of childhood asthma up to age 5 years in a retrospective cohort of more than 90,000 children,47 consistent with what has been found in a cohort of high risk children.48 In this study, the odds ratios for childhood asthma relative to no infant bronchiolitis visit were 1.9 (95% CI = 1.7–2.0), 2.4 (95% CI = 2.2–2.6), and 2.8 (95% CI = 2.6–3.0) in the outpatient, emergency department, and hospitalization groups, respectively.
Coherence: The observational data suggesting a causal role of early-life RSV ARI in the inception of childhood asthma is supported by both laboratory and semi-experimental data. In murine models, RSV ARI leads to acute and chronic changes similar to those found in asthma. Lukacs et al showed that infecting mice with a specific RSV strain resulted in increased airway reactivity during methacholine challenge, as well as an increased production of airway mucus.49 Similar results have been found in mice exposed to RSV and sensitized to ovalbumin or cockroaches.50–52 The semi-experimental data supporting early-life RSV ARI as a causal factor for childhood asthma is described below.
Biological plausibility: The biological mechanisms through which early-life RSV ARI could lead to childhood asthma have been extensively reviewed in the past.5, 53, 54 In brief, RSV is capable of modifying the host’s immune response, damaging the epithelial barrier function, and increasing airway hyperresponsiveness (see above), among others. In a study of newborn mice, Krishnamoorthy et al demonstrated that early-life RSV ARI increases the susceptibility to atopic asthma through impairment of regulatory T cell and promotion of a T helper type 2 (pro-allergic) immune response.52
Analogy: There is now ample evidence demonstrating the importance of early-life risk factors in the origins of chronic respiratory diseases, which reinforces the hypothesis that RSV ARI in infancy is a causal factor for childhood asthma. In addition to early-life RSV ARI, many other pre- and post-natal infectious (e.g., HRV ARI) and non-infectious risk factors (e.g., birth via cesarean section) for childhood asthma have been identified.5, 38, 55, 56 Furthermore, it is well established that certain viruses (e.g., human papillomavirus, hepatitis B virus, and hepatitis C virus) can lead to chronic diseases many years after the initial infection.57 Thus, it is conceivable that RSV ARI in infancy acts as a strong determinant of the onset of asthma later in life.
Experiment: Due to ethical reason, no direct human experiments will ever be conducted; however, indirect evidence does provide a proof-of-concept of the causal relationship between early-life RSV ARI and childhood asthma. In a randomized, double-blind, placebo-controlled trial, Blanken et al showed that otherwise healthy preterm infants who received palivizumab (a commercially available RSV monoclonal antibody indicated to prevent RSV in high-risk populations) in infancy had a relative reduction of 61% (95% CI = 56–65) in the total number of wheezing days and of 47% (95% CI = 14–80) in the proportion of RW episodes in the first year of life.58 In an observational study, the use of ribavirin (an antiviral agent against RSV ARI) in children younger than 2 hospitalized with RSV ARI decreased the odds of childhood asthma at school age by 80% (OR = 0.2, 95% CI = 0.1–0.7).59 Most other observational studies of RSV immunoprophylaxis have shown similar results.60–65 In contrast, in a randomized, double-blind, placebo-controlled trial of previously healthy Native American children, O’Brien et al found a protective effect of motavizumab (another RSV monoclonal antibody) on RSV-related hospitalizations in infancy, but not on the rates of RW between ages 1–3 years.65 However, the proportion of healthy children who developed RW was very low (16/641 [~3%]) compared to other populations, which may have led to low statistical power.
Specificity: Childhood asthma is a chronic pulmonary disease that arises from complex gene-by-environment interactions, and it is clear that there is no single risk factor that could explain the increasing prevalence of this disease.66 However, RSV ARI is a ubiquitous infection in early childhood with a large effect size and its population-attributable risk for childhood asthma development has been estimated in ~13%, with the phenotype of childhood asthma following RSV ARI accounting for ~30% of all early childhood asthma.5, 67 Thus, preventing early-life RSV ARI, particularly severe infection, could have a significant impact in the burden of childhood asthma.
Summary of the Con Position
There is strong evidence meeting criteria for causality that supports a causal role of respiratory viruses in the origins of childhood asthma, even in the absence of experimental human data. Such data do not currently exist for bacteria. However, as noted in the initial discussion, it is also clear that the different microorganisms that inhabit the respiratory tract (viruses, bacteria, and fungi) do not act in isolation but likely form networks that affect the response to infant respiratory viral infection and subsequent childhood respiratory outcomes. As our microbiome is in symbiotic relationship with us, it is very likely that respiratory bacterial networks interact with causal respiratory viruses on the development of childhood asthma, as we depict in Figure 1. Bacteria are meant to be there, respiratory viral pathogens are not, so we need to better understand how they interact with each other to modify the risk of developing childhood asthma.
Conclusion
Our understanding of the interactions between respiratory microorganisms is still fairly limited, but as respiratory viruses first enter through the upper airway, it seems likely that the existing host local microbial environment impacts the expression of the viral ARI and the subsequent risk of childhood asthma development. Newer sequencing technologies may shed light on how respiratory viruses, bacteria, and fungi interact with each other and how these interactions could affect pediatric respiratory outcomes. For respiratory bacteria, we need to next identify the most important protective and detrimental bacterial taxa, examine how these taxa relate to each other, determine protective microbial signatures that may alter the risk of asthma associated to causal environmental or infectious agents (including respiratory viruses or other “pathogenic” bacteria), and identify the biologic pathways underlying these associations. For respiratory viruses, we need to identify host factors that protect from asthma development following viral ARIs, further characterize asthmagenic viral strains to inform vaccine development, and develop feasible early-life preventive strategies.
Acknowledgments
Funding sources:
This work was supported in whole or in part with funds from the NIH/NIAID under award numbers U19AI095227, K24AI77930, HHSN272200900007C, and U19AI110819; the Vanderbilt Institute for Clinical and Translational Research grant support (NIH/NCATS under award numbers UL1 TR000445 and U54RR24975); the Vanderbilt Faculty Research Scholars Program, and the Parker B. Francis Fellowship Program.
Abbreviations
- ARI
Acute respiratory infection
- CI
Confidence interval
- HRV
Human rhinovirus
- LRTI
Lower respiratory tract infection
- OR
Odds ratio
- RSV
Respiratory syncytial virus
- RW
Recurrent wheeze
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration: The authors declare that they have no relevant conflicts of interest to disclose.
Disclosure: This was a Pro/Con debate presented at the American Academy of Allergy, Asthma, and Immunology Annual Meeting in March 2017 (Atlanta, GA). The authors disclose that their position for this Pro/Con debate was assigned to them. This position assignment does not necessarily reflect their personal opinion regarding the role of bacteria or viruses in childhood asthma.
References
- 1.Depner M, Fuchs O, Genuneit J, Karvonen AM, Hyvarinen A, Kaulek V, et al. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med. 2014;189:129–38. doi: 10.1164/rccm.201307-1198OC. [DOI] [PubMed] [Google Scholar]
- 2.Henderson J, Granell R, Heron J, Sherriff A, Simpson A, Woodcock A, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63:974–80. doi: 10.1136/thx.2007.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savenije OE, Granell R, Caudri D, Koppelman GH, Smit HA, Wijga A, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol. 2011;127:1505–12 e14. doi: 10.1016/j.jaci.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Martinez F, Wright A, Taussig L, Holberg C, Halonen M, Morgan W. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–8. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 5.Feldman AS, He Y, Moore ML, Hershenson MB, Hartert TV. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191:34–44. doi: 10.1164/rccm.201405-0901PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigelman A, Bacharier LB. Early-life respiratory infections and asthma development: role in disease pathogenesis and potential targets for disease prevention. Curr Opin Allergy Clin Immunol. 2016;16:172–8. doi: 10.1097/ACI.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beigelman A, Bacharier LB. Infection-induced wheezing in young children. J Allergy Clin Immunol. 2014;133:603–4 e4. doi: 10.1016/j.jaci.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 9.Larsen JM, Brix S, Thysen AH, Birch S, Rasmussen MA, Bisgaard H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J Allergy Clin Immunol. 2014;133:1008–13. doi: 10.1016/j.jaci.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Escobar GJ, Masaquel AS, Li SX, Walsh EM, Kipnis P. Persistent recurring wheezing in the fifth year of life after laboratory-confirmed, medically attended respiratory syncytial virus infection in infancy. BMC Pediatr. 2013;13:97. doi: 10.1186/1471-2431-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–15. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369–79. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson CJ, Vissing NH, Sevelsted A, Johnston SL, Bonnelykke K, Bisgaard H. Duration of wheezy episodes in early childhood is independent of the microbial trigger. J Allergy Clin Immunol. 2015;136:1208–14 e5. doi: 10.1016/j.jaci.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133:1301–7. 7 e1–3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Schobel S, Chappell JD, Larkin EK, et al. Differences in the Nasopharyngeal Microbiome During Acute Respiratory Tract Infection With Human Rhinovirus and Respiratory Syncytial Virus in Infancy. J Infect Dis. 2016;214:1924–8. doi: 10.1093/infdis/jiw456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beigelman A, Isaacson-Schmid M, Sajol G, Baty J, Rodriguez OM, Leege E, et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135:1171–8 e1. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedlander AL, Albert RK. Chronic macrolide therapy in inflammatory airways diseases. Chest. 2010;138:1202–12. doi: 10.1378/chest.10-0196. [DOI] [PubMed] [Google Scholar]
- 18.Beigelman A, Mikols CL, Gunsten SP, Cannon CL, Brody SL, Walter MJ. Azithromycin attenuates airway inflammation in a mouse model of viral bronchiolitis. Respir Res. 2010;11:90. doi: 10.1186/1465-9921-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azithromycin to Prevent Wheezing Following Severe RSV Bronchiolitis-II (APW-RSV-II) 2016 doi: 10.1016/j.conctc.2021.100798. Available from: https://clinicaltrials.gov/ct2/show/NCT02911935. [DOI] [PMC free article] [PubMed]
- 20.Bacharier LB, Guilbert TW, Mauger DT, Boehmer S, Beigelman A, Fitzpatrick AM, et al. Early Administration of Azithromycin and Prevention of Severe Lower Respiratory Tract Illnesses in Preschool Children With a History of Such Illnesses: A Randomized Clinical Trial. JAMA. 2015;314:2034–44. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokholm J, Chawes BL, Vissing NH, Bjarnadottir E, Pedersen TM, Vinding RK, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19–26. doi: 10.1016/S2213-2600(15)00500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandhane PJ, Paredes Zambrano de Silbernagel P, Aung YN, Williamson J, Lee BE, Spier S, et al. Treatment of preschool children presenting to the emergency department with wheeze with azithromycin: A placebo-controlled randomized trial. PLoS One. 2017;12:e0182411. doi: 10.1371/journal.pone.0182411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–68. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 24.Johnston SL, Szigeti M, Cross M, Brightling C, Chaudhuri R, Harrison T, et al. Azithromycin for Acute Exacerbations of Asthma : The AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630–7. doi: 10.1001/jamainternmed.2016.5664. [DOI] [PubMed] [Google Scholar]
- 25.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Schobel S, Chappell JD, Larkin EK, et al. Nasopharyngeal Lactobacillus is Associated with Childhood Wheezing Illnesses Following Respiratory Syncytial Virus Infection in Infancy. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.10.049. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmadizar F, Vijverberg SJH, Arets HGM, de Boer A, Turner S, Devereux G, et al. Early life antibiotic use and the risk of asthma and asthma exacerbations in children. Pediatr Allergy Immunol. 2017;28:430–7. doi: 10.1111/pai.12725. [DOI] [PubMed] [Google Scholar]
- 27.Schaad UB. OM-85 BV, an immunostimulant in pediatric recurrent respiratory tract infections: a systematic review. World J Pediatr. 2010;6:5–12. doi: 10.1007/s12519-010-0001-x. [DOI] [PubMed] [Google Scholar]
- 28.Razi CH, Harmanci K, Abaci A, Ozdemir O, Hizli S, Renda R, et al. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J Allergy Clin Immunol. 2010;126:763–9. doi: 10.1016/j.jaci.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 29.Oral Bacterial Extract for the Prevention of Wheezing Lower Respiratory Tract Illness (ORBEX) 2017 Available from: https://clinicaltrials.gov/ct2/show/NCT02148796.
- 30.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Chappell JD, Larkin EK, Nelson KE, et al. Nasopharyngeal Microbiome in Respiratory Syncytial Virus Resembles Profile Associated with Increased Childhood Asthma Risk. Am J Respir Crit Care Med. 2016;193:1180–3. doi: 10.1164/rccm.201512-2350LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyde ER, Petrosino JF, Piedra PA, Camargo CA, Jr, Espinola JA, Mansbach JM. Nasopharyngeal Proteobacteria are associated with viral etiology and acute wheezing in children with severe bronchiolitis. J Allergy Clin Immunol. 2014;133:1220–2. doi: 10.1016/j.jaci.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Steenhuijsen Piters WA, Heinonen S, Hasrat R, Bunsow E, Smith B, Suarez-Arrabal MC, et al. Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection. Am J Respir Crit Care Med. 2016;194:1104–15. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasegawa K, Mansbach JM, Ajami NJ, Espinola JA, Henke DM, Petrosino JF, et al. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur Respir J. 2016;48:1329–39. doi: 10.1183/13993003.00152-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasegawa K, Mansbach JM, Ajami NJ, Petrosino JF, Freishtat RJ, Teach SJ, et al. Serum cathelicidin, nasopharyngeal microbiota, and disease severity among infants hospitalized with bronchiolitis. J Allergy Clin Immunol. 2017;139:1383–6 e6. doi: 10.1016/j.jaci.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biesbroek G, Tsivtsivadze E, Sanders EA, Montijn R, Veenhoven RH, Keijser BJ, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190:1283–92. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 36.Korten I, Mika M, Klenja S, Kieninger E, Mack I, Barbani MT, et al. Interactions of Respiratory Viruses and the Nasal Microbiota during the First Year of Life in Healthy Infants. mSphere. 2016;1 doi: 10.1128/mSphere.00312-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Depner M, Ege MJ, Cox MJ, Dwyer S, Walker AW, Birzele LT, et al. Bacterial microbiota of the upper respiratory tract and childhood asthma. J Allergy Clin Immunol. 2017;139:826–34 e13. doi: 10.1016/j.jaci.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 38.Wu P, Feldman AS, Rosas-Salazar C, James K, Escobar G, Gebretsadik T, et al. Relative Importance and Additive Effects of Maternal and Infant Risk Factors on Childhood Asthma. PLoS One. 2016;11:e0151705. doi: 10.1371/journal.pone.0151705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 40.Hill AB. The Environment and Disease: Association or Causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 41.Regnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:820–6. doi: 10.1097/INF.0b013e31829061e8. [DOI] [PubMed] [Google Scholar]
- 42.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–52. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 43.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. American Journal of Respiratory and Critical Care Medicine. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 44.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–5. [PubMed] [Google Scholar]
- 45.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. American Journal of Respiratory and Critical Care Medicine. 2005;171:137–41. doi: 10.1164/rccm.200406-730OC. [DOI] [PubMed] [Google Scholar]
- 46.Wu P, Dupont WD, Griffin MR, Carroll KN, Mitchel EF, Gebretsadik T, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. Am J Respir Crit Care Med. 2008;178:1123–9. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carroll KN, Wu P, Gebretsadik T, Griffin MR, Dupont WD, Mitchel EF, et al. The severity-dependent relationship of infant bronchiolitis on the risk and morbidity of early childhood asthma. J Allergy Clin Immunol. 2009;123:1055–61. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 49.Lukacs NW, Moore ML, Rudd BD, Berlin AA, Collins RD, Olson SJ, et al. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am J Pathol. 2006;169:977–86. doi: 10.2353/ajpath.2006.051055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lukacs NW, Tekkanat KK, Berlin A, Hogaboam CM, Miller A, Evanoff H, et al. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J Immunol. 2001;167:1060–5. doi: 10.4049/jimmunol.167.2.1060. [DOI] [PubMed] [Google Scholar]
- 51.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2014;111:805–10. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, et al. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat Med. 2012;18:1525–30. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piedimonte G. Pathophysiological mechanisms for the respiratory syncytial virus-reactive airway disease link. Respir Res. 2002;3(Suppl 1):S21–5. doi: 10.1186/rr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Newcomb DC, Peebles RS., Jr Bugs and asthma: a different disease? Proc Am Thorac Soc. 2009;6:266–71. doi: 10.1513/pats.200806-056RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosas-Salazar C, Hartert TV. Prenatal exposures and the development of childhood wheezing illnesses. Curr Opin Allergy Clin Immunol. 2017 doi: 10.1097/ACI.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. 2015;386:1075–85. doi: 10.1016/S0140-6736(15)00156-7. [DOI] [PubMed] [Google Scholar]
- 57.O’Connor SM, Taylor CE, Hughes JM. Emerging infectious determinants of chronic diseases. Emerg Infect Dis. 2006;12:1051–7. doi: 10.3201/eid1207.060037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–9. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 59.Chen CH, Lin YT, Yang YH, Wang LC, Lee JH, Kao CL, et al. Ribavirin for respiratory syncytial virus bronchiolitis reduced the risk of asthma and allergen sensitization. Pediatr Allergy Immunol. 2008;19:166–72. doi: 10.1111/j.1399-3038.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 60.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. Journal of Pediatrics. 2007;151:34–42. e1. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 61.Prais D, Kaplan E, Klinger G, Mussaffi H, Mei-Zahav M, Bar-Yishay E, et al. Short- and Long-term Pulmonary Outcome of Palivizumab in Children Born Extremely Prematurely. Chest. 2016;149:801–8. doi: 10.1378/chest.15-0328. [DOI] [PubMed] [Google Scholar]
- 62.Wenzel SE, Gibbs RL, Lehr MV, Simoes EA. Respiratory outcomes in high-risk children 7 to 10 years after prophylaxis with respiratory syncytial virus immune globulin. Am J Med. 2002;112:627–33. doi: 10.1016/s0002-9343(02)01095-1. [DOI] [PubMed] [Google Scholar]
- 63.Yoshihara S, Kusuda S, Mochizuki H, Okada K, Nishima S, Simoes EA, et al. Effect of palivizumab prophylaxis on subsequent recurrent wheezing in preterm infants. Pediatrics. 2013;132:811–8. doi: 10.1542/peds.2013-0982. [DOI] [PubMed] [Google Scholar]
- 64.Carroll KN, Gebretsadik T, Escobar GJ, Wu P, Li SX, Walsh EM, et al. Respiratory syncytial virus immunoprophylaxis in high-risk infants and development of childhood asthma. J Allergy Clin Immunol. 2017;139:66–71 e3. doi: 10.1016/j.jaci.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Brien KL, Chandran A, Weatherholtz R, Jafri HS, Griffin MP, Bellamy T, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15:1398–408. doi: 10.1016/S1473-3099(15)00247-9. [DOI] [PubMed] [Google Scholar]
- 66.Martinez FD, Vercelli D. Asthma. Lancet. 2013;382:1360–72. doi: 10.1016/S0140-6736(13)61536-6. [DOI] [PubMed] [Google Scholar]
- 67.James KM, Gebretsadik T, Escobar GJ, Wu P, Carroll KN, Li SX, et al. Risk of childhood asthma following infant bronchiolitis during the respiratory syncytial virus season. J Allergy Clin Immunol. 2013;132:227–9. doi: 10.1016/j.jaci.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


