Abstract
The present study used cross-lagged panel analyses to test longitudinal associations among emotion regulation, prefrontal cortex (PFC) function, and depression severity in adolescent girls. The ventromedial and dorsomedial PFC (vmPFC; dmPFC) were regions of interest given their roles in depression pathophysiology, self-referential processing, and emotion regulation. At ages 16 and 17, 78 girls completed a neuroimaging scan to assess changes in vmPFC and dmPFC activation to sad faces, and measures of depressive symptom severity and emotion regulation. The one-year cross-lagged effects of dmPFC activity at age 16 on expressive suppression at age 17 and depressive symptomatology at age 17 were significant, demonstrating a predictive relation between dmPFC activity and both suppression and depressive severity.
Depression onset progressively increases across adolescence into early adulthood, and affects more adolescent girls than boys (Rohde, Beevers, Stice, & O’Neil, 2009). The marked biological, cognitive, and social changes of adolescence, such as puberty and brain maturation, are also linked to an uptick in negative emotionality, greater self- and other-awareness, and heightened focus on social experiences (Guyer, Silk, & Nelson, 2016). Etiological theories of depression propose that maladaptive aspects of emotional expression and regulation lead to depression in youths (Hyde, Mezulis, & Abramson, 2008; Kovacs, Joormann, & Gotlib, 2008). Neurodevelopmental models of adolescent depression further posit that the prefrontal cortex (PFC), which is implicated in depression pathophysiology and undergoes significant development in adolescence, plays a role in depression because of how medial regions of the PFC come to process and represent social-emotional information with maturation (Davey, Yucel, & Allen, 2008; Kilford, Garrett, & Blakemore, 2016). Guided by these models of depression, the present study examined longitudinal and joint contributions of emotion regulation strategies and medial PFC (mPFC) function in a social-emotional context to the development of depression in adolescent girls. Taking a longitudinal, multi-level approach in this way may help inform developmental theories of depression by accounting for both brain and behavior over time during a period in development when girls are vulnerable to depression onset.
Past work indicates that depressed vs. non-depressed adolescents draw on a more limited repertoire of emotion regulation strategies, use less effective strategies, and are less likely to trust their ability to use their strategies effectively (Garber, Braafladt, & Weiss, 1995, Yap, Allen, & Sheeber, 2007). The development and course of depression in adolescents has been associated further with less frequent use of cognitive reappraisal and increased use of expressive suppression (Hughes, Gullone, & Watson, 2011; Larsen et al., 2012), two widely studied types of strategies. Cognitive reappraisal is an antecedent-focused emotion regulation strategy by which an individual tries to cognitively re-evaluate the meaning of an emotional stimulus (Gross, 1998). Expressive suppression, on the other hand, is a response-focused strategy used after a situation has elicited an emotional response to hide overt expressions of affect, but also requires an element of cognitive control (Gross, 1998).
Developmental studies indicate that adolescents use reappraisal significantly less frequently when compared to adults (Garnefski, Legerstee, Kraaij, Van Den Kommer, & Teerds, 2002), although the frequency of reappraisal use shows no difference across adolescence (Gullone, Hughes, King, & Tonge, 2010). Variability in age-related changes have been found in expressive suppression, however. While no changes have been found during early adolescence (Sullivan, Helms, Kliewer, & Goodman, 2010), decreased expressive suppression usage has been reported for girls during mid-adolescence (Gullone et al., 2010) and increased expressive suppression usage for sadness has been found in youth during middle versus early and late adolescence (Zeman & Shipman, 1997). Furthermore, the use of emotion regulation strategies across adolescence may vary by the type of emotion experienced (Silk, Steinberg, & Morris, 2003). For example, age differences in expressive suppression usage were found for feelings of sadness and fear, but not anger (Zimmermann & Iwanski, 2014). The same study also reported considerable fluctuations in expressive suppression usage for sadness across adolescence and early adulthood, highlighting the transient nature of emotion regulation in adolescence and its potential varying influence on vulnerability for depression during this period.
One possible pathway linking difficulties in emotion regulation and depression is through increased experiences of negative affect, such as sad feelings, that may ultimately lead to depression. Poor or inefficient emotion regulation has been associated with excessive, inappropriate, or insufficient emotional responses (Aldao, Nolen-Hoeksema, & Schweizer, 2010; Etkin, Buchel, & Gross, 2015). Research in adults has shown that those who habitually seek to hide their emotions experience less positive affect (Gross & John, 2003), increased sympathetic nervous system activation (Campbell-Sills, Barlow, Brown, & Hofmann, 2006b), poorer memory performance (Hayes et al., 2010), disruptions in social relationships (Gross & John, 2003), and greater intensity and duration of negative emotions (Campbell-Sills, Barlow, Brown, & Hofmann, 2006a), all indicators of depression. Establishing whether specific emotion regulation strategies are simply symptoms of depression, a consequence of previously experienced depression or, alternatively, whether some strategies are risk factors that precede the onset of depression is necessary for determining precursors and trajectories of adolescent depression to inform intervention efforts. While evidence suggests that less reappraisal and more suppression are both associated with concurrent symptoms of depression in youth (e.g. Joormann & Gotlib, 2010), at least one study has shown that depression preceded increased use of suppression in adolescence (Larsen et al., 2012). Due to excessive negative affect or focus on negative information during a major depressive episode, individuals may rely more frequently on suppression as a strategy; or, are less able to recruit a reappraisal strategy. Equally so, individuals with major depressive disorder (MDD) tend to process information more negatively, leading to greater occurrence and intensity of negative mood and affect and in turn to less efficient emotion regulation strategies (Beck, 2008).
Emotion regulation may also confer risk for later depression through an increase in self-focus. Self-focus is multifaceted and includes the process by which one engages in self-referential processing, i.e., the appraisal of stimuli as strongly related to one’s own person (Northoff et al., 2006). Excessive self-focus may manifest in increased rumination, self-blame, negative emotional bias, and sense of failure in youth, and disrupts active engagement with the environment (Miller, 2007), thereby reducing attention to exogenous stimuli and diminishing cognitive control. This mechanism aligns with cognitive theories of depression that implicate negative cognitive schemas in biasing social-affective information processing (Beck, 2008). Children and adolescents with depression perform worse on executive function and attention tasks, particularly in the context of negatively valanced stimuli such as sad facial affect (Jacobs, Reinecke, Gollan, & Kane, 2008; Vilgis, Silk, & Vance, 2015). Memory for negative emotional stimuli is also affected; early adolescent girls with higher levels of depression are less accurate at recognizing sad faces (Guyer, Choate, Grimm, Pine, & Keenan, 2011) and show over-general autobiographical memory biases (Hipwell, Sapotichne, Klostermann, Battista, & Keenan, 2011).
Given that brain maturation continues and self-focus is heightened during adolescence, vulnerability to depression may relate to underlying neural mechanisms of self-focus. Yet, little is known about how these neural mechanisms relate to emotion dysregulation and depression in adolescents, particularly from a developmental perspective. To probe underlying neural mechanisms of the social-cognitive divergence of depression, emotional faces tasks paired with functional magnetic resonance imaging (fMRI) have been used widely and reliably (e.g. Stuhrmann, Suslow, & Dannlowski, 2011). The present study aimed to elicit neural response during self-focus by having adolescents explicitly assess their own state of sadness when viewing others depicting sadness. This self-referential process entails mentalizing about another’s emotional state and reflecting on one’s own internal state (Immordino-Yang, 2011). Three brain regions are predominantly involved in the processing of self-referential material: the dorsomedial PFC (dmPFC), ventromedial PFC (vmPFC), and posterior cingulate cortex comprising the default mode network (Northoff et al., 2006). In adults with MDD versus healthy controls, these medial regions show greater activation and are less well suppressed by more lateral PFC regions during cognitive tasks including reappraisal (Sheline et al., 2009), suggesting greater self-focus disrupts effective cognitive emotion regulation (Lemogne, Delaveau, Freton, Guionnet, & Fossati, 2012). This may be particularly relevant in the face of negative information such as mood congruent sad stimuli.
The current investigation focused on the vmPFC and dmPFC as both regions are relevant in the context of self-referential processing, emotion regulation, and depression. Several meta-analyses identify the dmPFC as having a role in effective emotion regulation during both suppression and reappraisal in adults (Buhle et al., 2014; Ochsner, Silvers, & Buhle, 2012). The role of the dmPFC in emotion regulation has been speculated to include emotional awareness and self-monitoring processes (Amodio & Frith, 2006). The dmPFC also reliably activates during self-referential processing (Northoff et al., 2006) and is abnormally active in adults with MDD, although both hyper- and hypo-activation have been observed (Lemogne et al., 2011). The role of the vmPFC in emotion regulation is less obvious, but due to its direct anatomical connections with the amygdala it is thought to control negative affect (Kim & Whalen, 2009). Greater severity of depressed mood correlates with increased vmPFC responses to negative emotional faces in children (Killgore & Yurgelun-Todd, 2006) and adolescents (Henderson et al., 2014). Although cross-sectional studies verify a correlation between mPFC response and behavior, they cannot address associations among brain function, behavior, and psychopathology over time.
To better understand how mPFC activity relates to emotion regulation and depression spanning middle adolescence, a critical period marked by high risk for depression, the current study measured mPFC responses while youth reflected on their feelings of sadness. Identifying whether contributions from the brain or from behavior precede one or the other may help to inform early detection of vulnerability for depression. The majority of neurobiological theories of adolescent development suggest that asynchronous maturation of the PFC and limbic areas may contribute to the increased vulnerability to psychopathology in adolescence, but this view has been challenged by another theory suggesting development of the PFC itself plays a critical role specifically in risk for depression (Davey et al., 2008). The present study was guided by this latter neurobiological theory of depression as well as by Beck’s cognitive theory of depression in adults, implicating PFC development and negative cognitive schemas that bias social-affective information processing and less efficient emotion regulation strategies in depression. We tested these propositions longitudinally in adolescent girls by modelling the relations of within-person change among mPFC function during self-focus, depression, and emotion regulation to depict the influence of each construct on the other and on brain function when making a self-referential judgment about sad faces. Sad facial expressions bias attention in youth at high risk for and with current depression (Hankin, Gibb, Abela, & Flory, 2010), and using a similar task design to the present one we have previously shown that behaviorally, higher depressive symptoms predicted difficulties encoding sad and happy faces (Guyer et al., 2011).
Our main research questions were as follows: 1) Do less effective emotion regulation strategies (more suppression; less reappraisal) lead to greater depression severity or vice versa?; 2) Does mPFC activity during self-referential processing of sadness predict greater depression or does depression predict subsequent mPFC activity?; and 3) Does mPFC activity predict subsequent emotion regulation strategies or vice versa? For each question, we tested the unique effects of vmPFC and dmPFC activity given the roles these regions play in self-focus, depression, and emotion regulation. We used a cross-lagged panel analysis (CLPA), which is a method of examining one-way or reciprocal causal inference between longitudinally changing variables. Using a CLPA, we examined the one-way causal relations among depression severity, emotion regulation, and mPFC activity over time in adolescent girls after controlling for initial levels of these three variables. We expected mPFC activity during self-referential processing of sadness to relate to aspects of emotion regulation and depression severity. We also tested the temporality of these associations to assess what precedes the other, but without existing longitudinal work, we lacked a basis for hypothesizing a specific direction of effects.
Method
Participants
The Pittsburgh Girls Study – Emotions (PGS-E) sub-study is part of the larger longitudinal Pittsburgh Girls Study (PGS; Keenan et al., 2010) in which 2450 girls and their caregivers have been interviewed annually beginning between the ages of 5 and 8 years. Girls in the PGS-E sub-study were recruited from the youngest sample of girls (age 8 years) in wave 4 of the PGS. All girls who screened high (upper quartile) by their own or caregiver report on a measure of depression symptoms were targeted for enrollment as well as a demographically matched comparison group (lower 75%). PGS-E participants (N=232) and their mothers underwent five yearly assessments from ages 9 to 13 years. Starting at age 16 (T6), 232 girls were invited to participate in further annual assessments of depression and fMRI scans; 194 girls participated with 146 completing the fMRI scan. FMRI data quality criteria resulted in 120 participants whose data passed quality control at T6. At T7, 78 participants met quality control at both time points and comprise the final study sample (see Supplementary Material for details on exclusions and attrition). Data were collected from November 2011–April 2014. All participants and their parents provided written assent or consent to take part in this study. 61% of participants identified as African American, 28% identified as Caucasian and 11% as multi-racial; 37% of participants’ households received public assistance when girls were age 9 and initially enrolled into PGS-E. All study procedures were approved by the study site’s Institutional Review Board.
Measures
Emotion regulation
Participants completed the Emotion-Regulation Questionnaire (ERQ; Gross & John, 2003) to measure the habitual use of two different emotion regulation strategies: cognitive reappraisal and expressive suppression. The ERQ consists of 10 items rated on a scale from 1 (strongly disagree) to 7 (strongly agree). An example item from the cognitive reappraisal reads ‘When I want to feel less negative emotion, I change the way I’m thinking about the situation’ and from the suppression scale: ‘When I am feeling negative emotions, I make sure not to express them’. Cronbach’s alphas for the six-item reappraisal scale were .84 (age 16) and .83 (age 17) and for the four-item suppression scale were .64 (age 16) and .67 (age 17), values consistent with past work (Gross & John, 2003; Gullone et al., 2010).
Depressive symptoms
The Adolescent Symptom Inventory - 4 (ASI-4; Gadow & Sprafkin, 1999) was used to assess depressive symptoms according to the Diagnostic and Statistical Manual of Mental Disorders 4th ed. (American Psychiatric Association, 1994). Symptoms were scored on 4-point scales from 0 (never) to 3 (very often) for all items except changes in appetite, sleep, and school functioning (scored .5 for no and 1.5 for yes). We used both parent- and self-reports on total symptom severity scores at age 16 and age 17. Severity scores measure the degree of behavioral deviance compared with a normative sample. The ASI-4 shows adequate concurrent validity, sensitivity and specificity of depression severity scores to clinicians’ diagnoses (Gadow & Sprafkin, 1999). Average internal consistency coefficients were .73 (age 16) and .77 (age 17) for parent reports and .82 (age 16) and .88 (age 17) for self-reports.
Neural activity during self-referential processing
A face processing neuroimaging task adapted from Guyer et al. (2011; 2008) was used to assess blood oxygenation level dependent (BOLD) effects while girls’ reflected on their feelings of sadness to sad facial stimuli. In this rapid, event-related design, participants viewed 12 sad, 12 angry, 12 happy, and 12 neutral faces portrayed by 48 unique actors. While viewing each picture, participants either judged “How sad does this face make you feel?” (self-referential judgment) or “How wide is the nose?” (judgment of physical feature). Responses from 1=Not at all to 5=Very much so were recorded via a button box with five buttons, one per finger. The task had participants reflect on their feelings of sadness rather than directly induce or regulate feelings of sadness. While we expected variability in rated degree of sadness felt, participants did report greater feelings of sadness to sad vs. other faces (Table S1, Supplementary Material). Order of task conditions and facial expressions was randomized across participants. The task had three runs of four 10-trial blocks.
MRI acquisition, preprocessing and registration
All MRI data were acquired with a 3.0 T Siemens (Erlangen, Germany) Tim Trio scanner. The functional scan comprised 280 contiguous echo planar imaging whole-brain functional volumes (repetition time [TR] = 2 s; echo time [TE] = 28 ms; flip angle = 90°, 39 slices, matrix = 64 × 64; field of view [FOV] = 205 mm; acquisition voxel size = 3.2×3.2×3.1 mm). A T1-weighted high-resolution anatomical image was acquired for co-registration and normalization of functional images with the following parameters: TR = 2.3 s; TE = 2.98 ms; flip angle = 9°; 160 slices; FOV = 256 mm; acquisition voxel size=1.0×1.0×1.2 mm.
Preprocessing and analysis of imaging data were conducted using Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm) and followed standard processing procedures as previously published (Casement et al., 2014; Romens et al., 2015). Functional images were slice time corrected to the middle volume in the time series, spatially realigned to the first volume to correct for head motion, spatially normalized to Montreal Neurological Institute (MNI) stereotaxic space using a 12-parameter affine model, and smoothed using a 6 mm full-width half maximum Gaussian filter. Voxel-wise signal was ratio-normalized to the whole brain global mean. The Artifact Detection Toolbox (ART; http://www.nitrc.org/projects/artifact_detect/) was used to detect functional movement more than three standard deviations from an individual’s mean, more than .5 mm translational, and more than .01 degrees of rotation scan-to-scan movement. Final analyses only included data with head movement in less than 20% of volumes. Temporal censoring based on ART output was used to remove motion artifacts. Second-level random effects models were used to estimate neural response while viewing faces. For each participant, we calculated task condition effects at each voxel with paired t-tests to create our contrast of interest: making a self-referential judgment while viewing a sad face versus making a judgment of a physical feature of a sad face. Supplementary Material presents results of the whole-brain analysis for this contrast (Table S3; Figure S1). To quantify dmPFC and vmPFC activity (Figure 1), MARSbar was used to create two 5 mm radius spheres, centered on the MNI coordinates of x = −6, y = 27, z = 42 (dmPFC) and x = −6, y = 42, z = 12 (vmPFC) adapted from Northoff et al. (2006) and Lemogne et al. (2009), from which parameters for the contrast of interest were extracted at T6 and T7.
Figure 1.
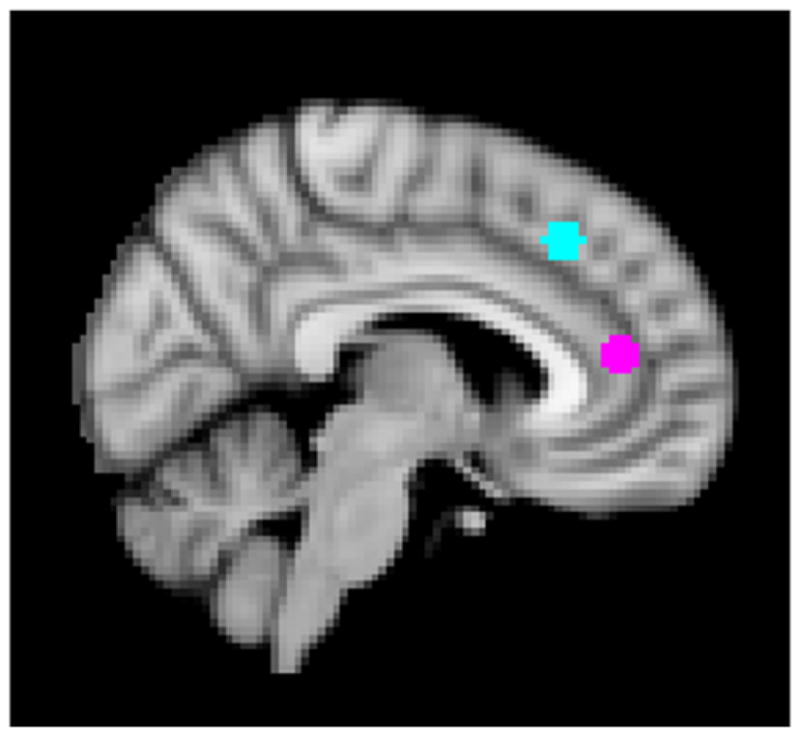
Regions of Interest (ROIs): Ventromedial prefrontal cortex (vmPFC) displayed in pink and dorsomedial prefrontal cortex (dmPFC) shown in blue. Both ROIs were created by drawing a 5 mm sphere around MNI coordinates x = −6, y = 27, z = 42 for vmPFC and x = −6, y = 42, z = 12 for dmPFC.
Statistical analysis
The present study utilized a cross-lagged structural equation model (Selig & Little, 2012) to test the significance of the longitudinal relations between variables of interest from age 16 (T6) to age 17 (T7). Variables included emotion regulation (reappraisal and suppression), depressive severity scores (separately from the mother and the child), and brain activation during self-referential processing of sad facial affect in cortical midline regions (vmPFC and dmPFC). In particular, the cross-lagged panel model simultaneously predicts variables at T7 using all variables from T6 (e.g., predicting brain activity, emotion regulation, and depressive severity at T7 from brain activity, emotion regulation, and depressive severity at T6). Figure 2 shows a conceptual depiction of the model. Eight different models were fitted to the data, with each model examining measures of emotion regulation, depressive severity, and brain activation. Each model contained autoregressive effects (e.g., predicting brain activity at T7 from brain activity at T6; predicting emotion regulation at T7 from emotion regulation at T6) and cross-lagged effects (e.g., predicting brain activity at T7 from emotion regulation and depressive severity at T6; predicting emotion regulation at T7 from brain activity and depressive severity at T6). The cross-lagged effects represent the focal tests of the current hypotheses because they summarize the degree to which the T6 variables predict the T7 variables while statistically controlling for the autoregressive effects. More specifically, the cross-lagged effects directly estimate the unique contribution of emotion regulation at T6 to brain activity and depressive symptoms at T7, the unique contribution of brain activity at T6 to emotion regulation and depressive symptoms at T7, and the unique contribution of brain activity at T6 to emotion regulation and depressive symptoms at T7. The model was fitted to the data using the lavaan package (Rosseel, 2012) within R (version 3.2.3; R Core Team, 2016). The ERQ was missing for one participant at T6 and four participants at T7. Parent-reported depression scores were missing for two participants at T6 and T7. Missing data were handled using full information maximum likelihood in lavaan.
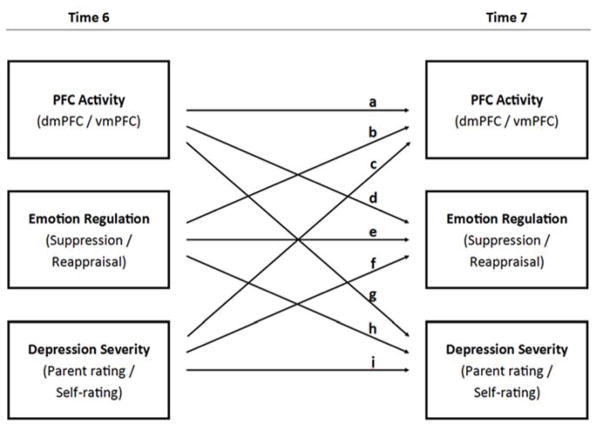
Figure 2.
Cross-lagged panel path model to test relations among emotion regulation, depression severity, and ventromedial prefrontal cortex (vmPFC) and dorsomedial prefrontal cortex (dmPFC) activity. Letters (a, b, c, …) correspond to path estimates in Table 2.
Results
Table 1 shows means, standard deviations, and correlations for the two emotion regulation subscales (expressive suppression and reappraisal), the two brain regions (vmPFC and dmPFC), and depression severity ratings (parent- and self-report) at T6 and T7. Both emotion regulation and depressive severity scores correlated significantly and positively from T6 to T7, indicating rank order stability among the variables across the measurement occasions. Brain-derived measures showed positive relations from T6 to T7, but these were a trend for vmPFC (p=0.05) and not significant for dmPFC (p=0.12). T6 parent-reported depressive severity was significantly and negatively associated with T7 reappraisal scores, but this pattern was not significant for T6 self-reported depressive symptoms. In contrast, self-reported depression correlated positively with concurrent vmPFC activity at T7 only. T6 dmPFC activity was positively associated with T7 suppression scores. Using paired sample t-tests, both suppression (t(72)=−2.35, p=.02) and self-reported depressive severity (t(75)=2.47, p=.02) scores decreased significantly from T6 to T7. Parent-reported depressive severity (t(75)=−1.00, p=n.s) and reappraisal (t(72)=.68, p=n.s.) scores did not change significantly over time. DmPFC activity did not change significantly over time (t(77)=.20, p=n.s.), but mean vmPFC activity decreased significantly from T6 to T7 (t(77)=3.92, p=.002).
Table 1.
Correlations and descriptive statistics for study variables at age 16 (Time 6) and age 17 (Time7)
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | M | SD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age 16 Suppression | 1 | 14.60 | 4.79 | ||||||||||
| 2. Age 17 Suppression | .49** | 1 | 15.95 | 4.47 | |||||||||
| 3. Age 16 Reappraisal | .09 | −.14 | 1 | 29.68 | 7.08 | ||||||||
| 4. Age 17 Reappraisal | .10 | −.21 | .39** | 1 | 28.97 | 6.23 | |||||||
| 5. Age 16 Depression (PR) | −.06 | .12 | −.13 | −.24* | 1 | 3.61 | 2.39 | ||||||
| 6. Age 17 Depression (PR) | .04 | .06 | −.06 | −.16 | .54** | 1 | 3.97 | 3.18 | |||||
| 7. Age 16 Depression (SR) | .21 | .22 | .00 | −.14 | .18 | .18 | 1 | 6.28 | 4.41 | ||||
| 8. Age 17 Depression (SR) | .15 | −.01 | .04 | −.01 | .02 | .22 | .59** | 1 | 5.22 | 4.36 | |||
| 9. Age 16 vmPFC | −.02 | .09 | −.13 | −.16 | .09 | .02 | .08 | .13 | 1 | .87 | 1.59 | ||
| 10. Age 17 vmPFC | .15 | .07 | .05 | −.09 | −.07 | .15 | .19 | .24* | .22 | 1 | .08 | 1.80 | |
| 11. Age 16 dmPFC | .08 | .26* | −.16 | −.16 | .03 | −.18 | .20 | −.09 | .28* | .02 | 1 | −.16 | 1.99 |
| 12. Age 17 dmPFC | .15 | .03 | .03 | −.03 | .08 | .17 | .07 | .05 | .06 | .28* | .12 | −.22 | 2.09 |
PR=parent report; SR=self-report; vmPFC = ventromedial prefrontal cortex; dmPFC = dorsomedial prefrontal cortex
Correlation is significant at the 0.05 level (2-tailed);
Correlation is significant at the 0.01 level (2-tailed).
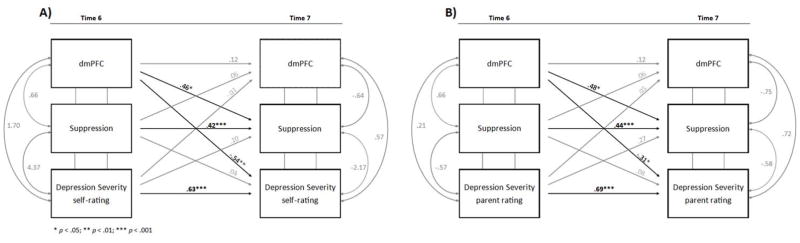
Next, cross-lagged models were fitted to examine the longitudinal relations among all variables of interest. The cross-lagged paths of interest (a – i, Figure 2) indicated the extent to which emotion regulation, brain activity, and depression severity at T6 predicted scores on the other measures at T7, above and beyond the autoregressive relations. Of the eight models, two showed significant cross-lagged associations between T6 brain activity and T7 emotion regulation and depression (Figure 3). Specifically, T6 dmPFC activity positively predicted T7 suppression scores and negatively predicted self-reported depression severity over and above the influence of these variables at T6. T6 dmPFC activity was also significantly negatively associated with T7 parent-reported depressive symptoms, despite weak correlations between parent- and self-reported depression. Therefore, higher levels of T6 dmPFC activity predicted greater emotional regulation (via suppression) and lower depression severity (for both self- and parent-ratings) at T7, after statistically controlling for T6 suppression and depression. Table 2 shows path estimates and significance levels of all eight models tested. Concurrent variables were allowed to co-vary, depicted with double-headed arrows (Figure 3). Supplementary Material shows concurrent path estimates and significance levels for all T6 and T7 models (Table S2).
Figure 3.
The two models showing significant paths from dmPFC activity at Time 6 (age 16) to suppression scores and self-ratings of depression severity at Time 7 (age 17) (A) and from dmPFC activity at Time 6 to suppression scores and parent ratings of depression severity at Time 7 (B). Significant paths are shown in black; non-significant paths are shown in grey.
Table 2.
Standardized parameter estimates for the cross-lagged model of the relations among prefrontal cortex (PFC) activity, emotion regulation and depression severity ratings at Time 6 and Time 7. Letters (a, b, c, …) refer to the paths depicted in Figure 2.
| PFC Region | Emotion Regulation | Depressive Severity | a | p (a) | b | p (b) | c | p (c) | d | p (d) | e | p (e) | f | p (f) | g | p (g) | h | p (h) | i | p (i) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dmPFC | Suppression | PR | .117 | .336 | .064 | .200 | .054 | .591 | .481 | .027 | .444 | < .001 | .273 | .133 | −.313 | .027 | .084 | .148 | .694 | < .001 |
| dmPFC | Suppression | SR | .125 | .313 | .065 | .203 | −.013 | .825 | .458 | .041 | .420 | < .001 | .098 | .354 | −.536 | .009 | .039 | .648 | .627 | < .001 |
| dmPFC | Reappraisal | PR | .135 | .278 | .013 | .708 | .053 | .606 | −.355 | .272 | .324 | < .001 | −.461 | .087 | −.315 | .029 | −.031 | .442 | .672 | < .001 |
| dmPFC | Reappraisal | SR | .135 | .285 | .011 | .760 | .002 | .976 | −.306 | .360 | .345 | < .001 | −.147 | .337 | −.533 | .011 | .000 | .997 | .635 | < .001 |
| vmPFC | Suppression | PR | .234 | .079 | .057 | .180 | −.070 | .425 | .175 | .543 | .458 | < .001 | .280 | .136 | −.087 | .640 | .075 | .211 | .687 | < .001 |
| vmPFC | Suppression | SR | .201 | .130 | .047 | .281 | .067 | .178 | .170 | .558 | .428 | < .001 | .130 | .228 | .163 | .548 | .035 | .695 | .572 | < .001 |
| vmPFC | Reappraisal | PR | .238 | .078 | .014 | .648 | −.070 | .433 | −.433 | .292 | .329 | < .001 | −.445 | .100 | −.100 | .597 | −.021 | .620 | .672 | < .001 |
| vmPFC | Reappraisal | SR | .204 | .128 | .018 | .546 | .078 | .107 | −.435 | .297 | .347 | < .001 | −.155 | .305 | .172 | .528 | .026 | .661 | .581 | < .001 |
Behavioral ratings of the face stimuli collected during the scan were analyzed to test differences in how participants perceived sadness in different expressions. Task performance was similar at T6 and T7 with no significant differences in mean reaction time (ms) to sad faces when making a self-referential judgement about feelings of sadness between (T6: M=1513.02, SD=260.31) and (T7: M=1493.51, SD=325.07; t(77)=0.61, p=n.s.) or when making a judgment about the physical feature of the face (T6: M=1571.08, SD=210.67; T7: M=1562.40, SD=219.27; t(77)=0.36, p=n.s.). No significant differences in ratings between T6 and T7 of the sad faces during the self-referential condition (T6: M=2.59, SD=1.08; T7: M=2.65, SD=1.04; t(77)=−0.55, p=n.s.) or during the nose-width rating condition (T6: M=2.49, SD=.49; T7: M=2.53, SD=.59; t(77)=−0.63, p=n.s.) were found. Sad faces on average were rated as inducing greater feelings of sadness than were neutral, happy, and angry faces at T6 and T7 indicating to some extent that sad facial affect was perceived as making participants feel sadder than other types of emotions (Table S1, Supplementary Material).
Discussion
Theories have linked cognitive emotion regulation and depression (Beck, 2008) and developmental changes in the PFC as a potential neurobiological risk marker for depression (Davey et al., 2008). The PFC plays a regulatory role that controls major psychological processes including regulation of emotion. While numerous studies have separately established links between emotion regulation and depression, PFC function and depression as well as PFC function and emotion regulation, few studies have examined how all three of these constructs operate over time, and particularly during adolescence. In the current study, we modeled these relations in a sample of girls over the period of one year to identify the direction of the associations between 1) emotion regulation strategies and depression severity, 2) mPFC activity and depression severity, 3) mPFC activity and emotion regulation strategies from age 16 to 17 while controlling for baseline levels of these variables.
We found that dmPFC activity while making a self-referential judgment about feelings of sadness to sad facial stimuli predicted depression severity ratings and expressive suppression scores a year later, over and above the contributions of initial depression ratings and suppression scores. More dmPFC activity was associated with both decreased parent- and self-ratings of girls’ depression severity. In contrast, less dmPFC activity was associated with reduced use of expressive suppression. The results were specific to the dmPFC and did not apply to the vmPFC. Furthermore, dmPFC activity only predicted suppression but did not predict reappraisal. Taken together, the results suggest that because heightened dmPFC activity typically indicates more top-down control over responses to emotion cues, girls’ with more dmPFC activity may exert more cognitive control during self-referential processing of sadness and this may buffer them from experiencing increased negative affect over time. In contrast, less dmPFC activity signifies less cognitive control in this self-referential context of processing sadness that may signify vulnerability to subsequent increases in depressive severity.
The dmPFC activates in response to a diverse set of experiences (e.g. Walter et al., 2009). Given the task we used, we favor two main explanations for the longitudinal associations of dmPFC activity, depression severity, and suppression. The first possibility is that dmPFC activity predicts depression by capturing an increase in symptom-relevant self-focus. The second possibility is that the negative correlation between depression severity and dmPFC activity reflects diminished top-down control of negative affect. With regard to the first possibility, studies in adults with MDD find abnormal activity in dmPFC during self-referential processing, although both hyper and hypoactivation has been observed (Lemogne et al., 2012). Lemogne et al. (2012) speculated that increased dmPFC activity in adults with MDD compared to healthy controls was most likely attributable to a methodological difference related to use of a block task design; reduced activity was more common in tasks that have participants switch from making a self-referential to a non-self-referential judgment on a trial-by-trial basis. Our task used blocks of event-related trials to distinguish between self-referential and other judgments; accordingly, we would have expected a positive association between dmPFC and depression severity. One explanation for the observed negative versus positive relation may be due to sample characteristics, as we focused on a non-clinical sample of adolescents with elevated risk for depression rather than adults with a current diagnosis of MDD. Only one study to date has examined self-referential processing in adolescents with MDD and found no activation differences in PFC regions between clinical and control participants (Bradley et al., 2016). Although the differences in task designs and sample characteristics could account for the observed negative relation, we suggest instead that the dmPFC serves as a domain general control region. In this context, diminished dmPFC activity at age 16 predicted greater depression severity one year later, and may represent an inflection point mechanism of depression.
Viewing the dmPFC as a domain-general control region is also supported by the observed positive association between dmPFC activity at age 16 and expressive suppression scores at age 17, with more activity linked to greater self-reported expressive suppression scores. In heathy adults, the dmPFC is engaged by monitoring regulation success and emotional awareness (Amodio & Frith, 2006). It is also recruited during reappraisal and suppression, and activates during uninstructed changes in affective experience (Silvers, Wager, Weber, & Ochsner, 2015). Greater activation of dmPFC (and dlPFC) while responding to negative stimuli corresponds to less negative affect in adults (Silvers, Shu, Hubbard, Weber, & Ochsner, 2015), suggesting that less activity contributes to rising negative affect and thereby potentially to increased risk for depression. Less dmPFC activity may also signify a reduced ability to shift attention away from negative stimuli leading to unsuccessful prevention of negative emotion entering and remaining in working memory (Joormann, 2010). Our dmPFC ROI fell within the posterior dmPFC and likely overlaps with dorsal anterior cingulate cortex (ACC) areas. This ROI converges with one of only a few regions identified across the most common mental health disorders (Goodkind et al., 2015), highlighting its role in domain general cognitive control processes compromised in various psychiatric disorders. Our study adds to this observation by showing that diminished dmPFC activity precedes subsequent difficulties in emotion regulation and indicates increased risk for depression among adolescent girls.
Whereas dmPFC activity positively predicted expressive suppression one year later, this relation was not observed for reappraisal. While suppression and reappraisal are conceptually distinct, relatively little evidence demonstrates that these strategies rely on different neural circuitry. Both activate top-down regions including ventrolateral, dorsolateral, medial PFC and dorsal ACC, but differ in their efficacy at diminishing activity in the insula and amygdala with reappraisal showing greater attenuation in these regions compared to suppression (Goldin, McRae, Ramel, & Gross, 2008; Vanderhasselt, Baeken, Van Schuerbeek, Luypaert, & De Raedt, 2013). One explanation for the lack of this association is that our task was not specifically designed to probe effortful emotion regulation. Effortful emotion regulation is viewed as the conscious modification of ongoing emotional responses and much research has focused on the deliberate deployment of these strategies, with the explicit goal to change one’s emotions (Ochsner et al., 2012). Viewing sad facial stimuli while making a self-referential judgment likely requires a more implicit, automatic form of emotion regulation, if not a ‘tuning in’ to one’s current affective experience. In addition, because facial affect was randomized throughout the task and participants could not know which emotion would appear on any given trial, antecedent-focused strategies such as reappraisal may not work or were not measureable in this context, whereas a response-focused strategy such as expressive suppression may be more representative of activation elicited in this region by this task.
We also examined whether vmPFC activity would show meaningful associations with depression severity and emotion regulation over one year. VmPFC did not predict subsequent depression or emotion regulation scores. However, self-reported depression severity at age 17 correlated positively with concurrent vmPFC activity. This aligns with findings from several neuroimaging studies showing elevated vmPFC activity at rest in adults with current depression (Sheline et al., 2009) and during facial affect processing tasks in adolescents and children (Henderson et al., 2014; Killgore & Yurgelun-Todd, 2006). Some propose that elevated vmPFC activity reflects increased self-focus and relates to rumination (Nejad, Fossati, & Lemogne, 2013). The role of the vmPFC in emotion regulation is thought to be indirect; lateral PFC regions engage the vmPFC, which in turn modifies activity in bottom-up structures such as the amygdala (Etkin et al., 2015; Ochsner et al., 2012). The reciprocal link between the amygdala and vmPFC has positioned the vmPFC as a control region of negative affect (e.g., fear, sadness) recruited by other more domain general regions such as the dlPFC and dmPFC (Buhle et al., 2014).
Emotion regulation and depression severity were not associated in the current study contrasting past work reporting an association between adolescent depression and specifically suppression (Aldao et al., 2010). As highlighted by a meta-analysis, suppression can reflect different forms of emotion regulation, namely suppression of overt emotion expression (as captured by the ERQ expressive suppression scale) and of subjective experience of the emotion (Webb, Miles, & Sheeran, 2012). The latter is considered ineffective, whereas the former can be effective in social situations where it is often necessary and perhaps deemed more mature to conceal one’s emotions. Most research on expressive suppression and depression studied individuals with clinically diagnosed depression or elevated symptoms (Aldao et al., 2010; Campbell-Sills et al., 2006a, 2006b; Hughes et al., 2011) suggesting this link may not apply to non-clinical samples. Indeed, a prospective study of a community sample of adolescents showed depressive symptoms preceded the use of suppression and not vice versa (Larsen et al., 2012).
The present results suggest that heightened dmPFC activity in middle adolescence contributes to a subsequent decrease in depressive symptom severity as well as increased use of expressive suppression as a strategy for emotion regulation. Rather than either of these two variables predicting activity, the brain-derived measure preceded subsequent behavior. Together, this indicates that top-down dmPFC activity is crucial for the successful development of cognitive control and emotion regulation. Skill building for adolescents to strengthen this type of control may be beneficial to prevent subsequent vulnerability to psychopathology and depression in particular. For example, an intervention using mindfulness-based stress reduction techniques in individuals with social anxiety disorder showed modulation of activity in several brain regions including one located in close proximity to the dmPFC ROI used in the current study (Goldin, Ramel, & Gross, 2009). The role of dmPFC as a predictor of subsequent vulnerability to psychopathology also aligns with Davey et al. (2008)’s model suggesting that development of the PFC itself may pose risk for subsequent depression.
We examined the temporal effects of brain and behavioral risk markers implicated in the pathway to depression in adolescent girls. This approach was used to help identify inflection points in development that would increase understanding about whether contributions from the brain or from behavior precede one or the other in the development of depression over time. As in childhood, brain development in adolescence supports protracted maturation of specific stage-dependent functions, such as the role of the PFC in cognitive control (Nelson, Jarcho, & Guyer, 2016). The protracted nature of adolescent brain development, particularly in the PFC, may elongate the window of time for potential vulnerabilities to impact behavior. The current study provides evidence for neurodevelopmental specificity whereby the dmPFC, but not vmPFC, contributes to changes in suppression of emotional expression, but not in cognitive reappraisal, and depression severity across 16 to 17 years of age, a period of heightened risk for girls to develop depression. This informs our understanding that, across this sensitive period, a region of the brain involved in domain general cognitive control is contributing to the development of the depression and emotion dysregulation phenotype.
Although the present study’s results showed general consistency with findings from the adult depression and emotion regulation literature, some study limitations warrant attention. One limitation is sample attrition. Recruitment of the full sample to complete the first neuroimaging assessment was hampered (e.g., MRI contraindications, loss of contact) and then followed by rigorous inclusion criteria (e.g., high quality MRI data at two time points). Thus, the final sample established for longitudinal analyses may have reduced statistical power and limits generalizability of the results. Nonetheless, analysis of two time points of high quality fMRI data is still relatively unique in the field and enabled tests of new hypotheses about within-person change in brain function and behavior. Second, associations between T6 and T7 dmPFC and vmPFC activity were relatively weak suggesting low stability over time in BOLD estimates elicited during the fMRI task. Thus, test-retest reliability of the faces task requires further determination especially for use in longitudinal analyses, as conducted for the first time in the current study. It is possible that activation in these two regions reflects current state and corresponds less to individual trait characteristics. Further, although habituation to task stimuli cannot be ruled out, the task was designed to mitigate this possibility through an event-related design, inclusion of a jittered inter-stimulus interval, and randomization of actor and emotion type pairings at each assessment. Third, although half of full PGS-E sample was originally recruited for elevated depression symptoms at age eight, rates of clinically diagnosed MDD in the current sample were relatively low. At age 16, 4 of 78 girls (5%) scored in the clinical range for depression (MDD n=2, minor depression n=2); at age 17, this held for 6 girls (8%; MDD n=1, minor depression n=5). Mean self-reported depressive symptoms also decreased significantly over time. Thus, although the two mPFC ROIs and depression severity were associated, the present results may not transfer to clinical populations or relate to MDD onset. Finally, because we used only two time points, lagged relations among variables may arise from a trend, within individual fluctuation around a trend, or both. At least three waves of data are needed to separate these potential sources of influence (Hamaker, Kuiper, & Grasman, 2015). Future studies should include more than two time points to understand better the relations observed here that refer to trends or fluctuations. Corrections for multiple comparisons are not commonly implemented within cross-lagged panel models, nor structural equation models more generally. Hence, the analyses in the current paper did not include an extra correction for Type I errors (as is common for other analyses; e.g. ANOVA). Future research should consider a similar set of models with a larger sample size to confirm (i.e., replicate) the results of the current study.
Results from the current study also highlight avenues for future research. First, although we selected these ROIs for their functional roles based on existing literature, each ROI unlikely constitutes a single unitary functional or structural region. Future work should more precisely quantify subregions within the prefrontal cortical midline and examine functional connectivity between mPFC ROIs and subcortical structures including the amygdala and insula to better understand the interplay between bottom-up and top-down regions. Second, future studies should examine whether neural probes of explicit emotion regulation using different fMRI tasks generate similar findings to speak to these processes when the brain is actively engaged in regulating emotions versus self-referential processing of emotion. Finally, other influences such as environmental stressors likely contribute to the complex associations between brain activity, depression, and emotion regulation; thus, future studies should account for contributions from a range of several different systems such as endocrine, parasympathetic and social systems that are highly relevant to the development of depression in adolescence.
Taken together, the current study showed that activity in dmPFC while making a self-referential judgement to sad faces at age 16 predicted increased self-reported depression severity and expressive suppression at age 17. The finding was replicated when examining parent-reported depression separately. The results highlight the likely role of the dmPFC in top-down control mechanisms, which increase vulnerability to psychopathology over time if control is low. DmPFC activity to social-affective cues may be an early precursor to vulnerability for psychopathology.
Supplementary Material
Acknowledgments
We are grateful to all the families who took part in this study, and to the Pittsburgh Girls Study team, which includes interviewers and their supervisors, data managers, student workers and volunteers. We would like to acknowledge Harvey Iwamoto and Deepak Yadav for their technical support and programming of the fMRI task. This research was funded by National Institutes of Health grants R01-MH093605 (Keenan, Guyer, Forbes), R01-MH066167 (Keenan), and R01-MH056630 (Loeber).
Footnotes
Financial Disclosures:
The authors report no financial interests or potential conflicts of interest.
References
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews: Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Beck AT. The Evolution of the Cognitive Model of Depression and Its Neurobiological Correlates. American Journal of Psychiatry. 2008;165:969–977. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Colcombe S, Henderson SE, Alonso CM, Milham MP, Gabbay V. Neural correlates of self-perceptions in adolescents with major depressive disorder. Developmental Cognitive Neuroscience. 2016;19:87–97. doi: 10.1016/j.dcn.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cerebral Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH, Brown TA, Hofmann SG. Acceptability and suppression of negative emotion in anxiety and mood disorders. Emotion. 2006a;6:587–595. doi: 10.1037/1528-3542.6.4.587. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH, Brown TA, Hofmann SG. Effects of suppression and acceptance on emotional responses of individuals with anxiety and mood disorders. Behaviour Research and Therapy. 2006b;44:1251–1263. doi: 10.1016/j.brat.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, Forbes EE. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nature Reviews: Neuroscience. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Garber J, Braafladt N, Weiss B. Affect regulation in depressed and nondepressed children and young adolescents. Development and Psychopathology. 1995;7:93–115. doi: 10.1017/S0954579400006362. [DOI] [Google Scholar]
- Gadow KD, Sprafkin J. Youth’s Inventory-4 manual. Stony Brook, NY: Checkmate Plus; 1999. [Google Scholar]
- Garnefski N, Legerstee J, Kraaij V, Van Den Kommer T, Teerds JAN. Cognitive coping strategies and symptoms of depression and anxiety: a comparison between adolescents and adults. Journal of Adolescence. 2002;25:603–611. doi: 10.1006/jado.2002.0507. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ramel W, Gross JJ. Mindfulness Meditation Training and Self-Referential Processing in Social Anxiety Disorder: Behavioral and Neural Effects. Journal of Cognitive Psychotherapy. 2009;23:242–257. doi: 10.1891/0889-8391.23.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, … Etkin A. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gullone E, Hughes EK, King NJ, Tonge B. The normative development of emotion regulation strategy use in children and adolescents: a 2-year follow-up study. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2010;51:567–574. doi: 10.1111/j.1469-7610.2009.02183.x. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Grimm KJ, Pine DS, Keenan K. Emerging depression is associated with face memory deficits in adolescent girls. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:180–190. doi: 10.1016/j.jaac.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, … Ernst M. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Gibb BE, Abela JR, Flory K. Selective attention to affective stimuli and clinical depression among youths: role of anxiety and specificity of emotion. Journal of Abnormal Psychology. 2010;119:491–501. doi: 10.1037/a0019609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Morey RA, Petty CM, Seth S, Smoski MJ, McCarthy G, Labar KS. Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Frontiers in Human Neuroscience. 2010;4:230. doi: 10.3389/fnhum.2010.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SE, Vallejo AI, Ely BA, Kang G, Roy AK, Pine DS, … Gabbay V. The neural correlates of emotional face-processing in adolescent depression: a dimensional approach focusing on anhedonia and illness severity. Psychiatry Research. 2014;224:234–241. doi: 10.1016/j.pscychresns.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell AE, Sapotichne B, Klostermann S, Battista D, Keenan K. Autobiographical memory as a predictor of depression vulnerability in girls. Journal of Clinical Child and Adolescent Psychology. 2011;40:254–265. doi: 10.1080/15374416.2011.546037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EK, Gullone E, Watson SD. Emotional Functioning in Children and Adolescents with Elevated Depressive Symptoms. Journal of Psychopathology and Behavioral Assessment. 2011;33:335–345. doi: 10.1007/s10862-011-9220-2. [DOI] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review. 2008;115:291–313. doi: 10.1037/0033-295x.115.2.291. [DOI] [PubMed] [Google Scholar]
- Immordino-Yang MH. Me, My “Self” and You: Neuropsychological Relations between Social Emotion, Self-Awareness, and Morality. Emotion Review. 2011;3:313–315. doi: 10.1177/1754073911402391. [DOI] [Google Scholar]
- Joormann J. Cognitive Inhibition and Emotion Regulation in Depression. Current Directions in Psychological Science. 2010;19:161–166. doi: 10.1177/0963721410370293. [DOI] [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: relation to cognitive inhibition. Cogn Emot. 2010;24:281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Chung T, Stepp S, Stouthamer-Loeber M, Loeber R, McTigue K. The Pittsburgh Girls Study: overview and initial findings. Journal of Clinical Child and Adolescent Psychology. 2010;39:506–521. doi: 10.1080/15374416.2010.486320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilford EJ, Garrett E, Blakemore SJ. The Development of Social Cognition in Adolescence: An Integrated Perspective. Neuroscience & Biobehavioral Reviews. 2016;70:106–120. doi: 10.1016/j.neubiorev.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Ventromedial prefrontal activity correlates with depressed mood in adolescent children. Neuroreport. 2006;17:167–171. doi: 10.1097/01.wnr.0000198951.30939.73. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Joormann J, Gotlib IH. Emotion (Dys)regulation and Links to Depressive Disorders. Child Development Perspectives. 2008;2:149–155. doi: 10.1111/j.1750-8606.2008.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JK, Vermulst AA, Geenen R, van Middendorp H, English T, Gross JJ, … Engels RCME. Emotion Regulation in Adolescence: A Prospective Study of Expressive Suppression and Depressive Symptoms. The Journal of Early Adolescence. 2012;33:184–200. doi: 10.1177/0272431611432712. [DOI] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. Journal of Affective Disorders. 2012;136:e1–e11. doi: 10.1016/j.jad.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Gorwood P, Bergouignan L, Pelissolo A, Lehericy S, Fossati P. Negative affectivity, self-referential processing and the cortical midline structures. Social Cognitive and Affective Neuroscience. 2011;6:426–433. doi: 10.1093/scan/nsq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. Social neuroscience of child and adolescent depression. Brain and Cognition. 2007;65:47–68. doi: 10.1016/j.bandc.2006.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Frontiers in Human Neuroscience. 2013;7:666. doi: 10.3389/fnhum.2013.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Jarcho JM, Guyer AE. Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience. 2016;17:118–127. doi: 10.1016/j.dcn.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Beevers CG, Stice E, O’Neil K. Major and Minor Depression in Female Adolescents: Onset, Course, Symptom Presentation, and Demographic Associations. Journal of Clinical Psychology. 2009;65:1339–1349. doi: 10.1002/jclp.20629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romens SE, Casement MD, McAloon R, Keenan K, Hipwell AE, Guyer AE, Forbes EE. Adolescent girls’ neural response to reward mediates the relation between childhood financial disadvantage and depression. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2015;56:1177–1184. doi: 10.1111/jcpp.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. lavaan: An R package for structural equation modeling. Journal of Statistical Software. 2012;48:1–36. [Google Scholar]
- Selig TD, Little JP. Autoregressive and cross-lagged panel analysis for longitudinal data. In: Laursen B, Little TD, Card NA, editors. Handbook of developmental research methods. New York: Guilford Press; 2012. p. 265. [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, … Raichle ME. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: links to depressive symptoms and problem behavior. Child Development. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Shu J, Hubbard AD, Weber J, Ochsner KN. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Developmental Science. 2015;18:771–784. doi: 10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Wager TD, Weber J, Ochsner KN. The neural bases of uninstructed negative emotion modulation. Social Cognitive and Affective Neuroscience. 2015;10:10–18. doi: 10.1093/scan/nsu016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biology of Mood & Anxiety Disorders. 2011;1:10–10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan TN, Helms SW, Kliewer W, Goodman KL. Associations between Sadness and Anger Regulation Coping, Emotional Expression, and Physical and Relational Aggression among Urban Adolescents. Social Development. 2010;19:30–51. doi: 10.1111/j.1467-9507.2008.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhasselt MA, Baeken C, Van Schuerbeek P, Luypaert R, De Raedt R. Inter-individual differences in the habitual use of cognitive reappraisal and expressive suppression are associated with variations in prefrontal cognitive control for emotional information: an event related fMRI study. Biological Psychology. 2013;92:433–439. doi: 10.1016/j.biopsycho.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Vilgis V, Silk TJ, Vance A. Executive function and attention in children and adolescents with depressive disorders: a systematic review. European Child and Adolescent Psychiatry. 2015;24:365–384. doi: 10.1007/s00787-015-0675-7. [DOI] [PubMed] [Google Scholar]
- Walter M, Matthia C, Wiebking C, Rotte M, Tempelmann C, Bogerts B, … Northoff G. Preceding attention and the dorsomedial prefrontal cortex: process specificity versus domain dependence. Human Brain Mapping. 2009;30:312–326. doi: 10.1002/hbm.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TL, Miles E, Sheeran P. Dealing with feeling: a meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin. 2012;138:775–808. doi: 10.1037/a0027600. [DOI] [PubMed] [Google Scholar]
- Yap MB, Allen NB, Sheeber L. Using an emotion regulation framework to understand the role of temperament and family processes in risk for adolescent depressive disorders. Clinical Child and Family Psychology Review. 2007;10:180–196. doi: 10.1007/s10567-006-0014-0. [DOI] [PubMed] [Google Scholar]
- Zeman J, Shipman K. Social-contextual influences on expectancies for managing anger and sadness: the transition from middle childhood to adolescence. Developmental Psychology. 1997;33:917–924. doi: 10.1037//0012-1649.33.6.917. doi: http://dx.doi.org/10.1037/0012-1649.33.6.917. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Iwanski A. Emotion regulation from early adolescence to emerging adulthood and middle adulthood. International Journal of Behavioral Development. 2014;38:182–194. doi: 10.1177/0165025413515405. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.