Abstract
The development of cognitive control during adolescence is paralleled by changes in the function of the lateral prefrontal cortex (PFC). Using a 3-wave longitudinal neuroimaging design (N = 22, mean age = 13.08 years at Wave 1), this study examined if youth’s stereotypes about teens modulate changes in their neural activation during cognitive control. Participants holding stereotypes of teens as irresponsible in the family context (i.e., ignoring family obligations) in middle school showed increases in bilateral ventrolateral PFC activation during cognitive control over the transition to high school, which was associated with increases in risk taking. These findings provide preliminary evidence that youth’s conceptions of adolescence play a role in neural plasticity over this phase of development.
Keywords: adolescence, neural development, prefrontal cortex, risk taking
Youth’s cognitive control is relatively flexible during adolescence in that it is sensitive to the social and motivational context (Crone & Dahl, 2012). Although this flexibility may heighten impulsive and risky behavior, it also provides an opportunity for adaptive adjustment, including learning and regulatory behavior. Thus, elucidating the development of divergent trajectories of cognitive control during adolescence is an important endeavor in understanding how to support adaptive adjustment among youth. Given that adolescence is a time of dramatic brain development, there has been keen interest in the neural changes that are involved in cognitive control over this phase (e.g., Andrews-Hanna et al., 2011; Crone & Dahl, 2012; Veroude, Jolles, Croiset, & Krabbendam, 2013). Notably, emerging evidence suggests that there is flexibility in the function of the prefrontal cortex (PFC) during adolescence, a brain region supporting cognitive control, which may underlie flexibility in youth’s behavior (Nelson & Guyer, 2011).
Neural regions in the PFC, which are involved in cognitive control, continue to develop throughout adolescence (Miller & Cohen, 2001; Luna, Padmanabhan, & O’Hearn, 2010; Sturman & Moghaddam, 2011). This prolonged maturation provides an extended window for social and motivational contexts to influence the development of the PFC (Nelson & Guyer, 2011). Although some neuroimaging studies on the development of cognitive control reveal age-related increases in PFC activity from childhood to adulthood, others reveal age-related decreases (e.g., Booth et al., 2003; Bunge et al., 2002; Crone & Dahl, 2012; Durston et al., 2006; Marsh et al., 2006; Rubia et al., 2007; Velanova et al., 2009). Resolving these seemingly discrepant findings, Crone and Dahl (2012) suggest that such mixed findings reflect the flexibility of the cognitive control system, in that the system responds to youth’s social and motivational context during adolescence. For example, the ventrolateral prefrontal cortex (VLPFC) is sensitive to youth’s social context and characteristics, influencing the development of valuation, inhibition, and rule use (Nelson & Guyer, 2011).
A growing number of neuroimaging studies underscore that youth’s social context and characteristics modulate the functional development of the PFC during adolescence (e.g., Guyer et al., 2015; Kerestes, Davey, Stephanou, Whittle, & Harrison, 2014; Qu, Fuligni, Galván, & Telzer, 2016; Telzer, Fuligni, Lieberman, & Galván, 2013). The goal of the current research was to further elucidate the development of divergent trajectories in adolescent neurodevelopment supporting cognitive control, specifically the PFC. To this end, we focused on understanding the modulating role of youth’s conceptions of adolescence. Youth hold views of teens that are distinct from their views of younger children: In contrast to elementary school children, teens are seen as more irresponsible in that they, for example, are rebellious (e.g., testing limits) and disregard family obligations (e.g., Buchanan & Holmbeck, 1998; Qu, Pomerantz, Cheung, Cimpian, & Wang, 2016). Although such stereotypes may be based on accurate base rate information to some extent, they also may be based on exaggerated media portrayals of teens as well as extreme, but memorable, instances of teen behavior (Gilliam & Bales, 2001; Nichols & Good, 2004). It is thus not surprising that many youth tend to see adolescence in a negative light (e.g., Galván, Spatzier, & Juvonen, 2011; Hines & Paulson, 2006), despite only mild storm and stress during this phase (e.g., Arnett, 1999; Steinberg, 2001).
Although negative conceptions of adolescence may be inaccurate, they often act as self-fulfilling prophecies in leading the youth who hold them to see irresponsible behavior as normative during this phase (Buchanan & Hughes, 2009). Stereotypes about teens may shape the expectations and standards youth hold for themselves, which ultimately guide their behavior (Buchanan & Hughes, 2009; Meece, Wigfield, & Eccles, 1990). For example, if youth see it as normative to be irresponsible—by disregarding their family obligations—during adolescence, they may come to hold expectations and standards for themselves that set the stage for irresponsible behavior as they navigate adolescence (e.g., Buchanan & Hughes, 2009; Madon, Guyll, Spoth, Cross, & Hilbert, 2003). Indeed, the more youth see teens as ignoring family obligations (e.g., they are less respectful of their parents), the less they maintain their engagement in school and the more they are involved in risk taking during early adolescence, over and above their earlier school engagement and risk taking, as well as other potential confounds (Qu, Pomerantz, et al., 2016; Qu, Pomerantz, Wang, & Ng, 2015).
Given the importance of cognitive control in inhibiting the heightened reward seeking that can increase risk taking during adolescence (e.g., Duell et al., 2016; Steinberg et al., 2008), a key question is whether youth’s conceptions of adolescence undermine the neural development of cognitive control. Youth who see the teen years as a time of irresponsibility may not exert the cognitive control involved in acting responsibly—for example, disregarding family obligations may mean that they do not refrain from risky behavior that may be rewarding but violates parents’ expectations. Youth’s infrequent exertion of cognitive control may lead to increases in PFC activation in the context of such control over time, as they need to recruit more PFC activation to regulate their impulsive behavior. Such altered neural development of cognitive control may make subsequent responsible behavior (e.g., risk taking) difficult. In essence, youth’s conceptions of adolescence may set off a series of neuro-behavior transactions. Two sets of finding are suggestive of these ideas. First, social contexts (e.g., parental depression and family conflict) that may foster irresponsible behavior are associated with increases in PFC activation over time (McCormick, Qu, & Telzer, 2016; Qu, Fuligni, et al., 2016). Second, longitudinal changes in PFC activation and risk taking co-occur over adolescence as youth who show longitudinal increases in PFC activation also exhibit longitudinal increases in risk taking (McCormick et al., 2016; Qu, Fuligni, et al., 2016; Qu, Galván, et al., 2015).
The Current Study
The goal of this research was to take a first step in examining the role of youth’s conceptions of adolescence in the neurodevelopment of their cognitive control that accompanies changes in their risk taking over adolescence. To this end, we used a three-wave longitudinal neuroimaging design, which allowed us to examine the link between youth’s conceptions and their neural trajectories of cognitive control and risk taking. Youth reported on their views of teens as ignoring family obligations at the first time point (T1), which took place in early adolescence (i.e., 7th grade) when youth may be particularly sensitive to information about teens given that they are taking on a new role about which they may be uncertain (Ruble, 1994). To examine changes over time in neural activation in the context of cognitive control, youth were scanned one year later (T2) in 8th grade as they completed a cognitive control task (i.e., the Go/Nogo task) and then again one year later (T3) in their first year of high school (i.e., 9th grade). At both of these latter time points, youth also reported on their risk taking.
The current study provides a preliminary test of three interrelated hypotheses. First, we investigated whether youth’s conceptions of adolescence as a time of dampened family obligation during middle school predicts changes in their risk taking as they move from middle to high school. Replicating prior research (Buchanan & Hughes, 2009; Qu, Pomerantz, et al., 2015), we anticipated that the more youth see the teen years as a time of irresponsibility in regards to the family, the greater the increase in their risk taking over the transition to high school. Second, and most centrally, we evaluated if a parallel trend exists for changes in neural activation in the PFC during cognitive control. Youth who hold stereotypes of teens as ignoring family obligations were hypothesized to show increases in PFC activation over the transition to high school. Third, increased PFC activation was expected to be associated with increased risk taking over the transition to high school.
Method
Participants
Participants were 23 (13 boys) youth. They completed self-report and observational measures in the spring of 7th grade (mean age = 13.08 years at T1) and underwent a functional MRI scan in the spring of 8th grade (T2; mean age = 14.39 years) and then again in the spring of 9th grade (T3; mean age = 15.20 years). One youth who showed excessive inter-slice head movement (> 2.0 mm) was excluded, yielding a final sample of 22 youth. Participants were primarily (64%) European American, with 22% being African American, and 14% other ethnicities (e.g., Asian American). A majority (62%) of mothers reported a college degree or higher.
Survey Measures
Conceptions of adolescence
At T1, participants reported on their conceptions of adolescence as a time of ignoring family obligations (Qu et al., 2016). Participants rated to what extent six behaviors or attitudes reflecting dampened family obligation (e.g., “care little about fulfilling family obligations” and “work hard to meet parents’ expectations” [reverse-scored], α = .80) are true during the teen years versus before the teen years (1 = more true before teen years, 5 = equally true before and during teen years, 9 = more true during teen years). The items were modified from Fuligni’s (1999) and Ng, Loong, Liu, and Weatherall’s (2000) scales of family obligation. The mean of the six items was taken, with lower numbers indicating that ignoring family obligations is viewed as more common before the teen years and higher numbers indicating that it is viewed as more common during the teen years.
Risk-taking behavior
At T2 and T3, the externalizing subscale from the Brief Problem Monitor Scale (Achenbach & Rescorla, 2001) was used to assess participants’ risk taking. Participants reported to what extent (1= not all true, 5 = very true) they engage in a variety of risky behaviors (e.g., “I stole things.” and “I hung around with peers who got in trouble.”; αs = .92). The mean of the 13 items was taken, with higher numbers indicating more risk taking. To examine changes over time, difference scores between T2 and T3 were calculated (i.e., T2 scores were subtracted from T3 scores), with more positive scores indicating greater increases in risk taking. Two participants did not provide self-report risk taking at T3 and were excluded from the analyses with risk taking.
Control measures
To ensure the unique role of conceptions of adolescence, data on potential confounds were also collected. First, because youth who view adolescence as a time of dampened family obligation may have poorer relationships with their parents, mother-child relationship quality was assessed. At T1, mothers and participants took part in a 15-min. video-recorded session in which participants were given a challenging set of cognitive problems to solve. The quality of the relationship between mothers and participants over the course of the interaction was coded (1 = negative, 5 = positive) by three coders (ICCs = .68 to .91, with an average of .83) using a coding system adapted from the Iowa Family Interaction Rating Scales (IFIRS; Melby et al., 1998). Visibly unhappy, conflicted, and brittle interactions were reflective of negative relationships and visibly satisfying, communicative, and warm interactions were reflective of positive relationships.
Second, participants reported on their pubertal development as puberty is linked to conceptions of adolescence as a time of ignoring family obligations (Qu et al., 2016) and risk taking (Icenogle et al., in press). At T1, participants completed the Pubertal Development Scale (PDS; Petersen, Crockett, Richards, & Boxer, 1988). The scale is comprised of five items (1 = no development, 4 = development is complete). Both boys and girls reported on growth spurt, hair growth, and skin changes; boys also reported on voice change and facial hair and girls on breast development and menarche status (1 = no, 4 = yes). The mean was taken with higher numbers indicating more advanced pubertal development (α = .79).
fMRI Task
At T2 and T3, participants completed a Go/NoGo task during an fMRI scan. The Go/Nogo task has been widely used in fMRI studies to measure neural reactivity underlying cognitive control; the PFC is reliably recruited in the task (e.g., Liddle, Kiehl, & Smith, 2001; Menon, Adleman, White, Glover, & Reiss, 2001). Participants were presented with brief (500 ms) trials in which they saw a single letter. They were instructed to press a button to all letters (go trials) with the exception of X (no-go trials). Xs were presented on 25% of the trials. Thus, participants developed a pre-potent response to press during go trials but had to inhibit during no-go trials. Each trial was separated by a fixation period that was jittered with a gamma distribution (M = 1000 ms). Participants completed the task four times across four separate blocks. Each block of the task consisted of 80-trials, comprising 20 nogo and 60 go trials. Each block was separated by a 60s rest period. Following previous studies using the Go/Nogo task (Liddle et al., 2001; Menon et al., 2001), behavioral performance on the task was measured via false alarm rate, an index of how often participants pressed the button on no-go trials, with higher scores indicating poorer behavioral inhibition.
fMRI Data Acquisition
Imaging data were collected using a 3 Tesla Siemens Trio MRI scanner. The Go/NoGo task included T2*-weighted echoplanar images (EPI) [slice thickness = 3mm; 38 slices; TR = 2s; matrix = 92×92; FOV = 230 mm; voxel size 2.5×2.5×3mm3]. Structural scans consisted of a T2 weighted, matched-bandwidth (MBW), high-resolution, anatomical scan (TR = 4s; TE = 64ms; FOV = 230; matrix = 192×192; slice thickness = 3mm; 38 slices) and a T1* magnetization-prepared rapid acquisition gradient echo (MPRAGE; TR = 1.9sec; TE = 2.3ms; FOV = 230; matrix = 256×256; sagittal plane; slice thickness = 1mm; 192 slices). The orientation for the MBW and EPI scans was oblique axial in order to maximize brain coverage.
fMRI Data Preprocessing and Analysis
Data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) software package. Preprocessing was conducted separately for the T2 and T3 scans, using the exact same parameters. Preprocessing included spatial realignment to correct for head motion, and coregistration with the high-resolution T1* MPRAGE structural scan, which was subsequently segmented into grey matter, white matter, and cerebrospinal fluid. The transformation matrix used to normalize the MPRAGE images was applied to the MBW and functional images to transform them into the standard stereotactic space defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping. Normalized functional images were smoothed using an 8mm Gaussian kernel, full-width-at-half maximum, to increase the signal-to-noise-ratio. The general linear model in SPM8 was used to perform statistical analyses, convolving each trial with a canonical hemodynamic response function. High-pass temporal filtering (cutoff 128s) was applied to remove low-frequency drift across the time series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm using an autoregressive model order of 1.
In each participant’s fixed-effects model, a general linear model (GLM) was created for each regressor of interest to separate the different events, including successful go trials, successful no-go trials, false alarms (i.e., pressing on no-go trials), and misses (i.e., not pressing on go trials). These regressors were modeled separately for T2 and T3. Null events consisted of the jittered inter-trial fixation periods plus the one minute rest period between blocks and were not explicitly modeled therefore constituting the implicit baseline. To examine longitudinal changes in neural reactivity, we used a difference score approach, and contrasts between T2 and T3 were computed at the individual level (i.e., Nogo T3 – Nogo T2).
Random effects, group-level analyses were performed on all individual subject contrasts using GLMFlex. GLMFlex corrects for variance-covariance inequality, partitions error terms, removes outliers and sudden activation changes in the brain, and analyzes all voxels containing data (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex). Given that the primary goal of the present study was to examine neural activation supporting effective cognitive control, the group-level analyses focused on trials where youth successfully inhibited their responses (no-go). To examine how youth’s conceptions of adolescence (i.e., seeing the teen years as a time of ignoring family obligation) are associated with changes in neural activation, whole-brain regression analyses were conducted by entering conceptions as a regressor on the contrast Nogo T3 > Nogo T2.
Correction for multiple comparisons was conducted using a Monte Carlo simulation through the updated 3dClustSim from the AFNI software package (Ward, 2000) using the group-level brain mask. The updated 3dClustSim uses the ACF (auto-correlation function) method that addresses the false positive issues raised by Eklund et al. (2016). The simulation resulted in a voxel-wise threshold of p < .005 and a minimum cluster size of 67 voxels for the whole brain, corresponding to p < .05 corrected. To plot significant effects, parameter estimates of signal intensity were extracted from the clusters using the MarsBar toolbox in SPM. These plots are not independent analyses and are presented for illustration purposes. For visualization, statistical maps of all analyses were projected onto a T2 template.
Results
Preliminary Analyses
Preliminary analyses using a dependent t-test indicated no significant group-level change in participants’ behavioral performance on the cognitive control task from T2 (M = 8.51%, SD = .04) to T3 (M = 8.47%, SD = .05), t(19) = .06, p > .95, and risk taking from T2 (M = 1.50, SD = .64) to T3 (M = 1.57, SD = .63), t(19) = -.70, p > .49. Moreover, participants’ behavioral performance (i.e., false alarm rate) on the cognitive control task and risk taking were relatively stable from T2 to T3 (ICC = .66 for cognitive control and .78 for risk taking). Conceptions of adolescence were not associated with changes in behavioral performance on the Go/Nogo task from T2 to T3, r = .34, p > .14 (For correlations between all the variables, see Table 1).
Table 1.
Correlations Between the Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Family obligation conceptions T1 | – | – | – | – | – | – | – | – | – |
| 2. | Mother-child relationship quality T1 | .12 | – | – | – | – | – | – | – | – |
| 3. | Participants’ pubertal status T1 | .05 | .23 | – | – | – | – | – | – | – |
| 4. | Risk taking T2 | −.20 | .10 | .59** | – | – | – | – | – | – |
| 5. | Risk taking T3 | −.04 | .08 | .50* | .78*** | – | – | – | – | – |
| 6. | False alarms T2 | −.08 | −.22 | .08 | .12 | −.06 | – | – | – | – |
| 7. | False alarms T3 | .19 | −.03 | .01 | .09 | −.31 | .68** | – | – | – |
| 8. | Maternal education | −.07 | .08 | .20 | .09 | .03 | .29 | .11 | – | – |
| 9. | Participants’ gender | .12 | .21 | .27 | .32 | .33 | .06 | −.16 | −.19 | – |
|
| ||||||||||
| Mean | 4.12 | 3.22 | 2.48 | 1.51 | 1.55 | .09 | .08 | 1.61 | – | |
| SD | 1.44 | .55 | .65 | .64 | .60 | .04 | .05 | .50 | – | |
| Range | 1.50–6.83 | 1.50–4.00 | 1.20–3.60 | 1.00–3.38 | 1.00–3.15 | .01–.14 | .00–.17 | 1.00–2.00 | – | |
Note. The family obligation conceptions measure uses a 9-point scale with 1 = more true before teen years, 4 = equally true before and during teen years, 9 = more true during teen years. For mothers’ education, −1 = less than a college degree and 1 = college degree or higher; for youth’s gender, −1 = male and 1 = female.
p < .05.
p < .01.
p < .001.
Do Conceptions of Adolescence Predict Changes in Risk Taking?
Neural activation during cognitive control (i.e., Nogo trials) at each time point (T2 and T3, respectively) are presented in Table 2. No brain regions showed longitudinal changes from T2 to T3. Our key analysis was to examine whether participants’ conceptions of adolescence in regards to family obligation during middle school (i.e., 7th grade) predict changes in their risk taking over the transition from middle (i.e., 8th grade) to high (i.e., 9th grade) school. Consistent with prior research, the more participants saw the teen years as a time of ignoring family obligations, the more their risk taking increased over the transition from middle to high school (see Figure 1), r = .64, p < .01. This association remained significant after controlling for risk taking at T2, pr = .62, p < .01, indicating that participants’ views of teens as irresponsible in the family context are associated with changes in their risk taking, above and beyond their risk taking at T2. Moreover, the association remained significant when analyses controlled for the quality of relationships between mothers and participants, participants’ pubertal status, participants’ gender, and mothers’ educational attainment, pr = .68, p < .01.
Table 2.
Brain Activation During Nogo Trials at T2 and T3
| Anatomical Region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Time 2 | ||||||
| Right VLPFC | 10 | 36 | 38 | −2 | 3.90 | 295 |
| ACC | 24/32 | 6 | 20 | 31 | 7.72 | 282 |
| Left insula | 13 | −36 | 2 | 7 | 5.60 | 187 |
| Right insula | 13 | 33 | 17 | −8 | 6.15 | 254 |
| Superior temporal gyrus | 60 | −40 | 13 | 6.23 | 282 | |
| Time 3 | ||||||
| Right VLPFC | 10 | 33 | 65 | −5 | 3.55 | 281 |
| Superior frontal gyrus | 0 | 2 | 52 | 4.59 | 103 | |
| Left insula | 13/22 | −45 | 8 | −5 | 4.32 | 125 |
| Right insula | 13/38 | 48 | 11 | −8 | 4.55 | 131 |
| Superior temporal gyrus | 63 | −37 | 19 | 3.63 | 120 | |
| Middle occipital gyrus | −45 | −76 | 1 | 4.20 | 160 |
Note. BA refers to putative Broadman’s areas. x, y, and z refer to MNI coordinates; t refers to the t-score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. VLPFC = ventrolateral prefrontal cortex. ACC = anterior cingulate cortex.
Figure 1.
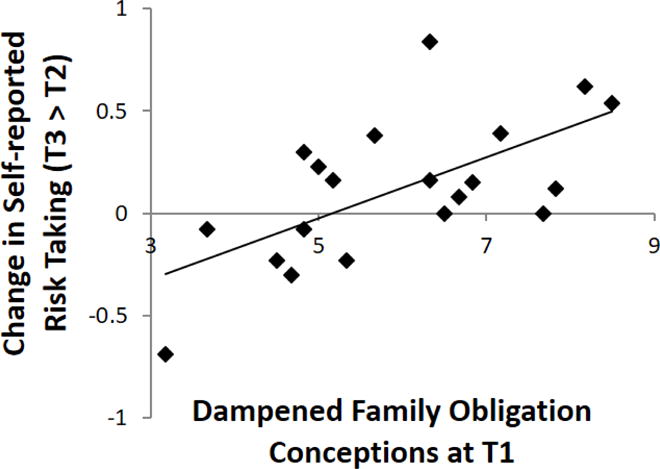
The more youth see teens as ignoring family obligation (T1), the more their risk taking increase over time (T2 to T3).
Do Conceptions of Adolescence Predict Changes in Neural Reactivity During Cognitive Control?
We next examined if participants’ conceptions of adolescence predict changes in their neural responses during cognitive control. Whole brain regression analyses were conducted with conceptions at T1 regressed onto changes in neural activation during successful Nogo trials (T3 – T2). As shown in Figure 2, the more participants viewed teens as ignoring family obligations, the more they showed an increase over time in bilateral ventrolateral prefrontal cortex (VLPFC) activation (left VLPFC: x = −36, y = 47, z = −5, t = 5.03, k = 72; right VLPFC: x = 36, y = 47, z = −2, t = 4.40, k = 99). No other neural regions showed associations with participants’ conceptions of adolescence.
Figure 2.
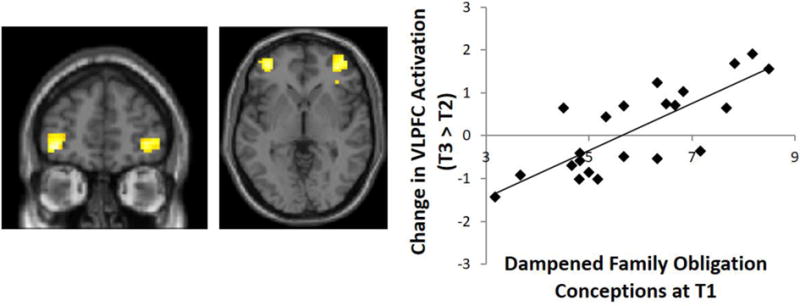
The more youth see teens as ignoring family obligation (T1), the more their bilateral VLPFC activation increases over time (T2 to T3). Parameter estimates of signal intensity were extracted and plotted for illustration purposes only and do not represent independent analyses.
To test whether this association holds after accounting for baseline VLPFC activation, we extracted parameter estimates of signal intensity from the same VLPFC region at T2. After controlling for T2 VLPFC activation, participants’ conceptions at T1 were still predictive of increases in VLPFC activation from T2 to T3. Moreover, the predictive effect of conceptions remained significant after controlling for mother-child relationship quality, participants’ pubertal status, participants’ gender, and mothers’ educational attainment (for additional analyses, see the online supplement).
Do Changes in Youth’s Neural Reactivity Predict Changes in Risk Taking?
To examine if changes in participants’ neural reactivity are associated with changes in their risk-taking behavior over the transition to high school, parameter estimates of signal intensity from the bilateral VLPFC clusters that showed significant changes as a function of family obligation conceptions were extracted. Participants showed substantial variation in the bilateral VLPFC changes from T2 to T3, ICC = .11. Correlation analyses using this functional ROI were conducted in SPSS. Consistent with prior research, participants who showed greater increases in VLPFC activation over time also showed greater increases in risk taking (see Figure 3), r = .54, p = .01. To eliminate the possibility that this association was driven by participants’ initial risk taking, we controlled for their risk-taking behavior at T2. The association between changes in VLPFC activation and changes in risk taking remained significant, pr = .55, p = .01. The other covariates also did not account for this association, pr = .56, p = .02.
Figure 3.
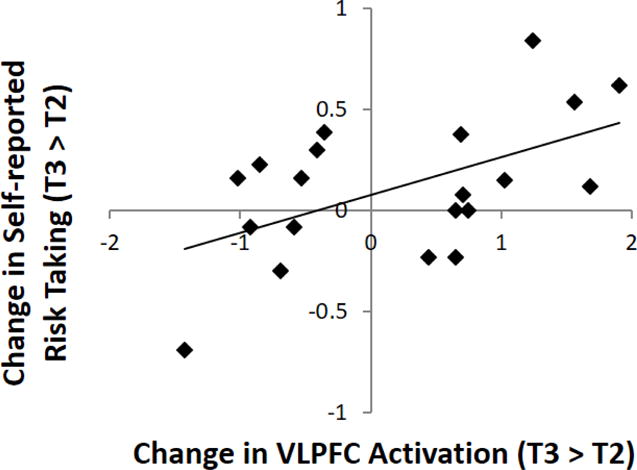
The greater the increase in the VLPFC over time (T2 to T3), the greater risk taking over time (T2 to T3).
Discussion
The current study adds to growing evidence that the functional development of the PFC is modulated by youth’s characteristics during adolescence. The more youth held conceptions of adolescence as a time of ignoring family obligations during middle school, the more their VLPFC activation during cognitive control increased over the transition to high school, which was related to increases in risk taking during this time. Notably, these effects of youth’s views of teens were evident above and beyond a variety of potential confounds such as the quality of youth’s relationships with mothers, youth’s pubertal maturation, youth’s gender, and mothers’ educational attainment, suggesting the unique role of conceptions in youth’s psychological adjustment. Taken together, the findings provide preliminary evidence that seeing the teen years as a time of ignoring family obligations may undermine the neural development involved in cognitive control, which accompanies increases in risk taking over adolescence.
The findings provide new insights into the neural development of the VLPFC during adolescence. The VLPFC is a relatively late developing neural region (Gogtay et al., 2004; Luna et al., 2010), which has been involved in behavioral inhibition and impulse control (Levy & Wagner, 2011; Wessel et al., 2013) and found in prior studies on adolescents (e.g., Batterink, Yokum, & Stice, 2010; Guyer et al., 2015). Importantly, the VLPFC appears to be responsive to youth’s social and motivational context (Crone & Dahl, 2012). For example, VLPFC activation is sensitive to the peer and parent environment as well as youth’s temperament (e.g., Guyer et al., 2015; Kerestes et al., 2014; Qu et al., 2016; Telzer et al., 2013). The current research adds to this perspective by suggesting that VLPFC activity may also be sensitive to youth’s conceptions of adolescence, such that holding stereotypes of teens as irresponsible in the family context is associated with increases in VLPFC activity during cognitive control over time, with both stereotypes and the increases in VLPFC activity being associated with increases in risk taking.
At first blush it may be surprising that increased VLPFC activation during cognitive control is associated both with conceptions of adolescence as a time of disregarding family obligations and risk taking, given some prior findings from cross-sectional studies identifying increases in such activity over adolescence (e.g., Bunge et al., 2002; Marsh et al., 2006), suggesting that increases may be adaptive. However, as Crone and Dahl (2012) highlight, neuroimaging studies on this issue yield inconsistent findings, such that although some show increases in PFC activation, others show decreases, and still others show curvilinear patterns from childhood to adulthood. However, recent research using a longitudinal approach, which allows for investigation of within-person changes in neural activation, suggests a decline in the VLPFC around mid-adolescence (Qu, Galván, et al., 2015). Along with evidence that longitudinal declines in PFC activation are associated with declines in risk taking (Qu, Galván, et al., 2015; Qu, Fuligni, et al., 2015), declines in PFC activation may reflect more mature neural development underlying cognitive control.
The idea that youth’s conceptions of adolescence shape their neural activity was based on Buchanan and Hughes (2009) argument that such conceptions act as self-fulfilling prophecies. Youth who view teens as irresponsible in the family context may see disregarding family obligations as normative among teens, which may shape the expectations and standards youth hold for themselves (Buchanan & Hughes, 2009; Meece, Wigfield, & Eccles, 1990). Thus, it is possible that youth become less likely to exert cognitive control to regulate their behavior (e.g., refraining from risk taking) so that they meet family obligations. Over time, this may alter youth’s neural processes, as they need to recruit more VLPFC activity to exert cognitive control. This may further undermine youth’s regulation of behavior. Therefore, conceptions of adolescence may set a foundation for risk taking and the neural processes involved in cognitive control to reinforce each other in a reciprocal process. Future research with additional longitudinal data points should examine the possibility that conceptions of adolescence set off reciprocal processes between risk taking—as well as other irresponsible behaviors—and VLPFC activity. In this context, attention should also be directed to why youth’s conceptions of adolescence were linked to risk taking and neural activity changes from middle school (i.e., 8th grade; T2) to high school (9th grade; T3), but not at either middle (T2) or high school (T3). Because conceptions of adolescence may set a foundation for the reciprocal processes between risk taking and neural activity, it may take time for conceptions of adolescence to exert influence.
Youth’s conceptions of adolescence as a time of irresponsibility predicted longitudinal changes in VLPFC activation on the Go/Nogo task, but not in behavioral performance on the task. Previous behavioral and fMRI studies have used the Go/Nogo task as a classic paradigm to examine the development of cognitive control from childhood to adulthood. In the Go/Nogo task, participants develop a pre-potent tendency to respond on go trials, but have to inhibit their responses during no-go trials. Behaviorally, a steep initial improvement in performance is observed from childhood to early adolescence (i.e., approximately 12 years), which then reaches adult-like performance and becomes stable (e.g., Bunge, Dudukovic, Thomason, Vaidya & Gabrieli, 2002; Casey et al., 1997; Rubia et al., 2000). However, behavioral similarity between adolescents and adults does not necessarily indicate similarity in neural function (Schlaggar, 2002), and the neural basis underlying cognitive control still develops over the course of adolescence. Indeed, a number of studies have shown that PFC activity in a cognitive control task continues to mature from late childhood through late adolescence even when task difficulty is controlled (Geier & Luna, 2009; Luna, et al., 2001; Rubia, et al., 2006; Rubia, Smith, Taylor, & Brammer, 2007). Therefore, as the PFC continues to mature, youth’s social context and individual characteristics may still play a role in the development of the neural processes underlying cognitive control, but the stability of behavioral performance in the Go/Nogo task after early adolescence may lead to no link between behavioral changes in the task and neural changes.
Limitations and Future Directions
The current study provides a preliminary examination of how conceptions of adolescence modulate neural development during adolescence. The findings should be taken with caution given several limitations, which can be addressed in future research. First, and perhaps most significantly, the small sample size warrants caution in interpreting the findings. Future research using larger samples is needed to examine the role of views about teens in youth’s neural development. However, the relation between conceptions of adolescence and changes over time in risk taking found in the current research is consistent with the results of survey studies using larger samples (e.g., Buchanan & Hughes, 2009; Qu, Pomerantz, et al., 2016). The fMRI findings linking longitudinal changes in VLPFC and longitudinal changes in risk taking are also consistent with prior research (e.g., Qu, Galván, et al., 2015, Qu, Fuligni, et al., 2015). Thus, the current findings are unlikely to simply be false positives.
Second, we examined the role of conceptions of adolescence in youth’s neural development underlying cognitive control, but did not investigate neural development underlying other processes (e.g., reward seeking). Other neural regions may also be influenced by how youth see the teen years. For example, recent evidence suggests that youth’s social environment (e.g., the presence of peers or parents) can modulate neural reactivity in reward-related regions (e.g., the ventral striatum), which are involved in sensation seeking and risk taking (e.g., Chein et al., 2011; Telzer, Ichien, & Qu, 2015). Moreover, it is possible that the longitudinal changes in the VLPFC activity that we found to be associated with conceptions of adolescence may be accompanied by compensatory responses in other neural regions (e.g., the ventral striatum). Future research is needed to identify if and how views about teens contribute to youth’s neural development of reward-related regions and their connectivity with the PFC using tasks involving reward seeking.
Third, although we took into account several potential confounds (e.g., mother-child relationship quality and youth’s pubertal status) and utilized a three-wave longitudinal design, causal conclusions cannot be made. By taking into account youth’s risk taking and VLPFC activation at T2, we ruled out the possibility that youth’s conceptions of adolescence predict changes in their neural and psychological adjustment because they reflect youth’s earlier adjustment. However, it will be useful to rule out other potential confounds. For example, it is possible that the stress youth experience, their family obligation values, their modeling of significant others’ (e.g., parents’, siblings’, or peers’) behavior, and parents’ conceptions of adolescence play a role in youth’s conceptions of adolescence, VLPFC activation, and risk taking such that they account for the relations among the three. In addition to taking into account such confounds in correlational research, it will be beneficial for future research to elucidate the causal role of conceptions of adolescence in youth’s neural development via experimental methods.
Conclusions
The current study provides novel, albeit preliminary, evidence that conceptions of adolescence may contribute to changes in youth’s neural development of cognitive control that accompany their risk taking during adolescence. Using a three-wave longitudinal neuroimaging approach, we found that youth’s views of teens as ignoring family obligations in middle school predict increases over the transition to high school in their bilateral ventrolateral PFC during cognitive control, which are accompanied by increases in their risk taking. These findings are in line with the view that adolescence is a time of neural plasticity, with the functional development of the PFC being sensitive to youth’s social and motivational context. They also point to the possibility that negative stereotypes about teens undermine youth’s neural and psychological development.
Supplementary Material
Acknowledgments
We greatly appreciate the assistance of the Biomedical Imaging Center at the University of Illinois. This research was supported by the National Science Foundation (BCS-1023170 to Pomerantz; SES-1459719 to Telzer), the National Institutes of Health (R01DA039923 to Telzer), and generous funds from the Department of Psychology at the University of Illinois.
Contributor Information
Yang Qu, Stanford University.
Eva M. Pomerantz, University of Illinois at Urbana-Champaign
Ethan McCormick, University of North Carolina at Chapel Hill.
Eva H. Telzer, University of North Carolina at Chapel Hill
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- Andrews-Hanna J, Seghete KLM, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: Neural underpinnings and relation to self-report behaviors. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ. Adolescent storm and stress, reconsidered. American Psychologist. 1999;54:317–326. doi: 10.1037/0003-066X.54.5.317. [DOI] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: An fMRI study. NeuroImage. 2010;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Mesulam MM. Neural development of selective attention and response inhibition. NeuroImage. 2003;20(2):737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Buchanan CM, Holmbeck GN. Measuring beliefs about adolescent personality and behavior. Journal of Youth and Adolescence. 1998;27:607–627. doi: 10.1023/A:1022835107795. [DOI] [Google Scholar]
- Buchanan CM, Hughes JL. Construction of social reality during early adolescence: Can expecting storm and stress increase real or perceived storm and stress? Journal of Research on Adolescence. 2009;19:261–285. doi: 10.1111/j.1532-7795.2009.00596.x. [DOI] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children. Neuron. 2002;33(2):301–311. doi: 10.1016/S0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chein J, Albert D, O’Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Duell N, Steinberg L, Chein J, Al-Hassan SM, Bacchini D, Chang L, Alampay LP. Interaction of reward seeking and self-regulation in the prediction of risk taking: A cross-national test of the dual systems model. Developmental Psychology. 2016;52:1593–1605. doi: 10.1037/dev0000152. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences USA. 2016;113(28):7900. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni AJ, Tseng V, Lam M. Attitudes toward family obligations among American adolescents with Asian, Latin American, and European backgrounds. Child Development. 1999;70:1030–1044. doi: 10.1111/1467-8624.00075. [DOI] [Google Scholar]
- Galván A, Spatzier A, Juvonen J. Perceived norms and social values to capture school culture in elementary and middle school. Journal of Applied Developmental Psychology. 2011;32:346–353. doi: 10.1016/j.appdev.2011.08.005. [DOI] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacology, Biochemistry and Behavior. 2009;93(3):212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam F, Bales S. Social Policy Report. Vol. 15. Ann Arbor, MI: Society for Research in Child Development; 2001. Strategic frame analysis: Reframing America’s youth; pp. 1–14. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Jarcho JM, Pérez-Edgar K, Degnan KA, Pine DS, Fox NA, Nelson EE. Temperament and parenting styles in early childhood differentially influence neural response to peer evaluation in adolescence. Journal of Abnormal Child Psychology. 2015;43(5):863–874. doi: 10.1007/s10802-015-9973-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines AR, Paulson SE. Parents’ and teachers’ perceptions of adolescent storm and stress: Relations with parenting and teaching styles. Adolescence. 2006;41:597–614. [PubMed] [Google Scholar]
- Icenogle G, Steinberg L, Olino TM, Shulman EP, Chein J, Alampay LP, Tirado LM. Puberty predicts approach but not avoidance on the Iowa gambling task in a multinational sample. Child Development. doi: 10.1111/cdev.12655. in press. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Davey CG, Stephanou K, Whittle S, Harrison BJ. Functional brain imaging studies of youth depression: A systematic review. NeuroImage: Clinical. 2014;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: Reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12(2):100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Madon S, Guyll M, Spoth RL, Cross SE, Hilbert SJ. The self-fulfilling influence of mother expectations on children’s underage drinking. Journal of Personality and Social Psychology. 2003;84:1188–1205. doi: 10.1037/0022-3514.84.6.1188. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick EM, Qu Y, Telzer EH. Adolescent neurodevelopment of cognitive control and risk-taking in negative family contexts. NeuroImage. 2016;124:989–996. doi: 10.1016/j.neuroimage.2015.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meece JL, Wigfield A, Eccles JS. Predictors of math anxiety and its influence on young adolescents’ course enrollment intentions and performance in mathematics. Journal of Educational Psychology. 1990;82:60–70. doi: 10.1037/0022-0663.82.1.60. [DOI] [Google Scholar]
- Melby J, Conger R, Book R, Rueter M, Lucy L, Repinski D, Scaramella L. The Iowa Family Interaction Rating Scales. 5th. Iowa State University, Institute for Social and Behavioral Research; 1998. Unpublished document. [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12(3):131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience. 2011;1(3):233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols SL, Good TL. America’s teenagers–myths and realities: Media images, schooling, and the social costs of careless indifference. Mahwah, NJ: Lawrence Erlbaum; 2004. [Google Scholar]
- Ng SH, Loong CSF, Liu JH, Weatherall A. Will the young support the old? An individual and family-level study of filial obligations in two New Zealand cultures. Asian Journal of Social Psychology. 2000;3:163–182. doi: 10.1111/1467-839X.00061. [DOI] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Qu Y, Fuligni AJ, Galván A, Lieberman MD, Telzer EH. Links between parental depression and longitudinal changes in youths’ neural sensitivity to rewards. Social Cognitive Affective Neuroscience. 2016;11(8):1262–1271. doi: 10.1093/scan/nsw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Fuligni AJ, Galván A, Telzer EH. Buffering effect of positive parent-child relationships on adolescent risk taking: A longitudinal neuroimaging investigation. Developmental Cognitive Neuroscience. 2015;15:26–34. doi: 10.1016/j.dcn.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Galván A, Fuligni AJ, Lieberman MD, Telzer EH. Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience. 2015;35(32):11308–11314. doi: 10.1523/JNEUROSCI.1553-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Pomerantz EM, Wang M, Cheung C, Cimpian A. Conceptions of adolescence: Implications for differences in engagement in school over early adolescence in the United States and China. Journal of Youth and Adolescence. 2016;45:1512–1526. doi: 10.1007/s10964-016-0492-4. [DOI] [PubMed] [Google Scholar]
- Qu Y, Pomerantz EM, Wang Q, Ng F. American and Chinese conceptions of adolescence: Implications for differences in adolescent pathways. Presentation at the Biennial Meeting of the Society for Research in Child Development; Philadelphia, PA. 2015. [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SCR, Simmons A, Bullmore ET. Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000;24(1):13–19. doi: 10.1016/S0149-7634(99)00055-X. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28(11):1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Human Brain Mapping. 2006;27(12):973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruble DN. Advances in experimental social psychology. Vol. 26. San Diego, CA: Academic Press; 1994. A phase model of transitions: Cognitive and motivational consequences; pp. 163–214. [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296(5572):1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Mumford JA, Congdon E, Trepel C, Poldrack RA. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: an fMRI investigation of the balloon analog risk task. Frontiers in Neuroscience. 2012;6:80. doi: 10.3389/fnins.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Steinberg L. We know some things: Parent–adolescent relationships in retrospect and prospect. Journal of Research on Adolescence. 2001;11(1):1–19. doi: 10.1111/1532-7795.00001. [DOI] [Google Scholar]
- Sturman DA, Moghaddam B. The neurobiology of adolescence: Changes in brain architecture, functional dynamics, and behavioral tendencies. Neuroscience and Biobehavioral Reviews. 2011;35(8):1704–1712. doi: 10.1016/j.neubiorev.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Fuligni AJ, Lieberman MD, Galván A. Meaningful family relationships: Neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience. 2013;25(3):374–387. doi: 10.1162/jocn_a_00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Ichien NI, Qu Y. Mothers know best: Redirecting adolescent reward sensitivity to promote safe behavior during risk taking. Social Cognitive Affective Neuroscience. 2015;10:1383–1391. doi: 10.1093/scan/nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. Journal of Neuroscience. 2009;29(40):12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veroude K, Jolles J, Croiset G, Krabbendam L. Changes in neural mechanisms of cognitive control during the transition from late adolescence to young adulthood. Developmental Cognitive Neuroscience. 2013;5:63–70. doi: 10.1016/j.dcn.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. 2000 Retrieved July 23, 2016, from http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim.
- Wessel JR, Conner CR, Aron AR, Tandon N. Chronometric electrical stimulation of right inferior frontal cortex increases motor braking. Journal of Neuroscience. 2013;33(50):19611–19619. doi: 10.1523/JNEUROSCI.3468-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


