Abstract
Defective Ca2+ regulation play a key role in the blunted force-frequency response in heart failure (HF). Since HF is commonly associated with oxidative stress, we studied whether oxidation of ryanodine receptor (RyR2) contributes to this defect. In control ventricular myocytes, oxidative stress induced formation of disulfide bonds between RyR2 subunits: intersubunit cross-linking (XL). Western blot analysis and Ca2+ imaging revealed a strong positive correlation between RyR2 XL and sarcoplasmic reticulum (SR) Ca2+ leak. These results illustrates that RyR2 XL can be used as a sensitive indicator of RyR2 dysfunction during oxidative stress. HF myocytes were in a state of oxidative stress since they exhibited an increase in reactive oxygen species (ROS) level, a decrease in ROS defense and an overall protein oxidation. These myocytes were also characterized by RyR2 XL and increased SR Ca2+ leak. Moreover, the frequency-dependent increase of Ca2+ transient amplitude was suppressed due to the inability of the SR to maintain Ca2+ load at high pacing rates. Because SR Ca2+ load is determined by the balance between SR Ca2+ uptake and leak, the blunted frequency-dependent inotropy in HF can be mediated by ROS-induced SR Ca2+ leak. Preventing RyR2 XL in HF myocytes decreased SR Ca2+ leak and increased Ca2+ transients at high pacing rate. We also studied whether RyR2 oxidation alone can cause the blunted frequency-dependent facilitation of Ca2+ transient amplitude in control myocytes. When RyR2 XL was induced in control myocytes to a similar level seen in HF, an increase of Ca2+ transient amplitude at high pacing rate was significantly suppressed. These results suggest that SR Ca2+ leak induced by RyR2 oxidation can play an important role in the blunted frequency-dependent inotropy of HF.
Keywords: Ca2+ homeostasis, ventricular myocytes, heart failure, sarcoplasmic reticulum, ryanodine receptor, oxidative stress
INTRODUCTION
Heart failure (HF) is a major cause of morbidity and mortality, particularly among the elderly. One of the mechanisms proposed to explain the impaired myocardial function of failing heart is a decline of the frequency-dependent positive inotropy. Described by Bowditch more than 140 years ago, this inotropic mechanism is essential for adjusting cardiac output during stress. Unlike in the healthy heart, where force generation increases as heart rate increases, force generation by the failing myocardium remains unchanged. The impaired frequency-dependent inotropic mechanism is a prominent characteristic of failing heart, and the degree of this defect correlates with the progression of HF [6;10;32]. It has been shown that defective sarcoplasmic reticulum (SR) Ca2+ handling plays a key role in the blunted frequency-dependent inotropy in HF [11;13;16;27].
The ryanodine receptor type-2 (RyR2) is the main SR Ca2+ release channel in ventricular myocytes. During systole, RyR2 activation generates the Ca2+ transient that initiates contraction. The force generated during contraction correlates directly with the amplitude of systolic Ca2+ transient. During diastole, RyR2s are not completely quiescent, providing a major pathway for SR Ca2+ leak [47]. Because the Ca2+ transient amplitude steeply depends on SR Ca2+ load [35], loss of the intra-SR [Ca2+] ([Ca2+]SR) via Ca2+ leak would reduce contractile force. By shortening diastole at higher heart rates, less Ca2+ would leak from the SR and more Ca2+ would enter into the cytosol via L-type Ca2+ channels, providing substrate for the SR Ca2+ pump (SERCA). Additionally, intracellular [Na+] accumulation at higher heart rates would decrease Ca2+ extrusion by Na+/Ca2+ exchanger (NCX). As a result, higher SR Ca2+ load can be built up at increased heart rate. By this fundamental mechanism, the heart is able to adjust the cardiac output to meet the body demand in oxygen and nutrient during stress. In HF myocytes this important inotropic mechanism is significantly suppressed, mainly because the SR has an impaired ability to retain Ca2+ at increased heart rate. While downregulation of SERCA has been recognized as an important factor in the blunted frequency-dependent inotropy in HF [12;18;24;31], augmentation of SR Ca2+ leak can also contribute to this defect by limiting SR Ca2+ load [37;39;47].
HF is commonly associated with oxidative stress as a result of increased reactive oxygen species (ROS) production and a decrease in ROS defense [21;30]. Among many ROS targets, RyR2 plays a particularly important role in an initial cellular response to oxidative stress [15;20;38;46]. RyR2 contains several highly redox-sensitive cysteine residues that can link oxidative stress and SR Ca2+ regulation [43]. Each free thiol residue can serve as a target for a number of oxidative modifications, including disulfide bond formation, S-nitrosylation and S-glutathionylation. It has been shown that oxidation of RyR2 cysteines increases the channel activity, whereas reduction of them makes the channel less active [49]. Although the functional significance of RyR2 redox modification is well documented, it remains less clear whether RyR2 oxidation contributes to the increased SR Ca2+ leak and the blunted frequency-dependent inotropy in HF.
MATERIALS AND METHODS
Rabbit heart failure model
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committees and comply with USA regulations on animal experimentation. New Zealand White rabbits (Harlan Laboratories, Indianapolis, USA) underwent two separate surgeries to induce hypertrophy and HF. A combined insult of aortic insufficiency and constriction was used to induce left ventricular volume and pressure overload, respectively [28]. Aortic insufficiency was performed by puncturing the aortic valve. Two-dimensional echocardiography was used to confirm and determine the degree of aortic valve insufficiency. Two weeks later, aortic constriction was performed on the abdominal aorta proximal to the renal arteries. The progression of hypertrophy and HF was monitored using echocardiography at 2 to 4 week intervals after both surgeries. All parameters were calculated relative to the baseline measurements taken prior to the surgeries. The development of hypertrophy and the subsequent onset of HF were carried out over the course of approximately 5–8 months. Once left ventricular end-diastolic diameter was increased by >50% and left ventricular fractional shortening was decreased by approximately 40% (depending on morbidity), hearts were categorized as HF.
Isolation of left ventricular myocytes
Ventricular myocytes were isolated from control and failing rabbit hearts. Total 26 animals (2–3 kg) were used in this study. Rabbits were anaesthetized with sodium pentobarbital (50 mg/kg I.V.). Following thoracotomy hearts were quickly excised, mounted on a Langendorff apparatus, and retrogradely perfused with collagenase-containing solution at 37°C according to the procedure described previously [7]. All chemicals and reagents were purchased from Sigma-Aldrich (St Louis, MO, USA).
Measurements of RyR2 XL
Alteration in the electrophoretic mobility of the 560 kDa subunit was used to detect RyR2 XL [1;23]. Control and HF myocytes were pelleted and lysed in non-reducing Laemmli buffer (“Sigma”, USA) containing N-ethylmaleimide (5 mM) to block free sulfhydryl groups. Dithiothreitol (DTT; 30 mM) was used to confirm reversibility of RyR2 XL and to estimate total RyR2. Lysate samples were incubated at 70°C for 10 min and ran on 3–15% SDS-PAGE. Then, the samples were transferred onto nitrocellulose membrane using Turbo-transfer (Bio-Rad, USA). Immunoblots against RyR2 were carried out using the monoclonal 34C primary antibody [3] (1:1000; “DSHB”, USA) and anti-mouse HRP-conjugated secondary antibody (1:5000; Santa Cruz, USA). Secondary HRP-conjugated antibodies were visualized using the Luminata Forte Western HRP Substrate (“Millipore”; USA). Western blots were quantified using the ChemiDoc XRS imaging system (BioRad, USA) and ImageJ software (NIH, USA). Relative RyR2 XL was analyzed as (Total RyR2 - Monomeric RyR2)/(Total RyR2).
Measurements of RyR2 phosphorylation
Control and HF myocytes were pelleted and lysed in Laemmli buffer (“Sigma”, USA) containing the reducing agent 2-mercaptoethanol. The same amount of total lysate from each sample was subjected to 4–15% SDS-PAGE. Then, the samples were transferred to nitrocellulose membranes. RyR2 phosphorylation level at the CaMKII site (Ser 2815) was quantified using phospho-specific antibody RyR-PS2815 [41] (kindly provided by Dr. Terentyev; Brown University, USA). The signal was normalized to total RyR2 level measured with the primary antibody C34 (“DSHB”, USA). Western blots were quantified using the ChemiDoc XRS imaging system and ImageJ software.
Free Thiol Content Measurement
The free thiol content of proteins was measured using the irreversible alkylating agent monobromobimane (mBB), which becomes fluorescent once it reacts with a free thiol [5]. To introduce mBB (400 μM) into the cytosol, myocytes were permeabilized with saponin (0.005%). Myocytes were incubated with mBB for one hour and then washed three times in order to remove unbound mBB. Myocytes were then lysed in reducing Laemmli Buffer (containing 10% 2-mercaptoethanol). The same amount of total lysate from each sample was subjected to 3–15% SDS-PAGE and transferred to nitrocellulose membranes. The gel was first imaged using UV light to measure mBB emission (490 nm) with the ChemiDoc XRS imaging system. Afterwards the gel was stained with Coomassie Blue in order to normalize the mBB signal to the total protein level. The maximal free thiol level was determined after treating myocytes with DTT (30 mM).
Measurements of the GSH/GSSG level
Ventricular myocytes were quickly settled down and homogenized using a bead-beater homogenizer (Biospec Products, USA). The addition of 5-sulfo-salicylic acid dihydrate (5% w/v) was used to precipitate protein from homogenate samples. Samples were centrifuged at 14,000 rpm at 4°C for 10 min. The resulting supernatant was used for analysis of the ratio between reduced (GSH) and oxidized (GSSG) glutathione (GSH/GSSG). The concentration of GSH was defined using fluorometric glutathione detection assay DetectX (Arbor Assays, USA), utilizing a fluorescent label that covalently binds to GSH. The sample fluorescence was measured using a fluorometer (OLIS DM 45, USA) at an excitation/emission of 390/510 nm. A standard plot of known GSH concentrations was developed before each set of experiments. After GSH fluorescence was determined for each sample, the subsequent reduction of GSSG by glutathione reductase would yield total GSH fluorescence. Absolute concentrations were determined based on the linear fit of the standard fluorescence. Absolute GSSG concentration was quantified as: [(Total GSH - Free GSH)/2]. Values presented are percent changes in GSH/GSSG.
Measurements of ROS production
ROS production was measured with a ROS-sensitive fluorescent dye, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA; Molecular Probes/Invitrogen, Carlsbad, USA) [5]. Myocytes were loaded with 20 μM H2DCFDA for 30 min at room temperature. H2DCFDA was measured at an excitation/emission of 488/>600 nm. Fluorescence intensity (F) was integrated over the entire volume of the cell. Changes of ROS level were presented as background-subtracted normalized fluorescence (F/F0), F0 is the initial fluorescence recorded at the beginning of an experiment. Because H2DCFDA irreversibly reacts with free oxygen radicals, ROS production was analyzed as the first derivative of H2DCFDA signal (d(F/F0)/dt). At the end of each experiments peroxide was applied to estimate the maximal rate of ROS production.
Intracellular Ca2+ Imaging
Changes in cytosolic [Ca2+] ([Ca2+]i) and intra-SR [Ca2+] ([Ca2+]SR) were measured with laser scanning confocal microscopy (Radiance 2000 MP, Bio-Rad, UK) equipped with a ×40 oil-immersion objective lens (N.A.=1.3).
Measurements of [Ca2+]i
To record [Ca2+]i we used the high affinity Ca2+ indicator Fluo-4 (Molecular Probes/Life Technologies, Grand Island, NY). Cells were incubated at room temperature with 10 μM Fluo-4/AM for 15 minutes in Tyrode solution (in mM: NaCl 140; KCl 4; CaCl2 2; MgCl2 1; glucose 10; HEPES 10; pH 7.4), followed by a 20 minute wash. Fluo-4 was measured at an excitation/emission of 488/>515 nm. Action potentials were induced by electrical field stimulation using a pair of platinum electrodes, which were connected to a Grass stimulator (Astro-Med. Inc., USA) set at a voltage ~50% above the threshold. Fluo-4 recordings were acquired in line-scan mode (3 ms per scan; pixel size 0.12 μm).
Simultaneous measurements of [Ca2+]i and [Ca2+]SR
For simultaneous imaging of [Ca2+]SR and [Ca2+]i dynamics, Rhod-2 (instead of Fluo-4) was used to record [Ca2+]i. To record [Ca2+]SR we used the low affinity Ca2+ indicator Fluo-5N (Molecular Probes/Life Technologies, Grand Island, NY). To load the SR with Ca2+ indicator, myocytes were incubated with 5 μM Fluo-5N/AM for 2.5 hours at 37°C. Then, the Fluo-5N loaded cells were incubated at room temperature with 10 μM Rhod-2/AM for 13 minutes in Tyrode solution. Fluo-5N was measured at an excitation/emission of 488/>515 nm. Rhod-2 was measured at an excitation/emission of 543/>580 nm. These measurements were made using line-sequential scanning to avoid any bleed-through in both channels. Recordings were acquired in line-scan mode (3 ms per scan; pixel size 0.12 μm).
SR Ca2+ leak measurements
SR Ca2+ leak was measured in cardiomyocytes loaded with Fluo-5N as described previously [47]. To improve signal-to-noise ratio of the low intensity Fluo-5N signal, fluorescence was collected with an open pinhole and averaged over the entire cellular width of an individual 2-D image. Changes in [Ca2+]SR were calculated by the formula: [Ca2+]SR = Kd × [(F − Fmin)/( Fmax − F), where F was the Fluo-5N fluorescence; Fmax and Fmin where the fluorescence level at 10 mM Ca2+/ionomycin and after depletion of the SR Ca2+ with caffeine (10 mM), respectively. The Fluo-5N Ca2+ dissociation constant (Kd ) was 390 μM based on in situ calibrations [47]. SR Ca2+ leak was calculated as changes of total [Ca2+]SR ([Ca2+]SRT) after SERCA inhibition with thapsigargin (TG): d[Ca2+]SRT/dt. [Ca2+]SRT was calculated as: [Ca2+]SRT = Bmax/(1 +Kd/[Ca2+]SR) + [Ca2+]SR; where Bmax and Kd were 2700 μM and 630 μM, respectively [36]. SR Ca2+ leak (d[Ca2+]SRT/dt) was plotted as a function of [Ca2+]SR. For all experimental groups, SR Ca2+ leak was compared at the same range of [Ca2+]SR: 400–500 μM. All images were analyzed with ImageJ software (NIH, USA).
Statistics
Data were presented as means ± S.E.M. of n measurements. In case of single cell experiments (such as ROS and Ca measurements), n represents the number of cells isolated from at least 3 different animals. In case of RyR2 XL and GSH/GSSG measurements, n represents the number of animals used in these experiments. When only two groups were compared, statistical significance was determined by Student’s t-test. Significance between multiple groups was determined by one-way ANOVA followed by a Newman-Keuls post-hoc test. P<0.05 was considered statistically significant. Statistical analysis and graphical representation of averaged data was carried out on OriginPro7.5 software (OriginLab, USA).
RESULTS
Correlation between RyR2 XL and SR Ca2+ leak in control myocytes
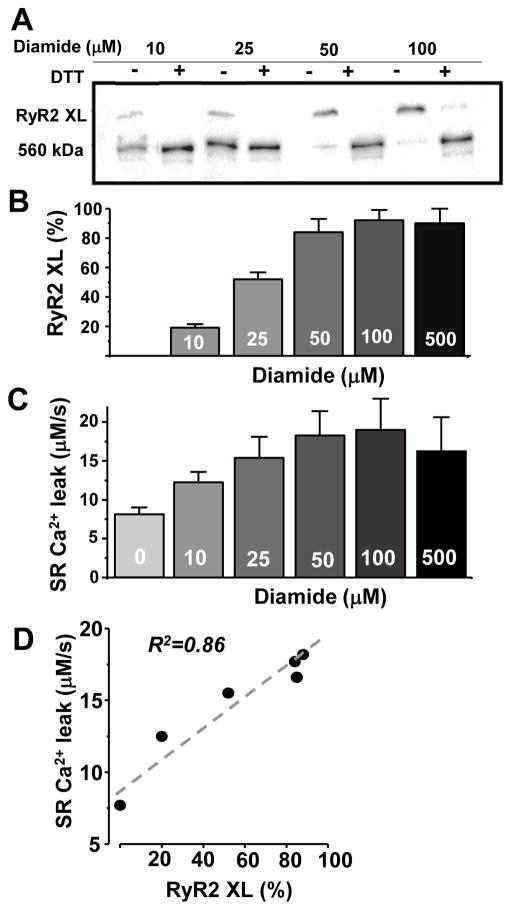
RyR2 as non-covalently assembled homotetramer is always detected as a single band in non-reducing SDS-PAGE (with MW ~560 kDa). However, after treatment of cardiomyocytes with oxidants (H2O2 or diamide) RyR2 can be detected as a polymer with MW >1MDa (Figs. 1A and 3). In the following experiments, diamide was used to selectively oxidize sulfhydryl groups of proteins. Dithiothreitol (DTT) was used to reduce disulfide bonds and restore the monomeric pattern of RyR2. Diamide increased RyR2 XL by a dose-dependent manner, causing a maximum RyR2 XL at 50 μM (Fig. 1B). We also found that diamide increased RyR2-mediated SR Ca2+ leak in control ventricular myocytes (Fig. 1C). In these experiments, SR Ca2+ leak was measured as the rate of decline of [Ca2+]SR after SERCA inhibition with thapsigargin (TG) [47]. Examples of SR Ca2+ leak experiment are shown on Fig. 4. Western blot analysis and Ca2+ imaging revealed a strong positive correlation (R2=0.89) between the degree of RyR2 XL and SR Ca2+ leak (Fig. 1D). When the XL reached the maximum level (at 50 μM diamide), further oxidation of RyR2 did not enhance SR Ca2+ leak. These results illustrate that RyR2 XL can be used as a sensitive indicator of RyR2 dysfunction in cardiac pathologies associated with oxidative stress.
Figure 1. Effect of diamide on RyR2 XL and SR Ca2+ leak in control rabbit ventricular myocytes.
A, representative western immunoblots (non-reducing) against RyR2 from control myocytes treated with different concentrations of diamide. For each sample, the reducing agent DTT (30 mM) was added in order to measure total RyR2. B, effects of different doses of diamide on RyR2 XL. Relative RyR2 XL was analyzed as (Total RyR2 - Monomeric RyR2)/(Total RyR2). The results were obtained from 4 animals. C, effects of different doses of diamide on SR Ca2+ leak. SR Ca2+ leak was measured at the same range of [Ca2+]SR (400–500 μM). The results were obtained from 31 cells; 6 animals. D, the correlation between RyR2 XL and SR Ca2+ leak rate.
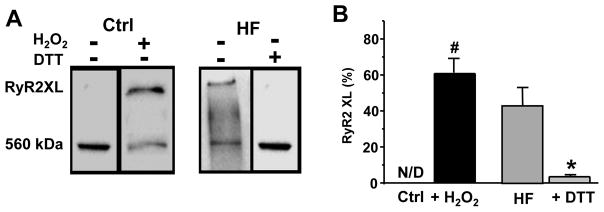
Figure 3. RyR2 XL in rabbit HF myocytes.
A, Representative western immunoblots (non-reducing) against RyR2 from control and HF myocytes. Control myocytes were treated with H2O2 (50 μM) to induce RyR2 XL. HF myocytes were treated with DTT (30 mM) to reduce disulfide bonds and restore the monomeric pattern of RyR2. B, Changes of RyR2 XL in control and HF myocytes. The results were obtained from 4 control and 4 HF animals. #P<0.05 vs control and *P<0.05 vs HF. N/D - Not Defined.
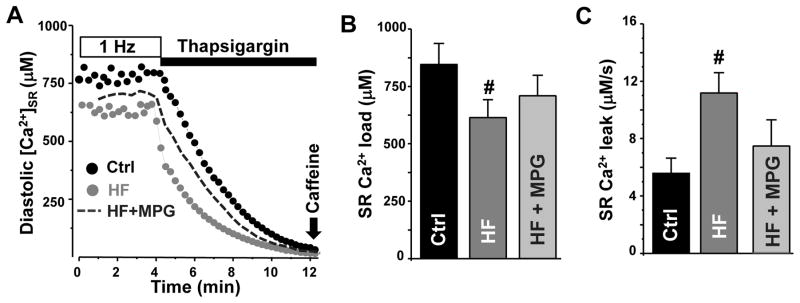
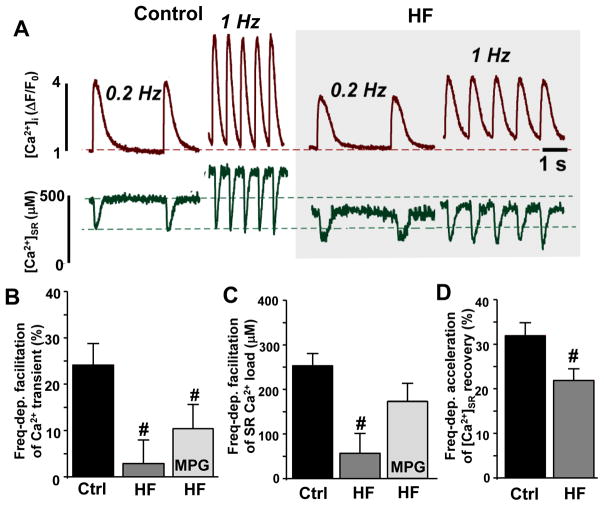
Figure 4. SR Ca2+ load and leak in control and failing myocytes.
A, changes of [Ca2+]SR during electrical stimulation at 1 Hz and during rest in the presence of thapsigargin in control (black circles), HF myocytes (light gray circles) and HF treated with MPG (dark gray dashed line). B, SR Ca2+ load in control (n=8) and HF myocytes (n=6). SR Ca2+ load was measured as diastolic [Ca2+]SR with the low-affinity Ca2+ dye Fluo-5N. C, SR Ca2+ leak in control (n=8) and HF myocytes (n=6). SR Ca2+ leak was measured as the rate of decline of [Ca2+]SR after SERCA inhibition with thapsigargin. Changes in SR Ca2+ leak between control and HF myocytes were compared at the same [Ca2+]SR: 400–500 μM. Mercapto-propionylglycine (MPG) was used to prevent RyR2 XL. #P<0.05 vs control.
Oxidative stress and RyR2 XL in HF myocytes
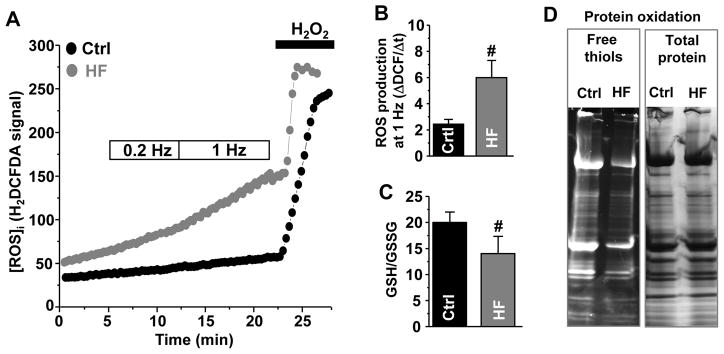
We studied whether HF induced by a combined insult of aortic insufficiency and stenosis is associated with oxidative stress and RyR2 XL. Ventricle myocytes isolated from rabbit failing hearts were in the state of oxidative stress since they exhibited an increase in reactive oxygen species (ROS) production and a decrease in ROS defense. The intracellular ROS level was measured using the ROS-sensitive fluorescent dye H2DCFDA in electrically stimulated myocytes (Fig. 2A). Because H2DCFDA irreversibly reacts with free oxygen radicals, ROS production was presented as the first derivative of H2DCFDA signal. We found that ROS level was substantially higher in HF myocytes, particularly at fast pacing rate (Fig. 2B). At the same time, the reducing power of the major antioxidant, glutathione, was decreased in HF myocytes (Fig. 2C). The glutathione redox status was estimated from the ratio between reduced (GSH) and oxidized (GSSG) glutathione. As a result of increased ROS production and reduced ROS defense, the overall protein oxidation was significantly higher in HF myocytes (Fig. 2D). Protein oxidation was measured as a decrease in free thiol content measured with the mBB assay. For each experimental group, the mBB signal was normalized to the total protein level. On average, the mBB signal decreased from 95% in control (n=4 animals) to 62% in HF (n=4 animals). Maximal free thiol level (100% reduced) was obtained after treating myocytes with DTT (30 mM). We also detected a significant level of RyR2 XL in HF (Fig. 3). We found that in HF myocytes 45% RyR2 subunits were cross-linked (n=4 animals). This post-translation modification was effectively reduced after treating HF myocytes with DTT.
Figure 2. Oxidative stress in control and HF myocytes.
A, changes of H2DCFDA signal during electrical stimulation at high pacing frequency (1 Hz) in control (black circles) and HF myocytes (gray circles). H2O2 (1 mM) was applied to estimate the maximal rate of ROS production. B, changes of ROS production at 1 Hz in control (n=9) and HF (n=7) myocytes. The rate of ROS production was presented as the first derivative of H2DCFDA signal (ΔF/Δt). C, changes of the GSH/GSSG ratio measured in 4 control and 4 HF animals. D, changes of free thiol content in proteins from control and HF myocytes. The free thiol content was measured using the mBB assay in 4 control and 4 HF animals. #P<0.05 vs control.
SR Ca2+ leak in HF myocytes
In the following experiments, we studied changes in SR Ca2+ handling that occur in HF myocytes. Fig. 4A shows original recordings of SR Ca2+ load and SR Ca2+ leak in control and HF myocytes. In these experiments, SR Ca2+ load was estimated from diastolic [Ca2+]SR measured between electrical stimulations at 1 Hz. SR Ca2+ leak was measured as the rate of decline of [Ca2+]SR after SERCA inhibition. In comparison with control myocytes, SR Ca2+ load was significantly depleted in HF myocytes (Fig. 4B). While downregulation of SERCA in HF [12;18;24;31] might contribute to the decreased SR Ca2+ load, we studied whether SR Ca2+ leak is also altered in HF myocytes. In these experiments, SR Ca2+ leak was analyzed as a function of [Ca2+]SR. Thus, SR Ca2+ leak in control and HF myocytes can be compared at the same SR Ca2+ load. Analysis of the [Ca2+]SR decline revealed that SR Ca2+ leak is nearly doubled in HF compared to control myocytes (Fig. 4C). In the following experiments mercapto-propionylglycine (MPG) was used to reduce disulfide bonds, because DTT significantly decreased the Fluo-5N fluorescence independent of [Ca2+]. Treating HF myocytes with MPG (10 mM) reduced SR Ca2+ leak and partially restored SR Ca2+ load (Fig 4B and C).
The frequency-dependent facilitation of Ca2+ transient amplitude in HF myocytes
Abnormal SR Ca2+ handling has been shown to play a key role in the blunted frequency-dependent inotropy in HF [11;13;16]. To define mechanisms of this defect, we simultaneously measured [Ca2+]i (dark red traces) and [Ca2+]SR (dark green traces) at low (0.2 Hz) and high (1 Hz) pacing frequencies. Before each recording, myocytes were given 2 min at each frequency so that Ca2+ cycling could reach steady state. Frequency-dependent facilitation from 0.2 to 1 Hz was calculated as the absolute change in SR Ca2+ load and the relative change in Ca2+ transient amplitude. We found that in control myocytes SR Ca2+ load and Ca2+ transients increased with increasing pacing frequency (Fig. 5A, left). These effects were associated with a faster [Ca2+]i transient decay and faster [Ca2+]SR recovery after Ca2+ release, presumably, due to an increase in SERCA activity. On average, an increase in pacing rate from 0.2 to 1 Hz increased the [Ca2+]SR recovery rate by 32% (n=6). In HF myocytes, an increase of Ca2+ transient amplitude at high pacing frequency was abolished (Fig. 5B) due to the inability of the SR to maintain SR Ca2+ load at high pacing frequency (Fig. 5C). At the same time, the frequency-dependent acceleration of [Ca2+]SR recovery was reduced only by ~30% (n=5; Fig. 5D). These results suggest that the increased SR Ca2+ leak might also contribute to the blunted frequency-dependent inotropy in HF. MPG only partially restored the frequency-dependent facilitation of Ca2+ transient amplitude and SR Ca2+ load in HF myocytes (Fig 5B and C).
Figure 5. Effects of stimulation frequency on SR Ca2+ load, Ca2+ transient amplitude and [Ca2+]SR recovery in control and HF myocytes.
A, Cytosolic Ca2+ transients (dark red traces) and corresponding [Ca2+]SR depletions (dark green traces) at low (0.2 Hz) and high (1 Hz) pacing frequencies in control and HF myocytes. Increase of Ca2+ transient amplitude (B), SR Ca2+ load (C) and [Ca2+]SR recovery (C) after changing pacing frequencies from 0.2 to 1 Hz in control (n=6) and in HF (n=5) myocytes. #P<0.05 vs control.
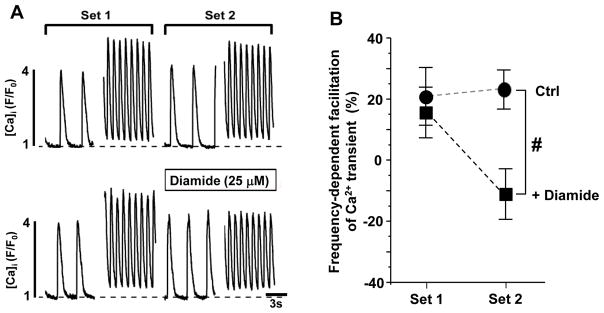
Effect of diamide on the frequency-dependent facilitation of Ca2+ transients in control myocytes
We used an alternative approach to test the hypothesis that the RyR2 oxidation contributes to the blunted frequency-dependent inotropy in HF. In the following experiments, the frequency-dependent facilitation of Ca2+ transients was studied in control myocytes exposed to diamide (25 μM). This concentration of diamide increased RyR2 XL and SR Ca2+ leak in control myocytes (Fig. 1) to similar level observed in HF (Figs. 3 and 4). Ca2+ transients were recorded from each myocyte at both 0.2 and 1 Hz in two consecutive sets (Fig. 6A). The myocytes subjected to oxidative stress were perfused with diamide during the second set (beginning 2 minutes prior to 0.2 Hz recording). Frequency facilitation from 0.2 to 1 Hz was calculated as the relative change in Ca2+ transient amplitude and was determined for each set. These experiments revealed that diamide (25 μM) significantly suppressed the frequency-dependent facilitation of Ca2+ transient amplitude (Fig. 6B). We had shown previously that diamide used at such concentration did not affect SR Ca2+ uptake [23], suggesting that the increased SR Ca2+ leak is a main contributor to the blunted frequency-dependent inotropy during oxidative stress.
Figure 6. Effect of diamide (25 μM) on the frequency-dependent facilitation of Ca2+ transient amplitude in control myocytes.
A, cytosolic Ca2+ transients at low (0.2 Hz) and high (1 Hz) pacing frequencies in control (n=10) and diamide-treated (n=8) myocytes. Ca2+ transients were recorded at 0.2 and 1 Hz in two consecutive sets (Set 1 and 2). B, increase of Ca2+ transient amplitude after changing pacing frequencies from 0.2 to 1 Hz in control and diamide-treated myocytes. #P<0.05 vs control.
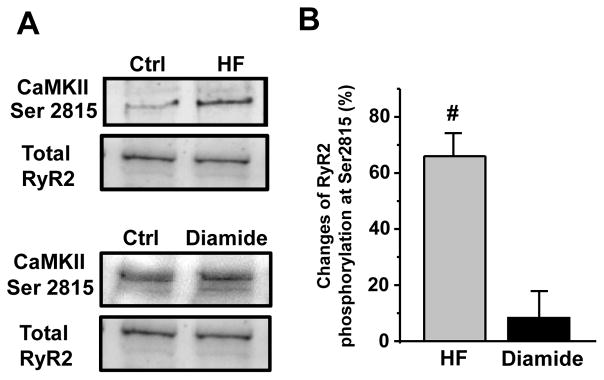
Effect of diamide and heart failure on RyR2 phosphorylation
RyR2 phosphorylation by the Ca2+/Calmodulin-dependent kinase type II (CaMKII) is known to increase SR Ca2+ leak [2;4;29]. In agreement with previous work [2], RyR2 phosphorylation at the CaMKII site (serine 2815) was significantly increased in HF (Fig 7). Because oxidative stress can activate CaMKII via a Ca2+-independent mechanism [8], we tested whether diamide (25 μM) increases RyR2 phosphorylation at the CaMKII site in control myocytes. We found that diamide (25 μM) did not affect RyR2 phosphorylation at serine 2815 (Fig 7). These results suggest that the observed effect of diamide on the frequency-dependent facilitation of Ca2+ transient amplitude in control myocytes was mainly mediated by RyR2 oxidation.
Figure 7. Changes of RyR2 phosphorylation in HF and control myocytes.
A, representative western immunoblots showing RyR2 phosphorylation at the CaMKII site in control, HF and diamide-treated control myocytes. The signal was normalized to total RyR2 level measured with the primary antibody 34C (“DSHB”, USA). B, changes in RyR2 phosphorylation level at the CaMKII sites in HF (n=4 animals) and diamide (25 μM)-treated control myocytes (n=4 animals). #P<0.05 vs control.
DISCUSSION
In the heart, SR Ca2+ release through RyR2 is essential for initiating a robust myocardial contraction. Consequently, defects in RyR2 regulation cause contractile dysfunction in a variety of cardiac pathologies. In particular, abnormal RyR2 activity contributes to SR Ca2+ mishandling, arrhythmias and contractile dysfunction in failing hearts [48]. Recent emphasis has been placed on the study of oxidative post translational modifications and their important role in the regulation of heart function [15;20;49]. It has been suggested that maladaptive changes in cellular metabolism that occur during HF progression decrease the ability of myocardium to resist oxidative stress [21]. In this study, we reported that the antioxidant defense (measured as the GSH/GSSG ratio) is decreased, whereas ROS production is increased in a rabbit HF model induced by left ventricular volume and pressure overload (Fig 2). Although specific sources of ROS production have not been identified in this study, it appears that several mechanisms are likely to be involved in oxidative stress in HF [30]. One of these mechanisms is related to mitochondrial respiration. It has been shown that in failing myocytes the mitochondrial electron transport chain (ETC) was more prone to uncoupling and subsequent ROS production [17]. We found that an increase of pacing rate increases ROS production only in HF myocytes (Fig 2). It seems that an increase in ATP consumption during higher pacing rate stimulates mitochondrial metabolism leading to electron leakage from the ETC and formation of superoxide radicals. The depleted antioxidant defense (including GSH [19]) and other ROS sources (including NADPH- [14] and xanthine- oxidases [9]) might also contribute to oxidative stress in HF.
We found that an unbalance between ROS production and defense leads to protein oxidation in HF myocytes (Fig 2). Among all cardiac ion transporters and channels, RyR2 appears to be the most sensitive to redox modification [15;46], thus linking oxidative stress to Ca2+ regulation. RyR2 has approximately 360 cysteine residues per tetrameric channel and 84 of those are estimated to be in a reduced free thiol state [43]. To date, a number of in vitro studies have shown that oxygen free radicals and other oxidants can modify RyR2 function. Lipid bilayer and isolated myocyte experiments have shown that RyR2 channel activity is increased in the presence of ROS and oxidants, whereas reducing agents decrease the RyR2 activity [46]. Thus, abnormally elevated ROS level can lead to RyR2 oxidation, RyR2 activation and SR Ca2+ leak in HF myocytes (Fig 4). Indeed, it has been shown previously that RyR2 in HF myocytes is partially oxidized [40]. However, mechanisms by which oxidative stress affect SR Ca2+ handling in HF are not yet fully understood.
High resolution cryo-EM studies revealed that a transition of RyR2 between close and open state is associated with a large-scale intersubunit dynamic [26;33;42;44]. Thus, any post-translational modifications that affect RyR2 intersubunit interaction would likely modify RyR2 function and SR Ca2+ release. We recently discovered that oxidation of RyR2 causes formation of disulfide bonds between two subunits within the channel: intersubunit XL [23]. Although redox-mediated RyR XL has been described previously [1], its functional significance remains unclear. Direct measurements of [Ca2+]SR using a fluorescent Ca2+ dye (Fluo-5N) entrapped within the SR demonstrated that RyR2 XL is associated with an increase in SR Ca2+ leak. We found a strong positive correlation between the degree of RyR2 XL and SR Ca2+ leak (Fig 1). When the XL reached the maximum level, further oxidation of RyR2 did not enhance SR Ca2+ leak. Therefore, these results suggest that RyR2 XL might be an important regulator of RyR2 function during oxidative stress. Although it remains to be determined whether XL is the most functionally important redox modification of RyR2, the level of XL can be used as a sensitive indicator of RyR2 dysfunction and SR Ca2+ mishandling during oxidative stress.
Since HF is commonly associated with oxidative stress (Fig 2 and [21]), we tested whether RyR2 in HF myocytes is also susceptible to intersubunit XL. Indeed, we found a significant level of RyR2 XL in HF (Fig 3). We suggested that RyR2 oxidation might contribute to the increased diastolic SR Ca2+ leak in HF myocytes (Fig 4 and [37;40;47]). Preventing RyR2 XL in HF myocytes decreased SR Ca2+ leak. Together with downregulation of SERCA function [12;18;24;31], the increased SR Ca2+ leak would cause a shift in Ca2+ balance that resulted in a depletion of SR Ca2+ content (Fig 4). Subsequently, this would decrease action potential-induced Ca2+ transients and contractions of failing heart. At low pacing frequencies, however, Ca2+ transients in HF myocytes had relatively similar amplitude to control myocytes (Fig 5A). The enhanced activity of RyR2 in HF can partially normalize Ca2+ transient amplitude at depleted SR Ca2+ load by increasing the fractional SR Ca2+ release [7]. The critical difference in Ca2+ regulation between control and HF myocytes appeared at a high pacing rate. Unlike in control myocytes, where an increase in pacing frequency increases Ca2+ transient amplitude (Fig 5C), Ca2+ transients in HF myocytes remains unchanged. It appears that the increased SR Ca2+ leak together with the decreased SR Ca2+ uptake in HF myocytes prevent the SR Ca2+ load buildup at high pacing rates. These defects in SR Ca2+ handling have been suggested to play an important role in reduced contractility of the failing heart during stress [11;13;27].
Although RyR2 oxidation increases SR Ca2+ leak in HF, other mechanisms may also contribute to aberrant SR Ca2+ regulation and the blunted frequency-dependent inotropy in failing heart. HF is commonly viewed as a disorder of cell signaling. For example, increased SR Ca2+ leak in HF has been attributed to abnormal RyR2 phosphorylation either by the protein kinase A [22;25;34] or CaMKII [2;29;45]. Furthermore, alterations in expression of other Ca2+ transporters or regulatory proteins, including SERCA, likely affect SR Ca2+ regulation as well. In agreement with previously published work [2], we detected a significant level of RyR2 phosphorylation at the CaMKII site (Fig 7). Instead of separating XL and CaMKII effect on RyR2 in HF (which would largely rely on unspecific pharmacological tools), we studied whether RyR2 oxidation alone can induce the blunted frequency-dependent inotropy in control myocytes. We reported previously, that in control myocytes diamide did not change cytosolic Ca2+ transient amplitude (recorded at a low pacing frequency) despite a decrease in SR Ca2+ load. Similar to HF, fractional SR Ca2+ release was significantly increased in the presence of diamide [23]. In this study, we found that a low dose of diamide (25 μM) that increases RyR2 XL in control myocytes to similar level observed in HF largely abolished the frequency-dependent facilitation of Ca2+ transient amplitude (Fig 6). At the same time, this dose of diamide did not affect RyR2 phosphorylation at the CaMKII site (Fig 7). Since this diamide concentration did not affect SR Ca2+ uptake [23], we suggest that the increased SR Ca2+ leak due to RyR2 oxidation should contribute to the blunted frequency-dependent inotropy during oxidative stress. Together with other maladaptive changes in structure and function of HF myocytes, this defect in SR Ca2+ handling contributes to contractile dysfunction of the failing heart.
Acknowledgments
This work was supported by the National Institutes of Health Grants HL130231 (to A.V.Z.). The authors would also like to thank Dr. Lothar Blatter and Dr. Jaime DeSantiago (Rush University Medical Center, Chicago, USA) for providing rabbit HF myocytes. We also would like to thank Dr. Dmitry Terentyev (Brown University, USA) for providing the phospho-specific antibody against Ser-2815 RyR2.
Footnotes
DISCLOSURES
None.
Reference List
- 1.Aghdasi B, Zhang JZ, Wu Y, Reid MB, Hamilton SL. Multiple classes of sulfhydryls modulate the skeletal muscle Ca2+ release channel. J Biol Chem. 1997;272:3739–3748. doi: 10.1074/jbc.272.6.3739. [DOI] [PubMed] [Google Scholar]
- 2.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 3.Airey JA, Beck CF, Murakami K, Tanksley SJ, Deerinck TJ, Ellisman MH, Sutko JL. Identification and localization of two triad junctional foot protein isoforms in mature avian fast twitch skeletal muscle. J Biol Chem. 1990;265:14187–14194. [PubMed] [Google Scholar]
- 4.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, Carnes CA, Gyorke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bovo E, Lipsius SL, Zima AV. Reactive oxygen species contribute to the development of arrhythmogenic Ca2+ waves during beta-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol. 2012;590:3291–3304. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies CH, Davia K, Bennett JG, Pepper JR, Poole-Wilson PA, Harding SE. Reduced contraction and altered frequency response of isolated ventricular myocytes from patients with heart failure. Circulation. 1995;92:2540–2549. doi: 10.1161/01.cir.92.9.2540. [DOI] [PubMed] [Google Scholar]
- 7.Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol. 2009;587:5197–5209. doi: 10.1113/jphysiol.2009.177576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285:28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987;61:70–76. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 11.Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM, Morgan JP. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest. 1990;85:1599–1613. doi: 10.1172/JCI114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 13.Hasenfuss G, Reinecke H, Studer R, Pieske B, Meyer M, Drexler H, Just H. Calcium cycling proteins and force-frequency relationship in heart failure. Basic Res Cardiol. 1996;91(Suppl 2):17–22. doi: 10.1007/BF00795357. [DOI] [PubMed] [Google Scholar]
- 14.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 15.Hool LC, Corry B. Redox control of calcium channels: from mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2007;9:409–435. doi: 10.1089/ars.2006.1446. [DOI] [PubMed] [Google Scholar]
- 16.Houser SR, Piacentino V, III, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 17.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 18.Kiss E, Ball NA, Kranias EG, Walsh RA. Differential changes in cardiac phospholamban and sarcoplasmic reticular Ca(2+)-ATPase protein levels. Effects on Ca2+ transport and mechanics in compensated pressure-overload hypertrophy and congestive heart failure. Circ Res. 1995;77:759–764. doi: 10.1161/01.res.77.4.759. [DOI] [PubMed] [Google Scholar]
- 19.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Bohm M, O’Rourke B, Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998;275:C1–24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- 21.Mak S, Newton GE. The oxidative stress hypothesis of congestive heart failure: radical thoughts. Chest. 2001;120:2035–2046. doi: 10.1378/chest.120.6.2035. [DOI] [PubMed] [Google Scholar]
- 22.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 23.Mazurek SR, Bovo E, Zima AV. Regulation of sarcoplasmic reticulum Ca(2+) release by cytosolic glutathione in rabbit ventricular myocytes. Free Radic Biol Med. 2014;68:159–167. doi: 10.1016/j.freeradbiomed.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 24.O’Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res. 1999;84:562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 25.Oda T, Yano M, Yamamoto T, Tokuhisa T, Okuda S, Doi M, Ohkusa T, Ikeda Y, Kobayashi S, Ikemoto N, Matsuzaki M. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation. 2005;111:3400–3410. doi: 10.1161/CIRCULATIONAHA.104.507921. [DOI] [PubMed] [Google Scholar]
- 26.Orlova EV, Serysheva II, van HM, Hamilton SL, Chiu W. Two structural configurations of the skeletal muscle calcium release channel. Nat Struct Biol. 1996;3:547–552. doi: 10.1038/nsb0696-547. [DOI] [PubMed] [Google Scholar]
- 27.Pieske B, Kretschmann B, Meyer M, Holubarsch C, Weirich J, Posival H, Minami K, Just H, Hasenfuss G. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–1178. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- 28.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92:1034–1048. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- 29.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, de Almeida A, Voigt N, Lawrence WS, Skapura DG, Skardal K, Wisloff U, Wieland T, Ai X, Pogwizd SM, Dobrev D, Wehrens XH. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos CX, Raza S, Shah AM. Redox signaling in the cardiomyocyte: From physiology to failure. Int J Biochem Cell Biol. 2016;74:145–151. doi: 10.1016/j.biocel.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- 32.Schwinger RH, Bohm M, Muller-Ehmsen J, Uhlmann R, Schmidt U, Stablein A, Uberfuhr P, Kreuzer E, Reichart B, Eissner HJ. Effect of inotropic stimulation on the negative force-frequency relationship in the failing human heart. Circulation. 1993;88:2267–2276. doi: 10.1161/01.cir.88.5.2267. [DOI] [PubMed] [Google Scholar]
- 33.Serysheva II, Ludtke SJ, Baker ML, Cong Y, Topf M, Eramian D, Sali A, Hamilton SL, Chiu W. Subnanometer-resolution electron cryomicroscopy-based domain models for the cytoplasmic region of skeletal muscle RyR channel. Proc Natl Acad Sci U S A. 2008;105:9610–9615. doi: 10.1073/pnas.0803189105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys J. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shannon TR, Ginsburg KS, Bers DM. Reverse mode of the sarcoplasmic reticulum calcium pump and load-dependent cytosolic calcium decline in voltage-clamped cardiac ventricular myocytes. Biophys J. 2000;78:322–333. doi: 10.1016/S0006-3495(00)76595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki YJ, Ford GD. Redox regulation of signal transduction in cardiac and smooth muscle. J Mol Cell Cardiol. 1999;31:345–353. doi: 10.1006/jmcc.1998.0872. [DOI] [PubMed] [Google Scholar]
- 39.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terentyev D, Rees CM, Li W, Cooper LL, Jindal HK, Peng X, Lu Y, Terentyeva R, Odening KE, Daley J, Bist K, Choi BR, Karma A, Koren G. Hyperphosphorylation of RyRs underlies triggered activity in transgenic rabbit model of LQT2 syndrome. Circ Res. 2014;115:919–928. doi: 10.1161/CIRCRESAHA.115.305146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tung CC, Lobo PA, Kimlicka L, Van PF. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010;468:585–588. doi: 10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- 43.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 44.Zalk R, Clarke OB, des GA, Grassucci RA, Reiken S, Mancia F, Hendrickson WA, Frank J, Marks AR. Structure of a mammalian ryanodine receptor. Nature. 2015;517:44–49. doi: 10.1038/nature13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 46.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 47.Zima AV, Bovo E, Bers DM, Blatter LA. Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J Physiol. 2010;588:4743–4757. doi: 10.1113/jphysiol.2010.197913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zima AV, Bovo E, Mazurek SR, Rochira JA, Li W, Terentyev D. Ca handling during excitation-contraction coupling in heart failure. Pflugers Arch. 2014;466:1129–1137. doi: 10.1007/s00424-014-1469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zima AV, Mazurek SR. Functional Impact of Ryanodine Receptor Oxidation on Intracellular Calcium Regulation in the Heart. Rev Physiol Biochem Pharmacol. 2016;171:39–62. doi: 10.1007/112_2016_2. [DOI] [PMC free article] [PubMed] [Google Scholar]