Understanding the environmental drivers of influenza transmissibility would contribute to the early intervention and long-term control strategies of seasonal influenza, a serious public health problem that causes considerable morbidity and mortality each year. Within the burgeoning literature on influenza transmission, there are conflicting lines of evidence on the role of the environment [1]. Besides meteorological factors, it is also uncertain how common air pollutants such as ozone (O3), sulphur dioxides (SO2), nitrogen dioxide (NO2), nitric oxide (NO), and particulate matter (PM) may affect influenza transmission [2]. The objective of our study was to examine the relationship of influenza transmissibility in Hong Kong with common air pollutants and other environmental factors including UV and absolute humidity.
A number of earlier studies on the environmental drivers of influenza transmission used absolute counts of influenza cases as the dependent variable in statistical models. However, the number of incident influenza cases is not an ideal representation of influenza transmission intensity [3]. We estimated the daily effective reproduction number (Rt), a real-time measure of transmissibility, for each influenza type/subtype using data from the subtropical city of Hong Kong which has excellent influenza surveillance data, near year-round circulation of influenza, and considerable variations in environmental factors and pollutant levels. We combined information on influenza-like illnesses in the community and laboratory surveillance data to estimate weekly incidence rates of influenza virus infections in the community, referred to as ILI+ rates [4]. In theory this time series should be a linear correlate of the incidence rate of infections in the community [4], and it was previously shown that there was a very close correlation between this measure and laboratory confirmed H1N1pdm09 hospitalizations in Hong Kong in 2009-10 [5]. Finally, we multiplied the weekly ILI+ rates by a large constant, representing the inverse of the coverage of the sentinel sites in Hong Kong, and rounded to the nearest integer to obtain a time series of weekly ILI+ counts (Figure 1a-d). This was then interpolated to daily ILI+ counts using splines. During the study period of January 1998 through December 2013, we identified 44 distinct influenza epidemics, including 16 epidemics of seasonal influenza A(H3N2), 10 of A(H1N1), 4 of A(H1N1pdm09), and 14 of influenza B (Figure 1a-d). Daily concentrations of major air pollutants in 10 local monitoring stations were used to calculate the territory-wide daily average concentrations for Hong Kong. Meteorological data were obtained from the Hong Kong Observatory.
Figure 1.
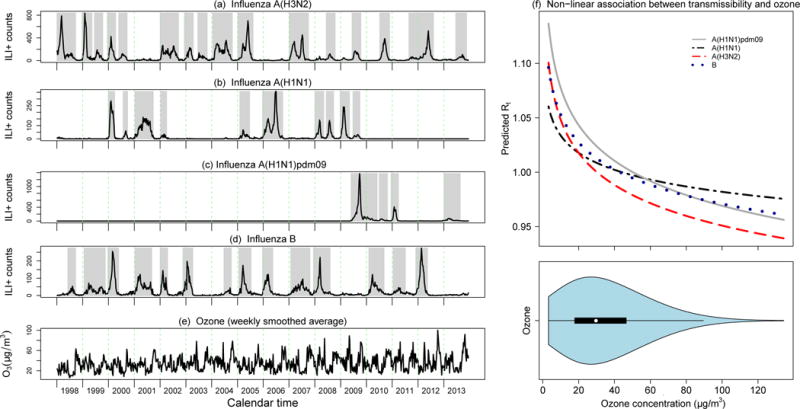
(a-e) weekly activity of influenza (ILI+ proxy) by virus type/subtype (black lines) along with the 44 predefined epidemics (gray bars), and the weekly smoothed average of ozone concentrations in Hong Kong from 1998 through 2013. (f) Estimated nonlinear relationship between the effective reproduction number Rt and ambient daily ozone concentrations in the regression analysis (based on the selected best-fitting lag of 5 days for A(H3N2), 6 days for A(H1N1), 7 days for A(H1N1)pdm09 and 4 days for influenza B) for influenza A(H3N2), A(H1N1) prior to 2009, A(H1N1)pdm09 from 2009 onwards, and influenza B. The violin plot shown in the lower panel indicates the distribution of daily ambient ozone concentrations; the median is indicated by the white circle, the interquartile range is indicated by the black rectangle, and the blue area displays a kernel density estimate of the distribution of values (i.e. a smoothed histogram).
Transmissibility can be measured by the effective (or instantaneous) reproduction number (Rt) as an unit-free index of outbreak intensity, defined as the average number of secondary infections caused by a typical single infectious person at time Rt from daily ILI+ counts for each influenza type/sub-type. We adopted a simple branching process model [6] to estimate daily Rt values. We assumed a Gamma distribution for the serial interval with mean values of 3.08 (SD=1.39) for influenza A(H1N1pdm09), 3.26 (SD=1.93) for A(H1N1), 3.48 (SD=1.88) for A(H3N2) and 3.72 (SD=1.95) for influenza B [7].
We used regression models to explore the association between influenza transmissibility, measured by the daily estimated effective reproductive numbers (Rt) for up to 8 weeks either side of each epidemic peak, and various pollutant factors with 0-7 days lag values. In non-linear univariate regression analysis, we found that Rt had statistically significant negative association with ambient O3 across all the types/sub-types; NO and CO had a weak positive association with influenza transmissibility, while other pollutants had no consistent patterns and the estimated effects were generally not statistically significant. The estimated non-linear effect of ozone on influenza transmissibility is shown in Figure 1f. The multivariable regression (DLM, dlmn package in R) model that included depletion of susceptibles, inter-epidemic factors, absolute humidity and ambient ozone could explain 40% of the observed variation in Rt for seasonal influenza A(H3N2), 35% for seasonal influenza A(H1N1), 60% for A(H1N1)pdm09 and 21% for influenza B. With a large proportion of the variance explained by the intrinsic factors and absolute humidity in the basic model for influenza transmissibility, the ambient ozone contributed only marginally, explaining a further 4% of the total variance in influenza transmissibility for H3N2 and up to 1% for the other three influenza types/subtypes. A permutation analysis indicated that the association was not likely to be due to chance (data not shown). While the proportion of variance in influenza transmissibility explained by ozone is modest, this could still correspond to a substantial effect on incidence in a single epidemic which includes many transmission events [8]. In Hong Kong, seasonal influenza often exhibits twice-annual peaks in periods from July to August (summer) and from January to March (late-winter/early-spring) which generally coincide with two troughs of ozone concentration seasonality (Figure 1a-e).
The association of ambient ozone with reduced influenza transmissibility may be related to ozone’s virucidal activity and the effect of ozone on the host defense. Ozone inactivation of influenza virus within a few hours has been reported in studies in vitro [9]. However, a more plausible mechanism underlying the association of ozone with a reduction in influenza transmissibility is ozone-primed immunity against influenza virus infection. Inhalation of ambient ozone can enhance pulmonary innate immunity that promote allergic responses in healthy human subjects and susceptible populations [10]. It is not likely for ozone as an oxidant gas to be directly recognized by a discrete receptor; ozone-induced inflammation is probably mediated by a secondary messenger. One such candidate is IL-33. Induced by ozone exposure, IL-33 further activates type 2 cytokines in the lung. IL-33 appears to be the common denominator for the list of asthma triggers including allergy, viral infection, and O3 [11]. As a multifaceted cytokine, however, IL-33 plays not just a pathogenic role in Th-2 mediated diseases but also drives TH 1 and CD8 T cell responses that induce protective immunity against viral infections [12]. In the case of influenza, IL-33 promotes lung tissue homeostasis during viral infection [13]. Used as an adjuvant in influenza vaccines, IL-33 increases the Ag-specific CD4 and CD8 T cell responses in preclinical settings [14].
One limitation of the present study was the interpolation of daily ILI+ counts from the weekly data. The day-to-day variation in transmissibility might have been reduced because of this interpolation, leading to underestimated effects of the drivers for influenza. If available, using ILI+ data at a daily scale would improve the estimates. Another limitation is that the territory-wide daily average calculation might introduce measurement errors for certain pollutants such as NO2 and CO which have a relatively large spatial variability. However, if the spatial variability did not change systematically with time, the aggregated exposure measurement should not bias the study findings based on territory-wide time-series data of both influenza and environmental drivers. As a highly reactive oxidant air pollutant, O3 may decrease host defenses against bacterial and fungal infections in the airways and aggravate pre-existing diseases such as asthma. In the case of influenza, however, ambient O3 had not been consistently associated with hospital admissions or emergency department visits for influenza virus infections according to the review by the United States Environmental Protection Agency in 2013 [10]. Our current findings of reduced influenza transmissibility associated with ambient ozone in Hong Kong warrants further study.
Acknowledgments
The authors thank Julie Au for technical assistance.
Funding
This work was financially supported by grants from the Health and Medical Research Fund (grant no. 17161212), the National Institute of General Medical Sciences (grant no. U54 GM088558), and the Hong Kong Research Grants Council (project no. T11-705/14 N). DH was supported by the Early Career Scheme from Hong Kong Research Grants Council (PolyU 251001/14M).
Potential conflicts of interest
BJC received research funding from Sanofi Pasteur for a study of influenza vaccine effectiveness.
Footnotes
Author contributions
BJC and LT designed the study. STA, PW, VJF and LT collected data. STA analysed data. STA wrote the first draft, and all authors contributed to review and revision and have seen and approved the final version.
References
- 1.Sooryanarain H, Elankumaran S. Environmental role in influenza virus outbreaks. Annu Rev Anim Biosci. 2015;3:347–373. doi: 10.1146/annurev-animal-022114-111017. [DOI] [PubMed] [Google Scholar]
- 2.Loveren HV, Rombout PJA, Fischer PH, Lebret E, Van Bree L. Modulation of host defenses by exposure to oxidant air pollutants. Inhal Toxicol. 1995;7 [Google Scholar]
- 3.Te Beest DE, Van Boven M, Hooiveld M, Van Den Dool C, Wallinga J. Driving factors of influenza transmission in the netherlands. Am J Epidemiol. 2013;178:1469–1477. doi: 10.1093/aje/kwt132. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein E, Viboud C, Charu V, Lipsitch M. Improving the estimation of influenza-related mortality over a seasonal baseline. Epidemiology. 2012;23:829–838. doi: 10.1097/EDE.0b013e31826c2dda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong JY, Wu P, Nishiura H, Goldstein E, Lau EHY, Yang L, Chuang SK, Tsang T, Peiris JSM, Wu JT, Cowling BJ. Brief Original Contribution Infection Fatality Risk of the Pandemic A (H1N1) 2009 Virus in Hong Kong. Am J Epidemiol. 2013;177:834–840. doi: 10.1093/aje/kws314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cori A, Ferguson NM, Fraser C, Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178:1505–1512. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy JW, Cowling BJ, Simmerman JM, Olsen SJ, Fang VJ, Suntarattiwong P, Jarman RG, Klick B, Chotipitayasunondh T. The serial intervals of seasonal and pandemic influenza viruses in households in Bangkok, Thailand. Am J Epidemiol. 2013;177:1443–1451. doi: 10.1093/aje/kws402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dushoff J, Plotkin JB, Levin SA, Earn DJD. Dynamical resonance can account for seasonality of influenza epidemics. Proc Natl Acad Sci. 2004;101:16915–16916. doi: 10.1073/pnas.0407293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng C-C, Li C-S. Ozone for inactivation of aerosolized bacteriophages. Aerosol Sci Technol. 2006;40:683–689. [Google Scholar]
- 10.USEPA. Integrated science assessment for ozone and related photochemical oxidants. Fed Regist. 2013;78:11172–11173. [Google Scholar]
- 11.Mathews J, Krishnamoorthy N, Kasahara DI, Cho Y, Wurmbrand AP, Ribeiro L, Smith D, Umetsu D, Levy BD, Shore SA. IL-33 Drives Augmented Responses to Ozone in Obese Mice. Environ Health Perspect. 2016;125:246–253. doi: 10.1289/EHP272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonilla WV, Fröhlich A, Senn K, Kallert S, Fernandez M, Fallon PG, Klemenz R, Nakae S, Adler H, Merkler D. The Alarmin Interleukin-33 Drives Protective Antiviral CD8+ T Cell Responses. Science (80-) 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 13.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung tissue homeostasis following acute influenza virus infection. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarreal DO, Weiner DB. IL-33 isoforms: their future as vaccine adjuvants? Expert Rev Vaccines. 2015;14:489–492. doi: 10.1586/14760584.2015.1011135. [DOI] [PMC free article] [PubMed] [Google Scholar]


