Abstract
Dysfunctional insulin signaling is a causative factor in type 2 diabetes. While insulin signal transduction has been well investigated in many tissues, less is known in retinal tissues. We have previously reported that toll-like receptor 4 (TLR4) is involved in retinal damage in diabetes. We used TLR4 retinal Müller cell specific knockout mice and Müller cells in culture to investigate the effects of loss of TLR4 on Müller cell insulin signal transduction. Loss of TLR4 in the mouse retinal Müller cells led to increased insulin receptor and Akt phosphorylation, with reduced insulin receptor substrate 1 (IRS-1) phosphorylation on serine 307, which was associated with reduced cleavage of caspase 3. In retinal Müller cells grown in high glucose, insulin signal transduction was impaired, but these responses were reduced with cells were transfected with TLR4 siRNA. Taken together, the data suggest that TLR4 regulates insulin signal transduction in retinal Müller cells.
Keywords: Insulin signaling, Müller cells, retina, knockout mice
Introduction
Impaired insulin signaling is a causative factor in type 2 diabetes. However, the effects of the loss of insulin signal transduction on specific diabetic complications is less clear. We have previously reported that insulin signaling is impaired in retinal endothelial cells (REC) and Müller cells grown in high glucose (1, 2). In both cell types, tumor necrosis factor alpha (TNFα) is detrimental to insulin signal transduction. Increasingly, a role for inflammatory factors has been recognized to contribute to insulin resistance (3). This led us to investigate other inflammatory players with a role in insulin signaling.
Others had reported toll-like receptor 4 (TLR4) is present in ocular tissues. For example, corneal epithelial cells have TLR2/4 that is potentially involved in keratitis (4). Additionally, studies have demonstrated that TLR4 decreases Wnt signaling, leading to photoreceptor apoptosis (5). Researchers demonstrated that diabetic rats have increased TLR4, TNFα, and interleukin-1beta (IL-1β) in the whole retina (6). Results from TLR2/4 and MyD88 chimeric mice have demonstrated a major role for MyD88 signaling in the pathogenesis of diabetic leukostasis and increased inflammation (7), suggesting that immune cells may regulate retinal inflammation. Additionally, studies recently demonstrated that high glucose causes increased TLR4 signaling in human retinal endothelial cells (REC) through TLR2/4 (8). We have previously demonstrated that loss of TLR4 signaling in endothelial cell specific knockout mice led to increased insulin signaling (9).
For this study, we focused on retinal Müller cells, as a number of TLRs have been localized to Müller cells (10). Additionally, we had previously shown that high glucose causes impaired insulin signaling in the rMC-1 cell line (2). We used Müller cell specific TLR4 knockout mice and rMC-1 cells in culture to investigate whether TLR4 is key to impaired insulin signaling in Müller cells.
Materials and Methods
Mice
All animal procedures were done according to the Association for Research in Vision and Ophthalmology requirements and were approved by the Institutional Animal Care and Use Committee at Wayne State University (A-08-07-15) and conform to NIH guidelines. TLR4 floxed mice (B6(Cg)-Tlr4tm1.1Karp/J mice) and PDGFRα-Cre (C57BL/6-Tg(Pdgfra-cre)1Clc/J ) mice were purchased from Jackson Laboratories. TLR4 floxed mice were bred with the TLR4-PDGFRα-Cre mice to generate conditional knockout mice in which TLR4 is eliminated in retinal Müller cells. Mice of both sexes were used at 3 months of age. Genotyping and verification of knockout has been previously published (11).
Rat retinal Müller cells
Retinal Müller Cells were kindly supplied by Dr. Vijay Sarthy at Northwestern University. Rat retinal Müller cells (rMC-1) were cultured and passaged in DMEM medium (Invitrogen, Carlsbad, CA) containing either 5mM glucose (low glucose) or 25mM glucose (high glucose) with 10% FBS. When the cells reached 80% confluence and after a minimum of 3 days in high glucose, TLR4 siRNA (10nM) was transfected into the cells using GenMute (SignaGen, Rockville, MD). TLR4 siRNA ON-TARGETplus SMART pool and the corresponding non-targeting siRNA were purchased from Dharmacon (Lafayette, CO). Cells were collected 24 hours after transfection for experiments.
Western blotting
After appropriate treatments, retinal Müller cells or retinal lysates were collected into lysis buffer containing protease and phosphatase inhibitors. Equal amounts of protein from the cell or tissue extracts were separated on the pre-cast tris-glycine gel (Invitrogen, Carlsbad, CA), blotted onto a nitrocellulose membrane. After blocking in TBST (10 mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (w/v) BSA, the membrane was treated with primary antibodies to insulin receptor, IRTyr1150/1151, IRS-1, IRS-1Ser307, Akt, AktSer473, and cleaved caspase 3 (Cell Signaling, Danvers MA) at a 1:500 dilution followed by incubation with horseradish peroxidase labeled secondary antibodies (1:5000). The antigen-antibody complexes were detected using chemilluminescence reagent kit (Thermo Scientific). Mean densitometry of immunoreactive bands was assessed using a C500 imager (Azure Biosystems), and results were expressed in densitometric units and compared to control groups for each individual experiment. For the phosphorylated antibodies, the ratio of phosphorylated to total protein levels are presented.
ELISA
A cleaved caspase 3 ELISA (Cell Signaling, Danvers, MA) was done according to manufacturer’s instructions. For the cleaved caspase 3 ELISA, equal protein concentrations were loaded into each well to allow for analyses based on the optical density measurement.
Statistics
All the experiments were repeated in triplicate, and the data are presented as mean ± SEM. Data was analyzed by Kruskal-Wallis non-parametric test followed by Dunn’s test with p-values <0.05 considered statistically significant. In the case of Western blotting, a representative blot is shown.
Results
Insulin signaling is increased in Müller cell specific TLR4 knockout mice
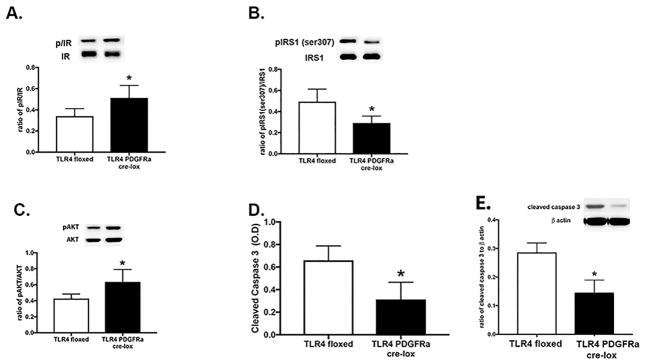
We recently reported that TLR4 signaling is reduced in retinal endothelial cell specific knockout mice (11). Due the link between inflammatory mediators and insulin signaling, we asked whether loss of TLR4 in Müller cells would alter insulin signaling proteins. Figure 1A shows the insulin receptor phosphorylation is significantly increased in retinal lysates from TLR4 knockout mice compared to floxed only mice. Subsequently, Akt phosphorylation is also increased in the TLR4 knockout mice (Figure 1C). IRS-1Ser307 phosphorylation was decreased (Figure 1B), as was the cleavage of caspase 3 (Figure 1D) in the TLR4 Müller cell knockout mice. Figure 1E confirms the ELISA data using Western blotting for cleaved caspase 3. Taken together, the data from mice suggest that TLR4 can regulate insulin signaling in retinal Müller cells.
Figure 1.
Western blot results for TLR4 floxed and TLR4 Müller cell specific Cre-Lox mice for insulin receptor (A), IRS-1Ser307 (B), Akt (C) phosphorylation ratios. Panel D is ELISA results for cleaved caspase 3 in the mice. Panel E is a Western blot for cleaved caspase 3 in the mice. *P<0.05 vs. TLR4 floxed. N=5 for all experiments. Data are mean± SEM.
TLR4 siRNA reduced TLR4 levels in retinal Müller cells
In order to confirm the data from mice, we used rMC-1 cells transfected with TLR4 siRNA. Figure 2 shows that high glucose increased TLR4 levels in the Müller cells. TLR4 siRNA was highly effective in reducing TLR4 levels in the cells.
Figure 2.
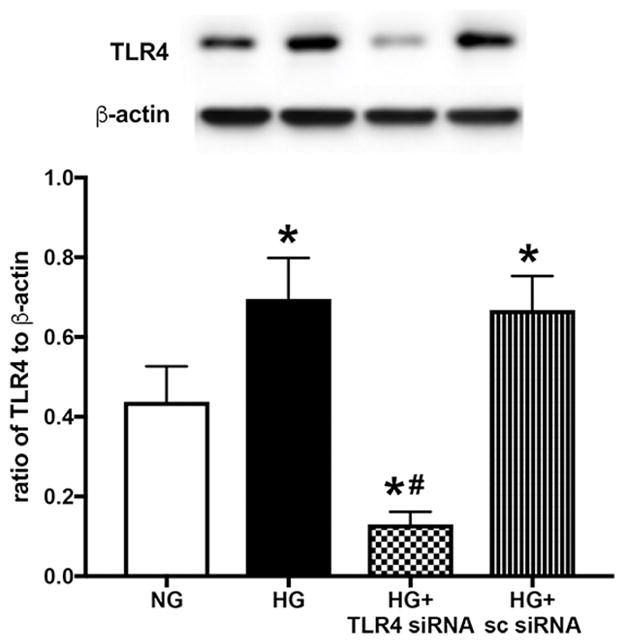
Western blot results for TLR4 in rMC-1 cells grown in normal glucose (NG) or high glucose (HG) or high glucose+TLR4 siRNA or scrambled siRNA. *P<0.05 vs. NG, #P<0.05 vs HG. N=3–4 for all groups. Data are mean± SEM.
Loss of TLR4 increased insulin receptor phosphorylation in vitro
In agreement with our data from the conditional knockout mice, we found that loss of TLR4 also increased insulin receptor phosphorylation in Müller cells grown in high glucose (Figure 3A). We also found that high glucose increased IRS-1Ser307 phosphorylation, which was reduced when cells had TLR4 levels blocked (Figure 3B). No changes in total insulin receptor or IRS-1 were noted.
Figure 3.
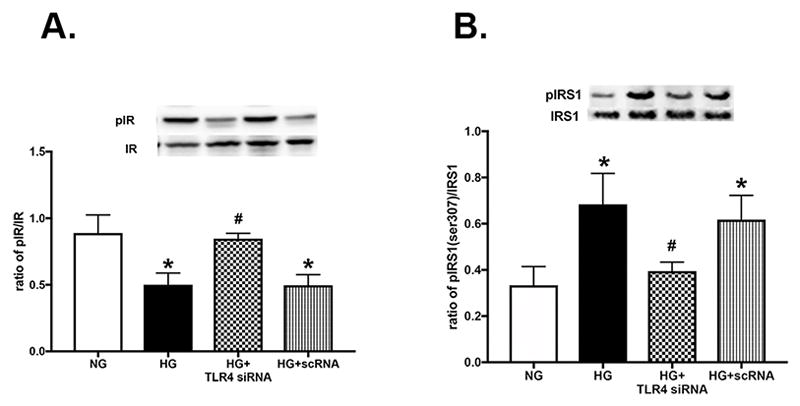
Western blot results for insulin receptor (A) and IRS-1Ser307 (B) phosphorylation in rMC-1 cells grown in normal glucose (NG) or high glucose (HG) or high glucose+TLR4 siRNA or scrambled siRNA. *P<0.05 vs. NG, #P<0.05 vs HG. N=3–4 for all groups. Data are mean± SEM.
TLR4 regulates Akt phosphorylation in Müller cells
Since insulin receptor activation should lead to increased Akt phosphorylation and decreased cleavage of caspase 3, we also measured Akt and caspase 3 in the Müller cells transfected with TLR4 siRNA. Figure 4A shows that loss of TLR4 increased Akt phosphorylation, which led to a decrease in the cleavage of caspase 3 (Figure 4B). Figure 4C confirms decreased cleaved caspase 3 levels in the TLR4 siRNA treated cells by Western blot. No changes in total Akt were observed. In conclusion, our findings suggest that TLR4 can inhibit insulin signaling, and upon blockade of TLR4 in Müller cells, insulin signaling is restored, despite high glucose culturing conditions.
Figure 4.
Western blot results for Akt phosphorylation (A) and cleaved caspase 3 (C) and ELISA data for cleaved caspase 3 (B) in rMC-1 cells grown in normal glucose (NG) or high glucose (HG) or high glucose+TLR4 siRNA or scrambled siRNA. *P<0.05 vs. NG, #P<0.05 vs HG. N=3–4 for all groups. Data are mean± SEM.
Discussion
Impaired insulin signaling is a key causative factor in type 2 diabetes (12, 13). Since the rates of type 2 diabetes-related complications are expected to reach epidemic levels in the next few decades, it is imperative that we develop a better understanding of the regulation of insulin signal transduction, particularly in organs with high diabetes complication rates. We have previously reported that high glucose culturing conditions decrease normal insulin signaling in retinal Müller cells, which were restored toward baseline by treatment with a β-adrenergic receptor agonist (2). We also demonstrated that blockade of IRS-1 caused apoptosis of retinal Müller cells, with a focus on IRS-1Ser307 as a key site for impaired insulin signaling (14). Our previous work suggested that retinal Müller cells were highly responsive to insulin signaling.
We had also observed that β-adrenergic receptors inhibited TLR4 signaling in both retinal endothelial cells and Müller cells (15). We recently reported that endothelial cell specific knockout of TLR4 led to improved insulin signaling (9), which was not observed in TLR4 overexpressing mice. For this study, we wanted to combine our previous studies on retinal Müller cells and insulin signaling to investigate the actions of TLR4 in retinal Müller cells. Using Müller cell specific knockout mice for TLR4, we found that loss of TLR4 increased insulin receptor and Akt phosphorylation. This was associated with a decrease in IRS-1Ser307 phosphorylation and decreased cleaved caspase 3 levels. These findings agree well with the work in REC. To extend the mouse work into cell culture, we treated rMC-1 cells in normal or high glucose with TLR4 siRNA. Work in rMC-1 cells in culture provided findings that matched the work in mice. High glucose culturing conditions significantly impaired insulin receptor and Akt phosphorylation, while increasing IRS-1Ser307 phosphorylation and the cleavage of caspase 3. All of the changes induced by high glucose were reduced/reversed when cells were treated with TLR4 siRNA, suggesting that high glucose induced TLR4 to mediate dysfunctional insulin signaling in retinal Müller cells. These findings agree with our previous work in Müller cells, as well as others work in other retinal cell types (9, 16). Future work will include making the Müller cell specific knockout mice diabetic with a high fat diet to better mimic type 2 diabetes in vivo.
Taken together, our findings show that loss of TLR4 improves insulin signal transduction in retinal Müller cells both in mouse retina and in cell culture. These findings suggest that cell-specific inhibition of TLR4 signaling may offer a new target for therapeutic development, particularly for type 2 diabetic retinal disease.
Acknowledgments
This work was supported by R01EY022330 (JJS), P30EY04068 (PI: Hazlett) and an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness (Kresge Eye Institute).
Footnotes
Conflict of Interest: No authors have any conflicts of interest with these studies.
Author Contributions: Data collection-LL; Mice maintenance-LL; experimental design-JS; wrote the paper-JS; reviewed final text-LL, JS
References
- 1.Jiang Y, Zhang Q, Soderland C, Steinle JJ. TNFalpha and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cell Signal. 2012;24:1086–92. doi: 10.1016/j.cellsig.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker RJ, Anderson NM, Jiang Y, Bahouth S, Steinle JJ. Role of beta-adrenergic receptors regulation of TNF-alpha and insulin signaling in retinal Muller cells. Invest Ophthalmol Vis Sci. 2011;52:9527–33. doi: 10.1167/iovs.11-8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan PS, Kanwar M, Kowluru RA. Resistance of retinal inflammatory mediators to suppress after reinstitution of good glycemic control: novel mechanism for metabolic memory. J Diabetes Complications. 2010;24:55–63. doi: 10.1016/j.jdiacomp.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jie Z, Wu XY, Yu FS. Activation of Toll-like receptors 2 and 4 in Aspergillus fumigatus keratitis. Innate Immun. 2009;15:155–68. doi: 10.1177/1753425908101521. [DOI] [PubMed] [Google Scholar]
- 5.Yi H, Patel AK, Sodhi CP, Hackam DJ, Hackam AS. Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis. PLoS One. 2012;7:e36560. doi: 10.1371/journal.pone.0036560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YL, Wang K, Yu SJ, Li Q, Li N, Lin PY, Li MM, Guo JY. Association of the TLR4 signaling pathway in the retina of streptozotocin-induced diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2015;253:389–98. doi: 10.1007/s00417-014-2832-y. [DOI] [PubMed] [Google Scholar]
- 7.Tang J, Allen Lee C, Du Y, Sun Y, Pearlman E, Sheibani N, Kern TS. MyD88-dependent pathways in leukocytes affect the retina in diabetes. PLoS One. 2013;8:e68871. doi: 10.1371/journal.pone.0068871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajamani U, Jialal I. Hyperglycemia induces Toll-like receptor-2 and -4 expression and activity in human microvascular retinal endothelial cells: implications for diabetic retinopathy. J Diabetes Res. 2014;2014:790902. doi: 10.1155/2014/790902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Liu L, Jiang Y, Curtiss E, Fukuchi KI, Steinle JJ. TLR4 regulates insulin-resistant proteins to increase apoptosis in the mouse retina. Inflamm Res. 2017 doi: 10.1007/s00011-017-1080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin X, Fang D, Zhou H, Su SB. The expression of Toll-like receptors in murine Muller cells, the glial cells in retina. Neurol Sci. 2013;34:1339–46. doi: 10.1007/s10072-012-1236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Steinle JJ. Loss of TLR4 in mouse Muller cells inhibits both MyD88-dependent and -independent signaling. PLoS One. 2017;12:e0190253. doi: 10.1371/journal.pone.0190253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajala RV, McClellan ME, Chan MD, Tsiokas L, Anderson RE. Interaction of the retinal insulin receptor beta-subunit with the p85 subunit of phosphoinositide 3-kinase. Biochemistry. 2004;43:5637–50. doi: 10.1021/bi035913v. [DOI] [PubMed] [Google Scholar]
- 13.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–91. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 14.Walker RJ, Anderson NM, Bahouth S, Steinle JJ. Silencing of insulin receptor substrate-1 increases cell death in retinal Muller cells. Mol Vis. 2012;18:271–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Berger EA, Carion TW, Jiang Y, Liu L, Chahine A, Walker RJ, Steinle JJ. beta-adrenergic receptor agonist, Compound 49b, inhibits TLR4 signaling pathway in diabetic retina. Immunol Cell Biol. 2016 doi: 10.1038/icb.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajala A, Tanito M, Le YZ, Kahn CR, Rajala RV. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J Biol Chem. 2008;283:19781–92. doi: 10.1074/jbc.M802374200. [DOI] [PMC free article] [PubMed] [Google Scholar]