Summary
This case describes an unusual presentation of prosthetic valve endocarditis (PVE): an acute coronary syndrome. A 67-year-old male presented with cardiac sounding chest pain on a background of a short history of night sweats, weight loss and general malaise. Four months previously, he had undergone bio-prosthetic aortic valve replacement for severe aortic stenosis and single vessel bypass grafting of the obtuse marginal. Whilst having chest pain, his ECG showed infero-lateral ST depression. Early coronary angiography revealed a new right coronary artery (RCA) lesion that was not present prior to his cardiac surgery. Using multi-modality cardiac imaging, the diagnosis of PVE was made. An aortic root abscess was demonstrated that was causing external compression of the RCA.
Learning points:
PVE accounts for up to 20% of all cases of infective endocarditis.
High clinical suspicion and early blood cultures before empirical antibiotics are key as the presentation of PVE can often be atypical.
PVE rarely presents as an acute coronary syndrome. Potential mechanisms by which PVE may result in an ACS include coronary embolization, obstruction of coronary ostia by a large mobile vegetation and external coronary artery compression from an infective aneurysms/abscess.
Repeat cardiac surgery is often required for high-risk PVE such as those caused by staphylococcal infection or severe prosthetic dysfunction.
Keywords: prosthetic valve endocarditis, acute coronary syndrome, aortic root abscess, aortic valve replacement
Background
This is a very unusual presentation of prosthetic valve endocarditis (PVE) as an acute coronary syndrome (ACS). This case highlights the importance of taking a detailed history to reach the diagnosis supported by blood cultures and echocardiography.
Case presentation
A 67-year-old male presented to our emergency department after a significant episode of cardiac chest pain. Four months prior to this presentation, he had undergone bio-prosthetic aortic valve replacement (21 mm perimount magna ease) for severe aortic stenosis with a single saphenous vein graft to the obtuse marginal (OM) artery. His other notable past medical history included treated hypertension, percutaneous intervention to the posterior descending artery (PDA) and OM two years previously and monoclonal gammopathy of undetermined significance.
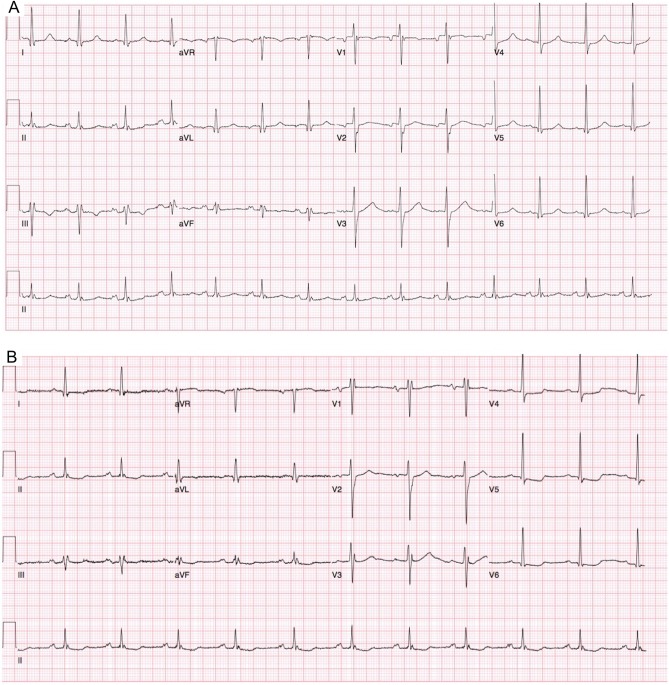
Clinical examination found him to be diaphoretic with soft systolic murmur in the aortic area. His resting 12-lead ECG on admission showed normal sinus rhythm (Fig. 1A). His high-sensitivity troponin assay was positive at 185 ng/L (normal values: 0–34 ng/L). He had further chest pain an hour later, whilst in the emergency department. His second ECG during this episode of chest pain showed ST depression in the infero-lateral leads (Fig. 1B).
Figure 1.
12-Lead ECG showing. (A) Normal sinus rhythm with no acute ST-T changes. (B) ST depression infero-laterally.
In view of ongoing chest pain and ischaemic changes on his ECG, he was transferred to the cardiac catheter laboratory for urgent coronary angiography.
A brief history prior to angiography revealed that the patient had been unwell for several weeks before this hospital attendance. He reported extreme fatigue, night sweats and weight loss. PVE was considered as a differential diagnosis for his presentation.
Investigation
Baseline haematology revealed an anaemia with a haemoglobin count of 95 g/L (normal values: 130–180 g/L), leucocytosis of 11.5 × 109/L (normal values 4–11). High-sensitivity troponin I was elevated at 185 ng/L (normal values: 0–34 ng/L) and the C-reactive protein was elevated at 167 (normal values: less than 5).
Pre-surgery, coronary angiography had revealed an unobstructed right coronary artery (RCA) with a patent stent in the PDA. There was no significant disease in the left main stem (LMS) or left anterior descending (LAD) artery, but there was significant in-stent restenosis within the stent in the OM.
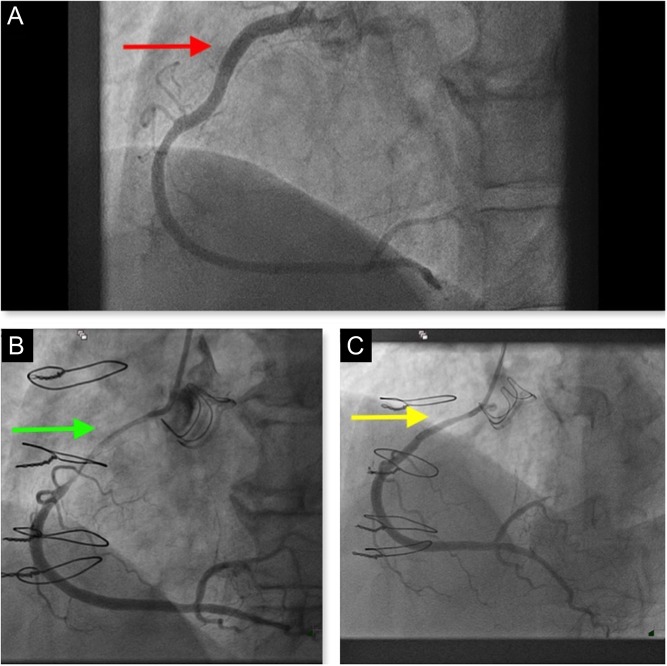
Now coronary angiography showed an unchanged appearance of the LMS, LAD and OM. There was a patent vein graft supplying the OM territory. Surprisingly, angiography of the RCA showed a new severe stenosis extending from the proximal to mid-vessel (Fig. 2A, B, Videos 1 and 2).
Figure 2.
Coronary angiogram showing. (A) Pre-AVR coronary appearance. (B) Severe stenotic appearance in the proximal to mid-RCA. (C) Stenotic appearance improved significantly following intracoronary nitrates.
Coronary angiogram (LAO view) showing severe stenosis in the RCA extending from the proximal to mid-vessel. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-1.
Download Video 1 (4.7MB, avi)
Coronary angiogram (pre-AVR) showing unobstructed RCA with a patent stent in the PDA. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-2.
Download Video 2 (12.6MB, avi)
Optical coherence tomography (OCT) of the RCA was performed. This showed a normal trilaminar appearance of the proximal and mid-RCA. There was no evidence of plaque rupture or intracoronary thrombosis (Video 3). The RCA appearance improved with intracoronary nitrate (Fig. 2C) but did not normalize. Coronary intervention was not performed. External compression of the proximal RCA by an aortic root abscess was suspected.
Optical coherence tomography (OCT) demonstrating normal trilaminar appearance of the proximal and mid-RCA with no evidence of plaque rupture or intracoronary thrombosis. View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-3.
Download Video 3 (163.2MB, avi)
Blood cultures were taken in the cardiac catheter laboratory and patient was admitted to coronary care unit.
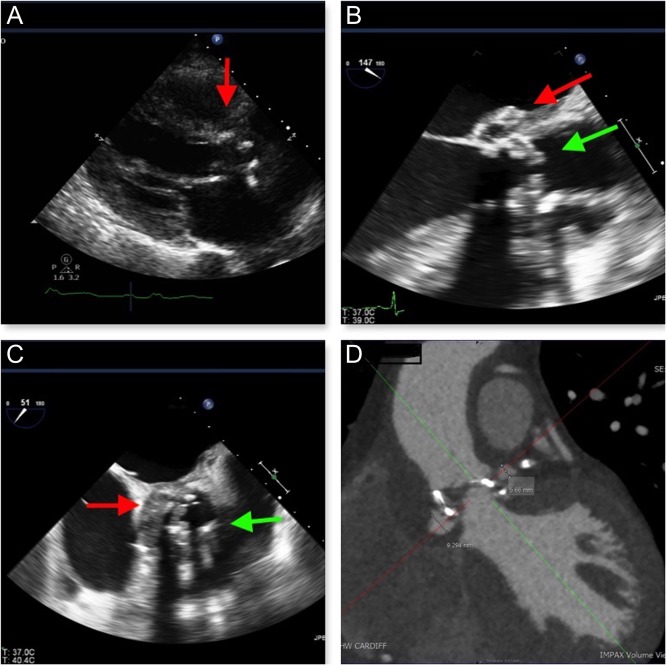
Transthoracic echocardiography (TTE) showed an abnormal appearance around the prosthetic AVR (Fig. 3A and Video 4).
Figure 3.
(A) TTE (PLAX view) demonstrating an abnormal appearance of the prosthetic AVR. (B and C) TOE demonstrating vegetation’s attached to the prosthetic AVR (green arrow) and an aortic root abscess (red arrow). (D) Cardiac CT demonstrating two false aneurysms anterior and posterior to the aortic root (yellow arrows).
TTE (PLAX view) showing an abnormal appearance of the prosthetic AVR. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-4.
Download Video 4 (1.5MB, mov)
Trans-oesophageal echocardiography (TOE) confirmed two small vegetation attached to two of the prosthetic aortic leaflets, but without significant aortic regurgitation and a large aortic root abscess (Fig. 3B, C and Video 5).
TOE demonstrating vegetation attached to the prosthetic AVR and a large aortic root abscess. View Video 5 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-5.
Download Video 5 (1.8MB, mov)
Blood cultures subsequently grew coagulase negative staphylococci sensitive to vancomycin and rifampicin.
Cardiac CT showed two false aneurysms measuring 6 mm and 9 mm anterior and posterior to the aortic root, respectively. The anterior false aneurysm cavity is situated to the right and inferior to the RCA origin and the more posterior false aneurysm cavity is seen just anterior to the left main stem/LAD junction (Fig. 3D).
Treatment and outcome
Initially, the patient responded well to antimicrobial therapy. After 2 weeks, he underwent aortic root replacement with coronary re-implantation and 19 mm Carpentier-Edwards magna ease aortic valve replacement. Cardiac surgery was complicated by extensive adhesions, bleeding and right ventricle dysfunction. Haemodynamic support with extracorporeal membrane oxygen support was required to successfully wean the patient from cardiopulmonary bypass. Unfortunately, post-operatively the patient deteriorated developing multi-organ failure. Despite increasing inotropic support and haemofiltration, he could not be stabilized. After multidisciplinary team discussion, it was decided to withdraw treatment as prognosis was extremely poor and the patient died.
Discussion
There has been a significant change in the epidemiology of endocarditis over the last decade. A retrospective, observational study conducted in 2015 found a significant rise in the incidence of staphylococcal, streptococcal, gram-negative and fungal endocarditis. The authors noted a 20% relative increase in staphylococcal endocarditis which was attributed to the steady increase in the number of invasive procedures and device implants now performed (1).
PVE is a severe form of endocarditis with a high in-hospital mortality rate (20–40%) (2). We believe that this could be an underestimate as many cases are not reported. PVE can be classified as early (endocarditis occurring within 1 year of surgery) or late (after 1 year). Staphylococci and fungi are the most common organisms associated with PVE. PVE often requires prolonged courses of antimicrobial therapy and surgery should be reserved for high-risk patient groups such as those complicated by heart failure or uncontrolled infection or to prevent systemic embolism.
The diagnosis of PVE in the setting of an ACS is difficult, as the presenting symptoms are often atypical. A high index of suspicion and careful history taking is crucial. Possible mechanisms for PVE to present as an ACS include coronary embolization, obstruction of coronary ostia by a large mobile vegetation and external coronary artery compression from an infective aneurysms/abscess (3). Of these, external coronary compression is the least common with only few case reports available in the literature (4, 5, 6, 7).
The treatment of ACS resulting from bacterial endocarditis is challenging and there is no consensus as to what represents optimum therapy. Conventional treatment for ACS includes dual anti-platelet therapy and anticoagulation; this can be hazardous in patients with PVE potentially resulting in haemorrhagic complications of embolism in remote organs (8). Percutaneous coronary intervention (PCI) and stent implantation may be complicated by infection seeding of the intracoronary stent, distant embolization or the development of coronary mycotic aneurysms (9). Generally, early cardiac surgery is recommended in patients with bacterial endocarditis who develop ACS, and PCI should be reserved for unstable patients with ongoing ischaemia.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this case report.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent has been obtained from the patient.
Author contribution statement
B S performed coronary angiogram and drafted the manuscript. R W performed TOE and helped in manuscript preparation. N M and S G are the cardiologists-in-charge of the patient and critically revised the manuscript.
References
- 1.Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the united states from 2000 to 2011. Journal of the American College of Cardiology 2015. 65 2070–2076. ( 10.1016/j.jacc.2015.03.518) [DOI] [PubMed] [Google Scholar]
- 2.Habib G, Lancellotti O, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, et al 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). European Heart Journal 2015. 36 3075–3128. ( 10.1093/eurheartj/ehv319) [DOI] [PubMed] [Google Scholar]
- 3.Murtaza G, Rahman ZU, Sitwala P, Ladia V, Barad B, Albalbissi K, Paul TK, Ramu V. Case of acute ST segment elevation myocardial infarction in infective endocarditis – management with intra coronary stenting. Clinics and Practice 2017. 7 950 ( 10.4081/cp.2017.950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke NR, Forfar JC. Aortic root abscess presenting as unstable angina due to extrinsic compression of the left coronary artery. Postgraduate Medical Journal 2002. 78 168–169. ( 10.1136/pmj.78.917.168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harinstein ME, Marroquin OC. External coronary artery compression due to prosthetic valve bacterial endocarditis. Catheterization and Cardiovascular Interventions 2014. 83 E168–E170. ( 10.1002/ccd.24578) [DOI] [PubMed] [Google Scholar]
- 6.Parashara DK, Jacobs LE, Kotler MN, Yazdanfar S, Spielman SR, Janzer SF, Bemis CE. Angina caused by systolic compression of the left coronary artery as a result of pseudo aneurysm of the mitral-aortic intervalvular fibrosa. American Heart Journal 1995. 129 417–421. ( 10.1016/0002-8703(95)90031-4) [DOI] [PubMed] [Google Scholar]
- 7.Rogers JG, McManus BM, Cassling RS, Dobry CA, Fleming WH. False aneurysm of the aortic root: mimic of malignancy, mediator of myocardial ischaemia. American Heart Journal 1987. 113 1237–1239. ( 10.1016/0002-8703(87)90944-6) [DOI] [PubMed] [Google Scholar]
- 8.Fabri J, Jr, Issa VS, Pomerantzeff PM, Grinberg M, Barretto AC, Mansur AJ. Time-related distribution, risk factors and prognostic influence of embolism in patients with left-sided infective endocarditis. International Journal of Cardiology 2006. 110 334–339. ( 10.1016/j.ijcard.2005.07.016) [DOI] [PubMed] [Google Scholar]
- 9.Singh M, Mishra A, Kaluski E. Acute ST-elevation myocardial infarction due to septic embolism: a case report and review of management options. Catheterization and Cardiovascular Interventions 2015. 85 E166–E171. ( 10.1002/ccd.25829) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronary angiogram (LAO view) showing severe stenosis in the RCA extending from the proximal to mid-vessel. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-1.
Download Video 1 (4.7MB, avi)
Coronary angiogram (pre-AVR) showing unobstructed RCA with a patent stent in the PDA. View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-2.
Download Video 2 (12.6MB, avi)
Optical coherence tomography (OCT) demonstrating normal trilaminar appearance of the proximal and mid-RCA with no evidence of plaque rupture or intracoronary thrombosis. View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-3.
Download Video 3 (163.2MB, avi)
TTE (PLAX view) showing an abnormal appearance of the prosthetic AVR. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-4.
Download Video 4 (1.5MB, mov)
TOE demonstrating vegetation attached to the prosthetic AVR and a large aortic root abscess. View Video 5 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-18-0022/video-5.
Download Video 5 (1.8MB, mov)



 This work is licensed under a
This work is licensed under a