Abstract
Adeno-associated virus (AAV) vector is a promising platform technology for ocular gene therapy. Recently clinical successes to treat choroidal neovascularization (CNV) in wet type age-related macular degeneration have been reported. However, because pathologic conditions of the retina may alter the tropism of viral vectors, it is necessary to evaluate the transduction efficiency of different serotypes of AAV vectors in the retinas with CNVs. Here, we show the patterns and efficacy of transduction of AAV2, -5, and -8 vectors in a laser-induced CNV mouse model. C57BL/6J mice were subjected to unilateral laser photocoagulation on the right eye to induce CNV 5 days prior to intravitreal injection of AAV2, -5, and -8 capsids expressing EGFP. Transduction was increased around CNV lesions for all AAV capsid types, and AAV2 resulted in the highest transduction efficiency. In the absence of CNV, the AAV2 vector transduced ganglion and inner nuclear layer (INL) cells, and AAV5 and AAV8 transduced only a small proportion of cells in the retinal ganglion cell layer. CNV increased AAV2 vector expression throughout the retina and in and around CNVs; the transduced cells included retinal ganglion cells, Müller cells, cells from the INL and outer nuclear layer (ONL), photoreceptors, and retinal pigment epithelium (RPE) cells. Inflammatory cells and endothelial cells in CNVs were also transduced by AAV2. AAV5 and AAV8 were transduced in retinal ganglion, Müller, INL, ONL, and RPE cells in a localized pattern, and only endothelial cells at the surface of CNV lesions showed EGFP expression. Taken together, CNV formation resulted in enhanced transduction of AAV2, -5, and -8, and AAV2 exhibited the highest transduction efficiency in cells in CNV lesions.
Keywords: adeno-associated virus, choroidal neovascularization, age-related macular generation, gene therapy
Introduction
Adeno-associated virus (AAV) is one of the most widely used viral vectors for ocular delivery of gene therapy. Since the first clinical success with AAV in treating Leber’s congenital amaurosis in 2008,1, 2, 3 the efficacy of gene therapy for other retinal diseases, such as diabetic retinopathy and age-related macular degeneration (AMD), has been investigated.4, 5, 6, 7, 8, 9
It is quite evident that AAV vectors are the most promising delivery method for gene therapy, and several serotypes can transduce genes into specific cell types organizing neural retina by intravitreal or subretinal administration. For example, intravitreal injection of AAV2 led to the transduction of retinal ganglion cells,10 and subretinal administration of AAV8 resulted in transduction of photoreceptor cells.11 The tropisms of AAV serotypes for chorioretinal tissues were established at the beginning of the ocular gene therapy era, and it is recently shown that certain diseases may enhance the transduction efficiency of specific AAV serotypes.5, 10, 12 Therefore, the optimum AAV serotype and route of administration for different retinal diseases must be determined to maximize the therapeutic effect.
Recently, neovascular AMD (nAMD) has gained much attention as a possible candidate for ocular gene therapy. Repeated injection of intravitreal anti-vascular endothelial growth factor (VEGF) agents is currently considered the optimum treatment for nAMD.13 However, the economic burden of monthly treatments and resistance to anti-VEGF therapy are issues in the management of nAMD; therefore, novel therapies need to be developed. We recently demonstrated that AAV-mediated mammalian target of rapamycin (mTOR) inhibition by short hairpin RNA (shRNA) (AAV-mTOR shRNA) resulted in increased autophagy and suppressed choroidal neovascularization (CNV) in the mouse retina, suggesting potential for nAMD gene therapy.14 Additionally, intravitreal administration of AAV2 resulted in transduction of CD31+ cells in CNV lesions. However, only AAV2 was evaluated in that study. When cell-specific therapeutic effects are taken into account, such as autophagy, the viral tropism that results in the highest efficiency of transduction of the target retinal cells should be ascertained.
In this study, we investigated the transduction efficacy of three commonly used AAV serotypes, AAV2, -5, and -8, for neuroretinal targeting in mouse retinas with CNV. In addition, we examined the ability of the three AAV serotypes to transduce various retinal cell types.
Results
Enhanced AAV Transduction Efficiencies in Retinas with CNV
To observe the AAV transduction efficiencies in CNV-induced mouse retina, laser photocoagulation was performed concentrically around the optic nerve head. CNV formation was confirmed by identifying dye leakage at the laser lesions in fundus fluorescein angiography (FFA) after 5 days of laser photocoagulation (Figure 1). The transduction efficiencies of intravitreally-delivered three AAV serotypes were determined by evaluating EGFP expression in posterior eyecup images. Among eyes not subjected to AAV injection, with or without CNV, weak background autofluorescence was detected around the optic nerve head (Figures 2A and 2E). Among eyes without CNV, AAV2-administered eyes exhibited diffuse, scattered EGFP expression throughout the fundus (Figure 2B), while AAV5- and AAV8-injected eyes showed scant EGFP expression around the optic nerve head and major vessels (Figures 2C and 2D). Among the eyes with CNV, the transduction efficiency of all AAV serotypes was increased around CNV lesions. AAV2 showed the highest transduction efficiency, as evidenced by robust EGFP expression throughout the fundus (Figure 2E). AAV5 and AAV8 transduction was enhanced when compared to that in eyes without CNV, and was concentrated around CNV lesions (Figures 2G and 2H).
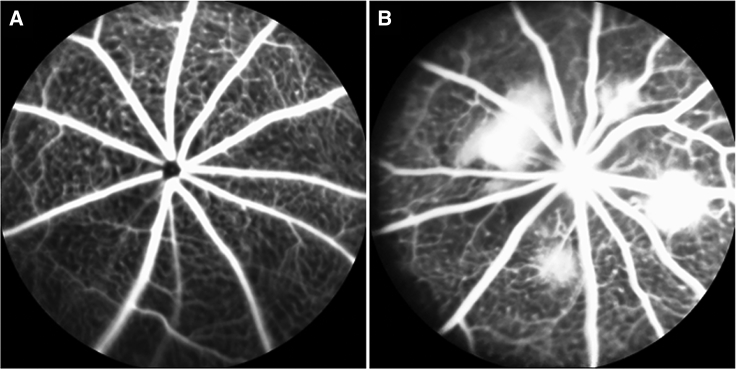
Figure 1.
Fundus Fluorescent Angiography Images Taken Pre- and Post-laser Photocoagulation
(A) Fundus fluorescent angiography (FFA) images captured before laser photocoagulation displayed no definite dye leakage around vessels. (B) FFA images taken after 5 days of laser photocoagulation showed extensive dye leakage at laser spots, indicating CNV formation.
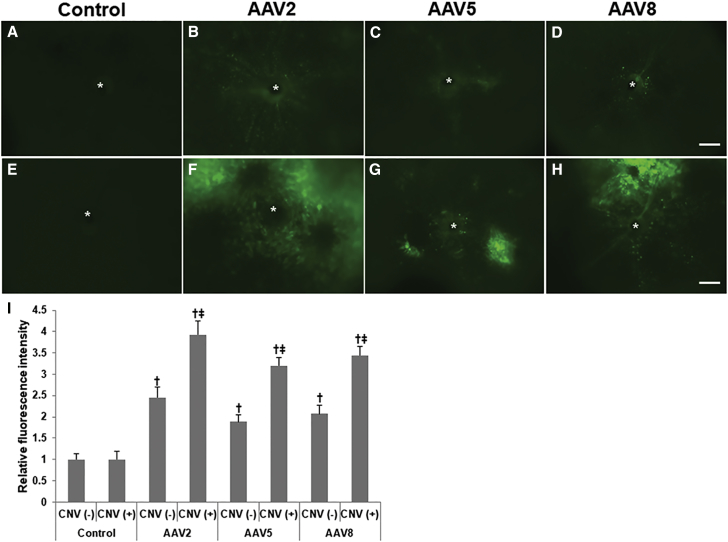
Figure 2.
Fluorescence Images of Control and AAV2-, AAV5-, and AAV-8-Injected Eyes
Whole mounts of control eyes with (E) or without (A) choroidal neovascularization (CNV) showed only faint autofluorescence around the optic nerve head. In the absence of CNV, eyes injected with AAV2 (B) demonstrated diffuse, scattered fluorescence spots throughout the fundus, while eyes injected with AAV5 (C) or AAV8 (D) showed less fluorescence. In eyes with CNV, transduction of AAV of all three serotypes—AAV2 (F), -5 (G), and -8 (H)—was markedly increased around CNV lesions. The increase in transduction of AAV2 was greater than that of the other two serotypes. (I) The relative fluorescence intensities were as follows: AAV2/CNV− (n = 5), 2.45 ± 0.24; AAV2/CNV+ (n = 10), 3.93 ± 0.32; AAV5/CNV− (n = 5), 1.88 ± 0.17; AAV5/CNV+ (n = 10), 3.20 ± 0.19; AAV8/CNV− (n = 5), 2.07 ± 0.21; AAV8/CNV+ (n = 10), 3.43 ± 0.21 (I). In the absence of CNV, there were significant differences in fluorescence intensity between control eyes and eyes injected with AAV (AAV2/CNV−, AAV5/CNV−, and AAV8/CNV−) (p < 0.05). The fluorescence intensity differed significantly between eyes with and without CNV injected with all of the three AAV serotypes (AAV2, p < 0.017; AAV5, p < 0.024; AAV8, p < 0.021). Data are expressed as mean ± SEM. Significant differences: †p < 0.05 versus control eyes, ‡p < 0.05 versus eyes without CNV. Asterisk (*), CNV lesion. Scale bars, 200 μm.
Fluorescence intensity was significantly higher in AAV-injected eyes compared to control eyes, irrespective of AAV serotype (AAV2, p = 0.016; AAV5, p = 0.041; AAV8, p = 0.032). Eyes with CNV had a significantly increased fluorescence intensity than those without CNV, again irrespective of AAV serotype (AAV2, p = 0.017; AAV5, p = 0.024; AAV8, p = 0.021). The relative fluorescence intensities were as follows: AAV2/CNV− (n = 5), 2.45 ± 0.24; AAV2/CNV+ (n = 10), 3.93 ± 0.32; AAV5/CNV− (n = 5), 1.88 ± 0.17; AAV5/CNV+ (n = 10), 3.20 ± 0.19; AAV8/CNV− (n = 5), 2.07 ± 0.21; and AAV8/CNV+ (n = 10), 3.43 ± 0.21 (Figure 2I).
AAV Transduction into Retinal Cells in the Presence of CNV
Transverse sections allowed identification of transduction patterns to different cell types by the vectors. In AAV2/CNV− eyes, vector transduction in the retinal nerve fiber layer and retinal ganglion cells was evident, together with sporadic transduction in the inner nuclear layer (INL) (Figure 3A). In AAV5/CNV− and AAV8/CNV− eyes, EGFP expression was negligible in the retinal ganglion cell layer and inner plexiform layer (Figures 3C and 3E). In eyes with CNV, the transduction pattern differed among the AAV serotypes. In AAV2/CNV+ eyes, CNV formation resulted in diffuse vector transduction into cells in retinal ganglion cell layer (GCL), INL cells, outer nuclear layer (ONL) cells, retinal pigment epithelium (RPE) cells, and cells in CNV lesions (Figure 3B). EGFP expression was dramatically increased in the RPE layer, even outside of CNV lesions. In contrast, in AAV5/CNV+ and AAV8/CNV+ eyes, AAV transduction was enhanced in GCL cells, INL cells, ONL cells, RPE cells, and cells in CNV lesions, more confined to the lesion area. Eyes injected with AAV8 showed a more diffuse transduction pattern compared than those injected with AAV5 (Figures 3D and 3E).
Figure 3.
Images of Transverse Sections of Fundi Transduced with AAV2, -5, and -8
In eyes without CNV, transduction of AAV2 (A), -5 (C), and -8 (E) was limited to the retinal nerve fiber layer and retinal ganglion cell layer. In the presence of CNV, AAV2 (B) transduced retinal ganglion cells, Müller cells, inner nuclear layer (INL) cells, outer nuclear layer (ONL) cells, and retinal pigment epithelium (RPE) cells with a diffuse pattern, as well as cells in CNV lesions. AAV5 (D) and -8 (F) transduced retinal ganglion cells, Müller cells, INL cells, ONL cells, RPE cells and cells localized to CNV lesions. Asterisk (*), CNV lesion. Scale bars, 100 μm.
AAV Transduction into Müller Cells in the Presence of CNV
AAV transduction into Müller cells was evaluated by double immunostaining using anti-EGFP and anti-GFAP antibodies. Induction of CNV resulted in extensive gliosis and upregulation of GFAP expression in Müller cells located close to CNV lesions. GFAP+ Müller cells were transduced by all AAV serotypes (Figure 4). Compared to eyes without CNV, AAV2 transduction in Müller cells was enhanced, and the tropism of AAV5 and AAV8 was altered, in the presence of CNV. AAV5 and AAV8 did not transduce Müller cells in the normal retina, but did transduce these cells in the presence of CNV. Among the three serotypes, the transduction efficiency of AAV2 exhibited the greatest increase in the presence of CNV compared to its absence (Figure 4).
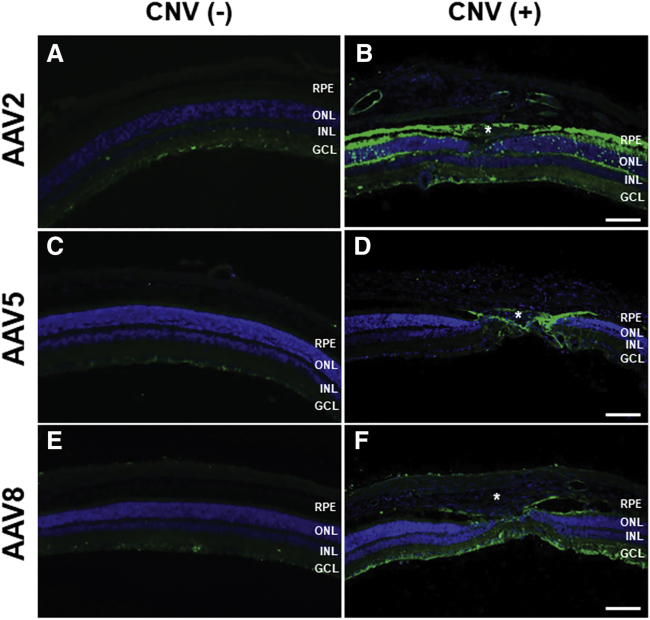
Figure 4.
Immunostaining of Anti-GFAP (Red) and EGFP (Green) in Müller Cells in the Presence of CNV
In control eyes without CNV, GFAP expression was limited to the ganglion cell layer without any vector transduction after AAV2 (A), -5 (B), and -8 (C) intravitreal injection. CNV resulted in substantial gliosis and upregulation of GFAP expression in Müller cells in the neural retina in all three serotypes, including AAV2 (D), -5 (E), and -8 (F). The majority of GFAP-expressing Müller cells were also positive for EGFP in eyes injected with AAV2, -5, and -8, indicating efficient transduction of Müller cells by all three AAV serotypes. Asterisk (*), CNV lesion. Scale bars, 50 μm.
Identification of Cell Types Transduced by AAV
To identify the cell types transduced by AAV, we performed further immunohistochemical analysis. CNV lesions comprise inflammatory cells and vascular tissues arising from beneath the choroid.15 EGFP+ cells were probed using antibodies against CD11b, F4/80, and CD31, which are markers of monocytes, macrophages, and endothelial cells, respectively.
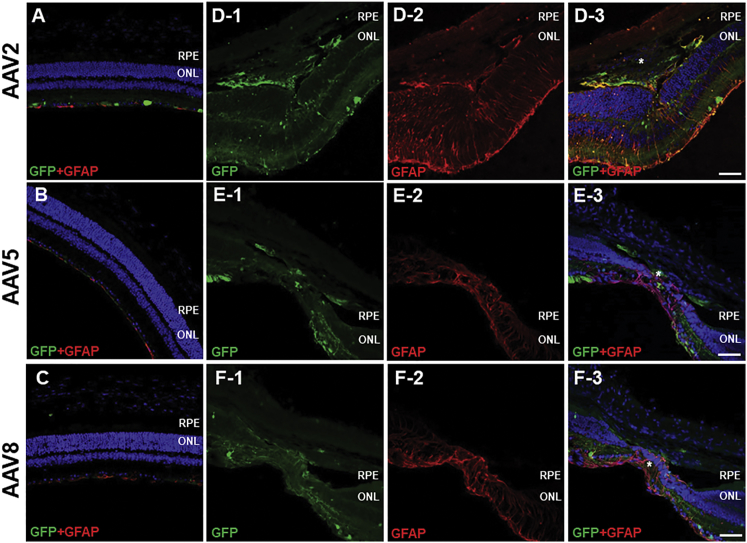
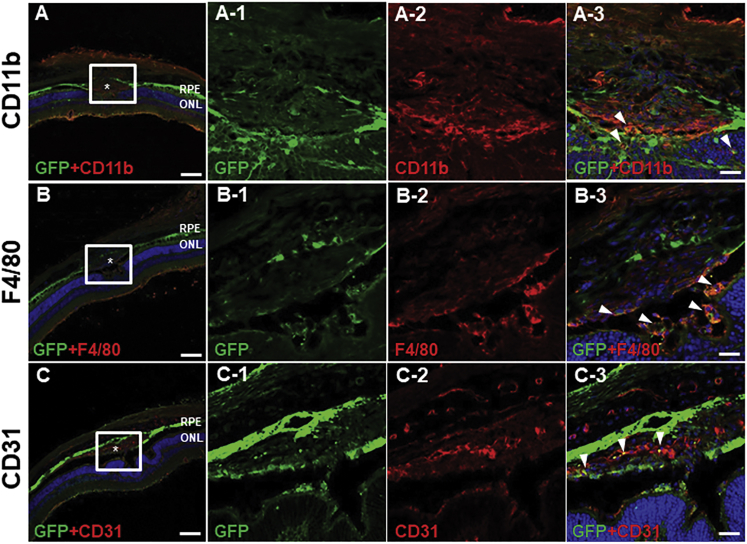
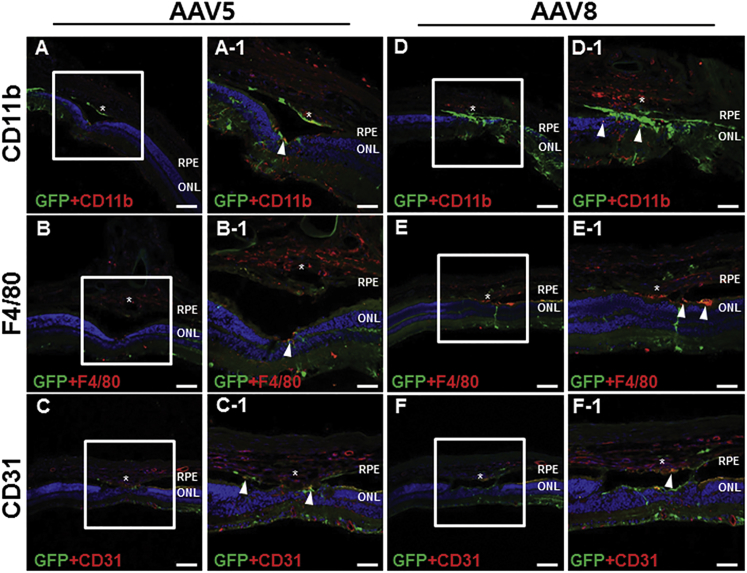
In eyes injected with AAV2, CD11b expression was detected in CNV lesions, as well as the ONL and INL (Figure 5A). Several EGFP+ cells in CNV lesions were also positive for CD11b, as well as those nearby to the lesions (Figure 5A-1–3). F4/80 immunostaining revealed a pattern similar to that of CD11b (Figure 5B). F4/80 expression was detected mainly in the GCL, ONL, subretinal space, and CNV lesions. F4/80+ cells were detected in the subretinal space and CNV lesions; in the latter, some F4/80+ cells were also EGFP+ (Figure 5B-1–3), indicating efficient transduction of AAV2. CD31 staining was localized in vascular tissue in the neural retina and choroid, as well as in CNV lesions (Figure 5C). Colocalization of EGFP and CD31 staining suggested a modest level of AAV transduction in vascular endothelial cells in CNV lesions (Figure 5C-1–3). In eyes injected with AAV5 or AAV8, vector transduced CD11b and F4/80+ cells were confined to the subretinal space and the INL (Figures 6A, 6B, 6D, and 6E). Colocalization of EGFP and CD31 expression revealed AAV5 and AAV8 transduction within vascular tissue in the superficial layer of CNV lesions (Figures 6C and 6F).
Figure 5.
Immunostaining of Transverse Sections from Eyes Injected for CD11b, F4/80, CD31, and EGFP
CD11b expression was detected in the neural retina as well as in CNV lesions (A). EGFP+ cells in and around CNV lesions also expressed CD11b (arrowheads in original magnification ×5 in A-3). Immunostaining of F4/80 showed a similar pattern (B). Several F4/80+ cells in the subretinal space and in CNV lesions expressed EGFP (arrowheads in original magnification ×5 in B-3). CD31+ endothelial cells were found in the neural retina and choroid, and in CNV lesions (C). AAV efficiently transduced vascular endothelial cells in CNV lesions (arrowheads in original magnification ×5 in C-3). Asterisk (*), CNV lesion. (A–C) Scale bars, 100 μm; and (A-1-3, B-1-3, C-1-3) scale bars, 20 μm.
Figure 6.
Colocalization of EGFP with CD11b, F4/80, and CD31 in Eyes Injected with AAV5 and AAV8
AAV5 (arrowheads in A, A-1, B, and B-1) and AAV8 (arrowheads in D, D-1, E, and E-1) transduction was observed in CD11b− and F4/80+ cells in the subretinal space and the INL, but not in these cells in CNV lesions. A small proportion of CD31+ endothelial cells was also positive for EGFP (C, C-1, F, and F-1). Asterisk (*), CNV lesion. (A–F) Scale bars, 100 μm; and (A-1–F-1) scale bars, 50 μm.
Discussion
To date, AAV-based gene therapy showed promise for various retinal diseases,16, 17 including nAMD. Recently, our group demonstrated the feasibility of gene therapy for nAMD using AAV-mTOR shRNA.14 Intravitreal administration of AAV2 facilitates targeting of CNV lesions, but we did not demonstrate vector transduction of specific retinal cell types. Because pathologic conditions of the retina may alter the vector transduction of retinal cells, there exists a great necessity to evaluate the transduction efficiency of viral vectors in the diseased retina. In this study, three AAV vectors exhibited different transduction patterns in mouse retinas with CNV. Eyes injected with AAV2 demonstrated diffuse, robust EGFP expression, while that in eyes injected with AAV5 and AAV8 was more modest. Substantial gliosis and Müller cell activation was observed after CNV, and activated Müller cells were transduced by AAV of all three serotypes. Inflammatory and endothelial cells in CNV lesions were effectively transduced by AAV2, while AAV5 and AAV8 transduced perilesional inflammatory cells and vascular tissues in the superficial layer of CNV lesions.
AAV vectors are typically administered subretinally or intravitreally. Subretinal injection requires an invasive procedure, in which localized retinal detachment is inevitable. Intravitreal injection is a safe and simple procedure, but results in limited vector transduction into retinal cells. To develop a safer and more efficient method of administration, several studies have sought to overcome the disadvantages of intravitreal AAV administration. Modification of the viral capsid reportedly enhances transduction of viral vectors into various cell types in the retina.18, 19, 20, 21, 22, 23, 24, 25 We previously reported significantly enhanced transduction of intravitreally administered AAV into retinal ganglion cells, Müller cells, photoreceptors, and RPE cells after laser photocoagulation.10, 26 Induction of CNV by laser photocoagulation involves rupturing Bruch’s membrane, followed by sprouting of abnormal new vessels and extensive inflammation.27, 28 In this study, CNV lesions exhibited enhanced vector transduction, supporting the notion that transduction of AAV vectors is enhanced in the diseased retina, and suggesting the potential of gene therapy for retinal diseases.
Currently, anti-VEGF therapy is the main strategy for AMD treatment, and there is ample evidence that it is effective in decreasing exudation and retinal thickness and improving visual acuity in patients with nAMD.29, 30 However, multiple studies have reported that anti-VEGF therapy has limited effects on eliminating abnormal blood vessels, especially when vascular tissues are in mature state.31, 32 In this study, AAVs were shown to transduce inflammatory cells and endothelial cells in and around CNV lesions with different transduction profiles according to the serotypes, and our group recently demonstrated that AAV-mTOR shRNA transduced endothelial cells in CNVs and showed that direct inhibition of mTOR effectively reduced the size of CNV, presumably due to the increased autophagy.14 Considering that inflammatory and endothelial cells are among the major components of CNV,33, 34, 35 directly transducing these cells therapeutic genes may address the limitations of current anti-VEGF therapy and enhanced therapeutic effects on eliminating CNV may be achieved.
The mechanism of enhanced transduction in laser-induced CNV is unclear. The vitreoretinal junction, also known as the inner limiting membrane (ILM), may act as a barrier to the transduction of AAV following intravitreal administration,36 as evidenced by enhanced transduction upon sub-ILM injection or surgical ILM peeling.37, 38 This barrier may play a role in our CNV model as the vitreoretinal junction could be compromised by inflammation and thermal disruption by the laser. Also, proliferation of Müller cells upon laser photocoagulation may lead to enhanced vector transduction; increased endfeet movement may lead to disruption of the ILM.39, 40 However, these mechanisms are not sufficient to explain the robust EGFP expression in the RPE layer detected in this study. Alternatively, the cellular stress and upregulated expression of receptors for viral capsid proteins induced by localized inflammation may be involved in the increased vector transduction seen in retinas with CNV,41, 42 and may explain robust EGFP expression outside of CNV lesions. Further studies should explore the mechanisms underlying enhanced AAV vector transduction in CNV retinas.
AAV-mediated gene therapy for AMD has shown recent progress. For example, subretinal delivery of soluble fms-like tyrosine kinase-1 (sFLT-1) using recombinant AAV has shown promise for treating nAMD,43, 44 and a recent phase 2a clinical trial and phase I dose-escalation trial reported successful results.45, 46 However, these previous studies, including ours, used AAV2 vector, and the tropism of each AAV serotypes in CNV induced retina have not been investigated thoroughly. In our previous study, we only managed to demonstrate that AAV2 effectively transduced endothelial cells in CNV, but not other cell components of CNV. In this study, we report that AAV5 and AAV8 transduced a small proportion of vascular endothelial cells in CNV lesions, as well as the inflammatory cells surrounding the lesions. These results suggest that AAV5 and AAV8 are also candidate viral vectors for gene delivery to the retina. However, AAV2 showed the greatest enhancement of transduction efficiency in the presence of CNV, and so is the optimum viral vector for gene therapy of nAMD.
There are several limitations to consider when interpreting the results of this study. First, although laser-induced CNV model is currently the most employed rodent model, the nature of CNV may be different from that seen in an AMD patient.47 The model involves acute injury and inflammation rather than long standing senescent degeneration and chronic inflammation found in AMD pathology.48, 49 Also, laser photocoagulation may cause greater damage to the overlying neural retina than typically seen in human AMD.47 However, CNV generated by laser photocoagulation follows development stages that are similar to human AMD,50 and the pathogenetic mechanism of CNV development, such as angiogenic cascade and cell types involved in CNV formation, and is not much different from that seen in human AMD.35, 47, 51, 52, 53, 54, 55 Therefore, the results from our study may have clinical implication on transduction profiles of different AAV serotypes in nAMD. Second, there may be discrepancies in AAV transduction patterns in rodents and humans. Previous studies on AAV transduction efficiencies in liver showed different transduction profiles between mouse and non-human primates or humans, and these differences may also exist regarding AAV transduction efficiencies in CNV. Further studies are needed to address this issue.
In summary, we assessed the transduction patterns of AAV2, -5, and -8 in mouse retinas with CNV; all three serotypes demonstrated enhanced transduction in and around CNV lesions. AAV2 was efficiently transduced into inflammatory and endothelial cells in CNV lesions, while AAV5 and AAV8 transduced only endothelial cells in superficial layer CNV lesions. Our results will facilitate future studies on ocular gene-therapy, particularly of nAMD, aiming to identify the optimum AAV serotypes for use as delivery vehicles in gene therapy for retinal diseases.
Materials and Methods
General Care of Animals
Forty-five male C57BL/6J mice, aged 8 weeks, (Orient Bio, Sungnam, Korea) were used in this study. All mice were reared in standard conditions under a 12-hr light/dark cycle. Animal care and experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research, and overseen by the Institutional Animal Care and Use Committee of Soonchunhyang University Hospital, Bucheon. For sedation and anesthesia, a mixture of 40 mg/kg zolazepam/tiletamine (Zoletil; Virbac, Carros Cedex, France) and 5 mg/kg xylazine (Rompun; Bayer Healthcare, Leverkusen, Germany) was injected intraperitoneally. Pupil dilation for CNV induction and intravitreal injection of EGFP-expressing AAV vectors was performed using a mixture of 0.5% tropicamide and 0.5% phenylephrine hydrochloric acid (HCl) (Tropherine Eye Drops; Hanmi Pharm, Seoul, South Korea).
Formation of Laser-Induced Choroidal Neovascularization
After anesthesia and pupil dilation, the mice were subjected to laser photocoagulation using a PASCAL diode ophthalmic laser system (neodymium-doped yttrium aluminium garnet [Nd:YAG], 532 nm; Topcon Medical Laser Systems, Santa Clara, CA) with the following parameters: 200-μm spot size, 0.02 s duration, and 100 mW power. Four to five laser spots were made around the optic nerve head of the right eye in each animal to induce CNV. Rupture of Bruch’s membrane was confirmed by observation of a bubble at each laser spot.
FFA
FFA was taken using a scanning laser ophthalmoscope (Heidelberg Retina Angiograph 2; Heidelberg Engineering, Heidelberg, Germany) at 5 days after laser photocoagulation before intravitreal injection. The animal was anesthetized and the pupil dilated to observe the retina. FFA images were captured 3–5 min after an intraperitoneal injection with 0.1 mL of 2% fluorescein sodium (Fluorescite; Akorn). From the images, the formation of CNV was confirmed by observing dye leakage around the laser photocoagulated lesion.
Intravitreal AAV Aministration
Five days after laser photocoagulation, AAV was injected intravitreally into the right eye under anesthesia with pupil dilation, using a 30G sharp needle and a 35G blunt needle fitted with NanoFil syringes (World Precision Instruments, Sarasota, FL). A sclerotomy was created approximately 0.5 mm posterior to the limbus using a sharp 30G needle tip prior to vector administration. One microliter of AAV2-EGFP, AAV5-EGFP, and AAV8-EGFP (1 × 1013 viral particles [vp]/ml) supplied by CdmoGen (Cheongju, Republic of Korea) were used for injection. Intravitreal injection was performed using a syringe fitted with a 35G blunt needle while visualizing the fundus directly using a surgical microscope and a small plastic ring filled with 0.5% methylcellulose on the cornea (GenTeal; Novartis, Basel, Switzerland). Ten and five mice with and without CNV, respectively, were injected with each AAV serotype; thus, a total of 45 mice underwent intravitreal vector administration.
Tissue Processing and Immunohistochemistry
Seven days after AAV administration, mice were deeply anesthetized and intracardially perfused with 0.1 M PBS containing 150 U/mL heparin, followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB). Eyes were enucleated, dissected into posterior eyecups, and post-fixed in 4% PFA. After the fixation process, eyecups were examined using an Axioplan microscope at 50× magnification (Carl Zeiss, Oberkochen, Germany) and a 1,500 ms exposure time. Images were obtained using a monochromatic charge-coupled device (CCD) camera (AxioCam MRm; Carl Zeiss) and Axiovision image-capture software (Carl Zeiss). Eyecups were then incubated in 30% sucrose in PBS overnight and embedded sagittally to prepare transverse sections. Ten-micrometer serial sections were cut from the embedded eyecups.
For immunohistochemistry, transverse tissue sections were blocked with 0.1% Triton X-100 in 5% goat serum for 1 hr. Subsequently, sections were incubated with primary antibodies overnight at 4°C. The following primary antibodies were used: anti-CD11b antibody (1:2,000; MCA711G; Serotec, Oxford, UK), anti-F4/80 antibody (1:2,000; MCA497GA; Serotec, Oxford, UK), anti-CD31 (1:200; 550274; BD PharMingen, San Diego, CA), and anti-glial fibrillary acidic protein (GFAP) antibody (1:2,000; NG1817590; Millipore, Temecula, CA). To visualize eGFP, an anti-GFP antibody (1:200; ab6556; Abcam, Cambridge, UK) was used. Next, the samples were incubated with the appropriate secondary antibodies for 1 hr at room temperature. The secondary antibodies used in this study were as follows: Alexa Fluor-488 anti-rabbit, Alexa Fluor-488 anti-mouse, and Alexa Fluor-568 anti-mouse (1:2,000; Molecular Probes, Grand Island, NY). The results were visualized and photographed using an Axioplan microscope at 200 × magnification.
Image Analysis and Statistical Analysis
Quantitative comparison of fluorescence intensity in retinal whole mounts was performed using relative fluorescence intensity obtained from ImageJ software (Bethesda, MD). The relative fluorescence intensity was determined by the ratio of mean fluorescence intensity for each image to mean fluorescence intensity of control images. The Wilcoxon signed-rank test was used to compare fluorescence intensities among the three experimental groups. Statistical analyses were conducted using SPSS for Windows software (v.20.0; SPSS, Chicago, IL). A p value < 0.05 was considered indicative of statistical significance.
Author Contributions
Conceptualization, T.K.P.; Methodology, T.K.P. and S.H.L.; Investigation, S.H.L., Y.S.K., S.K.N., H.J.K., H.Y.P., J.Y.Y., and K.P.; Writing – Original Draft, S.H.L.; Writing – Review and Editing, T.K.P. and S.H.L.; Supervision, T.K.P.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, (grant number HI17C0966), and also partially supported by the Soonchunhyang University research fund.
References
- 1.Bainbridge J.W., Smith A.J., Barker S.S., Robbie S., Henderson R., Balaggan K., Viswanathan A., Holder G.E., Stockman A., Tyler N. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 2.Hauswirth W.W., Aleman T.S., Kaushal S., Cideciyan A.V., Schwartz S.B., Wang L., Conlon T.J., Boye S.L., Flotte T.R., Byrne B.J. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H., Zhang L., Gu L., Lu L., Gao G., Li W., Xu G., Wang J., Gao F., Xu J.Y. Subretinal delivery of AAV2-mediated human erythropoietin gene is protective and safe in experimental diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2014;55:1519–1530. doi: 10.1167/iovs.13-13155. [DOI] [PubMed] [Google Scholar]
- 5.Díaz-Lezama N., Wu Z., Adán-Castro E., Arnold E., Vázquez-Membrillo M., Arredondo-Zamarripa D., Ledesma-Colunga M.G., Moreno-Carranza B., Martinez de la Escalera G., Colosi P. Diabetes enhances the efficacy of AAV2 vectors in the retina: therapeutic effect of AAV2 encoding vasoinhibin and soluble VEGF receptor 1. Lab. Invest. 2016;96:283–295. doi: 10.1038/labinvest.2015.135. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez J.M., 2nd, Hu P., Caballero S., Moldovan L., Verma A., Oudit G.Y., Li Q., Grant M.B. Adeno-associated virus overexpression of angiotensin-converting enzyme-2 reverses diabetic retinopathy in type 1 diabetes in mice. Am. J. Pathol. 2016;186:1688–1700. doi: 10.1016/j.ajpath.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birke M.T., Lipo E., Adhi M., Birke K., Kumar-Singh R. AAV-mediated expression of human PRELP inhibits complement activation, choroidal neovascularization and deposition of membrane attack complex in mice. Gene Ther. 2014;21:507–513. doi: 10.1038/gt.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askou A.L., Pournaras J.A., Pihlmann M., Svalgaard J.D., Arsenijevic Y., Kostic C., Bek T., Dagnaes-Hansen F., Mikkelsen J.G., Jensen T.G. Reduction of choroidal neovascularization in mice by adeno-associated virus-delivered anti-vascular endothelial growth factor short hairpin RNA. J. Gene Med. 2012;14:632–641. doi: 10.1002/jgm.2678. [DOI] [PubMed] [Google Scholar]
- 9.Maclachlan T.K., Lukason M., Collins M., Munger R., Isenberger E., Rogers C., Malatos S., Dufresne E., Morris J., Calcedo R. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol. Ther. 2011;19:326–334. doi: 10.1038/mt.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.H., Colosi P., Lee H., Ohn Y.H., Kim S.W., Kwak H.W., Park T.K. Laser photocoagulation enhances adeno-associated viral vector transduction of mouse retina. Hum. Gene Ther. Methods. 2014;25:83–91. doi: 10.1089/hgtb.2013.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igarashi T., Miyake K., Asakawa N., Miyake N., Shimada T., Takahashi H. Direct comparison of administration routes for AAV8-mediated ocular gene therapy. Curr. Eye Res. 2013;38:569–577. doi: 10.3109/02713683.2013.779720. [DOI] [PubMed] [Google Scholar]
- 12.Li H.L., Zheng X.Z., Wang H.P., Li F., Wu Y., Du L.F. Ultrasound-targeted microbubble destruction enhances AAV-mediated gene transfection in human RPE cells in vitro and rat retina in vivo. Gene Ther. 2009;16:1146–1153. doi: 10.1038/gt.2009.84. [DOI] [PubMed] [Google Scholar]
- 13.van Wijngaarden P., Qureshi S.H. Inhibitors of vascular endothelial growth factor (VEGF) in the management of neovascular age-related macular degeneration: a review of current practice. Clin. Exp. Optom. 2008;91:427–437. doi: 10.1111/j.1444-0938.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 14.Park T.K., Lee S.H., Choi J.S., Nah S.K., Kim H.J., Park H.Y., Lee H., Lee S.H.S., Park K. Adeno-Associated Viral Vector-Mediated mTOR Inhibition by Short Hairpin RNA Suppresses Laser-Induced Choroidal Neovascularization. Mol. Ther. Nucleic Acids. 2017;8:26–35. doi: 10.1016/j.omtn.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauppinen A., Paterno J.J., Blasiak J., Salminen A., Kaarniranta K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016;73:1765–1786. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochakovski G.A., Bartz-Schmidt K.U., Fischer M.D. Retinal Gene Therapy: Surgical Vector Delivery in the Translation to Clinical Trials. Front. Neurosci. 2017;11:174. doi: 10.3389/fnins.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petit L., Punzo C. Gene therapy approaches for the treatment of retinal disorders. Discov. Med. 2016;22:221–229. [PMC free article] [PubMed] [Google Scholar]
- 18.Petrs-Silva H., Dinculescu A., Li Q., Min S.H., Chiodo V., Pang J.J., Zhong L., Zolotukhin S., Srivastava A., Lewin A.S. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 2009;17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charbel Issa P., De Silva S.R., Lipinski D.M., Singh M.S., Mouravlev A., You Q., Barnard A.R., Hankins M.W., During M.J., Maclaren R.E. Assessment of tropism and effectiveness of new primate-derived hybrid recombinant AAV serotypes in the mouse and primate retina. PLoS ONE. 2013;8:e60361. doi: 10.1371/journal.pone.0060361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kay C.N., Ryals R.C., Aslanidi G.V., Min S.H., Ruan Q., Sun J., Dyka F.M., Kasuga D., Ayala A.E., Van Vliet K. Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS ONE. 2013;8:e62097. doi: 10.1371/journal.pone.0062097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mowat F.M., Gornik K.R., Dinculescu A., Boye S.L., Hauswirth W.W., Petersen-Jones S.M., Bartoe J.T. Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther. 2014;21:96–105. doi: 10.1038/gt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cronin T., Vandenberghe L.H., Hantz P., Juttner J., Reimann A., Kacsó A.E., Huckfeldt R.M., Busskamp V., Kohler H., Lagali P.S. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol. Med. 2014;6:1175–1190. doi: 10.15252/emmm.201404077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodard K.T., Liang K.J., Bennett W.C., Samulski R.J. Heparan sulfate binding promotes accumulation of intravitreally delivered adeno-associated viral vectors at the retina for enhanced transduction but weakly influences tropism. J. Virol. 2016;90:9878–9888. doi: 10.1128/JVI.01568-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khabou H., Desrosiers M., Winckler C., Fouquet S., Auregan G., Bemelmans A.P., Sahel J.A., Dalkara D. Insight into the mechanisms of enhanced retinal transduction by the engineered AAV2 capsid variant -7m8. Biotechnol. Bioeng. 2016;113:2712–2724. doi: 10.1002/bit.26031. [DOI] [PubMed] [Google Scholar]
- 25.De Silva S.R., Charbel Issa P., Singh M.S., Lipinski D.M., Barnea-Cramer A.O., Walker N.J., Barnard A.R., Hankins M.W., MacLaren R.E. Single residue AAV capsid mutation improves transduction of photoreceptors in the Abca4-/- mouse and bipolar cells in the rd1 mouse and human retina ex vivo. Gene Ther. 2016;23:767–774. doi: 10.1038/gt.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S.H., Kong Y.J., Lyu J., Lee H., Park K., Park T.K. Laser Photocoagulation Induces Transduction of Retinal Pigment Epithelial Cells by Intravitreally Administered Adeno-Associated Viral Vectors. Hum. Gene Ther. Methods. 2015;26:159–161. doi: 10.1089/hgtb.2015.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert V., Lecomte J., Hansen S., Blacher S., Gonzalez M.L., Struman I., Sounni N.E., Rozet E., de Tullio P., Foidart J.M. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat. Protoc. 2013;8:2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- 28.Zipfel P.F., Lauer N., Skerka C. The role of complement in AMD. Adv. Exp. Med. Biol. 2010;703:9–24. doi: 10.1007/978-1-4419-5635-4_2. [DOI] [PubMed] [Google Scholar]
- 29.Maguire M.G., Martin D.F., Ying G.S., Jaffe G.J., Daniel E., Grunwald J.E., Toth C.A., Ferris F.L., 3rd, Fine S.L., Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2016;123:1751–1761. doi: 10.1016/j.ophtha.2016.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaffe G.J., Martin D.F., Toth C.A., Daniel E., Maguire M.G., Ying G.S., Grunwald J.E., Huang J., Comparison of Age-related Macular Degeneration Treatments Trials Research Group Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120:1860–1870. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin L.E., Golijanin D., Itin A., Pode D., Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J. Clin. Invest. 1999;103:159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gee M.S., Procopio W.N., Makonnen S., Feldman M.D., Yeilding N.M., Lee W.M. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. Am. J. Pathol. 2003;162:183–193. doi: 10.1016/S0002-9440(10)63809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi X., Ogata N., Komada M., Yamamoto C., Takahashi K., Omori K., Uyama M. Vascular endothelial growth factor expression in choroidal neovascularization in rats. Graefes Arch. Clin. Exp. Ophthalmol. 1997;235:313–319. doi: 10.1007/BF01739641. [DOI] [PubMed] [Google Scholar]
- 34.Sakurai E., Anand A., Ambati B.K., van Rooijen N., Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 35.Dobi E.T., Puliafito C.A., Destro M. A new model of experimental choroidal neovascularization in the rat. Arch. Ophthalmol. 1989;107:264–269. doi: 10.1001/archopht.1989.01070010270035. [DOI] [PubMed] [Google Scholar]
- 36.Dalkara D., Kolstad K.D., Caporale N., Visel M., Klimczak R.R., Schaffer D.V., Flannery J.G. Inner limiting membrane barriers to AAV-mediated retinal transduction from the vitreous. Mol. Ther. 2009;17:2096–2102. doi: 10.1038/mt.2009.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boye S.E., Alexander J.J., Witherspoon C.D., Boye S.L., Peterson J.J., Clark M.E., Sandefer K.J., Girkin C.A., Hauswirth W.W., Gamlin P.D. Highly efficient delivery of adeno-associated viral vectors to the primate retina. Hum. Gene Ther. 2016;27:580–597. doi: 10.1089/hum.2016.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi K., Igarashi T., Miyake K., Kobayashi M., Yaguchi C., Iijima O., Yamazaki Y., Katakai Y., Miyake N., Kameya S. Improved intravitreal AAV-mediated inner retinal gene transduction after surgical internal limiting membrane peeling in cynomolgus monkeys. Mol. Ther. 2017;25:296–302. doi: 10.1016/j.ymthe.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulus Y.M., Jain A., Gariano R.F., Stanzel B.V., Marmor M., Blumenkranz M.S., Palanker D. Healing of retinal photocoagulation lesions. Invest. Ophthalmol. Vis. Sci. 2008;49:5540–5545. doi: 10.1167/iovs.08-1928. [DOI] [PubMed] [Google Scholar]
- 40.Tackenberg M.A., Tucker B.A., Swift J.S., Jiang C., Redenti S., Greenberg K.P., Flannery J.G., Reichenbach A., Young M.J. Müller cell activation, proliferation and migration following laser injury. Mol. Vis. 2009;15:1886–1896. [PMC free article] [PubMed] [Google Scholar]
- 41.Ozaki S., Radeke M.J., Anderson D.H. Rapid upregulation of fibroblast growth factor receptor 1 (flg) by rat photoreceptor cells after injury. Invest. Ophthalmol. Vis. Sci. 2000;41:568–579. [PubMed] [Google Scholar]
- 42.Nagineni C.N., Kutty V., Detrick B., Hooks J.J. Expression of PDGF and their receptors in human retinal pigment epithelial cells and fibroblasts: regulation by TGF-beta. J. Cell. Physiol. 2005;203:35–43. doi: 10.1002/jcp.20213. [DOI] [PubMed] [Google Scholar]
- 43.Lai C.M., Estcourt M.J., Wikstrom M., Himbeck R.P., Barnett N.L., Brankov M., Tee L.B., Dunlop S.A., Degli-Esposti M.A., Rakoczy E.P. rAAV.sFlt-1 gene therapy achieves lasting reversal of retinal neovascularization in the absence of a strong immune response to the viral vector. Invest. Ophthalmol. Vis. Sci. 2009;50:4279–4287. doi: 10.1167/iovs.08-3253. [DOI] [PubMed] [Google Scholar]
- 44.Rakoczy E.P., Lai C.M., Magno A.L., Wikstrom M.E., French M.A., Pierce C.M., Schwartz S.D., Blumenkranz M.S., Chalberg T.W., Degli-Esposti M.A. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet. 2015;386:2395–2403. doi: 10.1016/S0140-6736(15)00345-1. [DOI] [PubMed] [Google Scholar]
- 45.Constable I.J., Pierce C.M., Lai C.M., Magno A.L., Degli-Esposti M.A., French M.A., McAllister I.L., Butler S., Barone S.B., Schwartz S.D. Phase 2a randomized clinical trial: safety and post hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168–175. doi: 10.1016/j.ebiom.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Constable I.J., Lai C.M., Magno A.L., French M.A., Barone S.B., Schwartz S.D., Blumenkranz M.S., Degli-Esposti M.A., Rakoczy E.P. Gene therapy in neovascular age-related macular degeneration: three-year follow-up of a phase 1 randomized dose escalation trial. Am. J. Ophthalmol. 2017;177:150–158. doi: 10.1016/j.ajo.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Pennesi M.E., Neuringer M., Courtney R.J. Animal models of age related macular degeneration. Mol. Aspects Med. 2012;33:487–509. doi: 10.1016/j.mam.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edelman J.L., Castro M.R. Quantitative image analysis of laser-induced choroidal neovascularization in rat. Exp. Eye Res. 2000;71:523–533. doi: 10.1006/exer.2000.0907. [DOI] [PubMed] [Google Scholar]
- 49.Tobe T., Ortega S., Luna J.D., Ozaki H., Okamoto N., Derevjanik N.L., Vinores S.A., Basilico C., Campochiaro P.A. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am. J. Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller H., Miller B., Ishibashi T., Ryan S.J. Pathogenesis of laser-induced choroidal subretinal neovascularization. Invest. Ophthalmol. Vis. Sci. 1990;31:899–908. [PubMed] [Google Scholar]
- 51.Yamada H., Yamada E., Kwak N., Ando A., Suzuki A., Esumi N., Zack D.J., Campochiaro P.A. Cell injury unmasks a latent proangiogenic phenotype in mice with increased expression of FGF2 in the retina. J. Cell. Physiol. 2000;185:135–142. doi: 10.1002/1097-4652(200010)185:1<135::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 52.Oshima Y., Oshima S., Nambu H., Kachi S., Hackett S.F., Melia M., Kaleko M., Connelly S., Esumi N., Zack D.J. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J. Cell. Physiol. 2004;201:393–400. doi: 10.1002/jcp.20110. [DOI] [PubMed] [Google Scholar]
- 53.Ando A., Yang A., Mori K., Yamada H., Yamada E., Takahashi K., Saikia J., Kim M., Melia M., Fishman M. Nitric oxide is proangiogenic in the retina and choroid. J. Cell. Physiol. 2002;191:116–124. doi: 10.1002/jcp.10083. [DOI] [PubMed] [Google Scholar]
- 54.Berglin L., Sarman S., van der Ploeg I., Steen B., Ming Y., Itohara S., Seregard S., Kvanta A. Reduced choroidal neovascular membrane formation in matrix metalloproteinase-2-deficient mice. Invest. Ophthalmol. Vis. Sci. 2003;44:403–408. doi: 10.1167/iovs.02-0180. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Z.X., Wang Y.S., Shi Y.Y., Hou H.Y., Zhang C., Cai Y., Dou G.R., Yao L.B., Li F.Y. Hypoxia specific SDF-1 expression by retinal pigment epithelium initiates bone marrow-derived cells to participate in Choroidal neovascularization in a laser-induced mouse model. Curr. Eye Res. 2011;36:838–849. doi: 10.3109/02713683.2011.593107. [DOI] [PubMed] [Google Scholar]