Abstract
The ZPT2-2 gene belongs to the EPF gene family in petunia (Petunia hybrida), which encodes proteins with TFIIIA-type zinc-finger DNA-binding motifs. To elucidate a possible function for ZPT2-2, we analyzed its pattern of expression in relation to different developmental and physiological stress signals. The activity of the ZPT2-2 promoter was analyzed using a firefly luciferase (LUC) reporter gene, allowing for continuous measurements of transgene activity in planta. We show that ZPT2-2::LUC is active in all plant tissues, but is strongly modulated in cotyledons upon germination, in leaves in response to desiccation, cold treatment, wounding, or ultraviolet-B light, and in petal tissue in response to pollination of the stigma. Analysis of mRNA levels indicated that the modulations in ZPT2-2::LUC expression reflect modulations in endogenous ZPT2-2 gene expression. The change in ZPT2-2::LUC activity by cold treatment, wounding, desiccation, and ultraviolet-B light suggest that the phytohormones ethylene and jasmonic acid are involved in regulating the expression of ZPT2-2. Although up-regulation of expression of ZPT2-2 can be blocked by inhibitors of ethylene perception, expression in plants is not induced by exogenously applied ethylene. The application of jasmonic acid does result in an up-regulation of gene activity and, thus, ZPT2-2 may play a role in the realization of the jasmonic acid hormonal responses in petunia.
The petunia (Petunia hybrida) gene ZPT2-2 belongs to the EPF gene-family, which encodes transcription factors containing a canonical TFIIIA-type zinc finger motif (Kubo et al., 1998). More than 200 different cDNAs from different eukaryotic systems have been found to encode this type of zinc finger motif, and many of their products play an important role in development (Rosenberg et al., 1986; Tautz et al., 1987). The EPF family contains at least 21 members in petunia, and each member of this family is preferentially expressed in different floral organs (Takatsuji et al., 1994; Kubo et al., 1998). ZPT2-2 (renamed from EPF2-5) was first identified by its homology to ZPT2-1 (renamed from EPF1), which was originally isolated as a petal-specific DNA-binding protein for the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene (Takatsuji et al., 1992). ZPT2-2 and ZPT2-1 bind to the same DNA sequence in the promoter of the EPSPS gene (Takatsuji et al., 1996).
The EPSPS enzyme is part of the shikimate biosynthesis pathway, leading to synthesis of chorismate, which is a precursor for aromatic amino acids, lignin, flavonoids, and alkaloids (Hermann, 1995). The EPSPS gene is expressed at low levels in vegetative tissue and at high levels in floral petal tissues (Benfey et al., 1990). It is known that stress induces an increase in carbon flow through the shikimate pathway to provide precursor molecules for stress-related compounds such as lignin, flavonoids, and alkaloids (Hermann, 1995); however, it is not known whether the petunia EPSPS gene is transcriptionally activated by stress.
The expression patterns of ZPT2-1 and ZPT2-2 are very similar to that of EPSPS (Takatsuji et al., 1992, 1994). This, along with the DNA-binding activity in vitro, suggest that ZPT2-2 and ZPT2-1 might be involved in the transcriptional regulation of EPSPS. However, assigning a function to the ZPT2-2 has failed so far by lack of mutant plants for the ZPT2-2 gene. Ectopic expression or suppression of ZPT2-2 gene activity by co-suppression or antisense genes has yielded no viable plants (H. Takatsuji, unpublished data). Indirectly, this suggests that ZPT2-2 fulfills a crucial role in plant growth and development. An alternative way to elucidate the function of this gene could be a detailed analysis of its expression pattern. Both petal and stamen tissue contain high levels of ZPT2-2 mRNA, while ZPT2-2 mRNA level was undetectable in other plant tissues under normal growth conditions by northern-blot analysis (Takatsuji et al., 1994).
The apparent specific expression of ZPT2-2 in the second and third floral whorl suggested that this gene may be under control of B-type floral homeotic MADS box genes (Coen and Meyerowitz, 1991; van der Krol and Chua, 1993; Takatsuji et al., 1994). In petunia, second- and third-whorl organ identity has been shown to be under control of the B-type MADS box genes pMADS1 (van der Krol et al., 1993) and fbp1 (Angenent et al., 1992). Indeed, two putative MADS box DNA-binding sites have been identified around position −930 relative to the transcription start site within the ZPT2-2 promoter (Takatsuji et al., 1994). Thus, one possible function for the ZPT2-2 gene could be a role in the realization of petal and stamen identity.
A limited expression analysis of transgenic plants expressing a ZPT2-2::GUS reporter gene has previously been reported by Takatsuji et al. (1992, 1994). Here we continue the expression analysis of the 2.1-kb ZPT2-2 promoter activity, using the firefly luciferase (LUC) gene as a reporter. The high sensitivity by which photons can be detected allowed for expression analysis in tissues in which ZPT2-2 gene expression previously went unnoticed. Furthermore, the non-destructive monitoring of LUC activity allows for (semi)continuous measurements of gene activity at the level of whole plant or plant tissue. We could thus “film” transient modulations in LUC activity during germination and in petals in response to pollination, giving both a spatial and temporal resolution of the ZPT2-2 promoter activity. The LUC reporter system has thus led to the identification of new sites of ZPT2-2 gene expression and has revealed the dynamic behavior in the ZPT2-2 promoter activity under different physiological conditions.
MATERIALS AND METHODS
Construction of ZPT2-2::LUC Reporter Plants
The ZPT2-2::LUC reporter gene was constructed by fusing a 2.1-kb promoter fragment of the ZPT2-2 gene (Takatsuji et al., 1994) at the ATG initiation codon to the initiation codon of the LUC coding sequence. For the fusion an NdeI site was introduced at the site of the initiation codon in both the ZPT2-2 and the LUC+ gene (Promega, Madison, WI). The ZPT2-2::LUC chimeric reporter gene (pGM86) was introduced into petunia (Petunia hybrida) V26 plants by Agrobacterium tumefaciens-mediated transformation. Regeneration on selective medium resulted in the isolation of nine independently transformed petunia lines. Primary transformants were backcrossed to wild-type V26 and seeds were collected for the measurement of LUC activity in progeny plants.
Plant Growth, Seed Germination, and Pollination of Flowers
Plants were grown on soil in phytotrons at 25°C with 16 h of light, and at 20°C with 8 h of dark. For germination experiments, transgenic seeds were placed in a 8.5-cm Petri dish on two layers of filter paper with 3 mL of a 0.1 mm luciferin solution. The Petri dish was sealed and placed in a dark box under a 2D luminometer (Hamamatsu Photonics, Hamamatsu, Japan) for the measurement of LUC activity. For pollination experiments a freshly opened flower from a transgenic plant carrying the ZPT2-2::LUC reporter gene was emasculated, cut at the base of the pedicel, and placed in a flask containing a 0.1 mm luciferin solution either with or without 100 μm silver thiosulfate (STS). Pollination was performed 12 h later by applying fresh pollen of petunia line W115 on the stigma of the flower.
Measurements of in Planta LUC Activity with the 2D Luminometer
The LUC reporter plants were repeatedly sprayed with 1 mm luciferin (Promega) starting 1 d before imaging with the two-dimensional luminometer. Luciferin readily penetrates cells of most petunia tissues. It is a stable compound in plants and may persist for weeks. Repeated spraying with luciferin results in an equilibrium in luciferin influx and luciferin consumption by the LUC activity. Changes in photon production can therefore either be ascribed to a change in LUC reporter gene activity or to changes in the physiology of the cells that alter substrate (oxygen, luciferin, or ATP) influx. Changes in the physiology of the cell are not promoter specific and can be verified by comparison with other LUC reporter gene activity under similar conditions. Photon production by LUC was imaged in planta with an intensified CCD camera (Hamamatsu) in slice mode (gain 9.8). For expression studies in germinating seeds and pollinated flowers, each image was obtained by 30-min photon accumulation. Images were thus collected every 30 min over a period of 3 d, and analyzed with Argus 50 software (Hamamatsu). LUC activity in images is represented in false colors using the color scale shown in Figure 1. The range of relative LUC activities that the color scale spans is given in the legend of each figure.
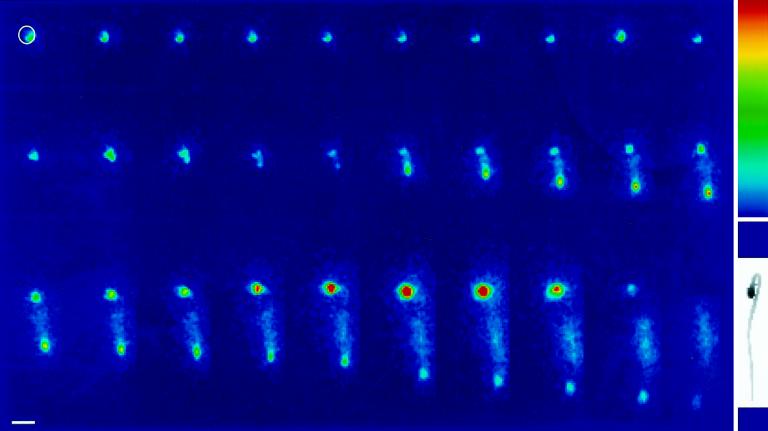
Figure 1.
ZPT2-2::LUC activity in a germinating petunia seed. A seed from a ZPT2-2::LUC transformant was imbibed in 0.5 mm luciferin. Individual images of in vivo LUC activity were obtained by integration of photon production during 30 min. Consecutive images were obtained over a 3-d period. Selected images are shown (time interval between images is 2 h). The white circle in the first image indicates the size of the petunia seed. Note the elevated LUC activity in the root tip and the transient increase in LUC activity in the cotyledons of the seedling. Inset top right, Color scale used for the representation of LUC activity. The color scale ranges from 0 (blue) to 256 (red) relative units of LUC activity. Inset bottom right, Light image of the seedling. Space bar =1 mm.
Wounding, Cold, and UV-B Light Treatment of Leaves
The LUC activity measurements in leaves of ZPT2-2::LUC plants in response to wounding were performed by making 3-mm discs (or by cutting leaves) of presprayed plants and placing them on two layers of filter paper with 0.5 mm luciferin. Photon production was registered in the dark with the 2D luminometer in 10- or 15-min intervals over a period of 1 to 2 d. For the cold-induction experiments, LUC activity in whole plants (presprayed with luciferin for 5 d) was imaged in a 20-min time interval, after which one set of plants was kept at 4°C and the other set at 24°C for 12 h in the dark. After 12 h, LUC activity was again imaged during a 20-min time interval. Note that no new luciferin was applied during the temperature treatments.
For the UV-B light treatment, isolated leaves from a ZPT2-2::LUC plant (presprayed with luciferin) were irradiated for 6 h with 15 μmol m−2 s−1 UV-B light (TL12 tubes, Philips, Eindhoven, The Netherlands) wrapped in a single layer of UV-C blocking cellulose acetate. The given flux density represents the emission in the spectral range between 295 and 345 nm. Control leaves were shielded from UV light by a glass sheet. Following the light treatment, LUC activity in the leaves was imaged during a 20-min interval.
Desiccation and Jasmonic Acid Treatment of ZPT2-2::LUC Plants
For desiccation experiments, two branches from a ZPT2-2::LUC plant (presprayed with luciferin for 5 d) were placed in a vase with or without water. After 24 h the LUC activity in both branches was monitored during a 30-min interval. For the jasmonic acid (JA) treatment two branches from a ZPT2-2::LUC plant were placed in a vase with water or water plus JA (50 μm). After 15 h, the LUC activity in the branches was imaged during a 30-min interval.
Analysis of ZPT2-2 mRNA Levels in Different Tissues
Isolation of plant RNA was as described previously (van der Krol et al., 1993). Total RNA (20 μg) was size-fractionated on formaldehyde agarose gels, transferred to nitrocellulose membranes, and hybridized with a 32P-labeled ZPT2-2 cDNA probe as described previously (van der Krol et al., 1993). Alternatively, mRNA was converted to cDNA and a semi-quantitative amplification by PCR was performed using ZPT2-2 specific primers and 1 μg of cDNA as template. As a control the same amount of cDNA was used in a PCR reaction using gapdh or ubiquitin specific primers (Souer et al., 1996). Samples were analyzed after 25, 30, or 35 cycles after fractionation on agarose gel or by probing Southern blots with a 32P-labeled ZPT2-2 cDNA probe.
RESULTS
ZPT2-2::LUC Activity during Germination
A ZPT2-2::LUC reporter gene was constructed by ligating a 2.1-kb promoter fragment from the ZPT2-2 gene to the ff-LUC coding sequence. The chimeric gene was cloned into a binary vector, and leaf discs from petunia line V26 were used for A. tumefaciens-mediated transformation. Nine independent transformed lines were obtained, and for each line seeds were obtained by backcrossing each line to wild-type V26 plants. Seeds were collected, and expression analysis of the ZPT2-2::LUC gene activity was performed on transgenic progeny plants. For expression analysis during germination the seeds were imbibed in the dark on wet filter paper with 0.5 mm luciferin. Each image of the LUC activity was obtained by integration of the photons emitted during a 30-min interval (gain 9.8), and sequential imaging was over a 3-d period. Selected images of this analysis are shown in the top of Figure 1.
LUC activity in the seeds could be detected as early as 3 h after imbibition, and seemed to be localized at the site of the root apex. Upon germination (approximately 29 h after imbibition), the LUC activity briefly declined but was subsequently re-established in the root tip, in which it stayed active but declined at the end of a 60-h period (Fig. 1, top). The LUC activity in cotyledons (still partly covered by the seed coat) transiently increased after germination, reaching a peak in expression 40 h after germination. The elevated LUC activity in the root tip was seen in seedlings of all nine transgenic lines carrying the ZPT2-2::LUC gene. The duration of the transient increase of expression in cotyledons varied among individual plants, varying from hours to days. This variation did not seem to depend on the site of transgene insertion, since the variation also occurred among genetically identical seeds (transgenic seeds from a backcross of a transgenic line carrying a single insert).
ZPT2-2::LUC Is Active in All Parts of Mature Transgenic Plants
One of the great advantages of the LUC reporter system is that the imaging of LUC activity with the 2D luminometer can give a global view of the reporter gene activity in whole, mature plants instead of in small, isolated plant tissues (Fig. 2). Image analysis of photon production in the ZPT2-2::LUC plants showed that the activity was detected in all parts of the plants during vegetative growth. The images of in planta LUC activity show a higher photon production/density in young leaves compared with mature, expanded leaves (Fig. 2, A and B). In both young and mature leaves, the expression of ZPT2-2::LUC was not evenly distributed, but showed localized patches of up to 15-fold higher photon production compared with the average activity in leaves. In mature, soil-grown roots, the ZPT2-2::LUC gene is active throughout the root at a low level, with elevated levels in the root tip (Fig. 2, C and D).
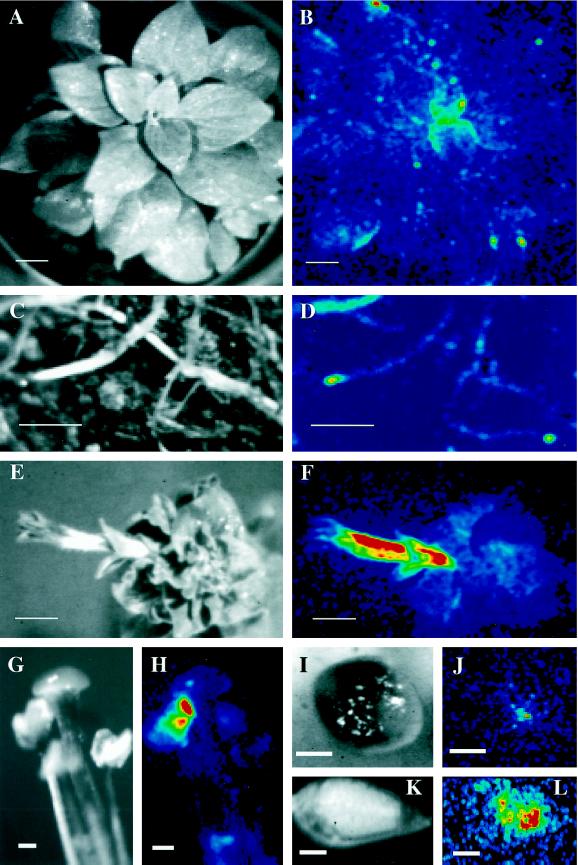
Figure 2.
In vivo ZPT2-2::LUC activity in vegetative and flowering plants. A plant expressing the ZPT2-2::LUC gene was repeatedly sprayed with luciferin, after which the in planta LUC activity was imaged during a 20-min interval. Light images, A, C, E, G, I, and K. ZPT2-2::LUC activity images, B, D, F, H, J, and L. The LUC activity is represented in a false color scale ranging from 0 to 256 (see legend to Fig. 1). A and B, Vegetative shoot (plant 4 weeks old); C and D, roots (plant 4 weeks old); E and F, inflorescence (plant 6 weeks old); G and H, stamen and style; I and J, transgenic pollen on non-transgenic stigma; K and L, developing seeds 5 d post pollination. Thick space bars = 1 mm; thin space bars = 1 cm. Note that the resolution of the images is limited by the resolution of the intensifier (350 × 350 dpi).
In flowering plants LUC activity was detected throughout the inflorescence (stem, bracts, floral bud; Fig. 2, E and F), and was clearly elevated in petal tissue. Detection of the LUC activity in intact mature stamen was not possible due to the lack of penetration of the luciferin substrate into locules (data not shown). When luciferin was applied on locules after anthesis, high LUC activity could be observed in the pollen (Fig. 2, G and H). Figure 2, I and J, shows the activity of ZPT2-2::LUC in pollen applied to a non-transgenic petunia stigma that was pre-treated with luciferin.
At 5 d after pollination of a non-transgenic petunia plant with ZPT2-2::LUC pollen the LUC activity could be imaged in developing seeds. For imaging of LUC activity the carpel leaves were removed (Fig. 2, K and L). Analysis of in vivo ZPT2-2::LUC gene expression during later stages of seed development was not possible due to the lack of penetration of the luciferin substrate into the seed (data not shown).
ZPT2-2::LUC Activity during 20 d of Flower Development
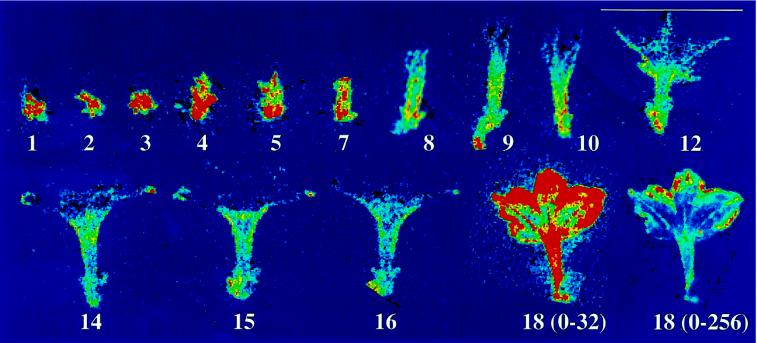
The change in ZPT2-2::LUC activity during floral development was monitored on the plant by imaging LUC activity in the same floral bud every day over an 18-d period (Fig. 3). The images show how the ZPT2-2::LUC promoter activity increases in the tube and limb tissues until opening of the petals. Prior to petal senescence at d 18 (non-pollinated flower), the activity of ZPT2-2::LUC shows a dramatic increase in the petals. As a control we also imaged the activity of a cauliflower mosaic virus 35S::LUC and a lipid transferase (LTP)::LUC reporter gene in transgenic petunia flowers. For both these reporter genes the LUC activity in petals declined steadily after opening of the petals, and the decline in LUC activity was accelerated at the onset of wilting (data not shown). It is therefore concluded that the increase in photon production in ZPT2-2::LUC petals prior to wilting is specific to the response of the ZPT2-2 promoter.
Figure 3.
ZPT2-2::LUC activity in the same flower during 18 d of development on the plant. A ZPT2-2::LUC plant was sprayed daily with luciferin and LUC activity was imaged in the same floral bud during a 30-min interval over an 18-d period. Numbers correspond to the day at which the image was taken. The LUC activity is represented in false colors (scale 0–16; see legend to Fig. 1). The last image (d 18) is shown twice, once in scale 0 to 16 and once in scale 0 to 256.
Pollination Induces Up-Regulation of ZPT2-2::LUC Activity in Petals
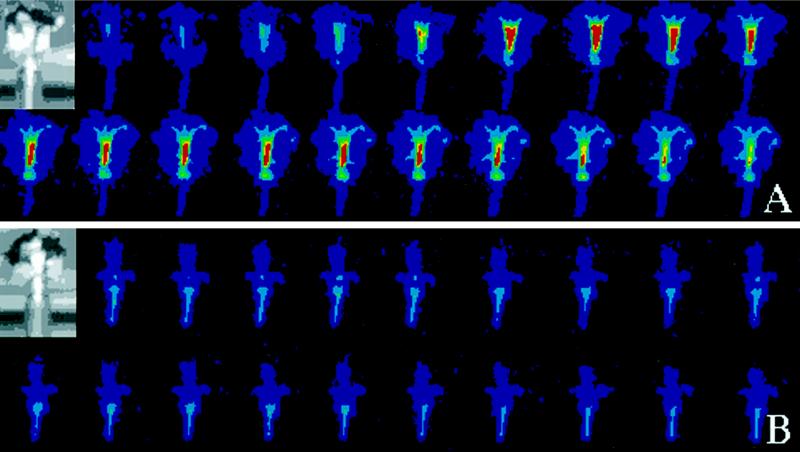
It has been shown previously that in petunia the developmental control of petal wilting is accelerated by pollination of the stigma (Gilissen and Hoekstra, 1984; Whitehead et al., 1984; Hoekstra and Weges, 1986). Without pollination, wilting of petals on isolated flowers commences approximately 8 d after anthesis. However, when the petunia stigma is pollinated at anthesis, the petals wilt within 2 to 3 d. We imaged the change in ZPT2-2::LUC gene activity in petals in response to pollination in 30-min intervals over a 3-d period. Figure 4A shows selected images of LUC activity in petals of a flower until 72 h post-pollination (time interval between images is 3.5 h). The figure shows that the ZPT2-2 promoter activity increases in response to pollination, reaching a peak in activity at 26 h post pollination. Quantification of the photon production in the petal tube shows that the up-regulation of ZPT2-2::LUC activity in petals can already be detected at 2 h post pollination (not shown).
Figure 4.
ZPT2-2::LUC activity in petals in response to pollination. A flower from a ZPT2-2::LUC plant was harvested prior to anthesis and put in a small vase with 0.1 mm luciferin (A) or 0.1 mm luciferin plus 0.1 mm STS (B). After 12 h of equilibration, the flowers were pollinated and in vivo LUC activity was imaged in 30-min intervals during a 3-d period. The time interval between the images was 3.5 h. The LUC activity is represented in false colors (color scale range from 0–256; see legend to Fig. 1). Note that the transient increase in LUC activity in the tube and corolla tissue (A) is blocked by the STS treatment (B). The insets show the light images of the flowers in the vase.
It has been shown before that the action of the phytohormone ethylene plays a role in the process of petunia petal wilting (Whitehead et al., 1984; Woltering et al., 1997). We tested whether ethylene signaling plays a role in the modulation of expression of the ZPT2-2 gene by testing the effect of a blocker of ethylene perception (STS) and the expression of ZPT2-2::LUC in petals. Figure 4B shows selected images of LUC activity in petals of a flower pre-treated with STS at different times after pollination (3.5-h interval between images). This figure shows that pollination did not induce any change in petal LUC activity; however, the basal level of expression of this reporter gene in the petals was not affected by the STS treatment. Similar results were obtained using the inhibitor MCP (data not shown).
Wounding-Induced Regulation of ZPT2-2::LUC Activity in Leaves
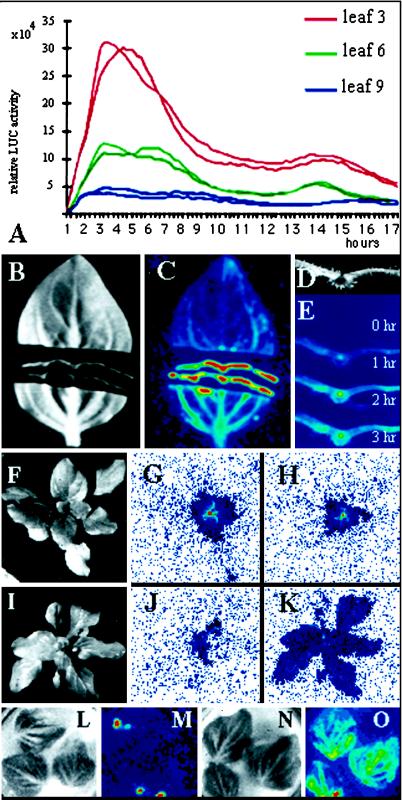
During our analysis of ZPT2-2::LUC plants, we noticed an elevated LUC activity at sites of injury, indicating that ZPT2-2 may be up-regulated by wounding. To test this, we quantified the ZPT2-2::LUC activity in leaf discs over a 17-h period. Figure 5A shows that, upon wounding, the LUC activity in the discs shows a dramatic increase, reaching a peak at 2 to 3 h after the wounding. At 17 h post wounding, the LUC activity was still elevated compared with the initial LUC activity in the discs. The maximum level and response dynamics in discs taken from the same leaf were always comparable, but the level of induction in older leaves was lower than in young leaves (Fig. 5A, compare response of the discs from leaf 3 and 9).
Figure 5.
ZPT2-2::LUC activity in response to different stress signals. A, ZPT2-2::LUC activity during a 24-h period in (2×) two small leaf discs taken from leaf 3, 6, or 9 from the top of the plant. B, Light image of sectioned leaf; C, ZPT2-2::LUC activity image acquired in 20-min interval 1 h after sectioning of the leaf. D, Light image of leaf section; E, ZPT2-2::LUC activity image acquired in 20-min interval in leaf section at 0, 1, 2, and 3 h after sectioning. F, Light image of plant treated at 24°C; G, ZPT2-2::LUC activity image acquired in 20-min interval in plant before treatment at 24°C. Note that here the background color has been changed from black to white. H, ZPT2-2::LUC activity image acquired in 20-min interval in plant after a 12-h treatment at 24°C. Note that here the background color has been changed from black to white. I, Light image of plant treated at 4°C. J, ZPT2-2::LUC activity image acquired in 20-min interval in plant before treatment at 4°C. Note that here the background color has been changed from black to white. K, ZPT2-2::LUC activity image acquired in 20-min interval in plant after a 12-h treatment at 4°C. Note that here the background color has been changed from black to white. L, Light image of leaves illuminated for 6 h with white light. M, ZPT2-2::LUC activity image acquired in 20-min interval in leaves illuminated for 6 h with white light. N, Light image of leaves illuminated for 6 h with white plus UV-B light. O, ZPT2-2::LUC activity image acquired during a 20-min interval in leaves illuminated for 6 h with white plus UV-B light.
Figure 5, B and C, shows an image of the induction of LUC activity in a sliced leaf. Besides the increase in LUC activity near the wound surface, an increased activity in the main veins, away from the wound surface, can also been seen. This indicates a transport of an inducing signal through the leaf. Figure 5, D and E, shows a closeup of the leaf slice and the change in the LUC activity over a time period of 3 h. The ZPT2-2::LUC gene is activated in all cells of the leaf (epidermis, mesophyll cells, cortex, and in vascular tissue).
Cold Stress Induces Up-Regulation of ZPT2-2::LUC Activity in Leaves
We also tested whether the ZPT2-2 gene expression could be induced by cold stress. Two transgenic plants expressing ZPT2-2::LUC were pre-sprayed with luciferin and LUC activity was imaged at room temperature over a 20-min period (Fig. 5, F and I, light image; G and J, LUC activity image). The plants were then placed in the dark at either 24°C or 4°C. After 12 h, the activity of the LUC reporter was imaged during a 20-min period at room temperature (Fig. 5, H and K). After 12 h at 24°C, the ZPT2-2::LUC shows a slight decrease in activity (Fig. 5H). In contrast, in the plant treated for 12 h at 4°C, the LUC activity had increased throughout the plant (Fig. 5K). This increased activity rapidly declined within 2 h at room temperature (not shown). Note that no new luciferin was applied during or after the 12-h temperature treatments.
UV-B Light Induces Up-Regulation of ZPT2-2::LUC Activity in Leaves
It has been well documented that UV-B light can impose stress on plant tissue (for review, see Jansen et al., 1998). We therefore tested the effect of UV-B irradiation on ZPT2-2::LUC expression in leaves. Isolated leaves were illuminated with white light either supplemented or not supplemented with UV-B light for 6 h. The expression of ZPT2-2::LUC was subsequently imaged during a 15-min period. The leaves illuminated with only white light show a low level of LUC activity throughout the leaf and a high level of LUC activity localized at the wound surface (Fig. 5, L and M). The leaves illuminated with white light supplemented with UV-B light showed elevated LUC activities throughout the leaf, mainly associated with the veins (Fig. 5, N and O). Quantification of the average LUC activity in the leaves shows that in UV-B-illuminated leaves LUC activity is about 5-fold higher than those in the control leaves.
Desiccation Induces Up-Regulation of ZPT2-2::LUC Activity in Shoots
Since the pollination-induced up-regulation of ZPT2-2::LUC activity in petals precedes the wilting of the petals, we tested whether this response could also be observed upon desiccation of leaf tissues. Two branches of a ZPT2-2::LUC plant (sprayed with luciferin) were placed in a vase either with or without water. The LUC activity was imaged in 30-min intervals over a 35-h period. Photon production in the shoots was quantified and expressed as a percentage of the initial value (Table I). In the control shoot (on water) the LUC activity remained more or less constant over the 35-h period (indicating that the pre-spray with luciferin was sufficient to maintain LUC activity for prolonged time). In the desiccating shoot the activity increased to up to 7-fold of the initial LUC activity. After 3 d these isolated leaves still look healthy and had retained full turgor. The leaves on this shoot had lost their turgidity and look completely wilted, but apparently the cells in these leaves had enough activity to show the increase in ZPT2-2 promoter activity. Shoots of a plant carrying a cauliflower mosaic virus 35S::LUC reporter gene only showed a decrease in LUC activity upon desiccation (data not shown), indicating that the up-regulation of LUC activity is specific for the ZPT2-2 promoter.
Table I.
Relative LUC activity in desiccating and non-desiccating shoots taken from the same plant
| Time | Relative LUC Activity
|
|
|---|---|---|
| Water | Dry | |
| h | % | |
| 0 | 100 | 100 |
| 5 | 117 | 163 |
| 10 | 114 | 260 |
| 15 | 112 | 415 |
| 20 | 109 | 519 |
| 25 | 117 | 602 |
| 30 | 115 | 671 |
| 35 | 109 | 763 |
Shoots from a ZPT2-2::LUC plant that was pre-sprayed with luciferin were placed in a vase with water (water) or without water (dry). LUC activity was quantified at t = 0 (set at 100%) and every subsequent 5 h for a period of 35 h. Note that no extra luciferin was added during the treatment.
Modulations of ZPT2-2::LUC Activity Are Comparable to Modulations in Endogenous ZPT2-2 Gene Expression
We tested whether the sites of ZPT2-2::LUC expression are a reflection of endogenous ZPT2-2- gene expression. Similarly, we tested whether the modulations in ZPT2-2::LUC expression are a reflection of changes in endogenous ZPT2-2 gene activity. To determine the expression of the endogenous ZPT2-2 gene in different tissues, we isolated RNA from different plant organs at different stages of development or under different abiotic stresses. ZPT2-2 mRNA levels were semi-quantified by reverse transcriptase (RT)-PCR using ZPT2-2 specific primers. RNA was isolated from seedling, leaf (wounded or desiccated), petal (before and after pollination), and stamen tissues. The mRNA converted to cDNA, which was used in the RT-PCR reactions.
The amount of ZPT2-2 specific PCR product was semi-quantified in relation to the amount of the gadph -specific PCR product from the same cDNA sample. From the PCR amplification experiment it can be concluded that ZPT2-2 transcripts are indeed present in all tissues sampled. The ZPT2-2 transcript level in non-stressed leaves was arbitrarily set at 100% (Table II). Quantification of the relative ZPT2-2 mRNA levels in leaves after wounding (2 h) or desiccation (24 h) showed a 7- and 27-fold increase in ZPT2-2 mRNA level. This increase is similar to what has been observed for the ZPT2-2::LUC reporter gene expression after wound or desiccation stress. Table II also shows that the induction of ZPT2-2::LUC in petals was up-regulated in response to pollination. These analyses indicate that the modulation in expression of the ZPT2-2::LUC reporter gene is a reflection of the changes in endogenous ZPT2-2 gene expression.
Table II.
Relative steady-state level of endogenous ZPT2-2 mRNA in different tissues
| Sample | ZPT2-2 mRNA |
|---|---|
| % | |
| Control leaf | 100 |
| Desiccated leaf | 709 |
| Wounded leaf | 2.758 |
| Petal (np.fl) | 6.260 |
| Petal (p.fl.) | 42.431 |
| Anther young | 2.912 |
| Anther mature | 3.460 |
| Seedlings | 707 |
The hybridization signal to ZPT2-2 specific RT-PCR products was quantified and related to the hybridization signal to gapdh specific RT-PCR product, amplified from the same cDNA pool. The relative signal of ZPT2-2 PCR product in unstressed leaves was arbitrarily set at 100%. p.fl., Pollinated flower; np.fl., non-pollinated flower.
Hormonal Regulation of ZPT2-2 Gene Expression
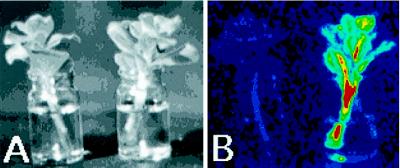
We tested whether ZPT2-2::LUC expression could be induced by application of endogenous ethylene or JA. When two shoots from the same transgenic plant were incubated with or without ethylene (10 nL L−1) for 12 h, no difference in LUC activity was observed (data not shown), indicating that ethylene alone is not sufficient for the modulation in ZPT2-2 gene expression. Figure 6 shows the LUC reporter gene activity in two shoots taken from the same reporter plant, with one shoot on water and one shoot on 50 μm JA (after 15 h). The figure shows that, in contrast to ethylene, JA is sufficient for the up-regulation of ZPT2-2::LUC gene expression.
Figure 6.
Induction of ZPT2-2::LUC activity in shoots by JA. Two shoots taken from the same ZPT2-2::LUC plant were placed in a vase with water (left) or water plus 50 μm JA (right). A, Light image; B, LUC activity image after 15 h, in a false color scale ranging from 0 to 256 (see legend to Fig. 1).
DISCUSSION
LUC Reporter Reveals Low Levels and Dynamic Behavior of ZPT2-2 Promoter Activity
The lack of ZPT2-2 mutant plants makes it difficult to assign a specific function to this gene. However, an extensive analysis of the expression pattern of ZPT2-2 during plant development and a comparison with the expression of other known genes may give clues about the developmental processes or physiological responses in which ZPT2-2 plays a role. Previous expression analysis identified the ZPT2-2 gene as floral specific. Expression on northern blots was detected only in the second and third floral whorls (A.R. van der Krol, unpublished data; Takatsuji et al., 1994), indicating that ZPT2-2 may be under control of the B-type floral homeotic MADS box genes fbp1 (Angenent et al., 1992) and pMADS1 (van der Krol et al., 1993).
We used the LUC reporter system to analyze the activity from the ZPT2-2 promoter in transgenic plants. The sensitivity by which photons can be detected allowed for the identification of sites with extremely low promoter activity that previously went unnoticed (e.g. the expression in leaves, root, pollen, and developing seeds). The non-destructive method of measuring gene activity through the LUC reporter enabled us to monitor gene expression in the same tissue over prolonged periods of time. We could thus follow the expression of ZPT2-2::LUC on the plant in the same flower over a period of 20 d (Fig. 3). In this manner we identified a strong up-regulation of ZPT2-2 gene activity at the onset of petal wilting. Our in planta studies also revealed transient modulation in ZPT2-2 activity during germination in cotyledons (Fig. 1) and petals in response to (pollination-induced) wilting (Figs. 3 and 4). At this time it is not known whether the transient nature of these modulations in ZPT2-2 activity are due to a transient occurrence of a signal, a desensitization to a continuous signal, or a combination of these two.
The ease by which LUC activity can be measured in plants using the two-dimensional luminometer makes it easy to test the effect of different stress conditions on the expression of ZPT2-2::LUC. We thus were able to identify up-regulation of ZPT2-2::LUC expression in leaves upon wounding, UV-B light stress, cold stress (see Fig. 5), or desiccation stress (Table I). Both the level and dynamics of the wound response were reproducible between discs taken from the same leaf (Fig. 5A). However, between leaves both the strength and dynamics of the response varied, with the youngest leaf showing the highest and fastest response (compare response discs from leaf 3, 6, and 9, Fig. 5A). Imaging of the wound response in leaf sections shows that the increase in ZPT2-2::LUC activity is observed in all cell layers of the leaf upon wounding (Fig. 5, D and E). The images that were obtained during the (semi) continuous measurements of LUC activity were compiled in stacks, which can be viewed as videos at http://www.spg.wau.nl/pf/movies.html.
Modulations in ZPT2-2::LUC Reporter Gene Activity Coincide with Modulations in Endogenous ZPT2-2 Gene Activity
The low ZPT2-2::LUC activity in transgenic plants suggested a low level of ZPT2-2 gene activity in vegetative tissues that previously went unnoticed. Expression of the endogenous ZPT2-2 gene in these tissues was confirmed using RT-PCR and RNA samples from leaf (Table II) and root (not shown). The steady-state levels of ZPT2-2 mRNA in the vegetative plant tissue were on average less than 3% of that observed in petals or stamen. We also demonstrated that the increase of ZPT2-2::LUC activity in leaves by desiccation and wound stress are a true reflection of modulations in endogenous ZPT2-2 gene activity. The (semi) quantification of ZPT2-2 mRNA steady-state levels in desiccated and wounded leaves showed a 7- and 27-fold increase, respectively, compared with the ZPT2-2 mRNA levels in control leaves (Table II). These induction levels coincide well with those observed for in vivo ZPT2-2::LUC reporter gene activity upon desiccation and wounding (9- and 30-fold, respectively, see Fig. 5A; Table II).
Complex Hormonal Control of ZPT2-2 Gene Expression
In flowering petunia plants, the petals wilt about 8 d after anthesis. This process of petal wilting can be accelerated by pollination of the style (Hoekstra and Weges, 1986; Hoekstra and van Roekel 1988; Tang et al., 1994; Tang and Woodson, 1996). It has been shown that removal of the style at 4 h after pollination can no longer inhibit this pollination-induced wilting of petals, indicating that 4 h after pollination a signal has reached the petals from the stigma. Once perceived by the petal, this signal is no longer required to complete the petal wilting process (Gilissen and Hoekstra, 1984). With our (semi) continuous measurement of ZPT2-2::LUC activity in the tube, we observed that at 2 h after pollination of the stigma, the LUC activity had already increased in the petal tube. This indicates that a pollination-induced signal is perceived by the petals within 2 h after pollination of the stigma. No modulation in petal LUC activity was seen in control, unpollinated flowers over a period of 3 d (data not shown).
Our analysis of the expression of ZPT2-2::LUC indicates that in petals there are two modes of ZPT2-2 gene expression: a low basal level and an inducible expression that may be dependent on endogenous phytohormone levels. Inhibition of ethylene perception blocks the up-regulation of ZPT2-2 gene expression in petals after pollination, suggesting that ethylene acts as an inducing signal in flowers in this regulatory system. However, incubation of whole plants or flowers in ethylene did not induce expression of the ZPT2-2::LUC gene within 24 h (data not shown), indicating that ethylene is required but not sufficient for induction.
We also tested whether the transient increase of ZPT2-2::LUC activity in cotyledons (Fig. 1) requires a functional ethylene signal transduction pathway. Seeds were germinated on wet filter papers in sealed Petri dishes either with or without 1 μL L−1 MCP. The effect of blocking ethylene receptors was evident from the lack of root hair development on MCP-treated seedlings. Imaging of the LUC activity in these seedlings showed that the transient up-regulation of ZPT2-2::LUC in cotyledons was not blocked and thus does not seem to require active ethylene sensing (data not shown).
It is known that the phytohormone JA mediates the response of plants to cold, wound, and UV-B light stresses (Sembdner and Parthier, 1993; Creelman and Mullet, 1995). Furthermore, the sites of the plant that show elevated levels of ZPT2-2 activity (i.e. root tip, young leaves, and petals) have been shown to be the sites of JA accumulation in plants (Meyer et al., 1984). These observations suggest that JA may be involved in the induction of ZPT2-2 gene activity and, indeed, JA can induce ZPT2-2 gene expression in shoots (Fig. 6). A further characterization of how the phytohormone signaling of ethylene and JA are integrated to modulate the activity of ZPT2-2 gene expression will be published elsewhere.
ACKNOWLEDGMENTS
We thank Leen Peters for excellent care of the plants and Ronny Joosen for technical assistance during some of the experiments.
Footnotes
This research has in part been paid for by the Research School Experimental Plant Science.
LITERATURE CITED
- Angenent GC, Busscher M, Franken J, Mol JNM, van Tunen AJ. Differential expression of two MADS box genes in wild type and mutant petunia flowers. Plant Cell. 1992;4:983–993. doi: 10.1105/tpc.4.8.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey PN, Takatsuji H, Ren L, Shah DM, Chua N-H. Sequence requirements of the 5-enolpyruvylshikimate-3-phosphate synthase 5′-upstream region for tissue specific expression in flowers and seedlings. Plant Cell. 1990;2:849–856. doi: 10.1105/tpc.2.9.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen ES, Meyerowitz E. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353:31–37. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet J. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen LJW, Hoekstra FA. Pollination-induced corolla wilting in Petunia hybrida: rapid transfer through the style of a wilting-inducing substance. Plant Physiol. 1984;75:496–498. doi: 10.1104/pp.75.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman KM. The shikimate pathway: early steps in the biosynthesis of aromatic compounds. Plant Cell. 1995;7:907–919. doi: 10.1105/tpc.7.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra FA, van Roekel T. Effects of previous pollination and stylar ethylene on pollen tube growth in Petunia hybrida styles. Plant Physiol. 1988;86:4–6. doi: 10.1104/pp.86.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra FA, Weges R. Lack of control by early pistillate ethylene of the accelerated wilting of Petunia hybrida flowers. Plant Physiol. 1986;80:403–408. doi: 10.1104/pp.80.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM. Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci. 1998;4:131–135. [Google Scholar]
- Kubo K, Sakamoto A, Kobayashi A, Rybka Z, Kann Y, Nakagawa H, Nishino T, Takatsuji H. Cys(2)/His(2) zinc finger protein family of petunia: evolution and general mechanism of target sequence recognition. Nucleic Acids Res. 1998;26:608–615. doi: 10.1093/nar/26.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Miersch O, Buttner C, Dathe W, Sembdner G. Occurrence of the plant growth regulator jasmonic acid in plants. J Plant Growth Regul. 1984;3:1–8. [Google Scholar]
- Rosenberg UB, Schroder C, Preiss A, Kienlin A, Cote S, Riede I, Jackle H. Structural homology of the product of the Drosophila Kruppel gene with Xenopus transcription factor IIIA. Nature. 1986;319:336–339. [Google Scholar]
- Sembdner G, Parthier B. The biochemistry and the physiological and molecular actions of jamonates. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:569–589. [Google Scholar]
- Souer E, van Houwelinge A, Kloos D, Mol J, Koes R. The no apical meristem gene of Petunia is required for pattern formation in embryo's and flowers, and is expressed at meristem and organ primordia boundaries. Cell. 1996;85:159–170. doi: 10.1016/s0092-8674(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Takatsuji H, Matsumoto T. Target sequence recognition by separate-type Cys2/His2 zinc finger proteins in plants. J Biol Chem. 1996;271:23368–23373. doi: 10.1074/jbc.271.38.23368. [DOI] [PubMed] [Google Scholar]
- Takatsuji H, Mori M, Benfey PN, Ren L, Chua N-H. Characterization of a zinc finger DNA binding protein expressed specifically in petunia petals and seedlings. EMBO J. 1992;11:241–249. doi: 10.1002/j.1460-2075.1992.tb05047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuji H, Nakamura N, Katsumoto Y. A new family of zinc finger proteins in petunia: structure, DNA sequence recognition, and floral organ-specific expression. Plant Cell. 1994;6:947–958. doi: 10.1105/tpc.6.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Gomes AMTR, Bhatia A, Woodson WR. Pistil-specific and ethylene-regulated expression of 1-aminocyclopropane-1-carboxylate oxidase genes in petunia flowers. Plant Cell. 1994;6:1227–1239. doi: 10.1105/tpc.6.9.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Woodson WR. Temporal and spatial expression of 1-ACC oxidase mRNA following pollination of immature and mature petunia flowers. Plant Physiol. 1996;112:503–511. doi: 10.1104/pp.112.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D, Lehmann R, Schnurch H, Schuh R, Seifert E, Kienlin A, Jones K, Jackle H. Finger protein of novel structure encoded by hunchback, a second member of the gap class of Drosophila segmentation genes. Nature. 1987;327:383–389. [Google Scholar]
- van der Krol AR, Brunell A, Tsuchimoto S, Chua N-H. Functional analysis of the petunia floral homeotic MADS box gene pMADS1. Genes Dev. 1993;7:1214–1228. doi: 10.1101/gad.7.7a.1214. [DOI] [PubMed] [Google Scholar]
- van der Krol AR, Chua N-H. Flower development in petunia. Plant Cell. 1993;5:1195–1203. doi: 10.1105/tpc.5.10.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead CS, Halevy AH, Reid MS. Roles of ethylene and 1-aminocyclopropane-1-carboxylic acid in pollination and wound-induced senescence of Petunia hybrida flowers. Physiol Plant. 1984;61:643–648. [Google Scholar]
- Woltering EJ, de Vrije T, Harren F, Hoekstra FA. Pollination and stigma wounding: same response, different signal. J Exp Bot. 1997;48:1027–1033. [Google Scholar]