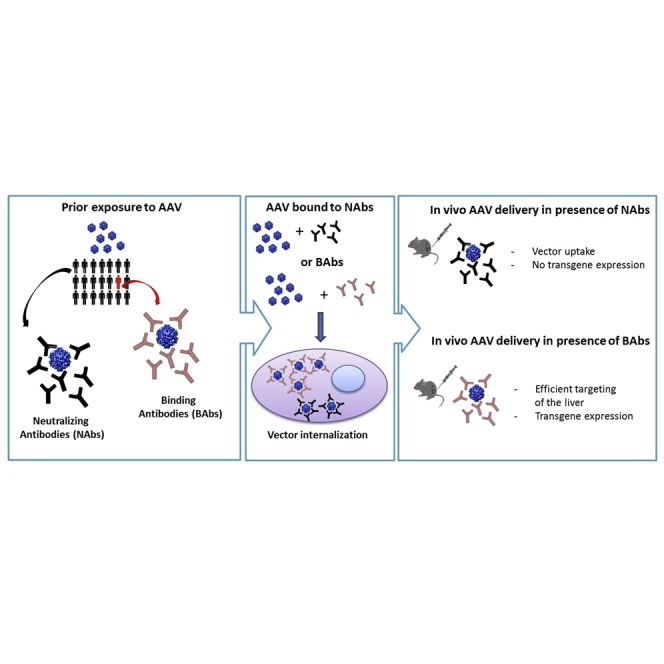
Abstract
Pre-existing immunity to adeno-associated virus (AAV) is highly prevalent in humans and can profoundly impact transduction efficiency. Despite the relevance to AAV-mediated gene transfer, relatively little is known about the fate of AAV vectors in the presence of neutralizing antibodies (NAbs). Similarly, the effect of binding antibodies (BAbs), with no detectable neutralizing activity, on AAV transduction is ill defined. Here, we delivered AAV8 vectors to mice carrying NAbs and demonstrated that AAV particles are taken up by both liver parenchymal and non-parenchymal cells; viral particles are then rapidly cleared, without resulting in transgene expression. In vitro, imaging of hepatocytes exposed to AAV vectors pre-incubated with either NAbs or BAbs revealed that virus is taken up by cells in both cases. Whereas no successful transduction was observed when AAV was pre-incubated with NAbs, an increased capsid internalization and transgene expression was observed in the presence of BAbs. Accordingly, AAV8 vectors administered to mice passively immunized with anti-AAV8 BAbs showed a more efficient liver transduction and a unique vector biodistribution profile compared to mice immunized with NAbs. These results highlight a virtually opposite effect of neutralizing and binding antibodies on AAV vectors transduction.
Keywords: AAV vector, pre-existing antibodies, neutralizing antibody, BAb, NAb, binding antibody, liver transduction, liver gene transfer, AAV immune-response, vector transduction
Graphical Abstract
Introduction
Systemic administration of adeno-associated virus (AAV) vectors has been utilized in preclinical studies to transduce a number of tissues, including liver parenchyma,1, 2, 3 skeletal muscle,4, 5 and CNS.6 Recent promising data from hemophilia clinical trials have confirmed that peripheral administration of AAV vectors can efficiently transduce the liver, resulting in therapeutic levels of transgene expression.7, 8, 9, 10, 11, 12 However, due to exposure to wild-type AAV, a relatively large proportion of humans carry circulating antibodies directed against the AAV capsid.13, 14 When anti-capsid antibodies target viral epitopes critical for cellular entry, they can block virus infectivity and are deemed neutralizing antibodies (NAbs).15, 16, 17 NAbs can have a profound effect on the efficiency of tissue transduction with AAV vectors, as even low titers of NAbs can result in lack of efficacy following gene transfer.9, 18 Depending on the AAV serotype and the age of the donor, the proportion of subjects positive for anti-AAV antibodies can be up to ∼60%, with only a small window of time after birth during which the vast majority of individuals are naive to the virus.19, 20, 21 Furthermore, after exposure to AAV vectors, humans become immunized against the capsid and develop high titers of NAbs that persist long term.22 The impact of pre-existing humoral immunity to AAV on the outcome of gene transfer in terms of safety and efficacy is only partially understood. Experience in human trials suggests that, above a certain level, NAbs simply prevent AAV vector transduction and consequently transgene expression.9 Beyond this observation, the clinical significance of pre-existing humoral immunity in influencing AAV vector biodistribution and clearance is still elusive. Studies in non-human primates indicate that low levels of capsid-specific NAbs can alter the tropism of AAV vector particles, directing them to the spleen.23 Here, we explored the role of NAbs on AAV liver transduction both in vitro and in vivo. We showed that AAV vectors complexed with NAbs can be found in both liver parenchymal and non-parenchymal cells, although they do not result in successful transduction. We also found that a relatively small proportion of adult human subjects carry binding antibodies (BAbs). These antibodies are capable of recognizing the AAV capsid but lack neutralizing capacity. We studied how BAbs influence systemic AAV-mediated gene delivery parameters, including transgene expression levels, vector persistence in the circulatory system, and vector immunogenicity in pre-immunized animals. BAbs have a markedly different effect on AAV vector liver transduction and tissue biodistribution compared to NAbs.
Results
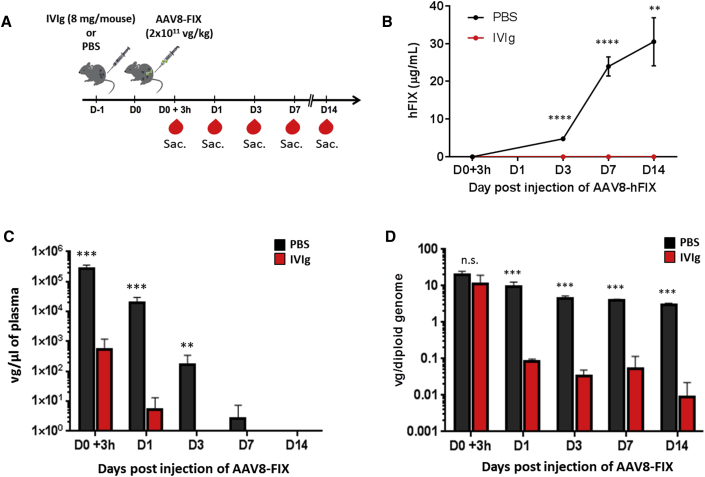
Antibody-Complexed Capsid Is Taken Up by Both Liver Parenchymal and Non-parenchymal Cells
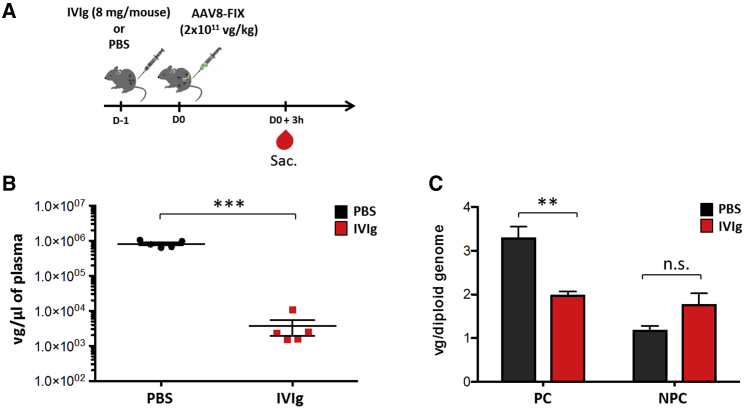
Circulating antigen-antibody complexes can be directed to the liver and influence the state of the immune reactivity toward a given antigen.24 Delineating whether and to what extent AAV capsid immune complexes reach this compartment can offer insight into the early stages of immune activation. To clarify this point, we investigated the role of NAbs both in vector uptake by the liver and in the clearance of AAV delivered into the bloodstream. As source of NAbs, we used human intravenous immunoglobulin (IVIg),25, 26 a reagent previously tested in an anti-AAV8 antibody neutralization assay and displaying high neutralization activity toward AAV vectors even at low concentrations (Table 1). Wild-type male C57BL/6 mice were passively immunized with IVIg (8 mg/mouse resulting in a NAb titer of ∼1:10; data not shown) or were infused with PBS as control. Twenty-four hours later, all animals received an AAV8 vector expressing the human factor IX transgene (AAV8-FIX) at a dose of 2 × 1011 vector genomes (vg)/kg via the intravenous (i.v.) route (Figure 1A). Viral particle persistence in plasma and circulating FIX transgene expression levels were quantified in samples collected at 3 hr on days 1, 3, 7, and 14 following vector administration (Figure 1A). Throughout the duration of the study, FIX transgene expression was undetectable in IVIg-immunized animals, indicating that the dose of IVIg used was sufficient to completely neutralize the AAV8-FIX vector (Figure 1B). In immunized mice, we also observed that viral particles were quickly removed from the circulation, as vector genomes were significantly diminished in blood 3 hr after vector administration and undetectable 3 days later (Figure 1C). Conversely, vector genomes remained detectable in the circulation of naive animals up to one week post-vector infusion (Figure 1C). In order to quantify the presence of AAV8 vector genomes in the liver, animals were sacrificed 3 hr and 1, 3, 7, and 14 days post-injection (n = 5/time point; Figure 1A). Three hours post-injection, the quantity of vector genomes in the liver of IVIg-immunized animals was not significantly different from that of PBS-injected controls (Figure 1D). However, 24 hr after vector administration, vector genomes were nearly undetectable in livers of pre-immunized mice (Figure 1D; limit of detection of the assay 0.001 vg/diploid genome). We next performed an experiment in which livers from passively immunized animals and PBS controls were harvested 3 hr post-AAV8-FIX vector delivery, and parenchymal (PC) and non-parenchymal (NPC) cells were analyzed separately (Figure 2A). As in the previous experiment, vector genomes in plasma were significantly decreased in IVIg-immunized animals following vector delivery (Figure 2B). Accordingly, vector genomes in PC cells were significantly decreased but still detectable (Figure 2C). Conversely, in NPC cells, AAV8-FIX genomes were slightly increased in IVIg pre-immunized animals, although not to statistically significant levels (Figure 2C). These results indicate that, in the presence of NAbs, a significant amount of AAV vector is initially captured in the liver by both PCs and NPCs; however, this does not result in successful hepatocyte transduction.
Table 1.
NAb Titer to AAV8 Measured at Increasing IVIg Concentrations
| IVIg Concentration | AAV8 NAb Titer (Reciprocal Dilution, 1:x) | % of Neutralization at a Given Reciprocal Dilution |
|---|---|---|
| 0 mg/mL | <1:1 | 0 |
| 0.0160 mg/mL | <1:1 | 6.1 |
| 0.0313 mg/mL | <1:1 | 9.3 |
| 0.0625 mg/mL | <1:1 | 24.4 |
| 0.125 mg/mL | 1:1 | 56.7 |
| 0.25 mg/mL | 1:1 | 81 |
| 0.5 mg/mL | 1:3.16 | 65.3 |
| 1 mg/mL | 1:3.16 | 86 |
| 50 mg/mL | 1:316 | 78 |
Figure 1.
Influence of Anti-AAV8 Neutralizing Antibodies on Transgene Expression and Vector Persistence in Blood and Liver
(A) Experimental design; red symbols represent timing of blood collection. Sac., sacrifice. (B) FIX transgene plasma levels at 3 hr and 1, 3, 7, and 14 days post-gene transfer (n = 5) are shown. (C and D) Vector persistence in plasma (C) and liver (D) of animals starting at 3 hr post-vector infusion (n = 5/time point) is shown. Statistical significance over the PBS control group was determined using the Mann-Whitney test. **p < 0.01; ***p < 0.001; ****p < 0.0001; n.s., not significant. Data are represented as mean ± SEM.
Figure 2.
AAV Vector Uptake by Both Liver Parenchymal and Non-parenchymal Cells
(A) Experimental design; red symbols represent timing of blood collection. (B) Vector persistence in plasma 3 hr post-vector delivery is shown. (C) Vector genomes determined in liver parenchymal (PC) and non-parenchymal (NPC) cells 3 hr post-AAV8-FIX delivery are shown. The statistical significance over the PBS group was determined by the Mann-Whitney test. **p < 0.01; ***p < 0.001; vg, vector genomes. Data are represented as mean ± SEM.
NAbs Do Not Completely Block AAV Capsid Uptake In Vitro
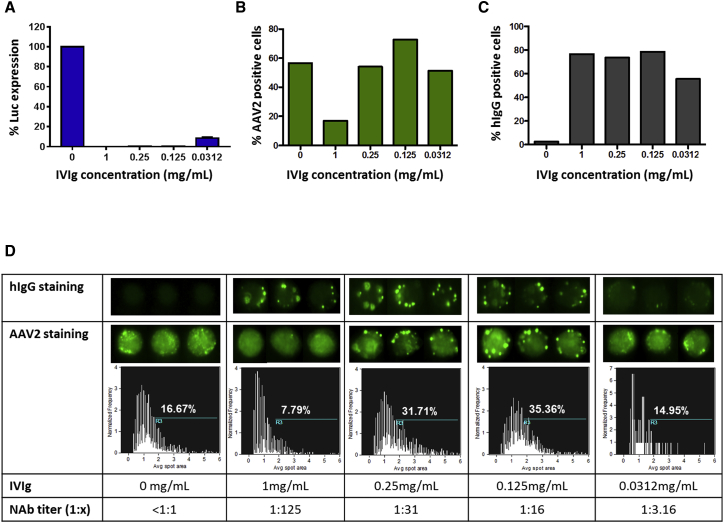
Results in vivo suggest that, in the presence of NAbs to AAV, the liver plays a role in clearing immune complexes. To better understand this phenomenon, we performed imaging flow cytometry in vitro to evaluate the cellular localization and internalization in hepatocytes of AAV vector particles complexed with antibodies. An AAV2 vector expressing luciferase (Luc) was used to infect the murine hepatocyte Hepa 1-6 cell line at an MOI of 103. Vector was pre-incubated with IVIg at concentrations ranging from 0 (NAbs negative control) to 1 mg/mL, resulting in residual Luc expression only at the lowest IVIg concentration (0.0312 mg/mL; Figure 3A). After one-hour incubation with the vector, cells were washed, fixed, permeabilized, and stained for human immunoglobulin G (hIgG) and AAV2 capsid. The frequency of cells that were positive for intracellular AAV2 was markedly reduced (17% of positive cells) with the highest concentration of IVIg (1 mg/mL). Conversely, when cells were transduced with AAV2 vector incubated without IVIg, they showed higher positivity to the capsid (57% of positive cells; Figure 3B). However, the frequency of AAV2-positive cells with the intermediate IVIg concentrations (0.25 and 0.125 mg/mL), which completely blocked Luc transgene expression (Figure 3A), was 54% and 73%, respectively (Figure 3B). These results indicate that internalization of AAV2 capsid bound to antibodies occurs in vitro, except when high concentrations of antibodies are present. The frequency of hIgG-positive cells was identical under all conditions, except for the lowest IVIg concentration (Figure 3C). This indicates that uncomplexed antibodies were efficiently taken up by the cells, particularly when incubated at the highest concentration. Also, results indicate that the concentrations of purified IgG used were saturating in most conditions (Figure 3C). Intracellular staining to detect AAV2 capsid resulted in a punctuated signal both in the presence or absence of IVIg (Figure 3D). Quantification of the area of the AAV capsid spots showed an increase in spot size in the presence of intermediate IVIg concentrations (Figure 3D), suggesting the presence of intracytoplasmic deposits of antibody-AAV2 immune complexes. In the presence of the highest level of IVIg, we observed a reduction in the size of the intracellular spots (7.79% of spots with a size >2 a.u.; Figure 3D). In this condition, the AAV2 epitopes recognized by the antibody used in the assay could be masked from the IVIg molecules. Also, uncomplexed antibodies could compete for the cellular entry with the antibodies bound to the AAV2 capsid. Conversely, with IVIg doses of 0.125 and 0.25 mg/mL, spots positive for AAV2 capsid were readily detectable. Thus, in vitro experiments confirm that hepatocytes can internalize the AAV capsid that is bound to neutralizing antibodies, although no transduction occurs.
Figure 3.
Internalization of AAV2 Immune Complexes in a Murine Hepatocyte Cell Line
(A) Luciferase transgene expression levels in the presence of different IVIg concentrations. Results are reported as % of maximum signal (0 mg/mL IVIg). (B and C) Frequency of cells positive for AAV2 (B) and human IgG (C) is shown. (D) Detection of the fluorescent spots representing the hIgG and AAV2 capsid internalized in hepatocytes is shown. Representative cell images of hIgG and AAV2 capsid presence in hepatocytes are shown for each IVIg concentration and the corresponding NAb titer. The percentage of the fluorescent spots representing AAV2 capsid that have an average area >2 (a.u.) is reported. Each experiment shown here was repeated and analyzed at least twice. IVIg, intravenous immunoglobulins. NAb, neutralizing antibody.
Adult Subjects Carry Circulating Neutralizing and Non-neutralizing Antibodies to AAV
The impact of NAbs on AAV vector transduction efficiency has already been described.9, 18 On the other hand, the effect of BAbs with no neutralizing activity is not yet fully characterized. Using a conventional in vitro NAb assay,27 we screened a collection of healthy donor sera (n = 100) and selected those with anti-AAV8 titers below or equal to 1:1 (n = 49/100; Figure S1A). Among these, using an ELISA for anti-AAV8 antibodies, we were able to find a subset of 10 subjects negative for NAbs but carrying anti-AAV8 antibodies at levels significantly higher than that of a cohort of 11 children, aged one year, that were naive to AAV (Table 2; Figure S1B). To further assess the specificity of these binding antibodies for the AAV8 capsid, we used an ELISA assay in which various lots of AAV8 capsid comprising full, empty, or mixed preparations produced by standard triple transfection,28 or baculovirus methods,29 were used to coat the plates (Figure S2A). We analyzed serum samples from three adult subjects containing anti-AAV8 BAbs (Ad-21, Ad-105, and Ad-32; Table 2) and a pool of these samples. Compared to a seronegative serum (C−) and a serum with high NAb titer (C+), intermediate levels of anti-AAV8 IgG were found in all the BAb samples (Figure S2B). Thus, we confirmed the presence of BAbs in samples from adult healthy donors. Their capacity of binding specifically to AAV8 capsid is unaffected by the virus preparation method.
Table 2.
NAb and IgG Titers to AAV2 and AAV8 in Adults and Children
| Subject ID No. | Age Group | NAb Titer (Reciprocal Dilution, 1:x) |
IgG (μg/mL) |
||
|---|---|---|---|---|---|
| AAV2 | AAV8 | AAV2 | AAV8 | ||
| Ad-58 | adult | <1:1 | <1:1 | 0.0 | 3.7 |
| Ad-56 | adult | 1:1 | <1:1 | 4.4 | 3.1 |
| Ad-105 | adult | 1:1 | <1:1 | 7.6 | 6.5 |
| Ad-32 | adult | 1:1 | <1:1 | 3.7 | 7.2 |
| Ad-89 | adult | 1:1 | <1:1 | 81.2 | 17.7 |
| Ad-109 | adult | 1:100 | <1:1 | 1.5 | 5.6 |
| Ad-118 | adult | <1:1 | <1:1 | 0.3 | 2.2 |
| Ad-93 | adult | 1:3.16 | <1:1 | 6.6 | 4.8 |
| Ad-101 | adult | 1:10 | 1:1 | 29.0 | 12.0 |
| Ad-21 | adult | 1:1 | 1:1 | 8.7 | 6.1 |
| Ch-1 | child | 1:3.16 | <1:1 | 0.58 | 0.41 |
| Ch-2 | child | <1:1 | <1:1 | 0.72 | 0.73 |
| Ch-3 | child | <1:1 | <1:1 | 2.01 | 1.47 |
| Ch-4 | child | 1:1 | <1:1 | 0.13 | 0.23 |
| Ch-5 | child | <1:1 | <1:1 | 0.07 | 0.15 |
| Ch-6 | child | 1:3.16 | <1:1 | 0.05 | 0.07 |
| Ch-7 | child | 1:1 | 1:1 | 0.2 | 1.10 |
| Ch-8 | child | 1:1 | <1:1 | 0.07 | 0.18 |
| Ch-9 | child | <1:1 | <1:1 | 0.25 | 0.34 |
| Ch-10 | child | <1:1 | <1:1 | 0.02 | 0.54 |
| Ch-11 | child | 1:1 | <1:1 | ND | 0.08 |
ND, not determined
Non-neutralizing BAbs Enhance In Vitro Uptake of AAV Vectors
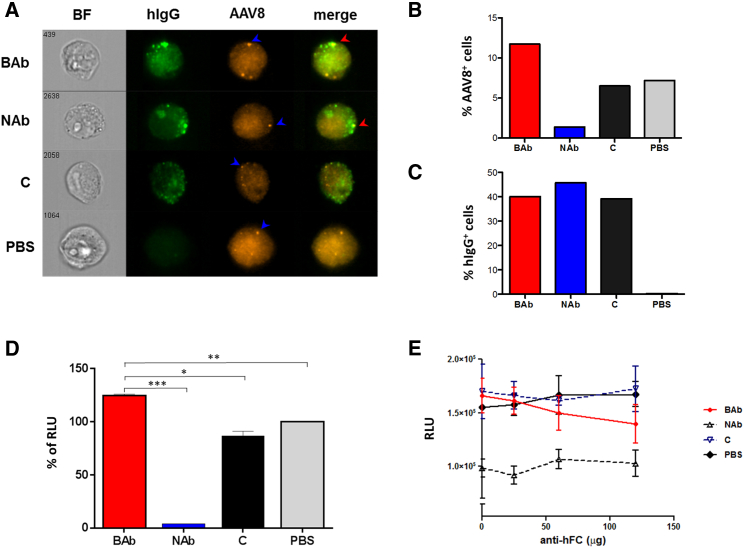
We next sought to compare the effect of anti-AAV BAbs and NAbs on vector internalization in vitro. To this aim, imaging flow cytometry was utilized to investigate AAV8 cell entry in the Hepa 1-6 cells in the presence of NAbs or BAbs. An AAV8-Luc vector was incubated one hour at 37°C with pools of human sera containing BAbs, NAbs, pooled sera from children (C), or PBS. Cells were then incubated for two hours with the corresponding immune complexes at an MOI of 104 and then washed, fixed, and stained intracellularly for hIgG and AAV8 capsid. Imaging flow cytometry revealed the presence of AAV8 capsid in all conditions (Figures 4A and 4B), although to a lower extent when the vector was pre-incubated with NAbs (Figure 4B). Conversely, in the presence of BAbs, we observed an increased uptake of the virus into the cells compared to that of the children’s serum (C) or PBS controls (Figures 4A and 4B). In all conditions in which human serum was added, similar levels of hIgG were detected intracellularly (Figure 4C). In a subsequent experiment, we pre-incubated HEK293 cells with NAbs, BAbs, C, or PBS before adding an AAV8 vector expressing luciferase. Data from this experiment showed the highest transgene expression in samples pre-incubated with BAbs compared to that of the other groups (Figure 4D). Finally, we blocked the Fc receptor using increasing amounts of an anti-human Fc antibody in HEK293 cells. At an MOI of 200, we observed a slight decrease in the cell transduction efficiency in the presence of BAbs with the highest Fc antibody concentration (120 μg; Figure 4E). Together, these results indicate that BAbs do not prevent cell uptake of AAV vectors; moreover, they suggest that BAbs enhance transduction efficiency in vitro.
Figure 4.
Effect of Neutralizing and Non-neutralizing Antibodies on the Entry of AAV8 Vector Particles in Hepa 1-6 and HEK293 Cells
(A) Representative images of cells analyzed by imaging flow cytometry. The blue arrows show the presence of AAV8 particles and the red arrows the colocalization of AAV8 particles with human IgG staining. (B) Frequency of cells positive for AAV8 capsid is shown. (C) Frequency of cells positive for human IgG is shown. (D) Luciferase transgene expression (% of RLU) is shown. Statistical significance of BAb over the NAb, C, and PBS group was determined using Student’s t test. *p < 0.05; **p < 0.01; ***p < 0.001. (E) Fc block experiment is shown. Serum samples diluted 1:36.1 were pre-incubated with increasing concentrations of an anti-hIgG antibody, Fc fragment specific, before adding to the mix an AAV8-luciferase vector. Residual luciferase transgene expression after transduction of HEK293 cells at an MOI of 200 is shown. Data derived from experiments were performed in duplicate. Data are represented as mean ± SEM. BAb, binding antibodies; BF, bright field; C, children naive to AAV8; RLU, relative light units.
Anti-AAV NABs and BAbs Have Opposite Effects on In Vivo Liver Transduction
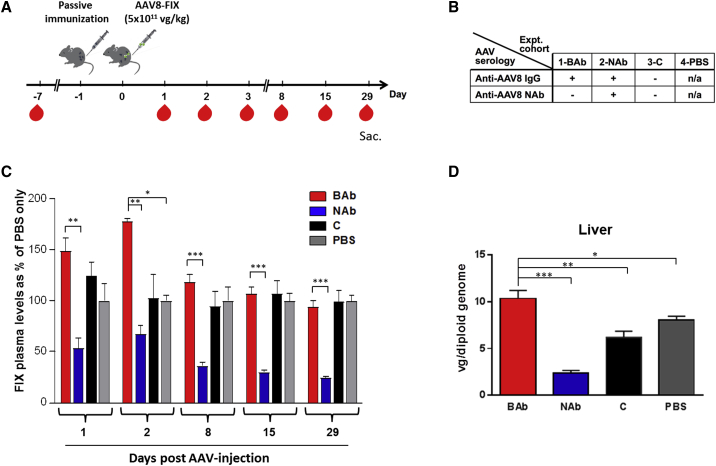
To explore the effect of NAbs and BAbs in vivo, we used a passive immunization model previously described.30 In this model, wild-type C57BL/6 mice were immunized 24 hr before the i.v. administration of an AAV8 vector encoding for the human FIX transgene, which was given with a dose of 5 × 1011 vg/kg (Figure 5A). Mice were assigned to 4 study cohorts (n = 8 to 10 mice/cohort) as outlined in Figure 5B. Animals in cohort 1 (BAb) received pooled serum containing anti-AAV8 BAbs; cohort 2 (NAb) received pooled serum with NAbs to AAV8; cohort 3 (C) received pooled serum from naive children (thus negative for both BAbs and Nabs); and cohort 4 (PBS) received PBS only. Animals were followed for four weeks after vector delivery, and plasma was collected at days −7, 1, 2, 3, 8, 15, and 29 in order to measure FIX transgene expression levels (Figure 5A). Animals carrying anti-AAV8 BAbs showed significantly higher levels of FIX transgene in plasma compared to that of animals pre-treated with NAbs (Figure 5C). All animals expressed similar levels of transgene at day 29, with the exception of animals pre-immunized with NAb-positive serum, which had levels significantly lower (Figure 5C). This was confirmed also by quantification of the human FIX transgene mRNA levels in livers harvested at day 29 (data not shown). We next evaluated levels of liver transduction by measuring vector genome copy number. Animals passively immunized with anti-AAV BAbs showed the highest vector genome copy number per diploid genome when compared with all other treatment cohorts (Figure 5D). Conversely, animals pre-immunized with serum positive for anti-AAV8 NAbs displayed the lowest levels of liver transduction (Figure 5D). An ∼20% decrease in liver transduction was observed in animals passively immunized with children’s sera (C) compared to the PBS control (Figure 5D). These results indicate that BAbs to AAV vectors do not affect AAV transduction of the liver and seem to skew vector genome biodistribution to this organ.
Figure 5.
Liver Transduction Profile in Mice Passively Immunized with Pre-existing Neutralizing or Non-neutralizing Antibodies
(A) Experimental design; red symbols represent timing of blood collection. (B) Treatment cohorts are shown. (C) FIX transgene expression levels assessed by ELISA are shown to be eliminated. Results are shown as % of PBS treated. (D) Vector genome copy number per diploid genome in the liver of animals by qPCR at the time of sacrifice is shown. Statistical significance of BAb over the NAb, C, and PBS group was determined using Student’s t test. *p < 0.05; **p < 0.01; ***p < 0.001. Data are represented as mean ± SEM.
NAbs and BAbs Have a Markedly Different Effect on AAV Vector Biodistribution
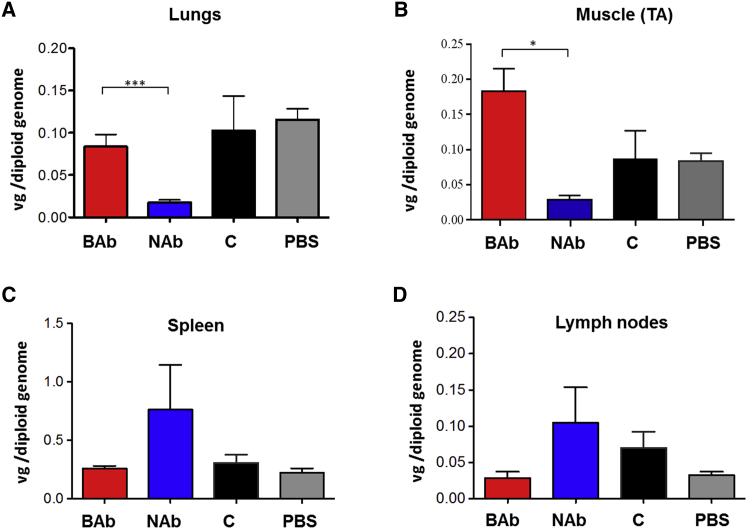
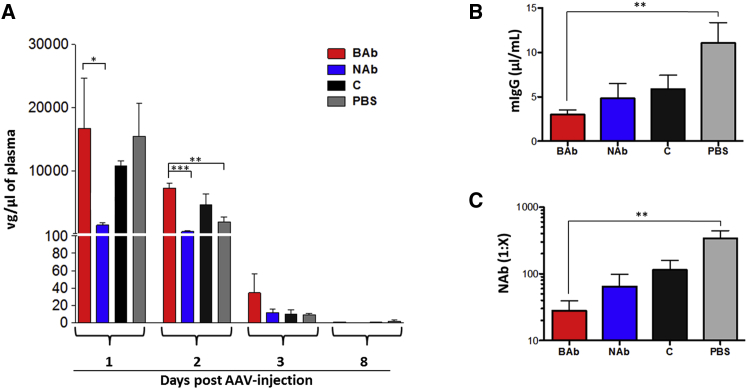
At the time of sacrifice (day 29; Figure 5A), mouse tissues were collected to evaluate the impact of anti-AAV NAbs and BAbs on vector biodistribution. Mice passively immunized with pooled serum containing anti-AAV8 NAbs showed lower levels of transduction in liver, lungs, and skeletal muscle than animals passively immunized with naive children’s serum (Figures 5D, 6A, and 6B). The presence of NAbs resulted in slightly elevated levels of vector genomes in spleen and lymph nodes (Figures 6C and 6D). Conversely, animals passively immunized with anti-AAV8 BAbs showed slightly higher vector genome copy number in skeletal muscle when compared with animals immunized with pooled serum from naive children (C) and PBS controls (Figure 6B). Together, these results indicate that NAbs and BAbs have opposite effects on vector biodistribution. Whereas NAbs direct the virus mostly toward lymphoid organs, BAbs appear to direct it mostly to liver and skeletal muscle. Next, we performed qPCR on blood samples from animals at days 1, 2, 3, and 8 post-AAV vector administration (Figure 5A) to evaluate vector genome presence in the bloodstream. We observed a longer persistence of vector genomes in the presence of BAbs compared with NAbs, similar to that of C and PBS controls (Figure 7A). Starting from day 3 post-AAV infusion, vector genome levels in plasma were dramatically reduced in all the groups and became undetectable one week post-injection (Figure 7A). To further investigate the effect of the pre-existing antibodies early after AAV vector administration, we pre-treated mice with pooled serum containing NAbs, BAbs, naive serum (C), or PBS in order to assess the vector biodistribution and clearance after 3 hr and one day post-injection (Figures S3A and S3B). Although we observed no differences in terms of vector clearance after 3 hr (Figure S3C), 24 hr later, vector was cleared from the circulation in the NAb group only, confirming previous results (Figure 7A). Moreover, biodistribution data after 24 hr post-injection showed AAV8 vector genome accumulation in the liver of animals pre-treated with BAbs (Figure S3D), similar to that in the group that received naive serum from children (C). In the other organs analyzed (Figures S3E–S3H), vector biodistribution followed a similar pattern to that observed on day 29 (Figure 6). These results show that BAbs, unlike NABs, are not detrimental to AAV transduction.
Figure 6.
Biodistribution of AAV8 Vector after Systemic Delivery in Mice Passively Immunized with Neutralizing or Non-neutralizing Antibodies
Vector genome copy number in various tissues was determined. (A) Lungs are shown. (B) Skeletal muscle is shown. (C) Spleen is shown. (D) Inguinal lymph nodes are shown. Statistical significance of BAb over the NAb, C, and PBS group was determined using Student’s t test. *p < 0.05; ***p < 0.0001. TA, tibialis anterior. Data are represented as mean ± SEM.
Figure 7.
Persistence of AAV8 Vector in the Bloodstream of Mice Harboring Pre-existing Neutralizing or Non-neutralizing Antibodies and Evaluation of the Anti-AAV8 Capsid Humoral Immune Response
(A) Clearance of vector genomes from plasma over time. (B) Total anti-AAV8 capsid murine IgG (mIgG) at the time of sacrifice is shown. (C) Anti-AAV8 neutralizing antibodies at the time of sacrifice are shown. Statistical significance of BAb over the NAb, C, and PBS group was determined using Student’s t test. *p < 0.05; **p < 0.01; ***p < 0.001. Data are represented as mean ± SEM.
Pre-existing Antibodies Result in Decreased Humoral Immune Responses following AAV Vector Administration
Immune responses mounted against a given antigen can be shaped by the presence of pre-existing antibodies reactive toward that antigen.31, 32 In mice passively immunized with pooled human sera samples prior to AAV8 administration (Figure 5A), we measured antibody-mediated immune response against the vector capsid by analyzing immunoglobulin content in plasma samples at the time of sacrifice (day 29). Total mouse IgG (mIgG) was quantified by an ELISA assay27 and appeared to be reduced in animals immunized with either NAbs or BAbs (Figure 7B). Similarly, we revealed that the presence of pre-existing antibodies was associated with decreased neutralizing antibody response following AAV vector administration (Figure 7C). The average NAb titer at one month post-vector infusion in naive animals was 1:339, whereas the average NAb titer in mice with pre-existing BAbs or NAbs was 1:28 and 1:65, respectively (Figure 7C). No apparent significant change in the frequency of CD4+ and CD8+ T cells infiltrating the liver of injected animals was noted across the different treatments (Figure S4). These results suggest that the presence of pre-existing anti-capsid antibodies can influence the immunogenicity of AAV vectors.
Discussion
Despite accumulating successes with AAV-mediated gene transfer,7, 10, 11, 12, 33, 34, 35 pre-existing humoral immunity to the viral capsid remains a critical obstacle to the widespread use of this technology. Here, we presented a detailed characterization of the effect of both neutralizing and binding antibodies on AAV transduction in vitro and in vivo. We focused on the liver as a target for gene therapy, based on the recent success of clinical trials targeting this organ.7, 8, 9, 10, 11, 12, 36 Previous studies were focused on the role of NAbs directed to AAV, showing that even low antibody titers result in lack of liver transduction18, 37 and localization of vector genomes to lymphoid organs.23 One caveat of these studies is that vector biodistribution was analyzed several weeks post-vector infusion, thus failing to provide details on early vector biodistribution and clearance. Here, we focused on the fate of AAV vectors shortly after intravenous administration to mice with circulating neutralizing antibodies. In this setting, we demonstrated that vector genome accumulates in the liver, both in parenchymal and non-parenchymal cells, within a few hours from vector infusion. Given the role of the liver in both innate and adaptive immunity, this result is not entirely surprising, as it may simply reflect the natural clearance of the antibody-bound virus.38, 39 Starting from one day post-injection, we observed a drastic reduction in vector genome copies in liver, probably due to the recruitment of the blood immune cells that mediate the clearance of the virus. We next explored the uptake of AAV-antibody complexes in vitro in Hepa 1-6 cells, showing that hepatocytes can internalize AAV vectors in the presence of NAbs, although this does not give rise to transgene expression. The lack of expression supports the possibility of an intracellular immunity that can mediate a post-entry virus neutralization, a mechanism previously described for other viruses.40
Here, we also identified a relatively small percentage of healthy humans naturally harboring low-titer antibodies that bind to AAV (BAbs), which are not neutralizing. Differently from what was observed in the presence of NAbs, AAV8 particles pre-incubated with BAbs showed enhanced transduction efficiency in vitro. This effect of BAbs was partially lost upon treatment of cells with an anti-Fc antibody. However, the possible role of the Fc receptor in the uptake of AAV-BAb complexes will need to be clarified in vivo, in the context of the immune system. In vivo, BAbs appeared to drive an increased localization of vector genomes in liver. Conversely, NAbs prevented vector transduction of liver and other tissues and, as with previous findings,23 resulted in a slight increase in AAV8 vector genome deposition in spleen and lymph nodes. Despite the higher vector gene copy number in liver, BAbs did not seem to have an impact on transgene expression levels. Whether this discrepancy is due to the accumulation of non-functional vector genomes in hepatocytes, uptake by non-parenchymal cells, or simply lack of sensitivity of the assay used to measure transgene expression remains to be studied. The origin of BAbs is unknown. We postulate that a non-neutralizing antibody response to AAV capsid could be generated by at least two means: (1) a primary encounter with wild-type AAV that would lead to the production of a BAb repertoire, non-neutralizing, as a consequence of the parameters of infection, or (2) the antibodies originating from a primary infection, capable of neutralizing the serotype encountered, could cross-react with other serotypes to a certain degree but would lack neutralizing capacity. The frequency of subjects harboring BAbs is hard to define, as also subjects with high-titer neutralizing antibodies may carry BAbs. The differential impact of anti-AAV8 NAbs and BAbs on vector transduction and biodistribution have clinical relevance, in particular for the prescreening of subjects prior to enrollment in AAV gene therapy trials. Our findings indicate that neutralizing assays22 should always be used to prescreen subjects at enrollment in clinical trials, particularly when the intended route of vector administration is systemic. The use of a dual-assay strategy based on neutralization and binding assays has been proposed to identify subjects seronegative to AAV.41 This is an acceptable approach to patient screening, as NAbs generally correlate to total IgG binding to AAV.42 However, our data indicate that NAb assays should be preferred over the use of binding assays, as they are better predictive of the potential outcome of gene transfer. An intriguing finding of our work is that anti-capsid antibody responses in mice harboring pre-existing AAV8 antibodies, either NAbs or BAbs, were attenuated upon AAV8-mediated gene transfer compared to PBS controls. This may be the result of virus-specific antibodies possessing the capacity of masking capsid epitopes from recognition by B cell antigen receptors.9, 22 The work presented here is limited to in vivo studies conducted with human antibodies in mice; future work will be aimed at conducting similar studies with species-specific antibodies, in order to better elucidate the effect of pre-existing antibodies in the context of a primed immune system. Future studies will have also to address whether AAV8-specific BAbs and NAbs retain distinct abilities to activate antibody-dependent complement, which can influence viral clearance from the blood,43 as well as neutralizing capacity of antibodies.44 Similarly, the impact of anti-AAV antibodies on the therapeutic efficacy of different AAV serotypes remains to be elucidated. Here, we focused our in vivo work on AAV8, as this serotype has been extensively studied in the setting of liver gene transfer.7, 8, 45 In conclusion, this study illustrates the diverse, opposite roles of anti-capsid neutralizing and binding antibodies to AAV vectors in systemic gene transfer. Delineating the distinct characteristics of these antibodies will help better understand the parameters influencing the efficiency of AAV vector transduction in patients.
Materials and Methods
AAV Vectors
AAV vectors were prepared as previously described.28 Genome-containing vectors were produced in roller bottles following a triple transfection protocol with cesium chloride gradient purification.28 The AAV vector titration was performed by real-time PCR (qPCR) performed in ABI PRISM 7900 HT Sequence Detector using Absolute ROX mix (Taqman, Thermo Fisher Scientific, Waltham, MA), except for empty capsids that were titered by SDS-PAGE followed by silver staining and quantification by densitometry against an AAV capsid used as a standard. Baculovirus vector preparations were produced as described and purified by immunoaffinity column.29 For the in vitro neutralization assay, an AAV8 vector encoding for luciferase (AAV8-Luc) was used as reporter as previously described.27 The AAV vectors used in the in vivo experiments expressed human FIX under the control of a liver-specific promoter.9, 46
Cell Culture
Hepa 1-6 (ATCC CRL-1830) and HEK293 cell line (ATCC CRL-1573) were maintained under 37°C, 5% CO2 condition in DMEM supplemented with 10% FBS, 2 mM GlutaMAX (Thermo Fisher Scientific, Waltham, MA). The 2V6.11 cells (ATCC CRL-2784) were maintained under the same conditions and used for the AAV neutralization assay.
Human Serum Samples
All samples from adult donors were obtained from the bio-collection (agreement no. DC-2014-2293) of Rouen University Hospital, Rouen (France). Serum samples from children aged one year were purchased from Seralab International (West Sussex, UK). All samples were de-identified and collected according to the local regulations.
Animal Studies
Animal protocols were approved by Genethon’s Ethical Committee and conducted by certified operators according to the guidelines and laws regulating animal experimentation under agreement number CE12-037. In all experiments, male C57BL/6 mice aged 6–8 weeks were used. Animals were passively immunized by intraperitoneal injection with 8 mg of IVIg (Octapharma, Lingolsheim, France) in a final volume of 100 μL. After 24 hr, mice received an AAV8 vector by tail vein route. For studies employing human serum, passive immunization was performed by delivering the test serum in a volume of 100 μL 24 hr prior to vector administration. Blood samples were collected from the retro-orbital plexus in heparin-coated capillary tubes (Sarstedt, Nümbrecht, Germany). At euthanasia, tissues were collected and snap frozen for additional studies. For the isolation of liver parenchymal and non-parenchymal cells, mouse livers were harvested 3 hr after AAV8 injection, and cells were obtained as previously described.47
Human FIX ELISA
Human FIX antigen and protein levels in mouse plasma were evaluated as previously described.30 The capture and secondary antibodies for the ELISA assay were purchased from Thermo Scientific (ref. MA1-43012) and Cedarlane (ref. CL20040APHP), respectively.
AAV Antibody Assays
The neutralizing antibody assay was performed as previously described.27 Anti-AAV Ig capture assay was performed as previously described.14, 30 Briefly, plates were coated with AAV2 or AAV8 capsid (1 × 1010 vg/mL; 50 μL per well), blocked with PBS with 6% non-fat dry milk, and serum or plasma samples added to the wells in duplicate.
Vector Genome Copy Number
Vector genome copy number was determined using a qPCR assay with primers and probes specific for the liver promoter and the FIX transgene for biodistribution studies and vector clearance, respectively. The promoter-specific primers and the probe (forward primer 5′-GGCGGGCGACTCAGATC-3′, reverse primer 5′-GGGAGGCTGCTGGTGAATATT-3′, probe [FAM] 5′-AGCCCCTGTTTGCTCCTCC GATAACTG-3′ [TAMRA]) were synthesized by Thermo Scientific (Waltham, MA, USA). The FIX-specific primers (forward primer 5′-ATGACTTCACTCGGGTTGTTGG-3′, reverse primer 5′-AGCCTCCACAGAATGCATCAAC-3′, probe [FAM] 5′-CCCTTGGCAG GTTGTTTTGA-3′ [TAMRA]) were all synthesized and purchased from Sigma Aldrich (Saint-Quentin Fallavier, France). Mouse Titin was used as a normalizing gene (forward primer 5′-AAAACGAGCAGTGACGTGAGC-3′, reverse primer 5′-TTCAGTCATGCT GCTAGCGC-3′, probe [VIC] 5′- TGCACGGAAGCGTCTCGTCTCAGTC-3′ [TAMRA] synthesized by Thermo Scientific [Waltham, MA, USA]). DNA from tissues was extracted after whole-organ homogenization using the automated system MagNA Pure MP96 instrument (Roche Diagnostics, Meylan, France). Vector DNA present in plasma was diluted (10 μL of plasma diluted 50-fold in RNase/DNase free water) prior to use in a Q-PCR (i.e., 0.2 μL of plasma per Q-PCR reaction). Each sample was tested in triplicate, and genome copy number per diploid genome was determined against a standard curve made of linearized plasmid or AAV8-hFIX vector diluted in mouse plasma for biodistribution and virus clearance, respectively.
Detection of T Cell Infiltrates in Liver Samples
Detection of CD4 and CD8 T cell infiltrates in the liver was performed by qPCR. Briefly, total RNA was extracted from frozen liver tissue by using the MagNA Pure 96 RNA extraction kit (Roche Diagnosis, Basel, Switzerland) according to manufacturer’s instructions. Total RNA was reverse-transcribed using random hexamers and the RevertAid H minus first strand cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA). qRT-PCR was performed using SybrGreen (Thermo Fisher Scientific, Waltham, MA) with primers specific for CD4, forward 5′-GGTTCGGCATGACACTCT-3′, reverse 5′-CTGACTCTCCCTCACTCTTATAG-3′; CD8, forward 5′-ATCACTCTCATCTG CTACC-3′, reverse 5′-GCCTTCCTGTCTGACTAG-3′; and GAPDH, forward 5′-CATGGC CTTCCGTGTTCCTA-3′, reverse 5′-GCGGCACGTCAGATCCA-3′. Expression levels were normalized for the level of expression of GAPDH (ΔCt) and then to the average level measured in control group (ΔΔCt). By using this method, a ΔΔCt of 1 corresponds to one cycle difference in the expression levels measured by RT-PCR, and this corresponds to a two-fold difference in the RNA expression.
Imaging Flow Cytometry
AAV vector was incubated with various amounts of IVIg (1, 0.25, 0.125, and 0.03125 mg/mL) or pooled serum samples and incubated at 37°C for 60 min. Immune complexes were added to Hepa 1-6 cells seeded on a 12-well plate at an MOI of either 103 (AAV2) or 104 (AAV8), and cells were placed at 37°C for 1 hr (AAV2) or 2 hr (AAV8). Cells were then trypsinized, washed, fixed, and permeabilized using the FIX and PERM kit from Thermo Scientific (Waltham, MA). The monoclonal antibodies A20 (Progen Biotechnik, Heidelberg, Germany) and ADK8/9 (Progen Biotechnik, Heidelberg, Germany) were used at a dilution of 1:50 in permeabilization buffer to detect intracellular AAV2 and AAV8 capsid, respectively. A rat anti-mouse IgG Alexa-Fluor-594-conjugated secondary antibody (Thermo Scientific, Waltham, MA) was used at a dilution of 1:200 in PBS 0.5% BSA. An Alexa-488-conjugated goat anti-human IgG antibody (Thermo Scientific, Waltham, MA) was used to detect internalized hIgG. After staining, cells were acquired on an Amnis Image Stream (EMD Millipore, Fontenay sous Bois, France) and analyzed using the INSPIRE software (EMD Millipore, Fontenay sous Bois, France). To quantify AAV capsid and hIgG internalization, the spot count feature was used to enumerate the number of fluorescent puncta per cell.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism v7 (GraphPad Software, La Jolla, CA). Data are presented as the mean ± SEM or SD. Statistical analyses were performed by using Mann-Whitney unpaired test or 1-way ANOVA, and significance was determined at p less than 0.05.
Author Contributions
Z.F., C.L., E.B., E.M., F.C., F.J., and G.R. contributed to experimental activities. Z.F., C.L., E.B., O.B., and F.M. provided critical insight on experimental design and data interpretation. Z.F., C.L., E.B., and F.M. wrote the manuscript.
Conflicts of Interest
F.M. and F.C. are inventors in patents describing the AAV technology. F.M. consulted for companies on topics unrelated to the content of this manuscript. F.M. received research support from Selecta Bioscience and Biomarin for projects unrelated to the content of this manuscript. All the other authors declare no conflict of interest associated with the current work.
Acknowledgments
This work was supported by Genethon; the European Union; FP7-PEOPLE-2012-CIG Career Integration Grant, grant agreement number 333628 (to F.M.); ERC-2013-CoG Consolidator Grant, grant agreement number 617432 (to F.M.); and the Bayer Early Career Investigator Award (to F.M.).
Footnotes
Supplemental Information includes four figures and can be found with this article online at https://doi.org/10.1016/j.omtm.2018.02.003.
Supplemental Information
References
- 1.Mount J.D., Herzog R.W., Tillson D.M., Goodman S.A., Robinson N., McCleland M.L., Bellinger D., Nichols T.C., Arruda V.R., Lothrop C.D., Jr., High K.A. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 2.Ronzitti G., Bortolussi G., van Dijk R., Collaud F., Charles S., Leborgne C., Vidal P., Martin S., Gjata B., Sola M.S. A translationally optimized AAV-UGT1A1 vector drives safe and long-lasting correction of Crigler-Najjar syndrome. Mol. Ther. Methods Clin. Dev. 2016;3:16049. doi: 10.1038/mtm.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathwani A.C., Rosales C., McIntosh J., Rastegarlari G., Nathwani D., Raj D., Nawathe S., Waddington S.N., Bronson R., Jackson S. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol. Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Guiner C., Montus M., Servais L., Cherel Y., Francois V., Thibaud J.-L., Wary C., Matot B., Larcher T., Guigand L. Forelimb treatment in a large cohort of dystrophic dogs supports delivery of a recombinant AAV for exon skipping in Duchenne patients. Mol. Ther. 2014;22:1923–1935. doi: 10.1038/mt.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arruda V.R., Stedman H.H., Haurigot V., Buchlis G., Baila S., Favaro P., Chen Y., Franck H.G., Zhou S., Wright J.F. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115:4678–4688. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathwani A.C., Tuddenham E.G.D., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathwani A.C., Reiss U.M., Tuddenham E.G.D., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 10.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miesbach W., Meijer K., Coppens M., Kampmann P., Klamroth R., Schutgens R., Tangelder M., Castaman G., Schwäble J., Bonig H. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2017 doi: 10.1182/blood-2017-09-804419. Published online December 15, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-Factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 13.Boutin S., Monteilhet V., Veron P., Leborgne C., Benveniste O., Montus M.F., Masurier C. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 14.Mingozzi F., Chen Y., Edmonson S.C., Zhou S., Thurlings R.M., Tak P.P., High K.A., Vervoordeldonk M.J. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013;20:417–424. doi: 10.1038/gt.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng Y.S., Vliet K.V., Rao L., McKenna R., Byrne B.J., Asokan A., Agbandje-McKenna M. Generation and characterization of anti-Adeno-associated virus serotype 8 (AAV8) and anti-AAV9 monoclonal antibodies. J. Virol. Methods. 2016;236:105–110. doi: 10.1016/j.jviromet.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurda B.L., Raupp C., Popa-Wagner R., Naumer M., Olson N.H., Ng R., McKenna R., Baker T.S., Kleinschmidt J.A., Agbandje-McKenna M. Mapping a neutralizing epitope onto the capsid of adeno-associated virus serotype 8. J. Virol. 2012;86:7739–7751. doi: 10.1128/JVI.00218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurda B.L., DiMattia M.A., Miller E.B., Bennett A., McKenna R., Weichert W.S., Nelson C.D., Chen W.J., Muzyczka N., Olson N.H. Capsid antibodies to different adeno-associated virus serotypes bind common regions. J. Virol. 2013;87:9111–9124. doi: 10.1128/JVI.00622-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H., Couto L.B., Patarroyo-White S., Liu T., Nagy D., Vargas J.A., Zhou S., Scallan C.D., Sommer J., Vijay S. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C., Narkbunnam N., Samulski R.J., Asokan A., Hu G., Jacobson L.J., Manco-Johnson M.J., Monahan P.E., Joint Outcome Study Investigators Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012;19:288–294. doi: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- 20.Erles K., Sebökovà P., Schlehofer J.R. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV) J. Med. Virol. 1999;59:406–411. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 21.Calcedo R., Morizono H., Wang L., McCarter R., He J., Jones D., Batshaw M.L., Wilson J.M. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy S.L., Li H., Mingozzi F., Sabatino D.E., Hui D.J., Edmonson S.A., High K.A. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J. Med. Virol. 2009;81:65–74. doi: 10.1002/jmv.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L., Calcedo R., Wang H., Bell P., Grant R., Vandenberghe L.H., Sanmiguel J., Morizono H., Batshaw M.L., Wilson J.M. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol. Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johansson A.G., Løvdal T., Magnusson K.E., Berg T., Skogh T. Liver cell uptake and degradation of soluble immunoglobulin G immune complexes in vivo and in vitro in rats. Hepatology. 1996;24:169–175. doi: 10.1002/hep.510240128. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Quintela A., Alende R., Gude F., Campos J., Rey J., Meijide L.M., Fernandez-Merino C., Vidal C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 2008;151:42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jolles S., Sewell W.A.C., Misbah S.A. Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 2005;142:1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meliani A., Leborgne C., Triffault S., Jeanson-Leh L., Veron P., Mingozzi F. Determination of anti-adeno-associated virus vector neutralizing antibody titer with an in vitro reporter system. Hum. Gene Ther. Methods. 2015;26:45–53. doi: 10.1089/hgtb.2015.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayuso E., Mingozzi F., Montane J., Leon X., Anguela X.M., Haurigot V., Edmonson S.A., Africa L., Zhou S., High K.A. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17:503–510. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- 29.Galibert L., Merten O.-W. Latest developments in the large-scale production of adeno-associated virus vectors in insect cells toward the treatment of neuromuscular diseases. J. Invertebr. Pathol. 2011;107(Suppl):S80–S93. doi: 10.1016/j.jip.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Mingozzi F., Anguela X.M., Pavani G., Chen Y., Davidson R.J., Hui D.J., Yazicioglu M., Elkouby L., Hinderer C.J., Faella A. Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci. Transl. Med. 2013;5:194ra92. doi: 10.1126/scitranslmed.3005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu. Rev. Immunol. 2000;18:709–737. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 32.Bouche F.B., Ertl O.T., Muller C.P. Neutralizing B cell response in measles. Viral Immunol. 2002;15:451–471. doi: 10.1089/088282402760312331. [DOI] [PubMed] [Google Scholar]
- 33.Manno C.S., Chew A.J., Hutchison S., Larson P.J., Herzog R.W., Arruda V.R., Tai S.J., Ragni M.V., Thompson A., Ozelo M. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 34.Bevan A.K., Duque S., Foust K.D., Morales P.R., Braun L., Schmelzer L., Chan C.M., McCrate M., Chicoine L.G., Coley B.D. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mingozzi F., High K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 36.D’Avola D., López-Franco E., Sangro B., Pañeda A., Grossios N., Gil-Farina I., Benito A., Twisk J., Paz M., Ruiz J. Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J. Hepatol. 2016;65:776–783. doi: 10.1016/j.jhep.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 37.Scallan C.D., Jiang H., Liu T., Patarroyo-White S., Sommer J.M., Zhou S., Couto L.B., Pierce G.F. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 38.Jenne C.N., Kubes P. Immune surveillance by the liver. Nat. Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 39.Crispe I.N. Liver antigen-presenting cells. J. Hepatol. 2011;54:357–365. doi: 10.1016/j.jhep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallery D.L., McEwan W.A., Bidgood S.R., Towers G.J., Johnson C.M., James L.C. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc. Natl. Acad. Sci. USA. 2010;107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falese L., Sandza K., Yates B., Triffault S., Gangar S., Long B., Tsuruda L., Carter B., Vettermann C., Zoog S.J., Fong S. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24:768–778. doi: 10.1038/gt.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veron P., Leborgne C., Monteilhet V., Boutin S., Martin S., Moullier P., Masurier C. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J. Immunol. 2012;188:6418–6424. doi: 10.4049/jimmunol.1200620. [DOI] [PubMed] [Google Scholar]
- 43.Evgin L., Acuna S.A., Tanese de Souza C., Marguerie M., Lemay C.G., Ilkow C.S., Findlay C.S., Falls T., Parato K.A., Hanwell D. Complement inhibition prevents oncolytic vaccinia virus neutralization in immune humans and cynomolgus macaques. Mol. Ther. 2015;23:1066–1076. doi: 10.1038/mt.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishimura Y., Igarashi T., Haigwood N., Sadjadpour R., Plishka R.J., Buckler-White A., Shibata R., Martin M.A. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 2002;76:2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calcedo R., Vandenberghe L.H., Gao G., Lin J., Wilson J.M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miao C.H., Ohashi K., Patijn G.A., Meuse L., Ye X., Thompson A.R., Kay M.A. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol. Ther. 2000;1:522–532. doi: 10.1006/mthe.2000.0075. [DOI] [PubMed] [Google Scholar]
- 47.Ferrand M., Da Rocha S., Corre G., Galy A., Boisgerault F. Serotype-specific binding properties and nanoparticle characteristics contribute to the immunogenicity of rAAV1 vectors. Mol. Ther. 2015;23:1022–1033. doi: 10.1038/mt.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.