Abstract
Summary
This study investigated the relationship between muscle and bone status in elderly individuals. Our results suggested links between sarcopenia and osteoporosis; impairment in muscle status (i.e., muscle mass, muscle strength, and physical performance) is associated with deterioration in bone mass and texture subsequently leading to an increased risk of fracture.
Introduction
Accumulating evidence has shown associations between sarcopenia and osteoporosis, but existing studies face inconsistencies in the clinical definition of both conditions. Thus, we sought to investigate bone health among older individuals with or without muscle health impairment.
Methods
We conducted an analysis of cross-sectional data available from the Sarcopenia and Physical Impairment with Advancing Age (SarcoPhAge) study. Sarcopenia was diagnosed according to the European Working Group on Sarcopenia in Older People (EWGSOP) (i.e., a low muscle mass plus either low muscle strength or low physical performance). Muscle mass and areal bone mineral density (aBMD) were determined using dual-energy X-ray absorptiometry (DEXA). Muscle strength was assessed using a hand dynamometer, and physical performance was assessed with the Short Physical Performance Battery test. Using the cutoff limits proposed by the EWGSOP, we have classified women in the “low SMI group” when its value was < 5.50 kg/m2, in the “low muscle strength group” when strength was < 20 kg, and in the “low physical performance group” when SPPB < 8 points. The thresholds of < 7.26 kg/m2 (for SMI), < 30 kg (for muscle strength), and SPPB < 8 points were used for men. The 10-year fracture risk was obtained using the FRAX® tool. Moreover, bone texture was determined using the trabecular bone score (TBS) method.
Results
The study sample consisted of 288 older subjects aged 74.7 ± 5.7 years, and 59.0% of the subjects were women. Sarcopenia was diagnosed in 43 individuals (14.9%), and osteoporosis was diagnosed in 36 subjects (12.5%). Moreover, aBMD values were, most of the time, lower in older men and women with muscle impairment (i.e., low muscle mass, low muscle strength, and low physical performance). For these subjects, we also noted a higher probability of fracture. When comparing bone quality, there were no significant differences in the TBS values between sarcopenic and non-sarcopenic older men and women or between those with low and high muscle mass. However, when controlling for confounders (i.e., age, BMI, number of co-morbidities, smoking status, and nutritional status), TBS values were lower in older women with low muscle strength (p = 0.04) and in older men with low physical performance (p = 0.01).
Conclusions
Our study showed interrelationships between components of sarcopenia and osteoporosis, with older subjects with muscle impairment having poorer bone health.
Keywords: Bone mineral density, Fracture risk, Sarcopenia, Trabecular bone score, Muscle mass, Osteoporosis
Introduction
During the senescence process, there is an increase in functional disability, which induces a higher risk of falls and fractures and predisposes older individuals to increased morbidity and mortality. These adverse health events may be related in part to the progressive wasting of muscle and bone tissue giving rise to sarcopenia and osteoporosis. The prevalence of the two disorders is expected to grow [1, 2] due to the increase in life expectancy responsible for major public health issues and significant healthcare costs.
There are striking relationships between sarcopenia and osteoporosis [3] leading to similar health consequences (e.g., falls and fracture). They also share putative etiological factors such as mechanical and functional influences, genetic aspects, nutritional disorders, chronic inflammation, lifestyle habits, and hormonal changes [4–6]. When one of the two tissues is subjected to the aging process, the functionality of the other seems to be prejudiced.
Many studies have provided evidence of a link between muscle and bone mass [7], but few have assessed the relationship between muscle and bone status or sarcopenia and osteoporosis. Moreover, the measurement methods and the definition of the two conditions are heterogeneous. In the case of sarcopenia, some studies defined the disease based on low muscle mass alone [8–12], but a growing consensus advocates for sarcopenia to be defined additionally on the basis of decreased muscle strength and/or impaired physical function [13, 14]. The definition of osteoporosis implies a decrease in bone mass and a deterioration of bone architecture. However, its operational definition is limited to the assessment of bone mineral density (BMD) with a reference standard at the femoral neck [15]. The evaluation of BMD as a prognostic tool lacks sensitivity to predict fracture over most reasonable assumptions [16]. However, there are other tools for assessing individual fracture risk. The FRAX® tool [17] allows the input of a set of clinical risk factors that affect fracture risk and provides a 10-year osteoporosis-related fracture probability with or without the inclusion of BMD. Additionally, trabecular bone score (TBS) is a new and non-invasive medical imaging technique that has been recently suggested to provide a measure of bone quality [18]. It reflects an index of bone texture derived from the analysis of two-dimensional lumbar spine bone densitometry images, and it captures a component of fracture risk that is independent of BMD [19].
The aim of the present study was to further characterize the relationships between sarcopenia and markers of skeletal fragility (i.e., BMD, FRAX and TBS) in a cohort of older subjects with a range of muscular disabilities (i.e., sarcopenia, decreased muscle mass, impaired muscle strength, and impaired physical performance).
Methods
Population
The present study is based on cross-sectional data obtained from the Sarcopenia and Physical Impairment with advancing Age (SarcoPhAge) study, which is an ongoing, prospective study that enrolled a convenience sample of 534 community-dwelling older subjects with the main objective of assessing the health and functional outcomes of sarcopenia. Methodological details of the study and baseline characteristics of the SarcoPhAge population have been previously described [20]. During the second year of follow-up (October 2015 to October 2016), we conducted additional investigations into skeletal status including BMD, FRAX, and TBS. Several subjects (n = 218) were not interviewed for the following reasons: physical or mental inability (n = 66), death (n = 22), refusal to participate again (n = 113), or inability to contact them (n = 17). A total of 316 consenting participants were then identified as being available for this ancillary analysis. Among them, individuals who had spinal surgery were excluded (n = 22), as along with those who had a body mass index (BMI) > 35 kg/m2 (n = 6) because a BMI beyond this threshold affects the accuracy of TBS [21]. The final sample size available for analysis comprised 288 individuals. Compared with the current sample, those excluded from the analysis were significantly older (p < 0.001) and had lower physical performance (p < 0.001) at the previous assessment.
All subjects were informed about the study objectives and procedures. Informed written consent was given by all participants, and the research protocol and subsequent amendments were approved by the Ethics Committee of the University Teaching Hospital of Liège.
Health examination
Clinical measurements
During the examination, subjects wore light cotton clothes without any jewelry, buttons, or metal objects and without shoes. Body weight and body height were measured using standardized scales to the nearest 0.1 kg and 0.1 cm, respectively. BMI was calculated as weight divided by height squared (kg/m2). Subjects were also asked to list all co-morbidities and all current medications. Smoking status (yes/no) and the daily consumption of 3 or more units of alcohol (yes/no) were also noted. The nutritional status of individuals was assessed by the Mini-Nutritional Assessment (MNA) [22]. The evaluation was scored on a 30-point scale. Subjects were classified as “well-nourished” when the score was equal to 24 points or more; those presenting with a score below 24 points were classified as “at risk of malnutrition or malnutrition.”
Muscle assessment
The skeletal muscle mass index (SMI) was determined by dual-energy X-ray absorptiometry (DXA) (Hologic Discovery A, USA) and calibrated daily in accordance with the manufacturer’s recommendations. The SMI was calculated as appendicular skeletal muscle mass (i.e., sum of the muscle mass in both arms and legs) divided by height squared. Muscle strength was assessed as hand grip strength using a hand dynamometer (Saehan Corporation, MSD Europe Bvba, Belgium) calibrated each year. Participants had to grip the device as hard as possible three times in each hand; the highest of the six values was recorded [23]. Physical performance was evaluated by the short physical performance battery (SPPB) test (12 points) [24].
Sarcopenia was diagnosed according to the European Working Group on Sarcopenia in Older People (EWGSOP) definition, comprising low muscle mass plus either low muscle strength and/or low physical performance [13]. To determine muscle impairment, we used the cutoff limits proposed by the EWGSOP [13], classifying women in the “low SMI group” when the value was < 5.50 kg/m2, the “low muscle strength group” when the strength was < 20 kg, and the “low physical performance group” when SPPB < 8 points. The thresholds of < 7.26 kg/m2 (for SMI), < 30 kg (for muscle strength), and < 8 points (for physical performance) were used for men.
Skeletal assessment
DXA scans to measure areal BMD (aBMD) were performed using the Hologic Discovery A device at three sites: lumbar spine (L2–L4), total hip, and femoral neck. The results of bone densitometry were expressed in grams per centimeter and as a T-score (sex-specific and derived from the National Health and Nutrition Examination Survey III normative data for hip sites and from the manufacturer’s reference range for the lumbar spine). Osteoporosis was defined as an aBMD T-score ≤− 2.5 on at least one of the three regions, and osteopenia was defined as an aBMD T-score between − 1 and − 2.5 [15]. Note that the femoral neck aBMD T-score is currently recommended as a reference standard [15]. Thus, we also reported the prevalence of osteoporosis using this criterion. The 10-year probability of major osteoporotic fracture (MOF) and hip fracture (HF) was determined using the Belgian version of the FRAX tool [25] (including aBMD measurement). We classified participants as having a high or a low 10-year probability of MOF according to the National Osteoporosis Guideline Group (NOGG) [26], calculated. Ten subjects with a hip prosthesis did not undergo DXA hip scans: we performed statistical analysis by including the maximum number of observations available in this case (n = 278) and, therefore, the FRAX probabilities (which includes hip aBMD measurement) were not established for 10 subjects. TBS values were calculated from the lumbar spine BMD using TBS iNsight® software (Medimaps Group, Geneva, Switzerland, version 3.0.1). The TBS technique provides an index of bone texture by analyzing gray-level variation in the DXA image. It reflects bone texture and subsequently bone strength [27]. A low TBS value reflects a poorer quality and fracture-prone bone microarchitecture and, conversely, a high TBS value is correlated with better, more fracture-resistant bone microstructure [19].
Statistical analyses
Statistical analysis was performed using Statistica® software (Dell Statistica, USA, version 13). The results are expressed as the means ± standard deviation (SD) for continuous variables and as absolute (n) and relative (%) frequencies for categorical variables. The distribution of the different variables was tested using the Shapiro-Wilk test. Student’s t test was used to test differences between groups in aBMD, FRAX® probabilities, and TBS values. Because bone and muscle variables are markedly different between men and women, analyses were performed separately. Differences in the proportions were compared using χ2 tests. To assess the association between two conditions, odds ratios (OR) were reported with 95% confidence intervals (95% CIs). To analyze the effect of several covariates on muscle health, stepwise logistic regression analysis was applied with adjustments for age, BMI, number of co-morbidities, smoking status, and nutritional status (model 1). These covariates were included in the multivariable model because they significantly differed between groups in the univariate analysis for at least one of the muscle measurements. To avoid over-adjustment, we only considered the number of co-morbidities in the model, because of collinearity between the number of co-morbidities and the number of drugs consumed. We also did not integrate alcohol consumption as a covariate because consumption was similar between the groups. To explore whether bone density is associated with bone texture, three further models, in addition to model 1, were developed for the TBS values. A second model (model 2) was additionally adjusted for lumbar spine aBMD. Sensitivity analysis was also performed using two other models: additional adjustment for total hip aBMD (model 3) and additional adjustment for femoral neck aBMD (model 4). To limit the loss of information that occurs when variables are treated dichotomously, a multivariable linear regression was also carried out with muscle measurements as continuous variables, including various potential explanatory variables: age, number of co-morbidities, smoking status, nutritional status, femoral neck aBMD, and TBS value. Two-tailed p values < 0.05 indicated a statistically significant association.
Results
Characteristics of the subjects studied
We studied 288 individuals aged 74.7 ± 5.7 years. The sample comprised 170 women (59.0%). A summary of baseline characteristics is given in Table 1. Of the 288 participants, 43 (14.9%) were diagnosed with sarcopenia, 81 (21.8%) had a low SMI, 98 (34.0%) showed weakness in muscle strength, and 57 (19.8%) had poor physical performance (Table 1). Some differences in clinical characteristics were observed between individuals with and without sarcopenia (i.e., sarcopenic subjects had higher age, higher prevalence of smoking habits, and poorer nutritional status), between individuals with a low SMI and those with a normal SMI (i.e., subjects with low SMI had a higher prevalence of smoking and poorer nutritional status), between participants presenting with poor and normal muscle strength (i.e., subjects with weakness in muscle strength had higher age, more co-morbidities, more drugs consumed, and poorer nutritional status), and between individuals with a decreased or normal physical performance (i.e., subjects with poor physical performance had higher age, more co-morbidities, more drugs consumed, and poorer nutritional status).
Table 1.
Characterization of the study population
| Variables | n | % | Mean (SD) |
|---|---|---|---|
| Age (years) | 288 | 74.7 (5.7) | |
| Sex | |||
| Women | 170 | 59.0 | |
| BMI (kg/m2) | |||
| Men | 118 | 27.5 ± 3.6 | |
| Women | 170 | 25.9 ± 4.5 | |
| Number of co-morbidities by subjects | 288 | 4.3 (2.5) | |
| Number of drugs by subjects | 288 | 6.4 (3.6) | |
| Current smoker | 22 | 7.6 | |
| Daily alcohol consumption ≥ 3 units | 32 | 11.1 | |
| All kind of self-reported previous fracture (adult life) | 79 | 27.4 | |
| Parental fractured hip | 40 | 13.9 | |
| Glucocorticoid use | 7 | 2.4 | |
| Rheumatoid arthritis | 13 | 4.5 | |
| Secondary osteoporosis | 44 | 15.3 | |
| Mini-Nutritional Assessment | |||
| Well-nourished | 268 | 93.1 | |
| Risk of malnutrition or malnutrition | 20 | 6.9 | |
| Lumbar spine aBMD (g/cm2) | |||
| Men | 118 | 1.200 ± 0.150 | |
| Women | 170 | 1.170 ± 0.145 | |
| Lumbar spine T-score | |||
| Men | 118 | 0.3 ± 1.9 | |
| Women | 170 | − 0.4 ± 1.8 | |
| Total hip aBMD (g/cm2) | |||
| Men | 116 | 0.920 ± 0.138 | |
| Women | 162 | 0.804 ± 0.124 | |
| Total hip T-score | |||
| Men | 116 | − 0.7 ± 1.1 | |
| Women | 162 | − 1.3 ± 2.8 | |
| Femoral neck aBMD (g/cm2) | |||
| Men | 116 | 0.761 ± 0.120 | |
| Women | 162 | 0.693 ± 0.119 | |
| Femoral neck T-score | |||
| Men | 116 | − 0.9 ± 1.4 | |
| Women | 162 | − 1.4 ± 1.1 | |
| 10-year MOF probability (%) | |||
| Men | 116 | 6.75 ± 4.1 | |
| Women | 162 | 13.6 ± 9.1 | |
| 10-year HF probability (%) | |||
| Men | 116 | 2.9 ± 2.7 | |
| Women | 162 | 6.0 ± 7.8 | |
| TBS value | |||
| Men | 118 | 1.27 ± 0.10 | |
| Women | 170 | 1.32 ± 0.11 | |
| Sarcopenia | 43 | 14.9 | |
| Low SMI | 81 | 21.8 | |
| Low muscle strength | 98 | 34.0 | |
| Low physical performance | 57 | 19.8 | |
| Osteoporosis | 36 | 12.5 | |
| Osteopenia | 188 | 65.2 | |
Relationship between sarcopenia and bone health
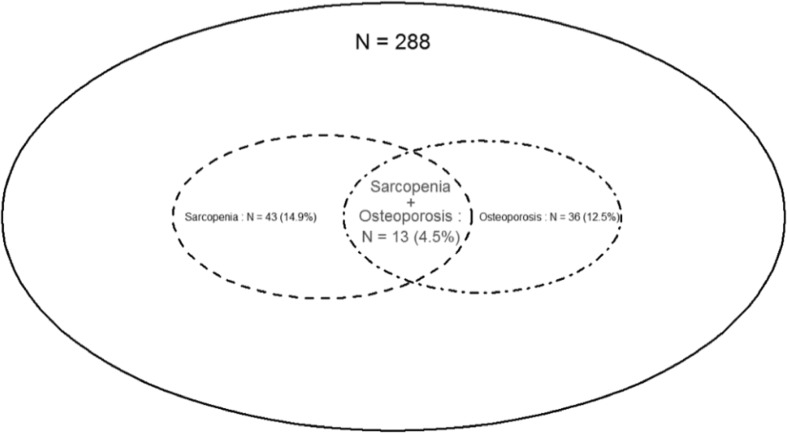
Among the 288 participants, 43 (14.9%) were diagnosed with sarcopenia (34.9% men, 65.1% women), and 36 (12.5%) were diagnosed with osteoporosis as judged by the minimum T-score at the three sites of aBMD measurements (22.2% men, 77.8% women) (Fig. 1). The prevalence of both sarcopenia and osteoporosis was 4.5% (Fig. 1). As shown in Table 2, a significantly higher prevalence of sarcopenia was found in subjects with deteriorated bone health. Indeed, there was a significantly higher number of people with sarcopenia among osteoporotic subjects or those with a high MOF probability (the latter also had osteopenia or were osteoporotic more often). Conversely, there was a higher number of osteoporotic or osteopenic individuals (including osteoporotic ones) with muscle impairment. Sarcopenic subjects had a 4-fold higher risk of having co-existing osteoporosis compared with non-sarcopenic individuals (OR = 4.18; 95% CI 1.92–9.12). When osteoporosis was defined using the femoral neck aBMD T-score alone, the prevalence of the condition rose to 9.0% (3.4% of men, 12.9% of women). Sarcopenic individuals had a 4.34 times higher chance of having osteoporosis (defined by the femoral neck aBMD T-score) when compared with non-sarcopenic subjects (OR = 4.34; 95% CI 1.82–10.36). Finally, we also noted that the prevalence of previous self-reported fracture occurring in adult life (no matter the site) was significantly higher in subjects presenting sarcopenia.
Fig. 1.
Relationship between sarcopenia and osteoporosis in the SarcoPhAge study
Table 2.
Differences in the prevalence of subjects presenting bone or muscle impairment between groups
| Presence of sarcopenia (%) | Presence of osteoporosis (%) | Presence of osteopenia (%) | |
|---|---|---|---|
| Sarcopenic subjects (n = 43) | – | 30.2 | 81.3 |
| Non-sarcopenic subjects (n = 245) | – | 9.4 | 38.1 |
| p value | – | < 0.001 | 0.02 |
| Osteoporotic subjects (n = 36) | 36.1 | – | – |
| Non-osteoporotic subjects (n = 252) | 11.9 | – | – |
| p value | < 0.001 | – | – |
| Osteopenic subjects (n = 188) | 18.1 | – | – |
| Non-osteopenic subjects (n = 100) | 8.0 | – | – |
| p value | 0.03 | – | – |
| High MOF probability (n = 24)a | 33.3 | 50.0 | 83.3 |
| Low MOF probability (n = 254)a | 13.8 | 9.0 | 10.0 |
| p value | 0.01 | < 0.001 | < 0.001 |
| Previous fracture (n = 134) | 36.4 | 34.0 | 39.2 |
| No previous fracture (n = 154) | 10.1 | 8.6 | 9.8 |
| p value | < 0.001 | < 0.001 | < 0.001 |
aAccording to the NOGG [26], calculated from age and FRAX probabilities of each subject
Skeletal status in sarcopenic subjects versus non-sarcopenic subjects
With regard to skeletal status in older men (Table 3), significantly higher MOF and HF fracture probabilities were observed in sarcopenic men, also after adjustments. All other bone variables were decreased in sarcopenic men, but this did not reach statistical significance (all p > 0.05). For older women, aBMD values at two of the three sites (i.e., lumbar spine and total hip) were significantly lower in sarcopenic subjects than in non-sarcopenic subjects (when adjusting for age, number of co-morbidities, smoking status, and nutritional status). Compared with non-sarcopenic subjects, we also observed that sarcopenic women had, after adjustments, a significantly higher fracture probability of major osteoporosis-related fracture and hip fracture. Regarding bone texture, there were statistically significant differences in TBS values between sarcopenic and non-sarcopenic subjects in the univariate analysis but not in the multivariate analysis. Additionally, when we performed further adjustments of TBS for lumbar spine aBMD (model 2), total hip aBMD (model 3), and femoral neck aBMD (model 4), we found no significant difference between sarcopenic and non-sarcopenic subjects in terms of TBS value (p = 0.25, 0.16, 0.18, respectively). In the multivariate model, nutritional status was significantly associated with sarcopenia (p = 0.001): risk of malnutrition or malnutrition was more prevalent among subjects with sarcopenia.
Table 3.
Comparisons of skeletal status between subjects with or without indices of muscle impairment in men (n = 118)
| Sarcopenic subjects (n = 15) Mean (SD) |
Non-sarcopenic subjects (n = 103) Mean (SD) |
Crude p value | p value after adjustmenta | |
|---|---|---|---|---|
| Lumbar spine aBMD (g/cm2) | 1.037 (0.149) | 1.110 (1.435) | 0.13 | 0.15 |
| Total hip aBMD (g/cm2) | 0.874 (0.101) | 0.932 (0.140) | 0.13 | 0.76 |
| Femoral neck aBMD (g/cm2) | 0.717 (0.103) | 0.768 (0.122) | 0.12 | 0.36 |
| 10-year MOF probability (%) | 9.04 (5.91) | 6.53 (3.88) | 0.01 | 0.04 |
| 10-year HF probability (%) | 4.23 (4.01) | 2.43 (2.06) | 0.01 | 0.03 |
| TBS value | 1.284 (0.089) | 1.298 (0.097) | 0.63 | 0.29 |
| Low SMI (n = 37) Mean (SD) |
Adequate SMI (n = 81) Mean (SD) |
Crude p value | p value after adjustmenta | |
| Lumbar spine aBMD (g/cm2) | 1.056 (0.158) | 1.121 (0.181) | 0.06 | 0.20 |
| Total hip aBMD (g/cm2) | 0.880 (0.159) | 0.945 (0.122) | 0.02 | 0.44 |
| Femoral neck aBMD (g/cm2) | 0.741 (0.124) | 0.771 (1.118) | 0.21 | 0.87 |
| 10-year MOF probability (%) | 8.64 (4.90) | 6.05 (2.43) | < 0.001 | 0.01 |
| 10-year HF probability (%) | 3.90 (3.34) | 2.11 (1.68) | < 0.001 | 0.01 |
| TBS value | 1.291 (0.097) | 1.30 (1.098) | 0.43 | 0.88 |
| Poor grip strength (n = 98) Mean (SD) |
Normal grip strength (n = 190) Mean (SD) |
Crude p value | p value after adjustmenta | |
| Lumbar spine aBMD (g/cm2) | 1.087 (0.192) | 1.104 (0.173) | 0.66 | 0.68 |
| Total hip aBMD (g/cm2) | 0.887 (0.108) | 0.935 (0.124) | 0.13 | 0.15 |
| Femoral neck aBMD (g/cm2) | 0.715 (0.102) | 0.789 (0.122) | 0.03 | 0.05 |
| 10-year MOF probability (%) | 8.41 (4.99) | 6.54 (3.01) | 0.01 | 0.03 |
| 10-year HF probability (%) | 3.91 (2.42) | 2.34 (2.03) | 0.01 | 0.02 |
| TBS value | 1.267 (0.098) | 1.305 (0.094) | 0.06 | 0.06 |
| Poor physical performance (n = 57) Mean (SD) |
Normal physical performance (n = 231) Mean (SD) |
Crude p value | p value after adjustmenta | |
| Lumbar spine aBMD (g/cm2) | 1.098 (0.149) | 1.110 (0.182) | 0.94 | 0.66 |
| Total hip aBMD (g/cm2) | 0.893 (0.106) | 0.932 (0.142) | 0.25 | 0.96 |
| Femoral neck aBMD (g/cm2) | 0.723 (0.073) | 0.780 (0.127) | 0.11 | 0.32 |
| 10-year MOF probability (%) | 8.17 (5.00) | 6.58 (3.17) | 0.07 | 0.09 |
| 10-year HF probability (%) | 3.78 (3.57) | 2.34 (2.01) | 0.02 | 0.04 |
| TBS value | 1.242 (0.097) | 1.307 (0.092) | 0.01 | 0.01 |
aAdjusted for age, BMI, number of co-morbidities, smoking status, and nutritional status
Skeletal status in subjects with low SMI versus subjects with adequate SMI
In older men, lower total hip aBMD was observed in subjects with low SMI as well as increased probabilities of fracture (adjusted p = 0.01) (Table 3). Other bone parameters, although lower in men with low SMI, do not differ significantly from those with adequate SMI (all p > 0.05). In older women, lower aBMD values for two of three sites (i.e., total hip and femoral neck) were noted in the low SMI group than in individuals with an adequate SMI (Table 4). Significantly higher major osteoporotic and hip fracture probabilities were also noted in those with low SMI. However, no significant difference was observed in TBS values in subjects with low SMI versus subjects with higher SMI when adjusting for covariates. Further adjustments of TBS values following models 2, 3, and 4 also demonstrated that there was no significant association between TBS value and SMI (p = 0.36, 0.78, 0.26, respectively). The logistic model showed that nutritional and smoking status as along with total hip aBMD were associated with weaker SMI (p = 0.01, 0.03, and 0.02, respectively).
Table 4.
Comparisons of skeletal status between subjects with or without indices of muscle impairment in women
| Sarcopenic subjects (n = 43) Mean (SD) |
Non-sarcopenic subjects (n = 245) Mean (SD) |
Crude p value | p value after adjustmenta | |
|---|---|---|---|---|
| Lumbar spine aBMD (g/cm2) | 0.940 (0.203) | 1.021 (0.181) | 0.03 | 0.04 |
| Total hip aBMD (g/cm2) | 0.738 (0.123) | 0.826 (0.129) | 0.01 | 0.01 |
| Femoral neck aBMD (g/cm2) | 0.647 (0.114) | 0.706 (0.124) | 0.02 | 0.07 |
| 10-year MOF probability (%) | 16.54 (9.68) | 12.02 (7.24) | 0.01 | 0.03 |
| 10-year HF probability (%) | 7.68 (7.25) | 4.13 (5.73) | 0.01 | 0.05 |
| TBS value | 1.062 (0.122) | 1.300 (0.112) | 0.04 | 0.08 |
| Low SMI (n = 81) Mean (SD) |
Adequate SMI (n = 207) Mean (SD) |
Crude p value | p value after adjustmenta | |
| Lumbar spine aBMD (g/cm2) | 0.982 (0.193) | 1.016 (0.184) | 0.28 | 0.39 |
| Total hip aBMD (g/cm2) | 0.760 (0.118) | 0.829 (0.132) | 0.01 | 0.03 |
| Femoral neck aBMD (g/cm2) | 0.655 (0.103) | 0.711 (0.127) | 0.01 | 0.03 |
| 10-year MOF probability (%) | 16.63 (8.42) | 12.15 (7.59) | 0.04 | 0.05 |
| 10-year HF probability (%) | 6.09 (4.34) | 4.26 (2.16) | 0.03 | 0.04 |
| TBS value | 1.109 (0.114) | 1.299 (0.115) | 0.02 | 0.07 |
| Poor grip strength (n = 98) Mean (SD) |
Normal grip strength (n = 190) Mean (SD) |
Crude p value | p value after adjustmenta | |
| Lumbar spine aBMD (g/cm2) | 0.982 (0.193) | 1.016 (0.184) | 0.29 | 0.05 |
| Total hip aBMD (g/cm2) | 0.761 (0.118) | 0.839 (0.123) | 0.01 | 0.03 |
| Femoral neck aBMD (g/cm2) | 0.655 (0.103) | 0.711 (0.128) | 0.01 | 0.01 |
| 10-year MOF probability (%) | 16.43 (8.42) | 12.14 (7.59) | 0.04 | 0.04 |
| 10-year HF probability (%) | 6.10 (5.34) | 4.26 (4.17) | 0.03 | 0.01 |
| TBS value | 1.079 (0.104) | 1.299 (1.019) | 0.03 | 0.04 |
| Poor physical performance (n = 57) Mean (SD) |
Normal physical performance (n = 231) Mean (SD) |
Crude p value | p value after adjustmenta | |
| Lumbar spine aBMD (g/cm2) | 1.004 (0.211) | 1.009 (0.180) | 0.87 | 0.67 |
| Total hip aBMD (g/cm2) | 0.779 (0.140) | 0.819 (0.129) | 0.11 | 0.82 |
| Femoral neck aBMD (g/cm2) | 0.607 (0.119) | 0.704 (0.124) | 0.01 | 0.11 |
| 10-(year MOF probability (%) | 15.79 (9.57) | 11.98 (6.76) | 0.01 | 0.15 |
| 10-year HF probability (%) | 7.48 (6.46) | 3.99 (5.13) | 0.01 | 0.06 |
| TBS value | 1.208 (0.132) | 1.301 (0.109) | 0.03 | 0.09 |
aAdjusted for age, BMI, number of co-morbidities, smoking status, and nutritional status
Skeletal status in subjects with or without weakness in muscle strength
We observed, in older men with impaired muscle strength, significantly lower femoral neck aBMD compared to those with normal muscle strength (Table 3). Moreover, significantly increased MOF and HF fracture probabilities were found (p < 0.05). Compared with older women with good muscle strength, we found significantly lower total hip and femoral neck aBMD, higher fracture risk, and lower TBS values in women with weakness in muscle strength (Table 4). Regarding the TBS value, the difference remained significant when adjusted for age, number of co-morbidities, smoking status, and nutritional status (model 1) (p = 0.04). However, the difference was not evident when also adjusting for lumbar spine aBMD (model 2) (p = 0.47). This observation was similar when adjusting for total hip and femoral neck aBMD (models 3 and 4) (p = 0.13 and 0.36, respectively). These results showed that when aBMD was considered a covariate, the association of the TBS values with muscle strength did not reach the level of statistical significance. When muscle strength was considered a continuous variable, there was also no evident influence of the TBS value in women (coefficient B = 4.52, p = 0.19).
Skeletal status in subjects with or without physical performance impairment
No significant difference regarding aBMD values was found in older men with impaired physical performance compared to men with good physical ability (all p > 0.05). However, we found, in this population with impaired physical performance, a significantly higher risk of HF fracture (adjusted p = 0.04) and a poorer bone texture (adjusted p value = 0.01). An extensive analysis showed that the significant relationship between TBS value and an impaired physical performance remained when adding lumbar spine aBMD as a covariate (models 2, 3, and 4; p = 0.001, 0.006, and 0.001, respectively). Multivariable linear regression confirmed this relationship (coefficient B = 0.96, p = 0.03). Univariate analysis indicated also that older women with low physical performance showed lower hip aBMD as well as higher fracture probabilities (Table 4). After adjusting for age, number of co-morbidities, smoking status, and nutritional status, these differences were, however, no longer significant. For TBS values, model 1 showed no significantly lower TBS value in subjects with decreased physical performance. Further investigation of the effect of the TBS value on physical performance tested as a continuous variable however demonstrated a significant association between the two components (coefficient B = 1.73, p = 0.01).
Discussion
Growing evidence shows that sarcopenia and osteoporosis share many common pathways [4], and that these conditions can be concomitantly found in a substantial number of individuals [28, 29]. The present cross-sectional study reports an increased proportion of sarcopenic individuals presenting BMD values corresponding to either osteoporosis or osteopenia. Conversely, in older participants with low BMD, we found an increased prevalence of sarcopenia, low muscle mass, decreased muscle strength, and impaired physical performance. We previously reported an increased risk of osteoporosis in subjects diagnosed with sarcopenia according to the definition of the EWGSOP [9, 30], and this observation was supported by other groups [31, 32]. However, the magnitude of the association between osteoporosis and sarcopenia varied between publications with reported risk increasing from 2- to 12-fold. These discrepancies are partly explained by the specificities of the investigated cohorts (e.g., age, sex, setting), but most likely, the major determinant of such differences in the association between the two diseases is the heterogeneity in the selected operational definition of sarcopenia [33–35]. The observation that low BMD is associated not only with sarcopenia as a global entity now recognized as a disease [36] but also with decreased values in muscle mass, strength, and performance, independent of several clinical parameters and lifestyle habits, is interesting. In older women, muscle strength was correlated with BMD but, except at the trochanter site, the significances disappeared when corrected for body weight [9]. Significant but marginal correlations between appendicular skeletal muscle mass and lumbar spine and total hip BMD were also observed in Japanese women aged 40–88 years [12]. In a cohort of healthy individuals, mean age ranging from 20 to 72 years, relative appendicular skeletal muscle mass, which includes upper and lower limb muscle mass, was more significantly correlated with femoral neck BMD than the relative upper limb muscle mass or the relative leg muscle mass [37]. However, our study is the first one to correlate BMD with all aspects of muscle mass, strength, and performance.
The International Osteoporosis Foundation (IOF) FRAX algorithm uses clinical risk factors, BMD values (optional), and country-specific fracture and mortality data to quantify a patient’s 10-year probability of a hip or major osteoporotic fracture [25, 38]. Few studies assessed the relationships between muscle wasting and an increase in individual 10-year facture probability. In a cross-sectional study of South Chinese women, fat mass and lean mass were negatively correlated with the predicted 10-year facture probability when FRAX included the BMD value [39]. However, the relative weight of BMD in the prediction of fracture risk was not assessed, and these data were not corrected for lifestyle habits including physical exercise. Most recently, analysis of a 3-year longitudinal study of community dwellers, with a mean age of 65 years, showed that low lean mass predicts fractures independently from FRAX [40], but there was no assessment of muscle strength or performance included in the study. In Chinese individuals, sarcopenia diagnosed according to the Asian Working Group for Sarcopenia algorithm added incremental value to FRAX in predicting incident fracture [41, 42]. However, this feature was not confirmed in a Caucasian population, and our study is the first one to show a relationship between fracture probability assessed by FRAX and muscle strength or performance in both men and women.
Factors other than bone mass influence bone strength and fracture risk. Trabecular bone score (TBS) is a novel imaging technique [19] that appears to constitute an index of bone architecture. TBS is not a direct measurement of bone architecture, but it is related to three-dimensional bone characteristics such as the trabecular number, the trabecular separation, and the connectivity density [43]. Our study is the first to investigate whether parameters reflecting muscle wasting is associated with TBS values. Although the TBS value was not significantly reduced in sarcopenic men and women after adjustments, we were able to show a relationship between muscle strength and physical performance, on the muscle side, and TBS value. However, after adjustment for bone mass, this relationship remained significant only in the case of physical performance in men, and of grip strength for women. Other investigations previously reported impaired bone texture in older men with low muscle mass, low grip strength, or impaired physical performance [44, 45]. These papers reported on results from the same cohort and used peripheral computed tomography of the distal radius.
In light of these findings, the strengths and limitations of our study should be addressed. The originality of this investigation relates to the comprehensive manner used to investigate the relationships between sarcopenia and the skeletal status of older subjects, with the systematic evaluation of the following three bone components: bone quantity, bone quality, and fracture risk. Specifically, fracture risk and bone texture have scarcely been studied in connection with muscle parameters. To our knowledge, this is the first time that the TBS technique was used to evaluate bone quality in sarcopenic individuals. It allows for an in-depth analysis of the determinants of muscle and bone health. Nevertheless, the transversal design of our study leads to a reflective interpretation of the results. We cannot determine whether the muscle impairment is a cause or a consequence of the deterioration of bone health, despite the observed relationship. Our study is prone to selection bias. Indeed, it should be noted that the sample used for the current study is likely composed of the healthier subjects from the SarcoPhAge study. Our population may therefore not be fully representative and is thus limited in its external validity. However, the prevalence of muscle and bone impairment found in our analysis is likely to be underestimated compared to the true prevalence because individuals who participated in our study still had acceptable musculoskeletal health. Our research may potentially also have uncontrolled confounding factors inherent in any epidemiological study, and more specifically, factors related to both muscle and bone status such as biochemical markers and physical activity.
In conclusion, we report the first study that exhaustively investigated the relationship between muscle (mass, strength, and performance) and bone (mass, architecture, fracture risk) wasting. We confirmed the decrease in bone mass and muscle mass in a subpopulation, previously called osteosarcopenic patients [28]. We also showed, for the first time in Caucasian individuals, that older men and women with major muscle wasting are characterized by an increased 10-year fracture risk and that poor physical performance is linked to a deficit in bone texture. Further longitudinal investigations are now needed to better understand the interaction between bone and muscle, and quantitative and qualitative loss, with the final objective of developing therapeutic strategies that are able to simultaneously prevent, reduce, or restore bone and muscle age-related wasting.
Compliance with ethical standards
Ethical approval
All procedures involving human participants were conducted in accordance with the ethical standards of the Ethics Committee of the University Teaching Hospital of Liège, Belgium, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
None.
References
- 1.Ethgen O, Beaudart C, Buckinx F, Bruyère O, Reginster JY. The future prevalence of sarcopenia in Europe: a claim for public health action. Calcif Tissue Int. 2016;100(3):229–234. doi: 10.1007/s00223-016-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone. 2006;38(2 Suppl 1):S4–S9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Reginster JY, Beaudart C, Buckinx F, Bruyère O. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care. 2016;19(1):31–36. doi: 10.1097/MCO.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V. Muscle and bone, two interconnected tissues. Ageing Res Rev. 2015;21:55–70. doi: 10.1016/j.arr.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Evans WJ. Protein nutrition, exercise and aging. J Am Coll Nutr. 2004;23(6 Suppl):601S–609S. doi: 10.1080/07315724.2004.10719430. [DOI] [PubMed] [Google Scholar]
- 6.Vitale G, Cesari M, Mari D. Aging of the endocrine system and its potential impact on sarcopenia. Eur J Intern Med. 2016;35:10–15. doi: 10.1016/j.ejim.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Ho-Pham LT, Nguyen UD, Nguyen TV. Association between lean mass, fat mass, and bone mineral density: a meta-analysis. J Clin Endocrinol Metab. 2014;99(1):30–38. doi: 10.1210/jc.2013-3190. [DOI] [PubMed] [Google Scholar]
- 8.Walsh MC, Hunter GR, Livingstone MD. Sarcopenia in premenopausal and postmenopausal women with osteopenia, osteoporosis and normal bone mineral density. Osteoporos Int. 2006;17(1):61–67. doi: 10.1007/s00198-005-1900-x. [DOI] [PubMed] [Google Scholar]
- 9.Lima RM, Bezerra LM, Rabelo HT, Silva MA, Silva AJ, Bottaro M, de Oliveira RJ. Fat-free mass, strength, and sarcopenia are related to bone mineral density in older women. J Clin Densitom. 2009;12(1):35–41. doi: 10.1016/j.jocd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Genaro PS, Pereira GA, Pinheiro MM, Szejnfeld VL, Martini LA. Influence of body composition on bone mass in postmenopausal osteoporotic women. Arch Gerontol Geriatri. 2010;51(3):295–298. doi: 10.1016/j.archger.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Di Monaco M, Vallero F, Di Monaco R, Tappero R. Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr. 2011;52(1):71–74. doi: 10.1016/j.archger.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Miyakoshi N, Hongo M, Mizutani Y, Shimada Y. Prevalence of sarcopenia in Japanese women with osteopenia and osteoporosis. J Bone Miner Metab. 2013;31(5):556–561. doi: 10.1007/s00774-013-0443-z. [DOI] [PubMed] [Google Scholar]
- 13.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaktaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD. Low bone mineral density and fracture burden in postmenopausal women. CMAJ. 2007;177(6):575–580. doi: 10.1503/cmaj.070234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pothuaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone. 2008;42(4):775–787. doi: 10.1016/j.bone.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Harvey NC, Glüer CC, Binkley N, McCloskey EV, Brandi ML, Cooper C, Kendler D, Lamy O, Laslop A, Camargos BM, Reginster JY, Rizzoli R, Kanis JA. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–224. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaudart C, Reginster J-Y, Petermans J, Gillain S, Quabron A, Locquet M, Slomian J, Buckinx G, Bruyère O. Quality of life and physical components linked to sarcopenia: the SarcoPhAge study. Exp Gerontol. 2015;69:103–110. doi: 10.1016/j.exger.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom. 2015;18(3):309–330. doi: 10.1016/j.jocd.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev. 1996;54(1 Pt 2):S59–S65. doi: 10.1111/j.1753-4887.1996.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Ferruci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.M221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson H, Kanis JA, McCloskey EV, Odén A, Devogelaer JP, Kaufman JM, Neuprez A, Hiligsmann M, Bruyère O, Reginster J-Y. A FRAX® model for the assessment of fracture probability in Belgium. Osteoporos Int. 2011;22(2):453–461. doi: 10.1007/s00198-010-1218-1. [DOI] [PubMed] [Google Scholar]
- 26.Compston J, Bowring C, Cooper A, Cooper C, Davies C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas 75(4):392–6 [DOI] [PubMed]
- 27.Bousson V, Bergot C, Sutter B, Thomas T, Bendavid S, Benhamou CL, Blain H, Brazier M, Breuil V, Briot K, Chapurlat R, Chapuis L, Cohen Solal M, Fardellone P, Feron JM, Gauvain JB, Laroche M, Legrand E, Lespessailles E, Linglart A, Marcelli C, Roux C, Souberbielle JC, Tremollieres F, Weryha G, Cortet B, Groupe de Recherche et d’Information sur les Ostéoporoses (GRIO) Trabecular bone score: where are we now? Joint Bone Spine. 2015;82(5):320–325. doi: 10.1016/j.jbspin.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Ormsbee MJ, Prado CM, Ilich JZ, Purcell S, Siervo M, Folsom A, Panton L. Osteosarcopenic obesity: the role of bone, muscle, and fat on health. J Cachexia Sarcopenia Muscle. 2014;5(3):183–192. doi: 10.1007/s13539-014-0146-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Monaco M, Castiglioni C, De Toma E, Gardin L, Giordano S, Di Monaco R, Tappero R. Presarcopenia and sarcopenia in hip-fracture women: prevalence and association with ability to function in activities of daily living. Aging Clin Exp Res. 2015;27(4):465–472. doi: 10.1007/s40520-014-0306-z. [DOI] [PubMed] [Google Scholar]
- 30.Locquet M, Beaudart C, Reginster J-Y, Petermans J, Gillain S, Quabron A, Slomian J, Buckinx F, Bruyère O. Prevalence of concomitant bone and muscle wasting in elderly women from the SarcoPhAge cohort: preliminary results. J Frailty Aging. 2017;6(1):18–23. doi: 10.14283/jfa.2016.111. [DOI] [PubMed] [Google Scholar]
- 31.Verschueren S, Gielen E, O'Neill TW, Pye SR, Adams JE, Ward KA, Wu FC, Szulc P, Laurent M, Claessens F, Vanderschueren D, Boonen S. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int. 2013;24(1):87–98. doi: 10.1007/s00198-012-2057-z. [DOI] [PubMed] [Google Scholar]
- 32.Sjöblom S, Suuronen J, Rikkonen T, Honkanen R, Kröger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75(2):175–180. doi: 10.1016/j.maturitas.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Beaudart C, Reginster J-Y, Slomian J, Buckinx F, Locquet M, Bruyère O. Prevalence of sarcopenia: the impact of different diagnostic cut-off limits. J Musculoskelet Neuronal Interact. 2014;14:425–431. [PubMed] [Google Scholar]
- 34.Di Monaco M, Castigliono C, Di Monaco R, Tappero R (2017) Association between low lean mass and low bone mineral density in 653 women with hip fracture: does the definition of low lean mass matter? Aging Clin Exp Res 29(6):1271–76 [DOI] [PubMed]
- 35.Di Monaco M, Castigliono C. Weakness and low lean mass in women with hip fracture: prevalence according to the FNIH criteria and association with the short-term functional recovery. J Geriatri Phys Ther. 2017;40(2):80–85. doi: 10.1519/JPT.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 36.Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512–514. doi: 10.1002/jcsm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blain H, Jaussent A, Thomas E, Micallef JP, Dupuy AM, Bernard PL, Mariano-Goulart D, Cristol JP, Sultan C, Rossi M, Picot MC. Appendicular skeletal muscle mass is the strongest independent factor associated with femoral neck bone mineral density in adult and older men. Exp Gerontol. 2010;45(9):679–684. doi: 10.1016/j.exger.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Neuprez A, Johansson H, Kanis JA, McCloskey EV, Oden A, Bruyère O, Hilligsmann M, Devogelaer JP, Kaufman JM, Reginster JY. A FRAX model for the assessment of fracture probability in Belgium. Rev Med Liege. 2009;64(12):612–619. [PubMed] [Google Scholar]
- 39.Zhang Z, Shen X, Zhang H, Li S, Zhou H, Wu X, Sheng Z, Liao E. The relationship between body composition and fracture risk using the FRAX model in central south Chinese postmenopausal women. Clin Endocrinol. 2012;77(4):524–530. doi: 10.1111/j.1365-2265.2012.04399.x. [DOI] [PubMed] [Google Scholar]
- 40.Hars M, Biver E, Chevally T, Herrmann F, Rizzoli R, Ferrari S, Trombetti A. Low lean mass predicts incident fractures independently from FRAX: a prospective cohort study of recent retirees. J Bone Miner Res. 2016;31(11):2048–2056. doi: 10.1002/jbmr.2878. [DOI] [PubMed] [Google Scholar]
- 41.Yu R, Leung J, Woo J. Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc. 2014;15(12):918–923. doi: 10.1016/j.jamda.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) study. J Am Med Dir Assoc. 2014;15(8):551–558. doi: 10.1016/j.jamda.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. J Bone Miner Res. 2011;26(11):2761–2769. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 44.Blaizot S, Boutroy S, Vilayphiou N, Boonen S, Chapurlat R, Szulc P. Poor bone microarchitecture in older men with impaired physical performance-the STRAMBO study. Osteoporos Int. 2012;23(12):2785–2796. doi: 10.1007/s00198-012-1906-0. [DOI] [PubMed] [Google Scholar]
- 45.Szulc P, Blaizot S, Boutroy S, Vilayphiou N, Boonen S, Chapurlat R. Impaired bone microarchitecture at the distal radius in older men with low muscle mass and grip strength: the STRAMBO study. J Bone Miner Res. 2013;28(1):169–178. doi: 10.1002/jbmr.1726. [DOI] [PubMed] [Google Scholar]