Abstract
Despite recent progress, the mechanisms governing shoot morphogenesis in higher plants are only partially understood. Classical physiological studies have suggested that gradients of the plant growth regulator auxin may play a role in controlling tissue differentiation in shoots. More recent molecular genetic studies have also identified knotted1 like homeobox (knox) genes as important regulators of shoot development. The maize (Zea mays L.) mutant rough sheath2 (rs2) displays ectopic expression of at least three knox genes and consequently conditions a range of shoot and leaf phenotypes, including aberrant vascular development, ligular displacements, and dwarfism (R. Schneeberger, M. Tsiantis, M. Freeling, J.A. Langdale [1998] Development 125: 2857–2865). In this report, we show that rs2 mutants also display decreased polar auxin transport in the shoot. We also demonstrate that germination of wild-type maize seedlings on agents known to inhibit polar auxin transport mimics aspects of the rs2 mutant phenotype. The phenotype elaborated in inhibitor-treated plants is not correlated with ectopic KNOX protein accumulation.
The majority of the aerial part of higher plants is derived from the shoot apical meristem (SAM). Despite recent progress, the exact process by which cells derived from the SAM give rise to the different parts of the vegetative plant body are still unclear. Molecular genetic analysis has suggested that the regulation of knox genes is instrumental both to maintenance of the SAM and to the initiation of lateral shoot organs (Kerstetter and Hake, 1997). Ectopic expression of knox genes in dicotyledonous plants results in a range of plant phenotypes, including lobed leaves, shoot vivipary, and decreased apical dominance. Intriguingly, these phenotypes are also observed in transgenic plants that either overexpress a cytokinin biosynthetic gene or underproduce auxin, and therefore have elevated cytokinin to auxin ratios (Estruch et al., 1991; Li et al., 1992; Klee and Lanahan, 1995). Such findings have led to the suggestion that the developmental pathways defined by plant growth regulators and knox genes are somehow interrelated (Kerstetter et al., 1997; Brutnell and Langdale, 1998; Tsiantis and Langdale, 1998).
Another tentative area of convergence between hormone- and homeobox-specified pathways is vascular development. It is known that exogenous auxin can induce vascular differentiation and affect the path of vascular strand differentiation in different plant systems (Aloni, 1995). In addition, correlations have been found in Arabidopsis between aberrations in vascular tissue development (twisting, midvein bifurcation) and decreased polar auxin transport (PAT) (Bennett et al., 1995; Carland and McHale, 1996). Notably, maize (Zea mays L.) mutants that ectopically express the homeobox genes kn1 and rough sheath1 (rs1) also display abnormalities in vascular differentiation patterns (Volbrecht et al., 1991; Becraft and Freeling, 1994). Moreover, in the stem of wild-type maize plants, both the rs1 and kn1 homeobox genes are expressed in close association with provascular strands (Smith et al., 1992; Jackson et al., 1994; Schneeberger et al., 1995). These observations suggest that auxin may be involved in mediating certain aspects of the phenotype that result from inappropriate knox gene expression.
The rough sheath 2 (rs2) mutant of maize displays ectopic expression of three knox genes due to loss of function of the rs2 gene that encodes a myb-like transcription factor (Schneeberger et al., 1998; Timmermans et al., 1999; Tsiantis et al., 1999). The resulting phenotype includes midrib duplication, leaf twisting, dwarfism, and vascular tissue aberrations. In this report, we assess whether perturbations in auxin homeostasis are a component of the maize rs2 mutant phenotype. Furthermore, we investigate the effects of PAT inhibitors on the growth of wild-type maize seedlings.
MATERIALS AND METHODS
Plant Material
Seeds of the maize (Zea mays L.) inbred line B73 were a gift from Pioneer Hi-Bred International (Des Moines, IA). The rs2-twd allele was isolated as in Schneeberger et al. (1998). The mutation was induced by transposon insertion into the region of the rs2 gene that encodes the myb domain (Tsiantis et al., 1999).
Measurement of PAT
Auxin transport measurements were conducted according to the method of Okada et al. (1991). Elongated mesocotyls were harvested from seedlings grown in the dark at 25°C for a week. Mesocotyl segments used were 2.2 to 2.4 cm. Tissue samples were incubated in a microfuge tube containing 40 μL of C-14 indole acetic acid for 16 h. After this time, the upper 2 mm of tissue was removed, placed in scintillant, and counted in a multipurpose scintillation counter (model LS6500, Beckman Instruments, Fullerton, CA).
Treatment of Plants with Inhibitors of PAT
Seeds of the inbred line B73 were sterilized and germinated on Murashige-Skoog medium in the presence or absence of 2,3,5-triiodobenzoic acid (TIBA) (28 μm) or naphthylphthamic acid (15 μm). Plants were grown in sterile pots at 25°C under a 16-h light/8-h dark photoperiod (100 μmol m−2 s−1), and after 2 weeks seedling morphology was examined.
Histology
Leaf samples were fixed in formalin acetic acid for 30 min, dehydrated through an ethanol series, paraffin embedded, and sectioned as in Langdale (1994). Sections (10 μm) were stained with Safranin/Fast Green as described in Schneeberger et al. (1998). Mutant and wild-type shoot apices were fixed in formalin acetic acid for 2 h, dehydrated, and embedded as above. Apices were sectioned completely and the number of axillary buds was noted per plant. Ten wild-type and seven mutant plants were examined.
Immunolocalization Assays
Tissue was fixed as described above and sections were reacted with anti-KNOX antibody as described in Schneeberger et al. (1998).
RESULTS AND DISCUSSION
Auxin Transport Aberrations in rs2 Mutant Plants
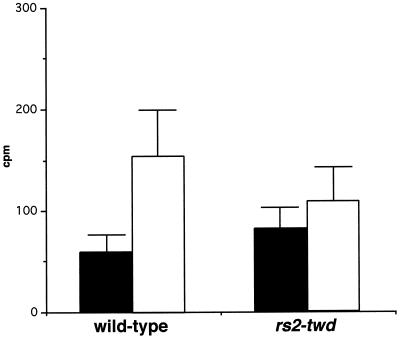
To assess the auxin transport capacity of rs2 mutant plants, PAT measurements were conducted on etiolated mesocotyls of wild-type and rs2 maize seedlings. These measurements revealed that there was a clear difference between basipetal and acropetal transport in wild-type plants, whereas in mutant seedlings such a difference was not apparent (Fig. 1). This indicates that auxin gradients may be perturbed in the shoots of rs2-twd seedlings. Auxin is generally thought to be produced in young emerging leaves and transported basipetally through the shoot (Sachs, 1991). A block in basipetal transport would be expected to result in the disruption of auxin gradients both within the leaves (where entrapment of excess auxin could occur) and across the vegetative axis (where less auxin could flow). The latter event would result in reduced internode elongation and could therefore explain the reduced stature of rs2 plants.
Figure 1.
Perturbed auxin transport in mesocotyls of rs2 mutant seedlings. Acropetal transport (black bars) and basipetal (white bars) transport of exogenously supplied C-14 IAA in wild-type and mutant (rs2-twd) seedlings. In wild-type plants, a significant difference is seen between the acropetal and basipetal measurements, demonstrating the presence of active polar basipetal transport mechanisms. In mutant plants, no significant difference is seen between basipetal and acropetal measurements, suggesting that the ability to basipetally transport auxin is significantly reduced in mutant tissue.
Auxin gradients are also believed to influence both vascular strand patterning and cytoskeletal organization. Thus, disruption of auxin gradients within the leaves could explain both the twisting growth pattern of rs2 leaves and the bifurcation of midribs. Indeed, it has recently been shown that two Arabidopsis mutants with deficiencies in PAT show similar characteristics. The lop1 mutant is both dwarfed and twisted (Carland and McHale, 1996), and the pin1 mutant often shows midrib bifurcation and leaf twisting (Bennett et al., 1995). Disrupted auxin gradients may also account for the changes of vascular patterning and overt vascularization of leaves that have been observed in both rs2 and Rs1 mutants (Becraft and Freeling, 1994; Schneeberger et al., 1995, 1998). Indeed, it has already been suggested that the Rs1 mutation, which conditions increased vascular size, could interfere with auxin-regulated developmental pathways (Becraft and Freeling, 1994). Thus, our findings suggest that perturbations to auxin physiology could mediate certain facets of the rs2 mutant phenotype.
Growth of Wild-Type Maize on Auxin Transport Inhibitors Mimics Aspects of the rs2 Phenotype
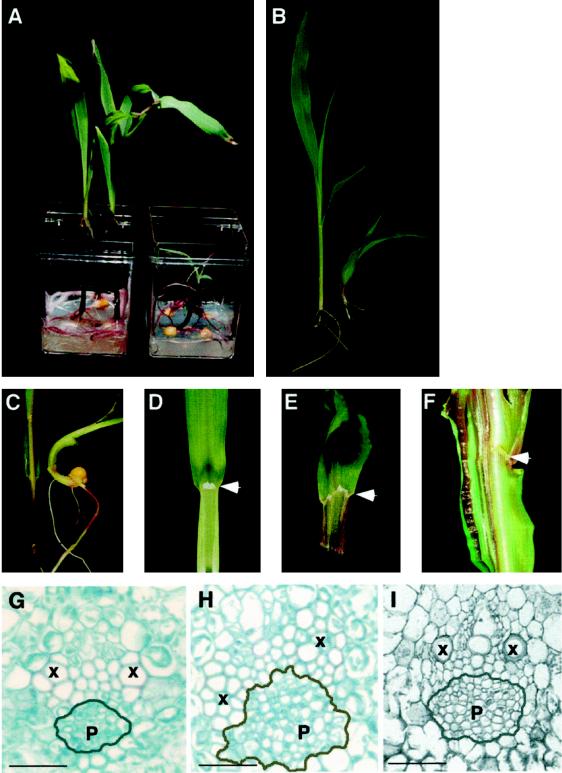
To ascertain whether reductions in PAT in wild-type maize could cause phenotypic perturbations similar to those seen in rs2 mutants, we germinated wild-type maize seedlings in the presence of compounds known to inhibit PAT. Treatment with TIBA (28 μm) resulted in pronounced effects on seedling development (Table I). Roots were agravitropic and showed inhibition of lateral root growth (data not shown). Treated seedlings showed similar phenotypes to rs2 mutant plants in that they were dwarfed, with compressed internodes and twisted leaves (Fig. 2, A–C). Occasionally, the second leaf to emerge exhibited a non-discrete blade/sheath boundary (Fig. 2E) as opposed to the discrete boundary defined by the ligule of untreated plants (Fig. 2D). Notably, rs2 mutant leaves show similar perturbations at the blade/sheath boundary (Fig. 2F). Histological examination of leaf sections revealed the presence of hypertrophic vascular bundles in both TIBA-treated wild-type plants and in rs2 mutant plants (for example, compare phloem tissue in Fig. 2, G–I). Qualitatively similar results were obtained after treatment of plants with naphthylphthamic acid (15 μm). Thus, aspects of the rs2 phenotype are phenocopied by treating wild-type maize seedlings with PAT inhibitors.
Table I.
Phenotypes exhibited by wild-type plants treated with the auxin transport inhibitor TIBA
| TIBA | Control | |
|---|---|---|
| no. | ||
| Agravitropic roots | 28 | 0 |
| Dwarfism | 25 | 4 |
| Twisted leaves/stem | 15 | 0 |
| Aberrant ligule | 3 | 0 |
| Feiled to germinate | 5 | 2 |
| Severe growth arrest | 3 | 1 |
Seventy-six plants were grown, half on control medium and half on medium containing TIBA. The number of plants showing specific phenotypes is indicated.
Figure 2.
Effects of PAT inhibitors on maize seedling growth. Dwarfism and twisting: A and C, Plants on the left have been germinated on control medium and allowed to grow in sterile pots for 2 weeks; plants on the right have been germinated in the presence of 28 μm TIBA and grown for the same time. B, The plant on the left is a wild-type sibling of the rs2 mutant plant shown on the right. Displaced ligule formation: D, Seedling leaf of an untreated wild-type plant. White arrow points to the ligule. Leaf twisting and aberrant ligular formation in a TIBA-treated wild-type plant (E) and in a rs2 mutant plant (F). White arrows point to the non-discrete ligular boundary. Hypertrophic vascularization: G, Vascular morphology of a lateral vein in an untreated wild-type seedling. Vascular hypertrophy seen in a TIBA-treated plant (H) and in a rs2 mutant (I) plant. Xylem and phloem are labeled X and P, respectively. Gray lines indicate the edge of the phloem in each case. Bar = 30 μm.
As discussed above, most of the phenotypic perturbations observed in treated plants can be rationalized on the basis of disrupted auxin gradients. However, little is known about the early signals involved in ligular formation so it is more difficult to establish why disrupted auxin gradients in emerging leaves led to the observed perturbations in the ligular area. Signals involved in ligule differentiation originate near the midrib at plastochron (P) 1–2 (Sylvester et al., 1990). Interestingly, the leaves in which we observed an abnormal blade/sheath boundary are established during embryogenesis and thus would be predicted to have already formed the blade/sheath boundary at the time of inhibitor treatment. Our data therefore suggest that there is a degree of plasticity in the formation of the ligule and that the boundary can be influenced somewhat later than P2. It is conceivable that auxin gradients may play a role in this process. For example, it was recently suggested that the steep radial gradient of auxin that exists in pine leaves acts as a morphogenetic field to direct the development of different cell types (Uggla et al., 1996). It is possible that similar gradients exist in leaves of other higher plants and that cellular differentiation within the leaf depends on such gradients.
Despite apparent similarities between wild-type maize seedlings treated with PAT inhibitors and rs2 mutant plants, there are also notable differences. Most obviously, the root phenotypes observed in TIBA-treated plants are not seen in rs2 mutants. This finding implies that at least some component of the PAT system is functional in rs2 mutants.
Decreased PAT in the rs2 Mutant Is Accompanied by Precocious Axillary Meristem Development
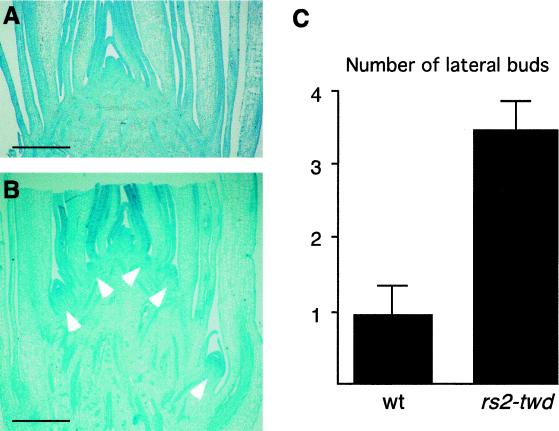
Basipetal auxin transport is believed to be at least partly responsible for axillary meristem arrest (Cline, 1994). Thus, we would predict that a reduction in PAT may lead to overdevelopment of axillary buds. Consistent with this idea, we observed overdevelopment of lateral buds in rs2-twd mutant apices (Fig. 3B). Detailed examination showed that 10 d after germination more axillary meristems were developed in rs2 mutants than in wild-type plants (Fig. 3C). This phenotype is consistent with the measured reduction in PAT since apical dominance is thought to involve basipetal flow of auxin across the vegetative axis. Notably, however, the number of lateral buds in wild-type and mutant plants was not significantly different 21 d after germination. Thus, the rs2-twd allele shows precocious rather than ectopic development of lateral buds. Although extra axillary meristems are initiated early in development, rs2 mutants do not produce increased numbers of tillars (side shoots) or ear shoots. This would suggest that the reduction in PAT is either transient or is not sufficient to fully derepress axillary bud development.
Figure 3.
Precocious axillary bud development in rs2 mutant seedlings. A, Median section through the apical region of a 10-d-old wild-type seedling. Bar = 1 mm. B, Median section through the apical region of a 10-d-old rs2 mutant seedling. Arrowheads point toward the axillary buds. Size bar = 1 mm. C, Number of axillary buds developed in 10-d-old wild-type (wt) and rs2 mutant seedlings.
Ectopic Expression of knox Genes and Disruptions of PAT
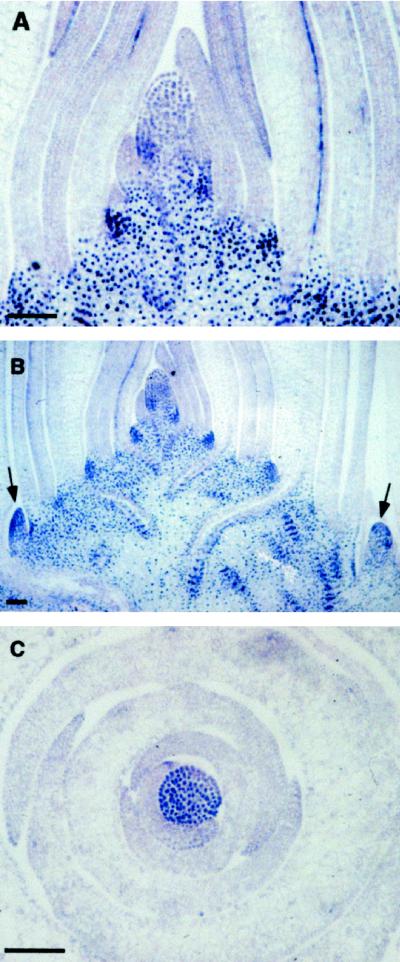
How does the observed reduction in PAT relate to the rs2 mutation and to ectopic knox gene expression? It is known that ectopic accumulation of KNOX proteins in rs2 mutants disrupts cell fate acquisition in the leaf and leads in particular to vascular-tissue-related aberrations. However, very little is known regarding the exact nature of the developmental pathways in which KNOX proteins operate. It is possible that genes involved in plant growth regulator function (including auxin) could be among the knox gene targets. Changes in expression patterns of such genes could impair auxin function, resulting in vascular tissue abnormalities. Alternatively, changes in auxin homeostasis could alter knox gene expression patterns. The latter possibility is suggested by a recent report showing that perturbations in growth regulator levels affect knox gene expression levels in Arabidopsis (Rupp et al., 1999). To distinguish these possibilities in our experimental system, KNOX protein accumulation patterns were examined in TIBA-treated wild-type plants. In both TIBA-treated and untreated wild-type plants, KNOX proteins accumulated in shoot meristems (both apical and axillary) (Fig. 4). No ectopic KNOX accumulation was observed in leaves. Thus, aberrant PAT in rs2 mutants is likely to result from rather than cause ectopic knox gene expression.
Figure 4.
KNOX protein accumulation patterns in TIBA-treated wild-type plants. A, Median section through the apical region of a 10-d-old untreated wild-type seedling. B, Median section through the apical region of a 10-d-old TIBA-treated wild-type seedling. Arrows denote the position of axillary meristems. C, Transverse section through the apical region of a 10-d-old TIBA-treated wild-type seedling. Bars = 100 μm.
Support for the idea that ectopic KNOX gene expression could alter hormonal function comes from studies in tobacco, rice, and lettuce (Tamaoki et al., 1997; Kusaba et al., 1998; Tanaka-Ueguchi et al., 1998; Frugis et al., 1999). In all cases, ectopic expression of KNOX protein was reported to drastically alter hormonal levels and in particular to lead to elevated cytokinin levels (Ori et al., 1999). The idea that KNOX proteins may directly affect hormonal production has been reinforced by a recent study demonstrating that targeted expression of kn1 in a novel developmental context increases cytokinin levels. Interestingy, disruptions in PAT would be predicted to condition phenotypic effects similar to those resulting from elevated cytokinin, since certain cells in PAT-inhibited plants would have reduced auxin and therefore an increased cytokinin to auxin ratio.
Despite the fact that a reasonable amount of evidence suggests tight connections between KNOX genes and hormonal function, an indirect link between ectopic KNOX protein accumulation and hormonal regulation cannot be ruled out. For example, it is possible that KNOX proteins alter cellular identities such that the normal transport canals of auxin are disrupted and therefore a reduction in PAT occurs as a secondary effect. To further this work, it will be essential to identify maize mutants that perturb auxin function. Analysis of double mutants obtained by crossing such lines and the already existing leaf development mutants (such as Rs1 and rs2) should help define the role of auxin in maize leaf development more accurately. Since we have now shown that PAT inhibitors affect maize seedling development, it may be possible to use these compounds as tools to screen for mutants impaired in auxin signaling. The validity of such screens has already been established for Arabidopsis (Ruegger et al., 1997).
ACKNOWLEDGMENTS
We thank R. Schneeberger and M. Freeling for the KNOX antibody and for helpful discussions.
Footnotes
This work was supported by grants from the Biotechnology and Biological Sciences Research Council and the Gatsby Charitable Foundation to J.A.L. M.T. is the recipient of a University of Oxford Glasstone Postdoctoral Fellowship. M.I.N.B. and G.S. were recipients of Nuffield Foundation Undergraduate Bursaries.
LITERATURE CITED
- Aloni R. The induction of vascular tissues by auxin and cytokinin. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 531–546. [Google Scholar]
- Becraft P, Freeling M. Genetic analysis of Rough sheath 1 developmental mutants of maize. Genetics. 1994;136:295–311. doi: 10.1093/genetics/136.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR. Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 1995;8:505–520. [Google Scholar]
- Brutnell TP, Langdale JA. Signals in leaf development. Adv Bot Res. 1998;28:162–187. [Google Scholar]
- Carland FM, McHale NA. LOP1: a gene involved in auxin transport and vascular patterning in Arabidopsis. Development. 1996;122:1811–1819. doi: 10.1242/dev.122.6.1811. [DOI] [PubMed] [Google Scholar]
- Cline MG. The role of hormones in apical dominance: new approaches to an old problem in plant development. Physiol Plant. 1994;90:230–237. [Google Scholar]
- Estruch JJ, Prinsen E, van Onckelen H, Schell J, Spena A. Viviparous leaves produced by somatic activation of an inactive cytokinin-synthesizing gene. Science. 1991;254:1364–1367. doi: 10.1126/science.254.5036.1364. [DOI] [PubMed] [Google Scholar]
- Frugis G, Giannino D, Mele G, Nicoladi C, Innocenti AM, Chiappetta A, Bitonti MB, Dewitte W, Van Onckelen H, Mariotti D. Are homeobox Knotted-like genes and cytokinins the leaf architects? Plant Physiol. 1999;119:371–373. doi: 10.1104/pp.119.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Veit B, Hake S. Expression of maize Knotted1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. 1994;120:404–413. [Google Scholar]
- Kerstetter RA, Hake S. Shoot meristem formation in vegetative development. Plant Cell. 1997;9:1001–1010. doi: 10.1105/tpc.9.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter RA, Laudencia-Chingcuanco D, Smith LG, Hake S. Loss of function mutations in the maize homeobox gene knotted1 are defective in shoot meristem maintenance. Development. 1997;124:3045–3054. doi: 10.1242/dev.124.16.3045. [DOI] [PubMed] [Google Scholar]
- Klee HJ, Lanahan MB. Transgenic plants in hormone biology. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 340–353. [Google Scholar]
- Kusaba S, Kano-Murakimi Y, Matsuoka M, Tamaoki M, Sakamoto T, Yamaguchi I, Fukumoto M. Alteration of hormone levels in transgenic tobacco plants overexpressing the rice homeobox gene OSH1. Plant Physiol. 1998;116:471–476. doi: 10.1104/pp.116.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale JA. In situ hybridization. In: Freeling M, Walbot V, editors. The Maize Handbook. New York: Springer Verlag; 1994. pp. 165–179. [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Dev Biol. 1992;153:386–395. doi: 10.1016/0012-1606(92)90123-x. [DOI] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Juarez MT, Jackson D, Yamaguchi J, Banowetz GM, Hake S. Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence activated promoter. Plant Cell. 1999;11:1073–1080. [PMC free article] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp H-M, Frank M, Werner T, Strnad M, Schmulling T. Increased steady state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. Plant J. 1999;18:557–563. doi: 10.1046/j.1365-313x.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- Sachs T. Cell polarity and tissue patterning in plants. Development. 1991;S1:833–893. [Google Scholar]
- Schneeberger R, Tsiantis M, Freeling M, Langdale JA. The rough sheath2 gene negatively regulates homeobox gene expression during maize leaf development. Development. 1998;125:2857–2865. doi: 10.1242/dev.125.15.2857. [DOI] [PubMed] [Google Scholar]
- Schneeberger RG, Becraft PW, Hake S, Freeling M. Ectopic expression of the knox homeobox gene rough sheath1 alters cell fate in maize leaf. Genes Dev. 1995;9:2292–2304. doi: 10.1101/gad.9.18.2292. [DOI] [PubMed] [Google Scholar]
- Smith LG, Greene B, Veit B, Hake S. A dominant mutation in the maize homeobox gene, Knotted-1, causes its ectopic expression in leaf cells with altered fates. Development. 1992;116:21–30. doi: 10.1242/dev.116.1.21. [DOI] [PubMed] [Google Scholar]
- Sylvester AW, Cande WZ, Freeling M. Division and differentiation during normal and liguleless-1 maize leaf development. Development. 1990;110:985–1000. doi: 10.1242/dev.110.3.985. [DOI] [PubMed] [Google Scholar]
- Tamaoki M, Kusaba S, Kano-Murakami Y, Matsuoka M. Ectopic expression of tobacco homeobox gene NTH15 dramatically alters leaf morphology and hormone levels in transgenic tobacco. Plant Cell Physiol. 1997;38:917–927. doi: 10.1093/oxfordjournals.pcp.a029252. [DOI] [PubMed] [Google Scholar]
- Tanaka-Ueguchi M, Itoh H, Oyama N, Koshioka M, Matsuoka M. Over-expression of a tobacco homeobox gene NTH15, decreases the expression of a gibberellin biosynthetic gene encoding GA 20-oxidase. Plant J. 1998;15:391–400. doi: 10.1046/j.1365-313x.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- Timmermans MCP, Hudson A, Becraft PW, Nelson T. ROUGH SHEATH2: a myb protein that represses knox homeobox genes in maize lateral organ primordia. Science. 1999;284:151–153. doi: 10.1126/science.284.5411.151. [DOI] [PubMed] [Google Scholar]
- Tsiantis M, Langdale JA. The formation of leaves. Curr Opin Plant Biol. 1998;1:43–48. doi: 10.1016/s1369-5266(98)80126-x. [DOI] [PubMed] [Google Scholar]
- Tsiantis M, Schneeberger R, Golz JF, Freeling M, Langdale JA. The maize rough sheath2 gene and leaf development programs in monocot and dicot plants. Science. 1999;284:154–156. doi: 10.1126/science.284.5411.154. [DOI] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B. Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci USA. 1996;93:9282–9286. doi: 10.1073/pnas.93.17.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volbrecht E, Veit B, Sinha N, Hake S. The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature. 1991;350:241–243. doi: 10.1038/350241a0. [DOI] [PubMed] [Google Scholar]