Abstract
Ectopic pancreas is an uncommon finding in the stomach. Complications are rare but can lead to significant morbidity and even mortality. We report a 49-year-old man who presented with upper abdominal pain, vomiting, and weight loss and was found to have a gastric wall abscess that developed a few weeks after endoscopic biopsy of a gastric ulcer. After medical treatment failed to resolve his symptoms, he underwent distal gastrectomy with Roux-en-Y gastrojejunostomy. Postoperatively, the gastric wall abscess was determined to have derived from a focus of ectopic pancreatic tissue with evidence of ectopic chronic pancreatitis.
Introduction
Ectopic pancreas is a congenital condition defined as pancreatic tissue that lacks anatomical or vascular communication with the normal body of the pancreas. It is relatively common and is reported in 0.5–13% of autopsy cases.1,2 It is typically an incidental finding during upper endoscopy, usually found in the distal stomach, duodenum, or proximal jejunum, but it can become clinically relevant when complications develop. Such complications are rare, but they can lead to significant morbidity and even mortality.
Case Report
A 49-year-old man presented with progressive epigastric pain, vomiting, and weight loss of 9 kg over the previous 2 months. He denied fevers or night sweats. He had a significant history of alcohol and tobacco abuse. Past medical history was significant for hypertension and well-managed gastroesophageal reflux disease. Physical exam showed normal vital signs and mild epigastric tenderness. Laboratory workup showed white blood cells 15 × 109/L, amylase 177 U/L, lipase 216 U/L, and alanine aminotransferase 44 U/L. Triglycerides were normal, and gallbladder ultrasound was unremarkable. An abdominal-pelvic computed tomography (CT) scan with contrast, done 2 months prior for evaluation of unrelated hip pain, was unremarkable. Esophagogastroduodenoscopy (EGD) revealed changes consistent with portal hypertensive gastropathy, slightly prominent gastric folds, a few sessile polyps suggestive of fundic gland polyps, and a small superficial ulcer (3-mm) in the antrum (Figure 1), which was biopsied. The largest polyp measured 1.3 cm and was removed.
Figure 1.
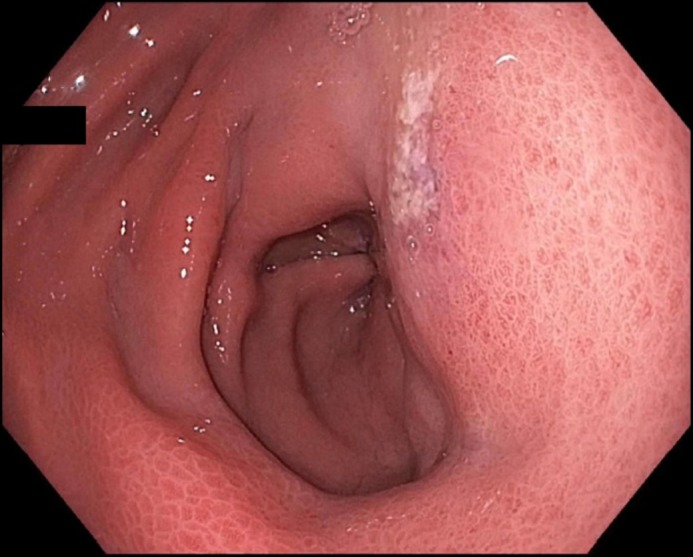
A 3-mm superficial ulcer in the prepyloric antrum.
Gastric biopsies showed active gastritis and chemical gastropathy, but no Helicobacter pylori. The excised polyp proved to be a gastric carcinoid tumor with clear margins. Sub-sequent gastrin and chromogranin A levels were normal, suggesting a type 3 gastric carcinoid tumor. An octreotide scan was found to be normal. The patient had worsening symptoms over the next 2 weeks and presented to the emergency department. Lipase was elevated at 514 U/L, and repeat abdominal CT scan showed a new, intramural low-density area (3.1 cm) with peripheral enhancement involving the antrum and pyloric area concerning for abscess (Figure 2), thrombosis in the middle hepatic vein, and enlarged lymph nodes at the gastrohepatic ligament and near the caudate lobe of the liver. The pancreas was unremarkable. CT-guided needle aspiration removed 15 mL of purulent fluid from the gastric wall abscess, and cultures grew Streptococcus mitis and Veillonella (both predominantly oral and airway flora). The patient was started on intravenous (IV) antibiotics and therapeutic anticoagulation, and he showed slow progressive improvement.
Figure 2.
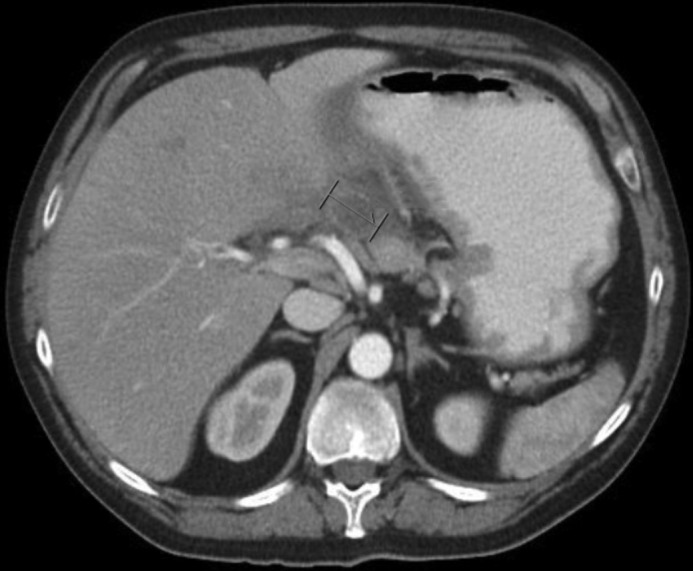
A 3.1-cm intramural low-density area with peripheral enhancement involving the antrum and pyloric area concerning for abscess.
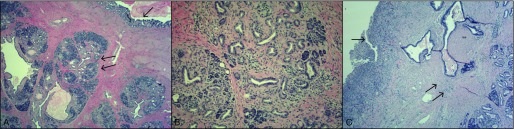
Despite prolonged IV antibiotics treatment, the patient continued to have upper abdominal pain with nausea and vomiting. Repeat abdominal CT scan 2 months later showed a persistent loculated cystic lesion in the anterior wall of the gastric antrum, which had increased in size since the previous CT scan (Figure 3). Given the failure of medical management, the patient was offered a distal gastrectomy with Roux-en-Y gastrojejunostomy. The distal gastrectomy specimen revealed a partially solid and cystic mass (5.0 × 4.5 × 2.4 cm) bulging into the gastric wall. Cut surface showed variegated gray and yellow tan solid areas and cyst-like cavities were visible, with the largest measuring 3.5 cm. This mass abutted the overlying gastric mucosa, which was normal and intact (Figure 4). Microscopic sections showed ectopic pancreatic tissue with a lobular architecture in the muscularis propria and subserosa of the gastric antrum. This was composed of a mixture of atrophic pancreatic acini, ducts, and islets of Langerhans. There was a mild lymphocytic infiltrate in the stroma, which was consistent with chronic pancreatitis (Figure 4). Adjacent to the ectopic pancreatic tissue, an organizing abscess cavity lined by granulation tissue was evident with dilated and inflamed pancreatic ductules opening into it (Figure 4). Additionally, there was an incidental gastrointestinal stromal tumor, measuring 0.6 cm, attached to the gastric serosal surface. No carcinoid tumors were found within the stomach or pancreatic tissue. The patient had an uneventful postoperative course with progressive clinical improvement.
Figure 3.
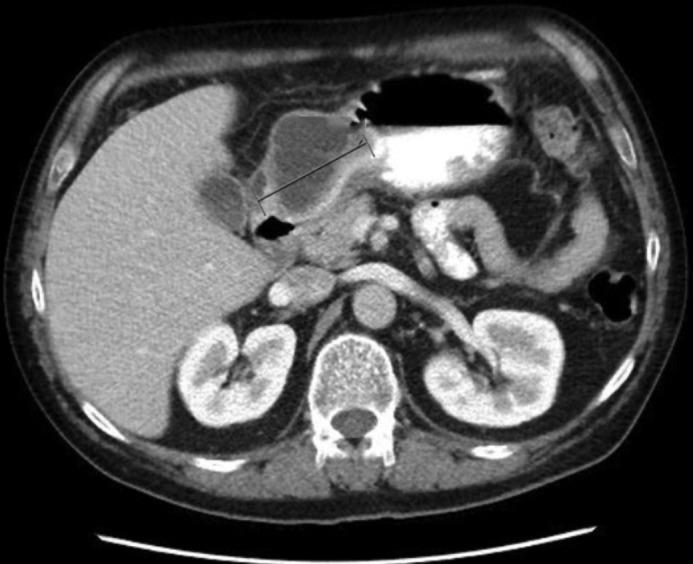
Persistent loculated abscess in the anterior wall of the gastric antrum measuring approximately 4.8 × 6.3 × 5.0 cm.
Figure 4.
(A) Hematoxylin and eosin (H&E) stain of the of stomach wall (×40). Intact gastric mucosa (single arrow) with ectopic pancreatic tissue in muscularis propria (double arrows). (B) Medium-power view (×100) of heterotopic pancreas with evidence of chronic pancreatitis. (C) H&E stain (×80) of an organizing abscess cavity lined with granulation tissue (single arrow), with adjacent dilated ducts and ectopic pancreatic tissue (double arrows).
Discussion
Although ectopic pancreatic tissue is usually asymptomatic, symptoms may develop depending on the anatomic location of the tissue. Such symptoms usually manifest as pain or obstructive sequelae. Ectopic pancreatic tissue usually needs to be 1.5 cm or larger to result in clinically meaningful symptoms. Endoscopic diagnosis can be challenging as the lesion can appear as a firm, round, subepithelial lesion with a central depression or umbilication, which makes differentiating it from other submucosal lesions difficult.3 However, an abscess related to chronic pancreatitis in ectopic pancreatic tissue is exceedingly rare. Indeed, we could only identify a handful of such cases in the literature.4-6
Our patient developed a persistent abscess within the gastric wall despite aggressive medical therapy, which eventually required surgical intervention. In the resected part of the stomach, ectopic pancreatic tissue was found within the muscular wall and the subserosa with evidence of chronic pancreatitis and pancreatic ductules opening into an abscess cavity. These findings indicate that the pancreatic tissue was draining directly into the abscess cavity, thus making it difficult to resolve. Indeed, this may have been analogous to a pseudocyst or walled-off pancreatic necrosis. The isolated bacteria from the abscess are both normal mouth and upper airway flora and may have been transmitted during biopsies of the gastric ulcer in the antrum on initial EGD.
On initial endoscopic evaluation, there was no evidence of a chronic inflammatory process anywhere in the examined stomach. The evaluation was generally unremarkable other than the findings mentioned in the Case Report section. As such, we believe that the endoscopic manipulation itself (biopsy) was the main route of bacterial access to the ectopic pancreatic tissue, which then led to the development of an abscess. We suspect that the pancreatitis in the ectopic tissue may have been related to heavy alcohol use, but this cannot be proven. The patient’s hyperlipasemia was identified without suggestion of involvement of the anatomic pancreas, which implies isolated involvement of the ectopic tissue. Whether there existed changes of chronic pancreatitis in the ectopic tissue at the time of presentation may never be known with certainty, but it should be noted that no previous radiological examinations had revealed anything to suggest this. However, if the focus of the ectopic pancreatic tissue was small, as is the case with most pancreatic rests, it probably could not be visualized radiologically given its small and submucosal nature. It was only later detected incidentally after surgical resection provided a large specimen for pathologic analysis. Similarly, the etiology of acute or chronic pancreatitis of ectopic pancreatic tissue is not fully understood and is seldom reported. Two case reports speculate that alcohol may have caused gastric ectopic pancreatitis without causing pancreatitis of the anatomical pancreas; however, this association could not be proven.7,8 This certainly seems to apply in our case as well.
According to a review of 6 case reports of gastric subepithelial lesions combined with gastric abscesses, 4 cases were associated with gastrointestinal stromal tumor (GIST) or leiomyosarcomas, while only 2 cases had ectopic pancreatic tissue associated with gastric abscesses.5,6 We report what appears to be the third such presentation. All 3 cases of ectopic pancreatic tissue were treated surgically and had a good postoperative course. Our case is unique because the patient also had incidental metachronous carcinoid and GIST tumors in other locations in his stomach. Whether these findings are related is unclear. While cases of a gastric neuroendocrine tumor arising directly from ectopic pancreatic tissue was previously reported in 2 cases in the literature, to our knowledge there are no case reports of metachronous gastric carcinoid tumor and gastric ectopic pancreas.9,10
Gastric wall abscess is a rare complication of ectopic pancreatic tissue in the stomach. This complication can be difficult to manage conservatively, and surgical treatment should be considered if medical therapy fails. Furthermore, although the most common cause of elevated lipase without associated pancreatitis is penetrating peptic ulcer disease, other less common etiologies should be considered and the index of suspicion should remain high when a clear cause is not readily identified.
Disclosures
Author contributions: Y. Alastal and B. Khalil wrote the manuscript. S. Singh provided the pathology images and interpretive information. S.B. Almadani edited the manuscript and is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Jiang LX, Xu J, Wang XW, et al. Gastric outlet obstruction caused by heterotopic pancreas: A case report and a quick review. World J Gastroenterol. 2008;14:6757–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SH, Han JK, Choi BI, Kim M, Kim YI, Yeon KM, Han MC. Heterotopic pancreas of the stomach: CT findings correlated with pathologic findings in six patients. Abdom Imaging. 2000;25(2):119–23. [DOI] [PubMed] [Google Scholar]
- 3.Elwir S, Glessing B, Amin K, Jensen E, Mallery S. Pancreatitis of ectopic pancreatic tissue: A rare cause of gastric outlet obstruction. Gastroenterol Rep (Oxf). 2017;5(3):237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uhrenholt L, Stimpel H. [Abscess formation in ectopic pancreatic tissue in the stomach]. Ugeskr Laeger. 1984;146(12):883–84. [PubMed] [Google Scholar]
- 5.Kim SB, Oh MJ, Lee SH. Gastric subepithelial lesion complicated with abscess: Case report and literature review. World J Gastroenterol. 2015;21(20):6398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneda M, Yano T, Yamamoto T, Suzuki T, Fujimori K, Itoh H, Mizumoto R. Ectopic pancreas in the stomach presenting as an inflammatory abdominal mass. Am J Gastroenterol. 1989;84(6):663–66. [PubMed] [Google Scholar]
- 7.Elwir S, Glessing B, Amin K, Jensen E, Mallery S. Pancreatitis of ectopic pancreatic tissue: A rare cause of gastric outlet obstruction. Gastroenterol Rep (Oxf). 2017;5(3):237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirasaki S, Tanimizu M, Moriwaki T, Nasu J. Acute pancreatitis occurring in gastric aberrant pancreas treated with surgery and proved by histological examination. Intern Med. 2005;44(11):1169–73. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Omote R, Okazaki N, Yanai H, Yoshino T. Gastric neuroendocrine tumor arising from heterotopic pancreas. Clin J Gastroenterol. 2018;11(1):34–37.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chetty R, Weinreb I. Gastric neuroendocrine carcinoma arising from heterotopic pancreatic tissue. J Clin Pathol. 2004;57(3):314–17. [DOI] [PMC free article] [PubMed] [Google Scholar]