Abstract
Aim
To define the optimal margin on MRI scans in the re-radiation planning of recurrent glioblastoma using methionine positron emission tomography (MET-PET).
Background
It would be very useful if the optimal margin on MRI to cover the uptake area on MET-PET is known.
Materials and Methods
CT, MRI, and MET-PET were performed separately over the course of 2 weeks. Among the MRI scans, we used the contrast-enhanced T1-weighted images (Gd-MRI) and T2-weighted images (T2-MRI). The Gd-MRI-based clinical target volume (CTV) (CTV-Gd) and the T2-MRI-based CTV (CTV-T2) were defined as the contrast-enhanced area on Gd-MRI and the high intensity area on T2-MRI, respectively. We defined CTV x mm (x = 5, 10, 15, 20) as x mm outside the CTV. MET-PET-based CTV (CTV-MPET) was defined as the area of accumulation of MET-PET. We calculated the sensitivity and specificity of CTV-Gd and CTV-T2 following comparison with CTV-MPET, which served as the gold standard in this study.
Results
The sensitivity of CTV-T2 5 mm (98%) was significantly higher than CTV-T2 (87%), and there was no significant difference in the sensitivity between CTV-T2 5 mm and CTV T2 10, 15, or 20 mm. The sensitivity of CTV-Gd 20 mm (97%) was lower than that of CTV-T2 5 mm (98%).
Conclusions
A margin of at least 5 mm around the high intensity area on T2-MRI is necessary in the target volume delineation of recurrent glioblastoma for the coverage of MET-PET findings in re-radiation therapy planning.
Keywords: Glioblastoma, Radiation therapy, Magnetic resonance imaging, 11C methionine positron emission tomography
1. Background
Glioma is one of the common primary brain tumors. Glioblastoma (GBM) is the most common among the gliomas. A landmark trial by Stupp et al. led to the acceptance that maximum safe surgical excision followed by adjuvant chemoradiotherapy and adjuvant chemotherapy is the best standard care protocol.1 Despite multidisciplinary treatment, early failure of local treatment is a common feature of this disease.2, 3 The median survival time is limited to approximately 10–15 months for GBM.1, 4 Most episodes of relapse occur within 2–3 cm of the margin of the original lesion.5, 6 The usefulness of hypofractionated stereotactic radiotherapy (SRT) was reported for recurrent GBM.7, 8, 9 SRT is often used to irradiate the gadolinium (Gd)-contrasted tumor edge on magnetic resonance imaging (MRI) for recurrent GBM. However, GBM often exists beyond the Gd-enhanced lesion because of its infiltrative character.10, 11, 12 Several articles reported that 11C methionine positron emission tomography (MET-PET) has greater accuracy than MRI in correctly outlining the true extent of gliomas.13, 14, 15, 16 Grosu et al. reported that patients who received SRT planned with a biological imaging technique, such as MET-PET, survived longer compared to patients who received SRT planned with MRI.17 Iuchi et al. reported that MET-PET could not only visualize the tumor extent but also predict the required irradiation dose to control tumors.18 Moreover, Yoo et al. reported that usefulness of MET-PET as a prognostic factor for progression-free survival.19 However, the number of institutes where MET-PET is available is limited.
2. Aim
It would be very useful if the optimal margin to add the target delineated on MRI to cover the uptake area on MET-PET is known. The purpose of this study was to investigate the recognition of the tumor extent and to define the optimal margin on MRI scans in the re-irradiation planning of recurrent GBM using MET-PET.
3. Materials and methods
3.1. Patients
A total of 25 patients participated in this study. All patients received an initial treatment consisting of surgery, adjuvant chemoradiotherapy, and adjuvant chemotherapy (temozolomide). The patient characteristics are shown in Table 1. This study was conducted with the approval of our institutional review board. Written informed consent was obtained from each patient before radiotherapy.
Table 1.
Patient characteristics.
| N (%) | ||
|---|---|---|
| Age (years) | ≥50 | 14 (56) |
| <50 | 11 (44) | |
| Sex | Male | 18 (72) |
| Female | 7 (28) | |
| KPS | ≥70 | 20 (80) |
| <70 | 5 (20) |
KPS: Karnofsky performance status.
3.2. Images
Computed tomography (CT), MRI and MET-PET were separately performed within a 2-week period. CT was performed using helical equipment (Light Speed; General Electric, Waukesha, WI). The head was immobilized in a commercially available stereotactic mask while acquiring CT images. The scan was performed with 2.5-mm slice-thickness, scanned without gaps.
MRI for radiation treatment planning was performed using a 1.5-T scanner (Genesis Signa; General Electric, Waukesha, WI). Images were acquired using a standard head coil without rigid immobilization. Axial, three-dimensional gradient echo T1-weighted native and after contrast administration (Gd-diethylenetriaminepentaacetic acid [Gd-DTPA; Magnevist, Schering, Berlin, Germany], 0.1 mmol/kg body weight) at 2.0-mm slice thickness were acquired from the foremen magnum to the vertex. The T2-weighted (2600/102 [effective]) images were acquired with a 512 × 224 matrix and a 24-cm field of view with a 6-mm slice thickness. We used contrast-enhanced T1-weighted images (Gd-MRI) and T2-weighted images (T2-MRI) for target delineation.
Patients fasted for at least 4 h before MET-PET to ensure standardized metabolic conditions. They were advised to have only a light breakfast on the morning of the examination day. The PET scanner used was an Advance NXi imaging system (General Electric Yokogawa Medical System, Hino-shi, Tokyo, Japan), which provides 35 axial images at 4.25 intervals. The crystal width is 4.0 mm (transaxial). The in-plane spatial resolution (full width at half-maximum) was 4.8 mm, and the standard 2-dimensional scan mode was used. Before the emission scans were performed, a 3-min transmission scan was performed to correct the photon attenuation, using a ring source containing 68 Ge. A dose of 7.0 MBq/kg 11C methionine was injected intravenously, depending on the examination. Emission scans were acquired for 30 min, beginning 5 min after injection of 11C methionine. During MET-PET data acquisition, the patient's head position was continuously monitored using laser beams projected onto ink markers drawn over the forehead skin, and the head position was corrected as necessary.
3.3. Target delineation
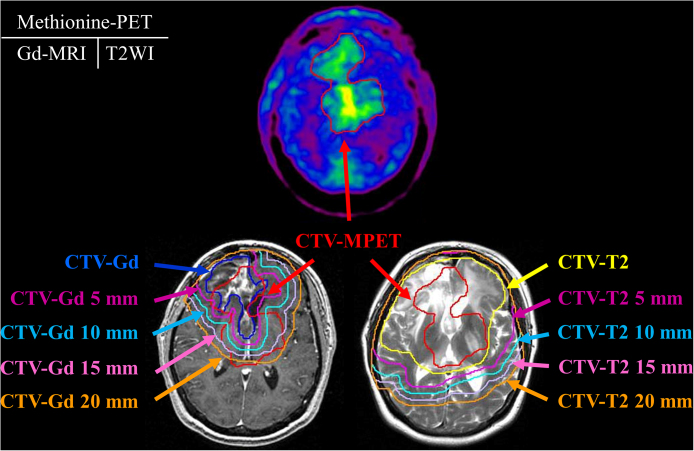
These images sets (CT/MRI and CT/MET-PET) were then fused utilizing the Pinnacle system. The MET-PET and MRI scans were analyzed separately in each patient. The target volumes were defined by three observers, including a neurosurgeon (JS), radiation oncologist (MM) and nuclear medicine specialist (HN). The Gd-MRI-based clinical target volume (CTV) (CTV-Gd) and the T2-MRI-based CTV (CTV-T2) were defined as the contrast-enhanced area on the Gd-MRI and high intensity area on the T2-MRI, respectively. We defined CTV x mm (x = 5, 10, 15, 20) as x mm outside the CTV. MET-PET-based CTV (CTV-MPET) was defined as the area of accumulation of MET-PET that was apparently higher than that of normal tissue on MET-PET. A threshold value of 1.3 of the CTV-MPET for the tumor/normal tissue index was considered indicative of malignant activity. The final determination of tumor delineation was obtained by consensus among three observers. The same window parameters were used for all patients included in this study. We calculated the sensitivity and specificity of CTV-Gd and CTV-T2 following comparison with CTV-MPET, which served as the gold standard in this study. The calculations of sensitivity and specificity are shown in Fig. 1.
Fig. 1.
The calculation of sensitivity and specificity.
4. Results
The typical CTVs are shown in Fig. 2. The average CTV-Gd, CTV-T2, and CTV-MPET volumes were 56, 222, and 59 mL, respectively (Table 2). There was no significant correlation between each type of CTV and its size. The sensitivities and the specificities of CTV-Gd and CTV-T2 are shown in Table 3. The sensitivity of CTV-Gd 5 mm (86%) was significantly higher than that of CTV-Gd (57%), and there was no significant difference in sensitivity between CTV-Gd 5 mm and CTV-Gd 10, 15, or 20 mm. The sensitivity of CTV-T2 5 mm (98%) was significantly higher than CTV-T2 (87%), and there was no significant difference in sensitivity between CTV-T2 5 mm and CTV T2 10, 15, or 20 mm. The sensitivity of CTV-Gd 20 mm (97%) was lower than that of CTV-T2 5 mm (98%). The specificity of CTV-Gd and CTV-T2 significantly decreased in accordance with increases in the margin outside the CTV-Gd and CTV-T2.
Fig. 2.
The typical contouring of the target volume on MET-PET, Gd-MRI, and T2-MRI.
Table 2.
Average and median CTV on MET-PET, Gd-MRI, and T2-MRI (mL).
| Average | Median | Standard deviation | |
|---|---|---|---|
| MET-PET | 59 | 29 | 64 |
| Gd-MRI | 56 | 38 | 58 |
| T2-MRI | 222 | 147 | 174 |
CTV: clinical target volume, MET-PET: 11C methionine positron emission tomography, Gd-MRI: contrast-enhanced T1-weighted image on MRI, T2-MRI: T2-weighted image on MRI.
Table 3.
Sensitivity and specificity of CTV-Gd and CTV-T2 (mean ± SD (%)).
| Sensitivity | Specificity | |
|---|---|---|
| CTV-Gd | 57 ± 25 | 98 ± 1 |
| CTV-Gd 5 mm | 86 ± 21 | 95 ± 3 |
| CTV-Gd 10 mm | 93 ± 15 | 90 ± 6 |
| CTV-Gd 15 mm | 96 ± 9 | 84 ± 9 |
| CTV-Gd 20 mm | 97 ± 5 | 78 ± 11 |
| CTV-T2 | 87 ± 15 | 89 ± 8 |
| CTV-T2 5 mm | 98 ± 6 | 80 ± 12 |
| CTV-T2 10 mm | 99 ± 3 | 72 ± 18 |
| CTV-T2 15 mm | 99 ± 1 | 67 ± 16 |
| CTV-T2 20 mm | 99 ± 0 | 61 ± 17 |
CTV-Gd x mm: x mm outside the clinical target volume based on contrast-enhanced magnetic resonance imaging, CTV-T2 x mm: x mm outside the clinical target volume based on T2-weighted image.
5. Discussion
Even if patients with GBM receive multidisciplinary treatment, the recurrence rate is high.2, 3 Approximately 80% of recurrence occurs within a 2–3-cm margin of the primary site.5, 6 The recurrence site is usually already irradiated. Therefore, the target of re-irradiation therapy is often limited to the Gd-enhanced lesion. However, GBM has an infiltrative character and can exist beyond the Gd-enhanced lesion.10, 11, 12 Several articles have reported that MET-PET has a greater accuracy than MRI in correctly outlining the true extent of gliomas.13, 14, 15, 16 MET is a natural amino acid avidly taken up by glioma cells, whereas its uptake by normal brain tissue is low. Although MET is incorporated into proteins, its uptake is probably not a measure of protein synthesis but mainly the activation of the L-mediated and A-mediated amino acid transport at the level of the blood–brain barrier.20 MET-PET demonstrates a higher amino acid transport in tumor cells compared with the normal tissue; therefore, they are very similar when used for tumor delineation.21 Autoradiographic findings reported by Kubota et al. demonstrated that the level of increased MET uptake correlated with the number of tumor cells, whereas no significant MET uptake occurs in the chronic inflammation of radiation necrosis.22 Grosu analyzed the extent of the residual tumor in patients with GBM who had undergone surgery.23 MET uptake was located outside the Gd enhancement in 74% patients. MET uptake was detected up to 45 mm beyond the Gd enhancement. MET uptake was located outside the high intensity area on T2-MRI in 50%. Voges et al. reported that 70% cases had an uptake on MET-PET that was observed outside the target delineated on MRI.24 Schinkelshoek et al. also reported that 65% cases were needed to modify the target delineated on MRI because the extent of uptake on MET-PET was outside the delineation on MRI.25 Kawai et al. reported that the uptake on MET-PET was usually larger than the area on the Gd enhancement and about 20–30% of the uptake on MET-PET extended outside the area of the enhancement.26 This fact indicated that the CTV-Gd or CTV-T2 is not sufficient as an irradiation target. When the irradiation is targeted to these CTVs, some part of the tumor would not be irradiated in most cases. The study in27 focused on patients with GBM receiving initial therapy; it is necessary to add at least a 2-cm margin for the high-intensity area on T2-weighted images. In this study, the sensitivity of the CTV-Gd was only 57%. The sensitivity and specificity of CTV-T2 were also not sufficient (87% and 89%, respectively). However, with an added 5-mm margin around CTV-T2 (CTV-T2 5 mm), there is sufficient sensitivity (98%). Sensitivity of CTV-T2 5 mm was significantly higher than CTV-T2, and there was no significant difference in sensitivity between CTV-T2 5 mm and CTV-T2 10, 15, or 20 mm, respectively. The sensitivity and specificity of CTV-Gd 20 mm (97% and 78%, respectively) were lower than those of CTV-T2 5 mm were. Therefore, CTV-T2 5 mm would be a suitable CTV for recurrent GBM in terms of tumor control. However, the specificity of CTV-T2 5 mm was not adequate (80%). Irradiation of CTV-T2 5 mm has a risk of increasing the rate of adverse events. A prospective trial is needed to investigate whether the rate of adverse events is increased by irradiation of CTV-T2 5 mm. In this situation, stereotactic radiotherapy should be used to decrease the toxicity.
6. Conclusions
In conclusion, CTV-Gd and CTV-T2 differed considerably from CTV-MPET in patients with recurrent GBM. It would be necessary for at least a 5 mm margin to be added to the high intensity area on T2-MRI in the target volume delineation of recurrent GBM to include MET-PET findings in re-irradiation therapy planning.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Stupp R., Hegi M.E., Mason W.P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R., Mason W.P., van den Bent M.J. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Curran W.J., Jr., Scott C.B., Horton J. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;58:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 4.Nieder C., Grosu A.L., Mehta M.P., Andratschke N., Molls M. Treatment of malignant gliomas: radiotherapy, chemotherapy and integration of new targeted agents. Exp Rev Neurother. 2004;4:691–703. doi: 10.1586/14737175.4.4.691. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar L.E., Fisher B.J., Macdonald D.R. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24:55–57. doi: 10.1016/0360-3016(92)91021-e. [DOI] [PubMed] [Google Scholar]
- 6.Sneed P.K., Gutin P.H., Larson D.A. Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys. 1994;29:719–727. doi: 10.1016/0360-3016(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd S.F., Laing R.W., Cosgrove V.P. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Phys. 1997;37:393–398. doi: 10.1016/s0360-3016(96)00455-5. [DOI] [PubMed] [Google Scholar]
- 8.Hudes R.S., Corn B.W., Werner-Wasik M. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 1999;43:293–298. doi: 10.1016/s0360-3016(98)00416-7. [DOI] [PubMed] [Google Scholar]
- 9.Fogh S.E., Andrews D.W., Glass J. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly P.J., Daumas-Duport C., Kispert D.B., Kall B.A., Scheithauer B.W., Illig J.J. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66:865–874. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- 11.Kelly P.J., Daumas-Duport C., Scheithauer B.W., Kall B.A., Kispert D.B. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62:450–459. doi: 10.1016/s0025-6196(12)65470-6. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain M.C., Murovic J.A., Levin V.A. Absence of contrast enhancement on CT brain scans of patients with supratentorial malignant gliomas. Neurology. 1988;38:1371–1374. doi: 10.1212/wnl.38.9.1371. [DOI] [PubMed] [Google Scholar]
- 13.Mosskin M., Ericson K., Hindmarsh T. Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference. Acta Radiol. 1989;30:225–232. [PubMed] [Google Scholar]
- 14.Ogawa T., Shishido F., Kanno I. Cerebral glioma: evaluation with methionine PET. Radiology. 1993;186:45–53. doi: 10.1148/radiology.186.1.8380108. [DOI] [PubMed] [Google Scholar]
- 15.Braun V., Dempf S., Weller R., Reske S.N., Schachenmayr W., Richter H.P. Cranial neuronavigation with direct integration of (11)C methionine positron emission tomography (PET) data – results of a pilot study in 32 surgical cases. Acta Neurochir. 2002;144:777–782. doi: 10.1007/s00701-002-0942-5. [DOI] [PubMed] [Google Scholar]
- 16.Bergström M.J., Collins V.P., Ehrin E. Discrepancies in brain tumor extent as shown by computed tomography and positron emission tomography using [68Ga]EDTA, [11C]glucose, and [11C]methionine. Comput Assist Tomogr. 1983;7:1062–1066. doi: 10.1097/00004728-198312000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Grosu A.L., Weber W.A., Franz M. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:511–519. doi: 10.1016/j.ijrobp.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 18.Iuchi T., Hatano K., Uchino Y. Methionine uptake and required radiation dose to control glioblastoma. Int J Radiat Oncol Biol Phys. 2015;93:133–140. doi: 10.1016/j.ijrobp.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Yoo M.Y., Paeng J.C., Cheon G.J. Prognostic value of metabolic tumor volume on (11)C-methionine PET in predicting progression-free survival in high-grade glioma. Nucl Med Mol Imaging. 2015;49:291–297. doi: 10.1007/s13139-015-0362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heiss P., Mayer S., Herz M., Wester H.J., Schwaiger M., Senekowitsch-Scmidtke R. Investigation of transport mechanism and uptake kinetics of O-(2-[18F]fluoroethyl)-l-tyrosine in vitro and in vivo. J Nucl Med. 1999;40:1367–1373. [PubMed] [Google Scholar]
- 21.Langen K.J., Ziemons K., Kiwit J.C. 3-[123I]iodo-alpha-methyltyrosine and [methyl-11C]-L-methionine uptake in cerebral gliomas: a comparative study using SPECT and PET. J Nucl Med. 1997;38:517–522. [PubMed] [Google Scholar]
- 22.Kubota R., Kubota K., Yamada S. Methionine uptake by tumor tissue: a microautoradiographic comparison with FDG. J Nucl Med. 1995;36:484–492. [PubMed] [Google Scholar]
- 23.Grosu A.L., Weber W.A., Riedel E. l-(Methyl-11C) methionine positron emission tomography for target delineation in resected high-grade gliomas before radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:64–74. doi: 10.1016/j.ijrobp.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 24.Voges J., Herholz K., Hölzer T. 11C-methionine and 18F-2-fuluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Steraotact Funct Neurosurg. 1997;69:129–135. doi: 10.1159/000099864. [DOI] [PubMed] [Google Scholar]
- 25.Schinkelshoek M., Lopci E., Clerici E. Impact of 11C-methionine positron emission tomography/computed tomography on radiation therapy planning and prognosis in patients with primary brain tumors. Tumori. 2014;100:636–644. doi: 10.1700/1778.19268. [DOI] [PubMed] [Google Scholar]
- 26.Kawai N., Maeda Y., Kudomi N. Correlation of biological aggressiveness assessed by 11C-methionine PET and hypoxic burden assessed by 18F-fluoromisonidazole PET in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. 2011;38:441–450. doi: 10.1007/s00259-010-1645-4. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo M., Miwa K., Tanaka O. Impact of [11C]methionine positron emission tomography for target delineation of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2012;82:83–89. doi: 10.1016/j.ijrobp.2010.09.020. [DOI] [PubMed] [Google Scholar]