Abstract
Head and neck squamous cell carcinomas (HNSCC) are in a group of cancers that are the most resistant to treatment. The survival rate of HNSCC patients has been still very low since last 20 years. The existence of relationship between oncogenic and surrounding cells is probably the reason for a poor response to treatment. Fibroblasts are an important element of tumor stroma which increases tumor cells ability to proliferate. Another highly resistance, tumorigenic and metastatic cell population in tumor microenvironment are cancer initiating cells (CICs). The population of cancer initiating cells can be found regardless of differentiation status of cancer and they seem to be crucial for HNSCC development.
In this review, we describe the current state of knowledge about HNSCC biological and physiological tumor microenvironment.
Keywords: Tumor microenvironment, Tumor initiating cells, Cancer stem cells, Metastasis, Resistance to therapy, HNSCC
1. Head and neck cancers
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy worldwide and represents 5% and up to 50% of all cancers in developed and developing countries, respectively.1, 2 According to the Polish National Cancer Registry (www.onkologia.org.pl), cancers of the oral cavity and lip account for approximately 4% in men and 1% in women of all cancers cases in Poland. The development of HNSCC is mainly caused by the carcinogens present in tobacco and alcohol as well as oncogenic viruses (HPV and EBV) and a wrong diet.3, 4, 5
The treatment method depends on the patient's status, clinical stage of the tumor, its localization and histological differentiation. Basic methods of HNSCC treatment are surgery, radiation and chemotherapy, used alone or in combination and recently also with targeted therapy agents. In the case of treatment involving conventional methods, the prognosis of HNSCC patients in advanced stages is largely unsatisfactory due to loco-regional recurrence.6, 7, 8, 9 The use of specific biomarkers, such as mRNA, lncRNA, miRNA or circulating RNA, is proposed as a good prognostic or predictive tool with clinical significance.10, 11, 12, 13, 14, 15, 16, 17 However, molecular characterization of patients’ tumor is only used to a limited extent in standard diagnostic procedures.
2. Tumor microenvironment
Tumor microenvironment (TME) differs essentially from the environment of normal tissues. The TME is a complex of cellular components such as cancer-associated fibroblasts (CAFs), myofibroblasts, adipocytes, endothelial cells, epithelial cells, immune inflammatory cells as well as extracellular components which surround tumor cells.18, 19 TME does not only surround the tumor cells, it also actively contributes to tumor development and progression, drug resistance and metastasis.20, 21 As far as tumor progress is concerned, the microenvironment of cancer cells is activated through constantly existing paracrine communication and promotes further changes in the microenvironment and expansion of tumor.19, 20, 21, 22, 23 Recent studies suggest that tumor microenvironment plays a crucial role not only in the progression but also in malignant transformation.20 The exact role of selected TME components and their interactions with cancer cells are described below.
Cancer-associated fibroblasts (CAFs) are heterogeneous population of irreversibly activated fibroblasts. They play various roles and are an important element of tumor stroma which increases tumor ability to proliferate, phenotype changes, resistance to therapy as well as metastasis.23, 24, 25 CAF secrets many factors (such as cytokines, chemokines, growth factors and other proteins) influencing both cancer cells and TME elements.24 Many cancers have altered cellular receptors such as members of fibroblast growth factor receptors (FGFR) family: FGFR1/2/3/4. They are transmembrane kinase receptors (RTKs) involved in basic cell cycle processes, such as differentiation, proliferation, cell survival, adult-tissue homeostasis and tumorigenesis26 as well as the formation of new blood vessels, wound repair and embryonic development.27 FGFR alterations can be divided into three main classes: gene amplification, gain-of-function coding mutation and gene fusion, with high impact in three most common malignancies worldwide.26 Aberrations in FGFR pathways play a critical role in cancer resistance to therapy. Chae et al. reported that FGFR1 amplification occurs in 10–17% of HNSCC patients.28 Additionally, Vairaktaris et al. demonstrated that a higher expression of FGFR1/2/3 contributes to early-stage cancer progression in HNSCC.29 FGFR2/3 high expression was found in the majority of HNSCC cell lines.28 Another interesting finding is that the reduction of FGFR3 level in HNSCC cell lines caused a 35% decrease of cell proliferation; furthermore – it caused their higher radiation sensitivity.30
Fibroblasts activation by tumor microenvironment, creating CAF phenotypes, depends on exogenous signals specific for a cancer type.31 However, mechanisms for their activation in HNSCC microenvironment remain ambiguous.32 Kellermann et al. revealed that oral fibroblasts transform to CAFs after co-culturing with oral squamous cell carcinoma (OSCC) cell lines. They demonstrated that cancer cell-derived TGF-β induced trans-differentiation of human gingival fibroblasts into CAF-line cells.33 Another group reported that TGF-β and IL-1 β might play a crucial role in CAF induction.34
In HNSCC, CAFs activation launches mechanisms involved in downregulation of tumor suppressor genes, such as p21 and CAV-1.32 They may also represent a resistant stromal cell type engaged in tumor relapse. CAFs are involved in remodeling and reprogramming the extracellular matrix (ECM) and tumor microenvironment. In primary tumor it may enhance cancer cell invasion leading to metastasis. Metabolic reprogramming of CAFs can contribute to enhancing cancer cells adaptation influencing tumor progression. They also play a crucial role in the secondary tumor growth because CAFs may enhance metastasis by releasing growth factors and cytokines into the circulation. That stimulates the growth and invasiveness of cancer cells at a distant site. Moreover, CAFs have also direct or indirect pleiotropic immunomodulatory functions. However, the most crucial role of CAFs is their impact on cancer therapy response caused mostly by modulations in CAF-ECM pathways interactions.31
Myofibroblasts are one of the carcinoma-associated fibroblasts which help to preserve stemness of cancer initiating cells (CICs). Myofibroblasts secrete cytokines, growth factors, chemokines, hormones, inflammatory mediators, adhesion and ECM proteins. This kind of fibroblasts is found in the stroma of carcinomas, particularly in the invasive front of a tumor. Their activity causes a rise of cancer cells proliferation, migration and invasion. The presence of myofibroblasts in cancer is correlated with lymph node involvement, disease recurrence, advance clinical staging and lower survival rates of HNSCC patients.35, 36 The analysis of all CAFs functions may give new insight into TME biology and offer a novel approach in cancer therapy.37
Mesenchymal stem cells (MSCs), another element of TME, are plastic, multipotential adherent cells similar to fibroblasts, but with a unique phenotype.38 They are characterized by strong tumor tropism.39 Liotta et al. showed that the number of MSCs (CD90+ cells) is correlated positively with tumor size but negatively with tumor-infiltrating leukocytes (CD45+ cells). Tumor-MSCs are able to inhibit proliferation and cytokine production of activated CD4+ and CD8+ T cells via IDO1 enzyme activity, causing impairment of anti-tumor immune response. Moreover, it was showed that MSCs can attract T-cells by CXCL10 chemokine.40 MSCs produce pro- and anti-inflammatory cytokines, chemotaxis, angiogenesis and growth factors. In vitro co-culture study indicated that MSCs influence HNSCC cells morphology and proliferation probably via MSCs-secreted IL-6 and activation and phosphorylation of ERK1/2 expression in tumor cells.39
Other components of TME are endothelial cells which create perivascular niches in HNSCC. Krishnamurthy et al. indicated that most of CICs (80%) are located close to blood vessels in tumor mass. Endothelial cells secrete the factors which promote CICs-self renewal, proliferation and survival. It was also observed that the ablation of endothelial cells leads to the reduction of CICs.41 The endothelial cells produce IL-6, CXCL8 and EGF which stimulate tumor cells. The reduction of secreted interleukins and epidermal growth factor causes the reduction of phosphorylation of STAT3, Akt and ERK in tumor cells and inhibits cell migration and anoikis.42 Probably, the HNSCC cells stimulate endothelial cells by VEGF and induce Bcl-2 signaling pathway, which, in turn, activates endothelial cells to secrete IL-6, CXCL8 and EGF.42, 43, 44
Tumor-associated macrophages (TAMs) are also an element of TME. They are a major population of inflammatory cells infiltrating tumors. Macrophages switch their own phenotypes in response to specific microenvironmental elements of the tumor. The TME-mediated signals determine the state of macrophages – M1 or M2. The M1 macrophages reveal cytotoxic activity toward cancer cells and produce toxic mediators, e.g. reactive oxygen intermediates, nitric oxide and TNF-α.45 The M2 macrophages show a poor antigen-presenting expanse and act as anti-inflammatory factor by suppressing Th1 adaptive immunity.46 In HNSCC, the M2 macrophages are reported as TAMs and can lead to cancer progression.32
When we talk about TME we should not only mention its biological, but also physiological aspects, such as oxygen tension. The areas of low oxygen tension (hypoxia) are created due to tumor growth, vascular disturbances and metabolic changes in solid cancers.47, 48 The phenotypes of different subpopulations of cancer cells and stromal cells are controlled through epigenetic mechanisms, which are regulated by hypoxia.49, 50
It was shown that hypoxia causes an aggressive phenotype of cancer with high treatment resistance and poor clinical prognosis.51, 52, 53, 54, 55 The specific hypoxic molecular panels were defined and they are supposed to be useful in clinical applications in HNSCC patients.56, 57, 58, 59, 60 It should be noted that HPV infection does not seem to change the expression of hypoxia-related genes nor HNSCC cell lines response to irradiation in low oxygen conditions compared to control (normoxic) cells.61
The cellular response to chemo- and radiotherapy is tightly connected with the hypoxia-inducible factor (HIF), family of transcription factors, which regulates the expression of many genes associated with cancer cells adaptation and progression.62 For example, stabilization of HIF-1 alpha by hypoxia (or other factors) enhances epithelial-to-mesenchymal (EMT) process in HNSCC cell lines in vitro.63, 64 It was showed that under hypoxic conditions the action of many drugs is overcome by changes in cell cycle (slower rate of cycle or G1 arrest),65 up-regulation of drug reflux system66, 67, 68 or changes in apoptosis.69, 70 Wiechec et al. reported that hypoxia in some cases could increase cell sensitivity to anti-EGFR (cetuximab) exposure in vitro. Moreover, knock-down of HIF-1 alpha reverses this sensitivity.71 It is supported by observation that the interaction between HIF-1 alpha and AKT signaling pathway depends on tumor type and its histological characterization. Under hypoxia condition, up-regulation of pAKT is induced by HIF-1 alpha only in some HNSCC lines. Similarly, only in some cases, inhibition of AKT leads to the reduction of HIF-1 alpha. Thus, the use of AKT inhibitors is not successful for all hypoxic HNSCC and it should be verified which patients could benefit from AKT inhibitor treatment.72 However, Lu et al. indicated that cetuximab down-regulates the LDH-A (lactate dehydrogenase A) and inhibits glycolysis in HNSCC cell lines through down-regulation of HIF-1 alpha. The cetuximab-induced resistant cells express high level of HIF-1 alpha and were highly glycolytic. The inhibition of LDH-A can reverse the resistance to cetuximab.73
Many reports indicated the role of hypoxia in the regulation of radioresistance. Sasabe et al. noticed that over-expression of HIF-1 alpha causes inhibition of reactive oxygen species (ROS) in OSCC cell lines, which are important in response to irradiation.69 Wozny et al. showed differences in cell survival after photon and carbon ion irradiations on different cell phenotypes in both normoxic and hypoxic conditions in vitro. The better in vitro therapeutic effect was connected with carbon ion irradiation method and cell phenotype marked as CICs. It seems, that HIF-1 alpha plays an important role in HNSCC resistance through its influence on ROS production. Probably, the use of HIF-1 alpha-knock-down adjuvant therapy with carbon ion irradiation method could effectively overcome cancer radioresistance and recurrence.74 As mentioned above, HIF-1 alpha influences the EMT process, which, in turn, causes cell resistance to irradiation by expression of high levels of free radical-scavenging proteins or by suppressing p53-mediated apoptosis.75, 76 One of the possible methods to overcome hypoxic resistance of HNSCC is targeting STAT3 pathway by molecular inhibitors such as Stattic.77 Moreover, the strong evidence of beneficial use of hypoxia modifiers in breaking radioresistance was shown by Overgaard in meta-analysis of 4805 HNSCC patients from 32 randomized clinical trials.48
To summarize, main cancer hallmarks, such as cell proliferation, apoptosis, immune response, metabolism, vascularization, genomic instability, invasion and metastasis are affected by cellular and no-cellular factors within tumor consisting of TME and should be taken into consideration under treatment strategies.
3. Cancer initiating cells (CICs)
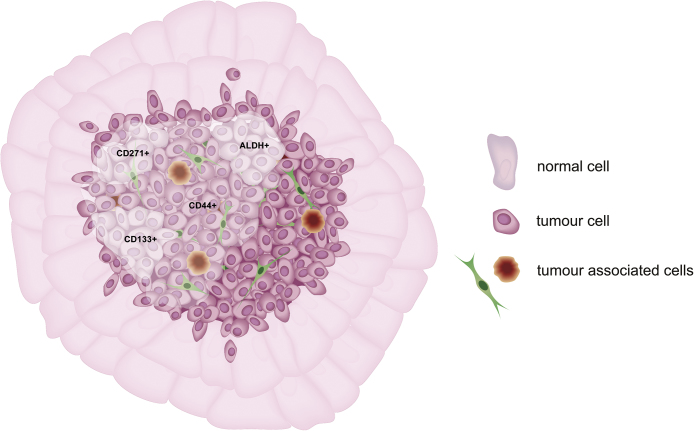
A solid tumor contains not only one type of cancer cells, but various cancer cells with different phenotypes and many different types of surrounding cells (Fig. 1). There are cancer initiating cells (CICs) among them , with unique genetic and behavior characteristics. The CICs are found in specific locations within tumor mass named “niches” which are, probably, the best microenvironment for them. In HNSCC, CICs reside close to blood vessels, where endothelial cell-secreted factors protect CICs against anoikis. Secreted VEGF enhances CICs’ proliferation and survival via phosphorylation of AKT in PI3K-AKT signaling pathway.78
Fig. 1.
The HNSCC contains small population of pluripotent cancer cells named cancer initiating cells (or cancer stem cells). Cancer initiating cells are found among other cells populations characterized by expression of markers such as CD44, CD133, CD27, ALDH (marked on the diagram by clouds).
The specific population of CICs can be found regardless of the differentiation status of cancer, even in the absence of visible distinction of tissue architecture.79 They are internal cell population characterized by expression of cell markers80, 81 (Table 1 and Fig. 1). It should be noted that the commonly used markers are not specific and can generate inconsistent results, denying CICs theory.82, 83 However, these results do not refute the theory as a whole but show the lack of specific detection methods of these cells.
Table 1.
Characteristics of common markers used to describe the cells population with high probability of cancer initiating cells presence in HNSCC.
| Marker | Population characteristic | Ref. |
|---|---|---|
| CD271 | • Only CD271+/CD44+ population contains mostly tumorigenic cells • Loss of CD271 reduces cell proliferation via inhibition of G2-M transition and inhibition of Erk1/2 phosphorylation |
103 |
| CD44 | • CD44+ cells are more tumorigenic than CD44− • CD44+ cells have primary cellular morphology and express Cytokeratin 5/14 (basal cell marker) • CD44− cells are differentiated squamous epithelial cells and express Involucrin (differentiation cell marker) • CD44+ cells express higher levels of BMI-1 protein than CD44− • There are several CD44 isoforms and only some of them can be used as CICs and radioresistance markers |
79, 83 |
| CD133 | • CD133+ cells are more invasive, with higher migration and clone-formation ability than CD133− cells; they possess differentiation capacity • CD133+ cells exhibit higher expression of anti-apoptosis genes, higher Bcl-2/Bax ratio and show dysregulated Hedgehog, Wnt signaling pathways and higher Bmi-1 expression • BMI-l seems to be central master of CIC-phenotype in the CD133+ cells through the regulation of p16(INK4A) and p14(ARF) • CD133+ expression is positively associated with more advanced TNM stage, pathological grade and lymph node metastasis • CD133+ patients have shorter overall survival and disease-free survival • CD133 expression negatively correlates with KAI1/CD82 protein and both could be used as independent prognostic factors • CD133+ cells have higher proliferation ratio than CD133− and possess higher expression of Glut-1 important in glucose transport • CD133 gene is over-expressed in SP cells (CD133+ SP) while down-regulation of CD133 reduces number of SP and increases chemosensitivity |
104, 105, 106, 107, 108, 109, 110 |
| CD24 | • CD24+ cells co-express OCT3/4 and CIP2A proteins • CD24+/CD44+ population is more invasive, more chemoresistant and creates larger tumor compared to CD24-/CD44+ population |
97, 111 |
| ALDH | • ALDH+ cells are more tumorigenic than ALDH−; most of ALDH+ cells are CD44+ • ALDH enzyme is probably required for cancer initiating cell activity • Expression of ALDH is observed in CD44+/CD24− cell population; CD44+/CD24-/ALDH+ subpopulation is more aggressive and resistant to therapy than other populations marked by these markers • CD44+/CD24-/ALDH+ subpopulation (as well as only ALDH+ cells) shows up-regulated stemness genes (OCT3/4, Nanog, SOX2, KLF4, BMI-1, Nestin) and the drug-resistant genes (MDR-1, MRP-1, ABCG2); CD44+/CD24-/ALDH1+ population has the most similar gene signature to mesenchymal stem cells (MSC) and MSC-related drift • EMT-related genes are activated in ALDH+ cells and Snail seems to be the most important regulator of CICs phenotype |
112, 113 |
| CD166 | • CD166+ cells have high capacity of sphere and tumor formation • Patients with high CD166 expression levels have poorer clinical outcome • CD166 expression is associated with tumor recurrence • CD166 enhanced phosphorylation of EGFR and (EGF)/EGFR pathway activation • Stimulation of CD166− cells by EGF causes increased cell tumorigenic ability in vitro and in vivo |
114, 115 |
CICs seem to be crucial for HNSCC development. It was observed that ALDH+ and CD133+ cells of oral leukoplakias have 4.17-fold and 2.86-fold increased risk of cancer transformation as compared to negative ones, respectively.84 Moreover, Sun et al. indicated that the expression of CD133 can be used to predict risk of developing oral cancer from lichen planus.85 The retrospective studies also indicated that oral leukoplakias with up-regulated ABCG2 and BMI-1 are associated with tumor development (3.24 and 4.03-fold increased risk, respectively), thus they could be used as a diagnostic marker.86
Some of HNSCCs are closely connected with HPV or EBV infections. Virus infection is one of the most important clinical factor in HNSCC and has also connection with CICs. It is well known that virus-related HNSCCs are less aggressive and HPV infection is a good prognostic factor. It was shown that HPV+ HNSCC (patients’ samples as well as cell lines) have a lower rate of CICs compared to HPV negative−. Moreover, HPV− cells are more sensitive to radio-induced dedifferentiation than HPV+, which highlights a distinct mechanism of maintenance CICs population in cancers caused by virus infection.87 Cai et al. showed that EBV-miR-BART7-3p targets PTEN and modulates PI3K/AKT/GSK-3beta pathway as well as influences the EMT process by changes in Snail and beta-Catenin expression. This phenomenon affects cell migration ability and is one of the known factors of nasopharyngeal carcinoma (NPC) metastasis.88
The main feature of CICs is the ability to self-renewal from a single cell. Prince and colleagues showed that CD44+ cells isolated from tumors of HNSCC patients restored tumor from a small number of cells. However, CD44+ cells proliferate asymmetrically and differentiate into CD44−, which are the main component of tumor mass. CD44− cells never form a tumor alone. The CD44+ cells express Cytokeratin 5/14, a marker of normal squamous epithelial stem and progenitor cells. They also express BMI-1 protein that plays a role in self-renewal and tumorigenesis.79
The CICs have some traits of embryonic stem cells or induced pluripotent stem cells, such as expression of KLF4, SOX2, OCT3/4 and c-MYC genes. Their unique phenotype is supported by changes in many important signaling pathways, epigenetic changes or mutations.89, 90, 91, 92, 93 Biological and clinical significance of these genes has been checked in HNSCC patients as well as in cell lines. Persistent KLF4 over-expression is detected only in some HNSCC patients and is correlated with a worse disease-specific survival, especially in a subgroup of advanced cancer. The up-regulation of KLF4 in SAS cell line leads to higher cell migration, invasion and higher resistance to some chemotherapeutic drugs. Moreover, KLF4 induces tumorigenicity of HNSCC cells injected to mice.94 Expression of SOX2 is higher in HNSCC metastasized to lymph nodes than in non-metastasized cancers. The over-expression of SOX2 is associated with poor outcome, but correlation between SOX2 expression and tumor grading, T-classification, Ki-67 expression or only cell-specific expression is not observed. These findings are not consistent with CICs theory, but SOX2 is probably a commonly activated oncogene affecting early step of the tumorigenesis. SOX2 is mostly associated with HPV-negative tumors and its over-expression activates the anti-apoptotic Bcl-2 preventing apoptosis.95 However, a meta-analysis of SOX2 revealed that it is significantly connected with more differentiated, advanced and metastatic tumors and could be used as a prognostic factor.96
OCT3/4, another marker of CICs, positively regulates CIP2A expression. The co-expression of these two genes in HNSCC is linked with CD44+/CD24+ cell population, tumor poor differentiation, enhanced aggressiveness, radioresistance and reduced 5-year survival of patients.97 Habu et al. showed that OCT3/4 and two other genes Nanog and ABCG2 were up-regulated in SP (side population) cells. SP cells are a class of CICs detected by low accumulation of Hoechst 33342 dye and characterized by high migration and invasion ability. It is not surprising that the analysis of patients’ samples showed that OCT3/4 can be used as a predictor of metastasis and advanced cancers.98 Moreover, Nanog/OCT3/4/CD133 triple-positive oral carcinoma patients have a worse survival prognosis compared to the triple-negative group.99 Tsai et al. proposed that OCT3/4 and Nanog could be used as potential predictive markers to distinguish resistant patients from those sensitive to cisplatin therapy.100 However, this statement is not confirmed on a large group of patients.
Chang et al. observed that HNSCC cells can be divided into 3 groups depending on the level of reactive oxygen species (ROS): (i) ROSlow, (ii) ROShigh and (iii) ROSmedi cells. This classification characterizes cells into groups with different chemoresistance, stemness and proliferation activity. The ROSlow cells have features of CICs (CD133+, memGrp78, Glut3, ALDH+) and their state may be supported by ROS-scavenging enzymes such as SOD2, CAT and PRDX3. The specific antioxidant phenotype probably allows CICs to protect their own genome and maintain the state of stemness. The microenvironment induces persistent ROS redox stress on CICs and causes generation of non-CICs populations in tumor mass.101
CICs are associated with cells ability to close and distant metastases. Some distinct cell populations with CICs features can be found circulating in blood and lymphatic vessels. They are referred as circulating tumor cells. Weller and colleagues observed cells in blood samples from HNSCC patients and distinguished three groups of cells: (i) mesenchymal, (ii) epithelial and (iii) a group with features of both types of these cells. Moreover, they detected circulating CD133+ cells subpopulations (probably CICs). It was shown that the number of epithelial-, mesenchymal- and stem cell-like circulating cells was decreased after the removal of tumor compared to the number before the surgery. This indicates that tumor mass is the main source of these circulating cells. Patients had significantly shortened survival rate when mesenchymal-like circulating cells were still present after surgery.102
It should be noted that CICs formation is also affected by some different biological and physical factors. The influence of chemo-physical factors is clearly visible in 3D cell culture in vitro where changes in the culture method causes the maintenance and enrichment of CICs.116
4. New therapeutic strategies
The CICs are able to survive after irradiation and chemical exposure.100, 117 Two of the most commonly used chemotherapeutic drugs in HNSCC treatment are cisplatin and 5-FU but it was proved that these drugs promote self-renewal and survival of CICs both in vitro and in vivo. They cause over-expression of BMI-1 as well as OCT3/4, CD44 and ALDH markers.120, 121 It was shown that cisplatin-resistant oral squamous carcinoma cell line has more aggressive phenotype than resistant parental cell line and stemness markers (Nanog, OCT3/4, BMI-1, CD117, CD133) and drug resistant ABCG2 protein are over-expressed.100 It should be noted that cisplatin can not overcome IL-6 induced STAT pathway that is involved in the maintaining of stemness.120 Moreover, the CICs resistance to cisplatin may be regulated by ROS-scavenging enzymes. Treatment HNSCC ROSlow cells with ROS-scavenging inhibitors chemo-sensitizes a tumor.101 Yang et al. indicated that CD133+ cells are more resistant to chemotherapy, probably because of high expression of ABCG2.122 Another study indicated significant correlation of high CD44 expression and positive lymph node with incomplete response to radio-chemotherapy and both of them could be used as independent predictive markers.117
The future therapies will target cancer cells and components of TME in the specific manner as well as reduce the number of CICs directly or indirectly. The depletion of cancer cells can be achieved by specific drugs (in most cases inhibitors), classical drugs with enhancement agent or specific cancer cell-related receptor, and by gene therapy–RNA interference.118, 119 There are many ongoing studies which are testing some stimulant agents with potential for therapeutic usage (Table 2). These “drugs” act as inhibitors for pro-cancer signaling pathways. Moreover, they may prevent the EMT process but also enhance chemotherapy effectiveness leading to cancer cell death.
Table 2.
Agents indicated as potentially useful in therapy of HNSCC based on CICs depletion and chemotherapy/radiotherapy.
| Agent | Description | Ref. |
|---|---|---|
| Salinomycin (livestock antibiotic) | Inhibition of cell viability and induction of apoptosis (elevation of Bax/Bcl-2 ratio); Synergistically action with cisplatin and paclitaxel causing higher cell mortality; Reduction of CICs – reduction of sphere formation capacity and suppression of CD44 and BMI-1 expression; Induction of EMT, phosforylation of Akt and up-regulation of miR-328, miR-199a-3p and down-regulation of miR-203. |
123 |
| ABT-737 (BH3 mimetic small molecule inhibitor) | Enhancement of apoptosis and delay of tumor growth in vivo in combination with irradiation; Reduction of CICs(–SP+/CD44high/ALDHhigh cells remain in the sub-G1 phase); cells are more sensitive to irradiation; increase the expression of Bcl-2 family members (except of Bak and PUMA). |
124 |
| Valproic acid (histone deacetylase inhibitor) | Reduction of CICs (reduction of sphere formation capacity, CD44+ population cells and expression of OCT3/4 and SOX2); Inhibition of tumor growth in vitro; Enhancement of cisplatin action – increase of cell mortality by suppressing ABCC2 and ABCC6 transporters, activation of Bax and Caspase3 in CICs population. |
125 |
| Suberoylanilide hydroxamic acid (histone deacetylase inhibitor) | Inhibition of cell proliferation; Reduction of CICs – reduction of sphere formation capacity and Nanog expression; inhibition of tumor growth and metastasis ability in vitro; Enhancement of cisplatin action in cisplatin resistance HPV+ and HPV− cell lines; Lack of additional toxicity in vivo. |
126 |
| Rapamycin (Sirolimus, mTOR inhibitor) | Inhibition of mTOR signaling and reduction of CICs – down-regulation of CD44 and SOX2 (no influence on OCT3/4); Reduction of tumor volume and weight; Reduction of tumor invasion by down-regulation of MMP-2 (no influence on MMP-9). |
127 |
| Curcumin (Diferuloylmethane) with cisplatin | Enhancement of CD133+ cells sensitivity to cisplatin; Reduction of cell colony formation; Decreasing expression of ABCG2 in CD133+ population. |
128 |
| Mesoporous silica nanoparticles (MSNs) with chemotherapeutic drug and siRNA against ABCG2 | Combination of classical drug (5-FU, cisplatin or paxlitaxel) with siRNA against ABCG2 loaded into nanocarrier; Enhancement of drug activity by blocking ABCG2 and breaking multidrug resistance; Enhancement CD133+ cells apoptosis; Reduction of tumor volume and weight. |
129 |
| Gene therapy (RNA restoration or RNA interference) | Restoration of IL-24 in CD133+ cells causing reduction of cell proliferation; Reduction of Snail expression improving sensitivity of ALDH+ cells to chemoradiotherapy. |
113, 130 |
5. Conclusions
Many different components have influence on cancer progression, invasiveness and metastasis. That provides evidence of the heterogeneity of cell types among the tumors and strong interaction between cancer cells and their environment. Each element is important on the way to the TME-targeting therapy in cancer cases. Cancer initiating cells rely on tumor microenvironment niche. Many researchers are trying to find a better solution for HNSCC patients by investigating signaling pathways existing in TME and understand CICs biology. The scientists are convinced that TME supports invasion of the tumor, its progression and metastasis. They also believe that TME may offer a broad spectrum of novel anti-cancer therapies, not only in HNSCC but also in other cancer types.
Authors’ contributions
TK and WP have contributed equally to this work.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Financial disclosure
This work was supported by Greater Poland Cancer Center – grant no.: 4/2012 (46), grant no.: 3/2015 (95) and grant no.: 24/2016 (139).
Acknowledgment
We are extremely grateful to Greater Poland Cancer Center in Poznan for it support in writing this publication.
Contributor Information
Tomasz Kolenda, Email: kolenda.tomek@gmail.com.
Weronika Przybyła, Email: weronika.przybyla@gmail.com.
Marta Kapałczyńska, Email: marta.kapalczynska@gmail.com.
Anna Teresiak, Email: anna.teresiak@wco.pl.
Maria Zajączkowska, Email: mariazajaczkowska@gmail.com.
Renata Bliźniak, Email: renata.blizniak@wco.pl.
Katarzyna M. Lamperska, Email: kasialam@o2.pl.
References
- 1.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(March–April (2)):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Marur S., Forastiere A.A. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(March (3)):386–396. doi: 10.1016/j.mayocp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Liu X., Gao X.L., Liang X.H., Tang Y.L. The etiologic spectrum of head and neck squamous cell carcinoma in young patients. Oncotarget. 2016;7(October (40)):66226–66238. doi: 10.18632/oncotarget.11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar M., Nanavati R., Modi T.G., Dobariya C. Oral cancer: etiology and risk factors: a review. J Cancer Res Ther. 2016;12(April–June (2)):458–463. doi: 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- 5.Gondivkar S.M., Parikh R.V., Gadbail A.R. Involvement of viral factors with head and neck cancers. Oral Oncol. 2012;48(March (3)):195–199. doi: 10.1016/j.oraloncology.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Gugić J., Strojan P. Squamous cell carcinoma of the head and neck in the elderly. Rep Pract Oncol Radiother. 2012;18(August (1)):16–25. doi: 10.1016/j.rpor.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao D., Ritter M.A., Oliver T., Browman G.P. Platinum-based concurrent chemoradiotherapy for tumors of the head and neck and theesophagus. Semin Radiat Oncol. 2006;16(January (1)):10–19. doi: 10.1016/j.semradonc.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Scully C., Bagan J.V. Recent advances in Oral Oncology 2007: imaging, treatment and treatment outcomes. Oral Oncol. 2008;44(March (3)):211–215. doi: 10.1016/j.oraloncology.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Palka K.T., Slebos R.J., Chung C.H. Update on molecular diagnostic tests in head and neck cancer. Semin Oncol. 2008;35(June (3)):198–210. doi: 10.1053/j.seminoncol.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhi X., Lamperska K., Golusinski P. Gene expression analysis of head and neck squamous cell carcinoma survival and recurrence. Oncotarget. 2015;6(1):547–555. doi: 10.18632/oncotarget.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamperska K.M., Kozlowski P., Kolenda T. Unpredictable changes of selected miRNA in expression profile of HNSCC. Cancer Biomark. 2016;16(1):55–64. doi: 10.3233/CBM-150540. [DOI] [PubMed] [Google Scholar]
- 12.Victoria Martinez B., Dhahbi J.M., Nunez Lopez Y.O. Circulating small non coding RNA signature in head and neck squamous cell carcinoma. Oncotarget. 2015;6(22):19246–19263. doi: 10.18632/oncotarget.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolenda T., Przybyła W., Teresiak A., Mackiewicz A., Lamperska K.M. The mystery of let-7d – a small RNA with great power. Contemp Oncol (Pozn) 2014;18(5):293–301. doi: 10.5114/wo.2014.44467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolenda T., Teresiak A., Kapałczyńska M. Let-7d and miR-18a as biomarkers of head and neck cancers. Zeszyty Naukowe WCO. Lett Oncol Sci. 2015;12:37–47. [Google Scholar]
- 15.Lamperska K.M., Kolenda T., Teresiak A. Different levels of let-7d expression modulate response of FaDu cells to irradiation and chemotherapeutics. PLOS ONE. 2017;12(June (6)):e0180265. doi: 10.1371/journal.pone.0180265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolenda T., Guglas K., Ryś M. Biological role of long non-coding RNA in head and neck cancers. Rep Pract Oncol Radiother. 2017;22(September–October (5)):378–388. doi: 10.1016/j.rpor.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guglas K., Bogaczyńska M., Kolenda T. lncRNA in HNSCC: challenges and potential. Contemp Oncol (Pozn) 2017;21(4) doi: 10.5114/wo.2017.72382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiteside T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(October (45)):5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cretu A., Brooks P.C. Impact of the non-cellular tumor microenvironment on metastasis: potential therapeutic and imaging opportunities. J Cell Physiol. 2007;213(November (2)):391–402. doi: 10.1002/jcp.21222. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann S., Bhola N.E., Grandis J.R. HGF/Met signaling in head and neck cancer: impact on the tumor microenvironment. Clin Cancer Res. 2016;22(16):4005–4013. doi: 10.1158/1078-0432.CCR-16-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu F., Hu C., Tai Z. Tumour microenvironment-responsive lipoic acid nanoparticles for targeted delivery of docetaxel to lung cancer. Sci Rep. 2016;6:36281. doi: 10.1038/srep36281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedl P., Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 23.Wu T., Hong Y., Jia L. Modulation of IL-1β reprogrammes the tumor microenvironment to interrupt oral carcinogenesis. Sci Rep. 2016;6:20208. doi: 10.1038/srep20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiga K., Hara M., Nagasaki T., Sato T., Takahashi H., Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers (Basel) 2015;7(December (4)):2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweeny L., Liu Z., Lancaster W., Hart J., Hartman Y.E., Rosenthal E.L. Inhibition of fibroblasts reduced head and neck cancer growth by targeting fibroblast growth factor receptor. Laryngoscope. 2012;122(7):1539–1544. doi: 10.1002/lary.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katoh M. FGFR inhibitors: effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review) Int J Mol Med. 2016;38(1):3–15. doi: 10.3892/ijmm.2016.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Luca A., Frezzetti D., Gallo M., Normanno N. FGFR-targeted therapeutics for the treatment of breast cancer. Expert Opin Investig Drugs. 2017;26(March (3)):303–311. doi: 10.1080/13543784.2017.1287173. Review. [DOI] [PubMed] [Google Scholar]
- 28.Chae Y.K., Pai S.G., Sun P. Fibroblast growth factor receptor (FGFR) as a therapeutic target in lung and head and neck cancer. Am J Hematol/Oncol. 2016;12(3) [Google Scholar]
- 29.Vairaktaris E., Ragos V., Yapijakis C. FGFR-2 and -3 play an important role in initial stages of oral oncogenesis. Anticancer Res. 2006;26(November–December (6B)):4217–4221. [PubMed] [Google Scholar]
- 30.Henson B.J., Gollin S.M. Overexpression of KLF13 and FGFR3 in oral cancer cells. Cytogenet Genome Res. 2010;128(June (4)):192–198. doi: 10.1159/000308303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 32.Utsipan K., Koontongkaew S. Fibroblasts and macrophages: key players in the head and neck cancer microenvironment. J Oral Biosci. 2017;59(February (1)):23–30. [Google Scholar]
- 33.Kellermann M.G., Sobral L.M., da Silva S.D. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44(May (5)):509–517. doi: 10.1016/j.oraloncology.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Dudás J., Bitsche M., Schartinger V., Falkeis C., Sprinzl G.M., Riechelmann H. Fibroblasts produce brain derived neurotrophic factor and induce mesenchymal transition of oraltumor cells. Oral Oncol. 2011;47(February (2)):98–103. doi: 10.1016/j.oraloncology.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekhon H.K., Sircar K., Kaur G., Marwah M. Evaluation of role of myofibroblasts in oral cancer: a systematic review. Int J Clin Pediatr Dent. 2016;9(July–September (3)):233–239. doi: 10.5005/jp-journals-10005-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lúcio P.S., Cavalcanti A.L., Alves P.M., Godoy G.P., Nonaka C.F. Myofibroblasts and their relationship with oral squamous cell carcinoma. Braz J Otorhinolaryngol. 2013;79(1):112–118. doi: 10.5935/1808-8694.20130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leef G., Thomas S.M. Molecular communication between tumor-associated fibroblasts and head and neck squamous cell carcinoma. Oral Oncol. 2013;49(5):381–386. doi: 10.1016/j.oraloncology.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haniffa M.A., Collin M.P., Buckley C.D., Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009;94(2):258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scherzad A., Steber M., Gehrke T. Human mesenchymal stem cells enhance cancer cell proliferation via IL-6 secretion and activation of ERK1/2. Int J Oncol. 2015;47(1):391–397. doi: 10.3892/ijo.2015.3009. [DOI] [PubMed] [Google Scholar]
- 40.Liotta F., Querci V., Mannelli G. Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br J Cancer. 2015;112(4):745–754. doi: 10.1038/bjc.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnamurthy S., Dong Z., Vodopyanov D. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70(23):9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neiva K.G., Zhang Z., Miyazawa M., Warner K.A., Karl E., Nör J.E. Cross talk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT3/Akt/ERK signaling. Neoplasia. 2009;11(6):583–593. doi: 10.1593/neo.09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nör J.E., Christensen J., Mooney D.J., Polverini P.J. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154(2):375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nör J.E., Christensen J., Liu J. Up-regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61(5):2183–2188. [PubMed] [Google Scholar]
- 45.Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(November (11)):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 46.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(October (10)):889–896. doi: 10.1038/ni.1937. Epub 2010 Sep 20. [DOI] [PubMed] [Google Scholar]
- 47.Bertout J.A., Patel S.A., Simon M.C. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck – a systematic review and meta-analysis. Radiother Oncol. 2011;100(July (1)):22–32. doi: 10.1016/j.radonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Biddle A., Liang X., Gammon L. Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res. 2011;71(August (15)):5317–5326. doi: 10.1158/0008-5472.CAN-11-1059. [DOI] [PubMed] [Google Scholar]
- 50.Chiavarina B., Martinez-Outschoorn U.E., Whitaker-Menezes D. Metabolic reprogramming and two-compartment tumor metabolism: opposing role(s) of HIF1α and HIF2α in tumor-associated fibroblasts and human breast cancer cells. Cell Cycle. 2012;11(September (17)):3280–3289. doi: 10.4161/cc.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong H., De Marzo A.M., Laughner E. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(November (22)):5830–5835. [PubMed] [Google Scholar]
- 52.Chang C.C., Lin B.R., Chen S.T., Hsieh T.H., Li Y.J., Kuo M.Y. HDAC2 promotes cell migration/invasion abilities through HIF-1α stabilization in human oral squamous cell carcinoma. J Oral Pathol Med. 2011;40(August (7)):567–575. doi: 10.1111/j.1600-0714.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 53.Beasley N.J., Leek R., Alam M. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha in head and neck cancer: relationship to tumor biology and treatment outcome in surgically resected patients. Cancer Res. 2002;62(May (9)):2493–2497. [PubMed] [Google Scholar]
- 54.Swartz J.E., Pothen A.J., Stegeman I., Willems S.M., Grolman W. Clinical implications of hypoxia biomarker expression in head and neck squamous cell carcinoma: a systematic review. Cancer Med. 2015;4(July (7)):1101–1116. doi: 10.1002/cam4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wigerup C., Påhlman S., Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152–169. doi: 10.1016/j.pharmthera.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Tawk B., Schwager C., Deffaa O. Comparative analysis of transcriptomics based hypoxia signatures in head- and neck squamous cell carcinoma. Radiother Oncol. 2016;118(February (2)):350–358. doi: 10.1016/j.radonc.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 57.Toustrup K., Sørensen B.S., Nordsmark M. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71(September (17)):5923–5931. doi: 10.1158/0008-5472.CAN-11-1182. [DOI] [PubMed] [Google Scholar]
- 58.Toustrup K., Sørensen B.S., Lassen P., Wiuf C., Alsner J., Overgaard J. Danish Head and Neck Cancer Group (DAHANCA). Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol. 2012;102(January (1)):122–129. doi: 10.1016/j.radonc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 59.Eustace A., Mani N., Span P.N. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin Cancer Res. 2013;19(September (17)):4879–4888. doi: 10.1158/1078-0432.CCR-13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lendahl U., Lee K.L., Yang H., Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 2009;10(December (12)):821–832. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 61.Sørensen B.S., Busk M., Olthof N. Radiosensitivity and effect of hypoxia in HPV positive head and neck cancer cells. Radiother Oncol. 2013;108(September (3)):500–505. doi: 10.1016/j.radonc.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Gammon L., Mackenzie I.C. Roles of hypoxia, stem cells and epithelial-mesenchymal transition in the spread and treatment resistance of head and neck cancer. J Oral Pathol Med. 2016;45(February (2)):77–82. doi: 10.1111/jop.12327. [DOI] [PubMed] [Google Scholar]
- 63.Gammon L., Biddle A., Heywood H.K., Johannessen A.C., Mackenzie I.C. Sub-sets of cancer stem cells differ intrinsically in their patterns of oxygen metabolism. PLoS ONE. 2013;8(April (4)):e62493. doi: 10.1371/journal.pone.0062493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang M.H., Wu M.Z., Chiou S.H. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10(March (3)):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 65.Moore N., Lyle S. Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol. 2011:2011. doi: 10.1155/2011/396076. pii:396076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sasabe E., Zhou X., Li D., Oku N., Yamamoto T., Osaki T. The involvement of hypoxia-inducible factor-1alpha in the susceptibility to gamma-rays and chemotherapeutic drugs of oral squamous cell carcinoma cells. Int J Cancer. 2007;120(January (2)):268–277. doi: 10.1002/ijc.22294. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan R., Paré G.C., Frederiksen L.J., Semenza G.L., Graham C.H. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol Cancer Ther. 2008;7(July (7)):1961–1973. doi: 10.1158/1535-7163.MCT-08-0198. [DOI] [PubMed] [Google Scholar]
- 68.Wartenberg M., Ling F.C., Müschen M. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 2003;17(March (3)):503–505. doi: 10.1096/fj.02-0358fje. [DOI] [PubMed] [Google Scholar]
- 69.Sasabe E., Tatemoto Y., Li D., Yamamoto T., Osaki T. Mechanism of HIF-1alpha-dependent suppression of hypoxia-induced apoptosis in squamous cell carcinoma cells. Cancer Sci. 2005;96(July (7)):394–402. doi: 10.1111/j.1349-7006.2005.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q., Zhang Z.F., Rao J.Y. Treatment with siRNA and antisense oligonucleotides targeted to HIF-1alpha induced apoptosis in human tongue squamous cell carcinomas. Int J Cancer. 2004;111(October (6)):849–857. doi: 10.1002/ijc.20334. [DOI] [PubMed] [Google Scholar]
- 71.Wiechec E., Hansson K.T., Alexandersson L., Jönsson J.I., Roberg K. Hypoxia mediates differential response to anti-EGFR therapy in HNSCC cells. Int J Mol Sci. 2017;18(April (5)) doi: 10.3390/ijms18050943. pii:E943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stegeman H., Span P.N., Peeters W.J. Interaction between hypoxia, AKT and HIF-1 signaling in HNSCC and NSCLC: implications for future treatment strategies. Future Sci OA. 2016;2(January (1)):FSO84. doi: 10.4155/fso.15.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu H., Li X., Luo Z., Liu J., Fan Z. Cetuximab reverses the Warburg effect by inhibiting HIF-1-regulated LDH-A. Mol Cancer Ther. 2013;12(October (10)):2187–2199. doi: 10.1158/1535-7163.MCT-12-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wozny A.S., Lauret A., Battiston-Montagne P. Differential pattern of HIF-1α expression in HNSCC cancer stem cells after carbon ion or photon irradiation: one molecular explanation of the oxygen effect. Br J Cancer. 2017;116(May (10)):1340–1349. doi: 10.1038/bjc.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nantajit D., Lin D., Li J.J. The network of epithelial–mesenchymal transition: potential new targets for tumor resistance. J Cancer Res Clin Oncol. 2014 doi: 10.1007/s00432-014-1840-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen-Jonathan E., Bernhard E.J., McKenna W.G. How does radiation kill cells? Curr Opin Chem Biol. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 77.Adachi M., Cui C., Dodge C.T., Bhayani M.K., Lai S.Y. Targeting STAT3 inhibits growth and enhances radiosensitivity in head and neck squamous cell carcinoma. Oral Oncol. 2012;48(December (12)):1220–1226. doi: 10.1016/j.oraloncology.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campos M.S., Neiva K.G., Meyers K.A., Krishnamurthy S., Nör J.E. Endothelial derived factors inhibit anoikis of head and neck cancer stem cells. Oral Oncol. 2012;48(January (1)):26–32. doi: 10.1016/j.oraloncology.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prince M.E., Sivanandan R., Kaczorowski A. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel S.S., Shah K.A., Shah M.J., Kothari K.C., Rawal R.M. Cancer stem cells and stemness markers in oral squamous cell carcinomas. Asian Pac J Cancer Prev. 2014;15(20):8549–8556. doi: 10.7314/apjcp.2014.15.20.8549. [DOI] [PubMed] [Google Scholar]
- 81.Satpute P.S., Hazarey V., Ahmed R., Yadav L. Cancer stem cells in head and neck squamous cell carcinoma: a review. Asian Pac J Cancer Prev. 2013;14(10):5579–5587. doi: 10.7314/apjcp.2013.14.10.5579. [DOI] [PubMed] [Google Scholar]
- 82.Pries R., Witrkopf N., Trenkle T., Nitsch S.M., Wollenberg B. Potential stem cell marker CD44 is constitutively expressed in permanent cell lines of head and neck cancer. In Vivo. 2008;22(1):89–92. [PubMed] [Google Scholar]
- 83.Spiegelberg D., Kuku G., Selvaraju R., Nestor M. Characterization of CD44 variant expression in head and neck squamous cell carcinomas. Tumour Biol. 2014;35(3):2053–2062. doi: 10.1007/s13277-013-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu W., Wu L., Shen X.M. Expression patterns of cancer stem cell markers ALDH1 and CD133 correlate with a high risk of malignant transformation of oral leukoplakia. Int J Cancer. 2013;132(4):868–874. doi: 10.1002/ijc.27720. [DOI] [PubMed] [Google Scholar]
- 85.Sun L., Feng J., Ma L., Liu W., Zhou Z. CD133 expression in oral lichen planus correlated with the risk for progression to oral squamous cell carcinoma. Ann Diagn Pathol. 2013;17(6):486–489. doi: 10.1016/j.anndiagpath.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 86.Liu W., Feng J.Q., Shen X.M., Wang H.Y., Liu Y., Zhou Z.T. Two stem cell markers, ATP-binding cassette, G2 subfamily (ABCG2) and BMI-1, predict the transformation of oral leukoplakia to cancer: a long-term follow-up study. Cancer. 2012;118(6):1693–1700. doi: 10.1002/cncr.26483. [DOI] [PubMed] [Google Scholar]
- 87.Vlashi E., Chen A.M., Boyrie S. Radiation-induced dedifferentiation of head and neck cancer cells into cancer stem cells depends on human papillomavirus status. Int J Radiat Oncol Biol Phys. 2016;94(5):1198–1206. doi: 10.1016/j.ijrobp.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai L.M., Lyu X.M., Luo W.R. EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene. 2015;34(April (17)):2156–2166. doi: 10.1038/onc.2014.341. [DOI] [PubMed] [Google Scholar]
- 89.Kulcenty K., Wróblewska J., Mazurek S., Liszewska E., Jaworski J. Molecular mechanisms of induced pluripotency. Contemp Oncol (Pozn) 2015;19(1A):A22–A29. doi: 10.5114/wo.2014.47134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klimczak M. Oncogenesis and induced pluripotency – commonalities of signalling pathways. Contemp Oncol (Pozn) 2015;19(1A):A16–A21. doi: 10.5114/wo.2014.47133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Czerwinska P., Kaminska B. Regulation of breast cancer stem cell features. Contemp Oncol (Pozn) 2015;19(1A):A7–A15. doi: 10.5114/wo.2014.47126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Czerwińska P., Mazurek S., Wiznerowicz M. The complexity of TRIM28 contribution to cancer. J Biomed Sci. 2017;24(August (1)):63. doi: 10.1186/s12929-017-0374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Czerwińska P., Shah P.K., Tomczak K. TRIM28 multi-domain protein regulates cancer stem cell population in breast tumor development. Oncotarget. 2017;8(January (1)):863–882. doi: 10.18632/oncotarget.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tai S.K., Yang M.H., Chang S.Y. Persistent Krüppel-like factor 4 expression predicts progression and poor prognosis of head and neck squamous cell carcinoma. Cancer Sci. 2011;102(4):895–902. doi: 10.1111/j.1349-7006.2011.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schröck A., Bode M., Göke F.J. Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis. 2014;35(7):1636–1642. doi: 10.1093/carcin/bgu094. [DOI] [PubMed] [Google Scholar]
- 96.Dong Z., Liu G., Huang B., Sun J., Wu D. Prognostic significance of SOX2 in head and neck cancer: a meta-analysis. Int J Clin Exp Med. 2014;7(12):5010–5020. eCollection 2014. [PMC free article] [PubMed] [Google Scholar]
- 97.Ventelä S., Sittig E., Mannermaa L. CIP2A is an Oct4 target gene involved in head and neck squamous cell cancer oncogenicity and radioresistance. Oncotarget. 2015;6(1):144–158. doi: 10.18632/oncotarget.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Habu N., Imanishi Y., Kameyama K. Expression of Oct3/4 and Nanog in the head and neck squamous carcinoma cells and its clinical implications for delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma. BMC Cancer. 2015;15:730. doi: 10.1186/s12885-015-1732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiou S.H., Yu C.C., Huang C.Y. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14(13):4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 100.Tsai L.L., Yu C.C., Chang Y.C., Yu C.H., Chou M.Y. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol Med. 2011;40(8):621–628. doi: 10.1111/j.1600-0714.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- 101.Chang C.W., Chen Y.S., Chou S.H. Distinct subpopulations of head and neck cancer cells with different levels of intracellular reactive oxygen species exhibit diverse stemness, proliferation, and chemosensitivity. Cancer Res. 2014;74(21):6291–6305. doi: 10.1158/0008-5472.CAN-14-0626. [DOI] [PubMed] [Google Scholar]
- 102.Weller P., Nel I., Hassenkamp P. Detection of circulating tumor cell subpopulations in patients with head and neck squamous cell carcinoma (HNSCC) PLoS ONE. 2014;9(12):e113706. doi: 10.1371/journal.pone.0113706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murillo-Sauca O., Chung M.K., Shin J.H. CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget. 2014;5(16):6854–6866. doi: 10.18632/oncotarget.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei X.D., Zhou L., Cheng L., Tian J., Jiang J.J., Maccallum J. In vivo investigation of CD133 as a putative marker of cancer stem cells in Hep-2 cell line. Head Neck. 2009;31(1):94–101. doi: 10.1002/hed.20935. [DOI] [PubMed] [Google Scholar]
- 105.Wei X., Wang J., He J., Ma B., Chen J. Biological characteristics of CD133(+) cancer stem cells derived from human laryngeal carcinoma cell line. Int J Clin Exp Med. 2014;7(9):2453–2462. [PMC free article] [PubMed] [Google Scholar]
- 106.Wei X., He J., Wang J., Yang X., Ma B. Bmi-1 is essential for the oncogenic potential in CD133(+) human laryngeal cancer cells. Tumour Biol. 2015;36(11):8931–8942. doi: 10.1007/s13277-015-3541-9. [DOI] [PubMed] [Google Scholar]
- 107.Yu C.C., Hu F.W., Yu C.H., Chou M.Y. Targeting CD133 in the enhancement of chemosensitivity in oral squamous cell carcinoma-derived side population cancer stem cells. Head Neck. 2016;38(Suppl. 1):E231–E238. doi: 10.1002/hed.23975. [DOI] [PubMed] [Google Scholar]
- 108.Yu L., Zhou L., Wu S. Clinicopathological significance of cancer stem cells marked by CD133 and KAI1/CD82 expression in laryngeal squamous cell carcinoma. World J Surg Oncol. 2014;12:118. doi: 10.1186/1477-7819-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu D., Liu Y., Yang J. Clinical implications of BMI-1 in cancer stem cells of laryngeal carcinoma. Cell Biochem Biophys. 2015;71(January (1)):261–269. doi: 10.1007/s12013-014-0194-z. [DOI] [PubMed] [Google Scholar]
- 110.Chen X.H., Bao Y.Y., Zhou S.H., Wang Q.Y., Wei Y., Fan J. Glucose transporter-1 expression in CD133+ laryngeal carcinoma Hep-2 cells. Mol Med Rep. 2013;8(6):1695–1700. doi: 10.3892/mmr.2013.1740. [DOI] [PubMed] [Google Scholar]
- 111.Han J., Fujisawa T., Husain S.R., Puri R.K. Identification and characterization of cancer stem cells in human head and neck squamous cell carcinoma. BMC Cancer. 2014;14:173. doi: 10.1186/1471-2407-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clay M.R., Tabor M., Owen J.H. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32(9):1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y.C., Chen Y.W., Hsu H.S. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385(3):307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 114.Yan M., Yang X., Wang L. Plasma membrane proteomics of tumor spheres identify CD166 as a novel marker for cancer stem-like cells in head and neck squamous cell carcinoma. Mol Cell Proteomics. 2013;12(11):3271–3284. doi: 10.1074/mcp.M112.025460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jia G., Wang X., Yan M., Chen W., Zhang P. CD166-mediated epidermal growth factor receptor phosphorylation promotes the growth of oral squamous cell carcinoma. Oral Oncol. 2016;59:1–11. doi: 10.1016/j.oraloncology.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 116.Kapałczyńska M., Kolenda T., Przybyła W. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch Med Sci. 2016 doi: 10.5114/aoms.2016.63743. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koukourakis M.I., Giatromanolaki A., Tsakmaki V., Danielidis V., Sivridis E. Cancer stem cell phenotype relates to radio-chemotherapy outcome in locally advanced squamous cell head-neck cancer. Br J Cancer. 2012;106(5):846–853. doi: 10.1038/bjc.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kozlowska A.K., Florczak A., Smialek M. Functionalized bioengineered spider silk spheres improve nuclease resistance and activity of oligonucleotide therapeutics providing a strategy for cancer treatment. Acta Biomater. 2017;59(September):221–233. doi: 10.1016/j.actbio.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Florczak A., Mackiewicz A., Dams-Kozlowska H. Functionalized spider silk spheres as drug carriers for targeted cancer therapy. Biomacromolecules. 2014;15(August (8)):2971–2981. doi: 10.1021/bm500591p. [DOI] [PubMed] [Google Scholar]
- 120.Nör C., Zhang Z., Warner K.A. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia. 2014;16(2):137–146. doi: 10.1593/neo.131744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu D., Liu Y., Yang J. Clinical implications of BMI-1 in cancer stem cells of laryngeal carcinoma. Cell Biochem Biophys. 2015;71(1):261–269. doi: 10.1007/s12013-014-0194-z. [DOI] [PubMed] [Google Scholar]
- 122.Yang J.P., Liu Y., Zhong W., Yu D., Wen L.J., Jin C.S. Chemoresistance of CD133+ cancer stem cells in laryngeal carcinoma. Chin Med J (Engl) 2011;124(7):1055–1060. [PubMed] [Google Scholar]
- 123.Kuo S.Z., Blair K.J., Rahimy E. Salinomycin induces cell death and differentiation in head and neck squamous cell carcinoma stem cells despite activation of epithelial-mesenchymal transition and Akt. BMC Cancer. 2012;12:556. doi: 10.1186/1471-2407-12-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gilormini M., Malesys C., Armandy E. Preferential targeting of cancer stem cells in the radiosensitizing effect of ABT-737 on HNSCC. Oncotarget. 2016;7(13):16731–16744. doi: 10.18632/oncotarget.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee S.H., Nam H.J., Kang H.J., Samuels T.L., Johnston N., Lim Y.C. Valproic acid suppresses the self-renewal and proliferation of head and neck cancer stem cells. Oncol Rep. 2015;34(4):2065–2071. doi: 10.3892/or.2015.4145. [DOI] [PubMed] [Google Scholar]
- 126.Kumar B., Yadav A., Lang J.C., Teknos T.N., Kumar P. Suberoylanilide hydroxamic acid (SAHA) reverses chemoresistance in head and neck cancer cells by targeting cancer stem cells via the downregulation of nanog. Genes Cancer. 2015;6(3–4):169–181. doi: 10.18632/genesandcancer.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang C., Zhang Y., Zhang Y. Downregulation of cancer stem cell properties via mTOR signaling pathway inhibition by rapamycin in nasopharyngeal carcinoma. Int J Oncol. 2015;47(3):909–917. doi: 10.3892/ijo.2015.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang H., Yu T., Wen L., Wang H., Fei D., Jin C. Curcumin enhances the effectiveness of cisplatin by suppressing CD133+ cancer stem cells in laryngeal carcinoma treatment. Exp Ther Med. 2013;6(5):1317–1321. doi: 10.3892/etm.2013.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qi X., Yu D., Jia B. Targeting CD133(+) laryngeal carcinoma cells with chemotherapeutic drugs and siRNA against ABCG2 mediated by thermo/pH-sensitive mesoporous silica nanoparticles. Tumour Biol. 2016;37(2):2209–2217. doi: 10.1007/s13277-015-4007-9. [DOI] [PubMed] [Google Scholar]
- 130.Cheng J.Z., Yu D., Zhang H. Inhibitive effect of IL-24 gene on CD133(+) laryngeal cancer cells. Asian Pac J Trop Med. 2014;7(November (11)):867–872. doi: 10.1016/S1995-7645(14)60151-6. [DOI] [PubMed] [Google Scholar]