Abstract
Background
Intensity-modulated radiotherapy (IMRT) improves dose distribution in head and neck (HN) radiation therapy. Volumetric-modulated arc therapy (VMAT), a new form of IMRT, delivers radiation in single or multiple arcs, varying dose rates (VDR-VMAT) and gantry speeds, has gained considerable attention. Constant dose rate VMAT (CDR-VMAT) associated with a fixed gantry speed does not require a dedicated linear accelerator like VDR-VMAT. The present study explored the feasibility, efficiency and delivery accuracy of CDR-VMAT, by comparing it with IMRT and VDR-VMAT in treatment planning for HN cancer.
Methods and materials
Step and shoot IMRT (SS-IMRT), CDR-VMAT and VDR-VMAT plans were created for 15 HN cancer patients and were generated by Pinnacle3 TPS (v 9.8) using 6 MV photon energy. Three PTVs were defined to receive respectively prescribed doses of 66 Gy, 60 Gy and 54 Gy, in 30 fractions. Organs at risk (OARs) included the mandible, spinal cord, brain stem, parotids, salivary glands, esophagus, larynx and thyroid. SS-IMRT plans were based on 7 co-planar beams at fixed gantry angles. CDR-VMAT and VDR-VMAT plans, generated by the SmartArc module, used a 2-arc technique: one clockwise from 182° to 178° and the other one anti-clockwise from 178° to 182°. Comparison parameters included dose distribution to PTVs (Dmean, D2%, D50%, D95%, D98% and Homogeneity Index), maximum or mean doses to OARs, specific dose-volume data, the monitor units and treatment delivery times.
Results
Compared with SS-IMRT, CDR-VMAT significantly reduced the maximum doses to PTV1 and PTV2 and significantly improved all PTV3 parameters, except D98% and D95%. It significantly spared parotid and submandibular glands and was associated with a lower Dmean to the larynx. Compared with VDR-VMAT, CDR-VMAT was linked to a significantly better Dmean, to the PTV3 but results were worse for the parotids, left submandibular gland, esophagus and mandible. Furthermore, the Dmean to the larynx was also worse. Compared with SS-IMRT and VDR-VMAT, CDR-VMAT was associated with higher average monitor unit values and significantly shorter average delivery times.
Conclusions
CDR-VMAT appeared to be a valid option in Radiation Therapy Centers that lack a dedicated linear accelerator for volumetric arc therapy with variable dose-rates and gantry velocities, and are unwilling or unable to sanction major expenditure at present but want to adopt volumetric techniques.
Keywords: Head and neck radiation therapy, Intensity modulated radiotherapy, Constant dose rate VMAT, Volumetric-modulated arc therapy
1. Background
Radiation therapy for head and neck cancer is challenging because of the complex anatomy of the region as tumors are often located in close proximity to crucial structures at high risk of toxicity. Therefore, reducing the dose to these organs needs to be balanced against appropriate coverage of target volumes. Head and neck tumors were conventionally treated with 3-dimensional (3D) conformal radiotherapy which has been replaced by intensity-modulated radiotherapy (IMRT). The latter improves target volume coverage sparing organs at risk (OARs) of toxicity.
In recent years, a new form of IMRT, i.e. volumetric-modulated arc therapy (VMAT), has gained considerable attention. It delivers radiation in single or multiple arcs, at varying dose rates (VDR-VMAT) and gantry speeds. VMAT was reported to be as good as IMRT in terms of target volume coverage and OAR sparing with the advantages of using fewer monitor units (MU) and taking less time to deliver treatments.1, 2, 3, 4, 5, 6, 7, 8 Consequently, the patient undergoes a shorter restriction time in the thermoplastic mask and treatment is safer with less risk of intra-fractional error. Furthermore, more patients can be treated in the Radiation Oncology Center.
VDR-VMAT requires a dedicated linear accelerator and treatment planning modules, all of which are costly. A cheaper option is constant dose rate VMAT (CDR-VMAT) associated with a fixed gantry speed because a dedicated linear accelerator is not required, although specific treatment planning software is.
The present study explored the feasibility, efficiency and delivery accuracy of CDR-VMAT and compared it with IMRT and VDR-VMAT in radiotherapy treatment plans for patients with head and neck cancer.
2. Methods and materials
A sample of patients with stage III–IV head and neck cancer was retrospectively selected from among those who had undergone IMRT at our Radiation Oncology Unit. Treatment plans were re-calculated (see below) for 15 patients (13 male, 2 female; age range 46–79 years of age, mean age 63 years, median 64; 5 had oropharyngeal cancer, 5 hypo-pharyngeal cancer and 5 larynx cancer).
2.1. Contouring and dose prescription
Computed tomography (CT) scans with 2.5 mm slice thicknesses were acquired from the top of the head to sternoclavicular junction. In the supine position with arms by their sides, each patient was wearing a customized head and neck immobilization thermoplastic mask. All CT scans were transmitted to the Pinnacle3 treatment planning system (TPS) V9.8 (Philips Radiation Oncology Systems, Fitchburg, WI). Gross tumor volumes (GTVs, corresponding to the primary tumor and lymph node metastases) and clinical target volumes (CTVs) were contoured. OARs included the mandible, spinal cord, brain stem, parotid and submandibular glands, esophagus, thyroid and larynx (except for 5 patients with larynx cancer).
To obtain the planning target volumes (PTVs), GTVs were expanded by 1 cm and each CTV by 3 mm. Overlapping areas between PTVs and OARs were attributed to the PTV. To avoid the dose build-up effect, PTVs were restricted to 5 mm depth of the skin surface. Two rings surrounded each PTV. The 10 mm thick Ring1 constrained dose fall-off from the PTV while the 30 mm thick Ring 2 prevented hot-spots outside the targets.
Of all the OARs, only the spinal cord and brain stem were expanded by 5 mm to create planning risk volumes (PRV).
In accordance with RTOG guidelines,9 treatment was delivered in 30 fractions. Prescribed doses in each patient were 66 Gy (2.20 Gy/fraction) for the high-risk volume (PTV1), 60 Gy (2.00 Gy/fraction) for the intermediate-risk volume (PTV2) and 54 Gy (1.8 Gy/fraction) for the low-risk volume (PTV3).
2.2. Treatment planning
Each patient was re-planned by the same physicist (AD) with CDR-VMAT and then with VDR-VMAT. The original IMRT plan and the new CDR-VMAT and VDR-VMAT plans were generated using 6 MV photon beam commissioned for a Varian Clinac 2100 DHX-S linear accelerator (Varian Medical Systems, Palo Alto, CA) equipped with the Millennium 120-leaves multi-leaf collimator (MLC). Version 9.8 Pinnacle3 TPS calculated all treatment plans using the Pinnacle3 SmartArc module for the CDR-VMAT and VDR-VMAT plans. The dose grid resolution was set at 2 mm for all plans. CDR-VMAT and VDR-VMAT plans used the same dose-volume objectives as the original IMRT plan (Table 1). All plans ensured PTV coverage was optimally 98% of the PTV receiving 95% of the prescribed dose and acceptably 95% receiving 95% prescribed dose. To reduce OAR doses without compromising target coverage, dose-volume objectives were adopted as in Table 1. All plans were evaluated and accepted by four radiation oncologists (BMP, AF, VL, SS).
Table 1.
Dose objectives for all regions of interest.
| ROI | Objectives |
|---|---|
| Brain stem | D0.1cc = 54 Gy |
| Spinal cord | D0.1cc ≤ 44–45 Gy |
| Mandible | D1cc = 70–73.5 Gy |
| Larynx |
Dmean = 44 Gy D1cc = 73.5 Gy |
| Thyroid | V45 Gy < 50% |
| Esophagus | D1cc = 45 Gy |
| Parotids |
Dmean ≤ 26 Gy V30 Gy < 50% |
| Salivary glands | Dmean ≤ 35 Gy |
| Ring 1 | D2% = 95% prescribed dose to PTV3 |
| Ring 2 | D2% = 80% prescribed dose to PTV3 |
| PTVn | Dmin = 80% prescribed dose to PTVn |
| PTVn eval | D95% = 95% prescribed dose to PTVn |
| Dmin = 90% prescribed dose to PTVn | |
| D2% = 105% prescribed dose to PTVn | |
| Uniform dose = prescribed dose to PTVn |
Abbreviations: ROI = region of interest; PTV = planning target volume.
Step and shoot IMRT (SS-IMRT) plans were based on 7 co-planar beams at fixed gantry angles of 180°, 210°, 290°, 315°, 45°, 70° and 150°. Collimator angles were chosen to obtain optimal MLC orientation and travel direction in accordance with PTV and OAR positions and shapes. Maximum iterations were 40 and maximum segments 50, with an 8 cm2 minimum segment area and at least 5 MU for each segment.
CDR-VMAT and VDR-VMAT plans used a 2-arc technique: one clockwise from 182° to 178° and the other anti-clockwise from 178° to 182°. The collimator angle was set at 45° with a leaf motion constraint of 0.46 cm/° and final arc spacing of 4°.
Maximum iterations were 40 as for SS-IMRT.
The linear accelerator delivery parameters that were inserted into the TPS were: 2.25 cm/s maximum MLC motion speed, 4.8 deg/s maximum gantry rotation speed, as recommended by the manufacturer for Varian linear accelerators. SS-IMRT, CDR-VMAT and VDR-VMAT plans were optimized using the Direct Machine Parameter Optimization (DMPO) algorithm.
At delivery, the dose rate was 300 MU/min for SS-IMRT and CDR-VMAT and 50–600 MU/min for VDR-VMAT.
2.3. Dosimetries
Dose-volume histograms (DVHs) for SS-IMRT, CDR-VMAT and VDR-VMAT were compared for PTV coverage and OAR doses. PTV and OAR dosimetry parameters are reported in Table 1. The homogeneity index (HI) was calculated as the difference between the dose to 2% (D2%) and 98% (D98%) of PTV divided by the median dose (D50%): HI = (D2%–D98%)/D50%.
2.4. MU and delivery times
The SS-IMRT delivery time was calculated as the number of MUs for each field divided by the dose rate plus one minute for the time the gantry needed to move from one configuration to the next. CDR-VMAT and VDR-VMAT delivery times were automatically provided by the TPS as it indicated the estimated time for each arc.
2.5. CDR-VMAT treatment plan verification
All 15 CDR-VMAT plans were checked by the cylindrical Octavius® 4D phantom (PTW, Germany)10 that rotates synchronously with the gantry, taking time- and gantry angle-resolved dose measurements. Phantom-measured and TPS dose distribution were compared using local and global gamma index function evaluations for each arc in accordance with the following criteria: 10% maximum dose threshold, 3% dose difference and 3 mm distance to agreement. The optimal gamma function passing rate was ≥95% while the acceptable one was ≥90%.
Data were collected and evaluated by the PTW-VeriSoft software package.
2.6. Statistics
Distribution of variables was checked by the Shapiro–Wilk test. Friedman's test analyzed differences in the three techniques. When a significant difference emerged, the post hoc Conover test determined which pair-wise comparisons differed. All statistical analyses were conducted with the SPSS 20.0 software. Differences were considered significant at p < 0.05.
3. Results
Plans were generated for SS-IMRT, CDR-VMAT and VDR-VMAT for all 15 cases. Global gamma analysis was done only for CDR-VMAT plans, as VDR-VMAT cannot be delivered by our linear accelerator. For each CDR-VMAT arc the average passing rates were respectively, 98.4% (range: 96.7–99.1%) and 98.2% (range: 96.9–99.1%). Overall, the average passing rate was 98.0% (range: 96.1–99.2%).
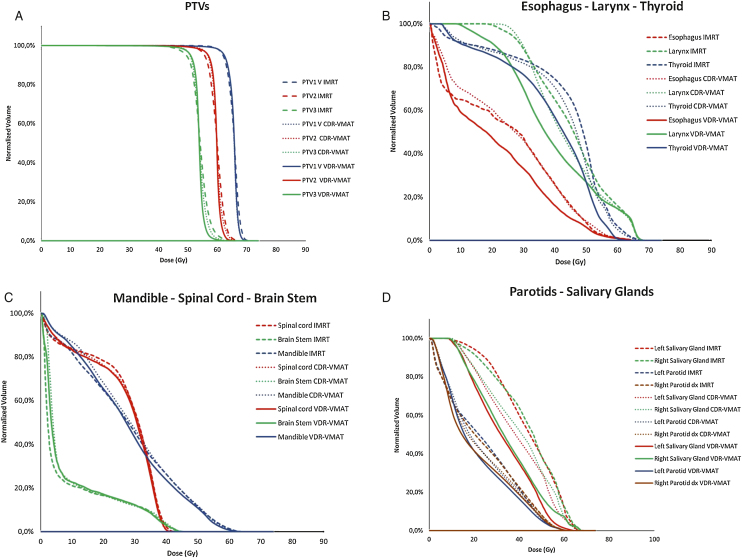
Fig. 1 (Panels A–D) illustrates dose volume histograms for each technique, each PTV and each OAR in a representative patient.
Fig. 1.
Dose volume histograms for each technique in a representative patient. PTVs (panel A) and OARs (Panel B larynx, thyroid and esophagus; Panel C mandible, spinal cord and brain stem; panel D parotid and salivary glands).
Table 2 reports mean PTV coverage parameters. Compared with SS-IMRT, CDR-VMAT significantly (p < 0.0001) reduced only the maximum doses to PTV1 and PTV2. Except for D98% and D95%, all PTV3 parameters improved significantly (see Table 2 for p values).
Table 2.
Dosimetric results with SS-IMRT, CDR-VMAT and VDR-VMAT for the PTVs.
| IMRT | CDR-VMAT | VDR-VMAT | p Value | |
|---|---|---|---|---|
| PTV1 | ||||
| D98% | 60.08 Gy (57.09–62.68) Gy | 60.06 Gy (55.88–63.39) Gy | 59.41 Gy (49.29–63.06) Gy | p = 0.4643 |
| D95% | 62.01 Gy (60.3–63.37) Gy | 62.31 Gy (61.36–64) Gy | 62.26 Gy (59.3–63.85) Gy | p = 0.9843 |
| D50% | 65.78 Gy (64.75–66.73) Gy | 65.74 Gy (64.95–66.05) Gy | 65.85 Gy (65.45–66.65) Gy | p = 0.8233 |
| D2% | 68.63 Gy (67.03–69.68) Gy | 67.74 Gy (66.49–68.97) Gy | 67.82 Gy (66.77–69.38) Gy | IMRT vs. CDR-VMAT p < 0.0001 |
| IMRT vs. VDR-VMAT p < 0.0001 | ||||
| CDR-VMAT vs. VDR-VMAT p = 0.3647 | ||||
| Dmean | 65.52 Gy (64.57–66.37) Gy | 65.42 Gy (64.9–65.73) Gy | 65.45 Gy (64.88–66.42) Gy | p = 0.7508 |
| HI | 0.13 (0.07–0.18) | 0.12 (0.05–0.2) | 0.13(0.08–0.29) | p = 0.4643 |
| PTV2 | ||||
| D98% | 53.76 Gy (48.58–57.17) Gy | 54.21 Gy (51.03–58.24) Gy | 56.67 Gy (49.31–58.65) Gy | p = 0.9395 |
| D95% | 55.87 Gy (52.13–58.26) Gy | 56.81 Gy (55.26–58.59) Gy | 56.92 Gy (55.05–59) Gy | p = 0.1023 |
| D50% | 65.78 Gy (64.75–66.73) Gy | 59.82 Gy (59.19–60.9) Gy | 59.95y (59.5–61.5) Gy | p = 0.4504 |
| D2% | 64.19 Gy (62.36–66) Gy | 62.7 Gy (60.87–65.56) Gy | 62.24 Gy (60.59–64.83) Gy | IMRT vs. CDR-VMAT p < 0.0001 |
| IMRT vs. VDR-VMAT p < 0.0001 | ||||
| CDR-VMAT vs. VDR-VMAT p = 0.3647 | ||||
| Dmean | 59.77 Gy (58.44–60.41) Gy | 59.67 Gy (59.28–60.53) Gy | 59.6 Gy (59.06–61.28) Gy | p = 0.1255 |
| HI | 0.17 (0.09–0.3) | 0.14 (0.07–0.24) | 0.14(0.04–0.22) | p = 0.1922 |
| PTV3 | ||||
| D98% | 48.44 Gy (46.81–51.02) Gy | 48.59 Gy (43.54–51.66) Gy | 48.22 Gy (41.32–51.38) Gy | p = 0.5647 |
| D95% | 50.32 Gy (48.58–51.8) Gy | 50.88 Gy (49.25–52.35) Gy | 51.06 Gy (47.91–52.97) Gy | p = 0.0563 |
| D50% | 54.21 (53.2–55.18) Gy | 53.68 Gy (50–55.4) Gy | 53.89 Gy (53.33–55.25) Gy | IMRT vs. CDR-VMAT p = 0.0021 |
| 59.76 (56.78–63.22) Gy | 58.15 Gy (54–62.48) Gy | 57.84 Gy (55.79–60.52) Gy | IMRT vs. VDR-VMAT p = 0.0036 | |
| CDR-VMAT vs. VDR-VMAT p = 0.8283 | ||||
| D2% | 54.22 Gy (53.04–55.38) Gy 0.20 (0.11–0.27) |
53.98 Gy (53.55–55.17) Gy 0.16 (0.06–0.27) |
53.76 Gy (53.18–55.06) Gy 0.17(0.06–0.36) |
IMRT vs. CDR-VMAT p = 0.0052 |
| IMRT vs. VDR-VMAT p = 0.0017 | ||||
| CDR-VMAT vs. VDR-VMAT p = 0.668 | ||||
| Dmean | IMRT vs. CDR-VMAT p = 0.0251 | |||
| IMRT vs. VDR-VMAT p < 0.0001 | ||||
| CDR-VMAT vs. VDR-VMAT p = 0.0251 | ||||
| HI | IMRT vs. CDR-VMAT p = 0.0001 | |||
| IMRT vs. VDR-VMAT p = 0.0028 | ||||
| CDR-VMAT vs. VDR-VMAT p = 0.2522 | ||||
Abbreviations: SS-IMRT: step and shoot intensity-modulated radiotherapy; VDR-VMAT: varying dose rates volumetric-modulated arc therapy; CDR-VMAT: constant dose rate volumetric-modulated arc therapy; PTV: planning target volume; HI: homogeneity index.
Compared with VDR-VMAT only PTV3 Dmean was significantly better (p < 0.025) with CDR-VMAT.
Table 3 shows all OAR dose volume data. Results reported here were significant when CDR-VMAT was compared with SS-IMRT and VDR-VMAT
Table 3.
Dosimetric results with SS-IMRT, CDR-VMAT and VDR-VMAT for organs at risk (OARs).
| Mean range | ||||
|---|---|---|---|---|
| SS-IMRT | CDR-VMAT | VDR-VMAT | p Value | |
| Mandible | ||||
| D1cc | 50.5 Gy (32.2–62.9) | 52.15 (34.6–61.7) | 51.45 (32–63) | IMRT vs. CDR-VMAT p > 0.9999 IMRT vs. VDR-VMAT p = 0.0133 CDR-VMAT vs. VDR-VMAT p = 0.0133 |
| Right parotid | ||||
| Dmean | 23.6 Gy (16.2–27.6) | 22.7 Gy (14.8–25.9) | 21.5 Gy (12.9–25.4) | IMRT vs. CDR-VMAT p < 0.0001 IMRT vs. VDR-VMAT p < 0.0001 CDR-VMAT vs. VDR-VMAT p < 0.001 |
| V30Gy | 39% (30–46) | 34.50% (19–40) | 31.50% (17–37) | IMRT vs. CDR-VMAT p = 0.0057 IMRT vs. VDR-VMAT p < 0.0001 CDR-VMAT vs. VDR-VMAT p = 0.0057 |
| Left parotid | ||||
| Dmean | 23.6 Gy (19.8–26.4) | 22.6 Gy (15.4–24.7) | 21.2 Gy (15.1–24.5) | IMRT vs. CDR-VMAT p < 0.0001 IMRT vs. VDR-VMAT p < 0.0001 CDR-VMAT vs. VDR-VMAT p < 0.001 |
| V30Gy | 40% (27–46) | 33.50% (18–37) | 29.50% (21–36) | IMRT vs. CDR-VMAT p < 0.0001 IMRT vs. VDR-VMAT p < 0.0001 CDR-VMAT vs. VDR-VMAT p = 0.0065 |
| Right submandibular gland | ||||
| Dmean | 44.2 Gy (24.7–62) | 37.6 Gy (24.1–61.3) | 34.5 Gy (24.1–63.1) | IMRT vs. CDR-VMAT p < 0.0001 IMRT vs. VDR-VMAT p < 0.0001 CDR-VMAT vs. VDR-VMAT p = 0.0663 |
| Left submandibular gland | ||||
| Dmean | 44.1 Gy (24.8–61.7) | 36.7 Gy (24.6–67.9) | 32.9 Gy (25.1–50.3) | IMRT vs. CDR-VMAT p = 0.0003 IMRT vs. VDR-VMAT p < 0.0001 CDR-VMAT vs. VDR-VMAT p = 0.0237 |
| Spinal cord | ||||
| D0.1cc | 40.8 Gy (36–55) | 40.1 Gy (38.1–58.1) | 39.5 Gy (37.8– 60.8) | IMRT vs. CDR-VMAT p = 0.5807 IMRT vs. VDR-VMAT p = 0.2735 CDR-VMAT vs. VDR-VMAT p = 0.5807 |
| Brainstem | ||||
| D0.1cc | 35.4 Gy (2.9–43.3) | 30.9 Gy (5–45.1) | 32.2 Gy (5–44.6) | IMRT vs. CDR-VMAT p = 0.8965 IMRT vs. VDR-VMAT p = 0.3708 CDR-VMAT vs. VDR-VMAT p = 0.3088 |
| Larynx | ||||
| Dmean | 46.5 Gy (38.9–59.1) | 43.1 Gy (35.4–51.3) | 39.5 Gy (35.2–46) | IMRT vs. CDR-VMAT p = 0.0045 IMRT vs. VDR-VMAT p < 0.0001 CDR-VMAT vs. VDR-VMAT p = 0.0045 |
| D1cc | 63.5 Gy (53.3–67.7) | 64.2 Gy (53.6–68.2) | 65.7 Gy (52.9–67.5) | IMRT vs. CDR-VMAT p = 0.8965 IMRT vs. VDR-VMAT p = 0.3708 CDR-VMAT vs. VDR-VMAT p = 0.3088 |
| Thyroid | ||||
| V45Gy | 72.5% (20–100) | 55% (6–100) | 38% (7–100) | IMRT vs. CDR-VMAT p = 0.056 IMRT vs. VDR-VMAT p = 0.0005 CDR-VMAT vs. VDR-VMAT p = 0.56 |
| Esophagus | ||||
| D1cc | 40.5 Gy (20.7–61) | 41 Gy (31.3–60.3) | 34.9 Gy (23.9–58.8) | IMRT vs. CDR-VMAT p > 0.9999 IMRT vs. VDR-VMAT p = 0.0133 CDR-VMAT vs. VDR-VMAT p = 0.0133 |
Abbreviations: SS-IMRT: step and shoot intensity-modulated radiotherapy; VDR-VMAT: varying dose rates volumetric-modulated arc therapy; CDR-VMAT: constant dose rate volumetric-modulated arc therapy.
Parotids, Submandibular glands and Larynx: Dmeans were significantly lower with CDR-VMAT vs. SS-IMRT but significantly higher with CDR-VMAT vs. VDR-VMAT. The same significance was reached for V30 Gy in the parotid.
Esophagus and Mandible: CDR-VMAT was associated with a significantly higher D1cc than VDR-VMAT.
Brain stem, spinal cord and thyroid: no significant differences emerged with any technique.
CDR-VMAT was associated with higher average MU values (752 ± 88 vs. 613 ± 92 SS-IMRT p = 0.0125; vs. 704 ± 57 VDR-VMA p = 0.0554) and significantly shorter average delivery times (148 ± 21 s vs. 197 ± 18 s SS-IMRT p = 0.0001; vs. 168 ± 15 s VDR-VMAT p = 0.0085).
4. Discussion
Present results support using CDR-VMAT for head and neck radiotherapy as it emerged as a valid option when a dedicated linear accelerator is not available for VMAT therapy with variable dose-rates and gantry velocities. To date, few studies have focused on CDR-VMAT in treatment planning11, 12, 13, 14, 15 with only three assessing the implications of using it in clinical practice. Two investigated its role in the pelvic area13, 14 and one in nasopharyngeal cancer.15 Comparing observations by Yu et al.15 with the present series is difficult because the former focused only on the nasopharyngeal area, while our series only included patients with oropharyngeal, hypo-pharyngeal and laryngeal cancer. Consequently, in the study by Yu et al.,15 some OARs such as the brainstem and parotids were closer to the PTV than in the present investigation so that maximum and mean doses were higher.
In our view, the strength of the present study lies in our use of only one TPS to calculate all SS-IMRT and VMAT treatment plans for one linear accelerator. In fact, comparing results from other in silico studies is arduous as dosimetric results vary with the TPS and the linear accelerator model that was programmed.8, 16 Our results showed that, compared with SS-IMRT, CDR-VMAT provided lower maximum doses (D2%) to all three PTVs. However, as the dose differences were small (0.89 Gy for PTV1, 1.49 Gy for PTV2 and 1.61 Gy for PTV3), some doubts arose as to whether the reductions could impact clinical outcomes. Dose distribution was more homogeneous only in PTV3 as HI was significantly lower, confirming observations on the PTV-CTV by Yu et al.15
Compared with SS-IMRT, CDR-VMAT significantly spared the parotid, submandibular glands and delivered a lower mean dose to the larynx. Consequently, dose values were maintained within the predefined objectives to all OARs except the submandibular glands and thyroid. In all instances, these minimal dose increases over the thresholds did not appear sufficient to play a role in radiotherapy-related toxicity.
The SmartArc module is programmed to generate CDR-VMAT and VDR-VMAT treatment plans, the standard delivery modality in VMAT techniques. The present study showed CDR-VMAT provided a significantly higher mean dose to PTV3 than VDR-VMAT (53.98 vs. 53.76 Gy, prescribed dose 54 Gy) but results were worse for the parotids, left submandibular gland, esophagus and mandible. Furthermore, the Dmean to the larynx was also worse.
Surprisingly, in the present study, unlike other reports,2, 3, 5, 6, 8, 14, 16, 17, 18 VMAT techniques always needed more monitor units than IMRT. Poorer results with the VMAT techniques could have depended on the Pinnacle settings for the Constraint Leaf Motion Parameter. Although 0.46 cm/° was suggested as default by the manufacturer, it might not have been fully suitable for the head and neck region as they required highly modulated treatment planning. When the MLC is pushed to its limit to achieve the required modulation in highly modulated treatments, the constraint leaf motion parameter may be lowered. We speculated that a leaf motion parameter of 0.40–0.35 would reduce the number of monitor units without impacting dose distribution.
Delivery times with VMAT techniques were significantly shorter than with SS-IMRT, with the CDR being shorter than the VDR. Although present VMAT times may have been linked to arch rather than step and shoot delivery, both were shorter than reported elsewhere in head and neck cancer treatment planning.3, 8, 13, 16, 17 The advantages of shorter delivery times, particularly with CDR, include less risk of intra-fractional error, shorter patient restriction times and time to treat more patients every day, thus delivering a better service to individual patients and the health service.
5. Conclusions
Present results indicated that the Pinnacle SmartArc module generated appropriate CDR-VMAT treatment plans for head and neck cancer patients. Radiotherapy equipment is being up-dated in many EU countries and technology is evolving in RT Units, so, in fact, VDR-MAT linear accelerators are widely distributed. They are not yet found, however, in all RT Units EU-wide.19 As CDR-VMAT treatment plans were better than IMRT and approached VDR-MAT, they may be a valid option in Radiation Therapy Centers that are unwilling or unable to sanction major expenditure at present but want to adopt volumetric techniques. However, as the present results are only dosimetric data, clinical trials with sufficiently large patient cohorts are needed to confirm the usefulness, feasibility and accuracy of CDR-VMAT.
Financial disclosure
None declared.
Conflict of interest
None declared.
References
- 1.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 2.Bertelsen A., Hansen C.R., Johansen J., Brink C. Single arc volumetric modulated arc therapy of head and neck cancer. Radiother Oncol. 2010;95:142–148. doi: 10.1016/j.radonc.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Stieler F., Wolff D., Schmid H., Welzel G., Wenz F., Lohr F. A comparison of several modulated radiotherapy techniques for head and neck cancer and dosimetric validation of VMAT. Radiother Oncol. 2011;101:388–393. doi: 10.1016/j.radonc.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Wiehle R., Knippen S., Grosu A.L. VMAT and step-and-shoot IMRT in head and neck cancer: a comparative plan analysis. Strahlenther Onkol. 2011;187:820–825. doi: 10.1007/s00066-011-2267-x. [DOI] [PubMed] [Google Scholar]
- 5.Van Gestel D., van Vliet-Vroegindeweij C., Van den Heuvel F. RapidArc, SmartArc and TomoHD compared with classical step and shoot and sliding window intensity modulated radiotherapy in an oropharyngeal cancer treatment plan comparison. Radiat Oncol. 2013;8:37. doi: 10.1186/1748-717X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holt A., Van Gestel D., Arends M.P. Multi-institutional comparison of volumetric modulated arc therapy vs. intensity-modulated radiation therapy for head-and-neck cancer: a planning study. Radiat Oncol. 2013;8:26. doi: 10.1186/1748-717X-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin X., Yi J., Zhou Y., Yan H., Han C., Xie C. Comparing of whole-field simultaneous integrated boost VMAT and IMRT in the treatment of nasopharyngeal cancer. Med Dosim. 2013;38:418–423. doi: 10.1016/j.meddos.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Broggi S., Perna L., Bonsignore F. Static and rotational intensity modulated techniques for head-neck cancer radiotherapy: a planning comparison. Phys Med. 2014;30:973–979. doi: 10.1016/j.ejmp.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Eisbruch A., Harris J., Garden A.S. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22) Int J Radiat Oncol Biol Phys. 2010;5:1333–1338. doi: 10.1016/j.ijrobp.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stathalis S., Myers P., Esquivel C., Mavroidis P., Papanikolaou N. Characterization of a novel 2D array dosimeter for patient specific quality assurance whit volumetric arc therapy. Med Phys. 2013;40:071731. doi: 10.1118/1.4812415. [DOI] [PubMed] [Google Scholar]
- 11.Tang G., Earl M.A., Yu C.X. Variable dose rate single-arc IMAT delivered with a constant dose rate and variable angular spacing. Phys Med Biol. 2009;54:6439–6456. doi: 10.1088/0031-9155/54/21/001. [DOI] [PubMed] [Google Scholar]
- 12.Peng F., Jiang S.B., Romeijn H.E., Epelman M.A. VMATc: VMAT with constant gantry speed and dose rate. Phys Med Biol. 2015;60:2955–2979. doi: 10.1088/0031-9155/60/7/2955. [DOI] [PubMed] [Google Scholar]
- 13.Hatanaka S., Tamaki S., Endo H., Mizuno N., Nakamura N. Utility of Smart Arc CDR for intensity-modulated radiation therapy for prostate cancer. J Radiat Res. 2014;55:774–779. doi: 10.1093/jrr/rrt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang R., Wang J., Xu F., Li H., Zhang X. Feasibility of volumetric modulated arc therapy with constant dose rate for endometrial cancer. Med Dosim. 2013;38:351–355. doi: 10.1016/j.meddos.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Yu W., Shang H., Xie C. Feasibility of constant dose rate VMAT in the treatment of nasopharyngeal cancer patients. Radiat Oncol. 2014;9:235. doi: 10.1186/s13014-014-0235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guckenberg M., Richter A., Krieger T., Wilbert J., Baier K., Flentje M. Is a single arc sufficient in volumetric-modulated arc therapy (VMAT) for complex-shaped target volumes? Radiother Oncol. 2009;93:259–265. doi: 10.1016/j.radonc.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Vanetti E., Clivio A., Nicolini G. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: a treatment planning comparison with fixed field IMRT. Radiother Oncol. 2009;92:111–117. doi: 10.1016/j.radonc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Verbakel W.F., Cuijpers J.P., Hoffmans D. Volumetric intensity-modulated arc therapy vs. conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys. 2009;74:252–259. doi: 10.1016/j.ijrobp.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Reinfuss M., Byrski E., Malicki J. Radiotherapy facilities, equipment, and staffing in Poland: 2005–2011. Rep Pract Oncol Radiother. 2013;18:159–172. doi: 10.1016/j.rpor.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]