Abstract
The type III secretion system (T3SS) is a key virulence mechanism of many Gram-negative bacterial pathogens. Upon contact between bacteria and host cells, T3SS transfers a series of effectors from the bacterial cytosol to host cells. It is widely known that a mutation in T3SS does not impair bacterial growth, thereby avoiding any subsequent development of resistance. Thus, T3SS is expected to be a candidate therapeutic target. While developing the T3SS screening method, we discovered that sanguinarine chloride, a natural compound, could decrease the production of the SPI-1 type III secretion system main virulence proteins SipA and SipB and prevent the invasion of HeLa cells by Salmonella enterica serovar Typhimurium without affecting the growth of Salmonella. Furthermore, sanguinarine chloride downregulated the transcription of HilA and consequently regulated the expression of the SPI-1 apparatus and effector genes. In summary, our study directly demonstrated that this putative SPI-1 inhibitor belongs to a novel class of anti-Salmonella compounds.
Keywords: Type III secretion, Salmonella, Anti-virulence, Natural compound, Sanguinarine chloride
Highlights
-
•
Sanguinarine chloride effectively inhibits the translocation of a SipA-Lactamase fusion into mammalian cells.
-
•
Sanguinarine chloride inhibits the invasion of Hela cells by Salmonella enterica serovar Typhimurium.
-
•
Sanguinarine chloride inhibits the secretion of SPI-1 virulence proteins.
-
•
Sanguinarine chloride inhibits SPI-1 effectors through SPI-1 transcription regulate.
1. Introduction
The nontyphoidal Salmonella (NTS) strain Salmonella enterica serovar Typhimurium is a facultative intracellular pathogen capable of surviving within phagocytic cells [1], [2]. It can infect a broad spectrum of warm- and cold-blooded hosts [3]. Salmonella enterica serovar Typhimurium can cause gastrointestinal symptoms and severe systemic infections [4]. S. Typhimurium infection has exerted great influence by its high mortality rate. In the past several decades, Salmonella has had a widespread distribution in the environment.
Certain host factors make humans particularly susceptible to Salmonella infection. The increasing antimicrobial resistance, prevalence, virulence, and adaptability to Salmonella infections are a challenge worldwide. Multiple-antibiotic-resistance (Mar) mutants of S. Typhimurium are resistant to a wide variety of antibiotics [5], Therefore, it is necessary to find new approaches to antimicrobial therapy, including the identification of inhibitors of infectious diseases.
Type III secretion system (T3SS) is highly conserved in many Gram-negative pathogenic bacteria [6], [7]. The SPI-1 T3SS forms a needle-like complex that is responsible for delivering a series of effectors into host cells [8], [9]. SPI-1 is the best characterized of the Salmonella pathogenicity islands (SPIs), which encodes type III secretion system (T3SS-1) that includes regulatory proteins, effector proteins, and chaperone proteins [10]. A previous study showed that SPI-1 is virulent, and that deletion of SPI-1 or mutants of its secreted effector proteins could be developed as vaccines, which are viewed as effective ways to control salmonellosis [11], [12]. Thus, T3SS-1 was an ideal target for small molecule inhibitor screening of this infectious disease.
Natural compounds with anti-microbial properties continue to provide a source of novel drug leads. Since less than 10% of the world's biodiversity has been evaluated for potential biological activity, many more useful natural lead compounds await discovery with the challenge of how to access this natural chemical diversity [13]. In this context, our drug screening result showed that sanguinarine chloride, a natural compound, was capable of inhibiting the invasion of HeLa cells by S. Typhimurium. Sanguinarine chloride decreased the production of SPI-1 T3SS effector proteins SipA and SipB and regulator protein HilA [14]. The mechanism of sanguinarine chloride inhibition of SPI-1 T3SS expression will also be discussed in this study.
2. Materials and methods
Chemicals and Reagents—Unless otherwise noted, all chemicals used in this study were from Sigma. the purity of Sanguinarine chloride used in all experiments was higher than 98% (Sigma, cat# S5890-50MG).
2.1. Bacterial strains and plasmid construction
To generate S. Typhimurium SipA-3×FLAG and S. Typhimurium SipB- 3×FLAG mutants, the first 15 and the last 15 amino acids of the genes of interest were amplified from the genome of the wild type Salmonella Typhimurium [15]. The two fragments were digested with the proper enzymes and a ligation reaction with gel purified plasmid pSR47S was conducted. The reconstructed plasmids were transformed into E. coli DH5αλpir. After verification by sequencing, the plasmid was introduced into SL1344 by mixing pSR47S, SL1344 and E. coli HB101 (pRK600), a helper strain. The mixture was set on nonselective LB plate and incubated for 3 h at 37 °C. The cells were scraped and streaked onto LB plate containing 50 μg/ml kanamycin and 100 μg/ml streptomycin, followed by incubation at 37 °C overnight. Four to five colonies were transferred on non-selective plate and incubated at 37 °C overnight. The cells were then heavily seeded onto LB plate containing 15% sucrose. Then, 48 colonies were transferred onto nonselective LB plate with tooth picks. PCR was conducted to screen for the mutants. Strains harboring hila::lacZ, sopa::lacZ, sica::lacZ, prgh::lacZ fusion [16] was from Dr. James Slauch (University of Illinois at Urbana-Champaign).
2.2. Drug screening and flow cytometry analysis
Hela cells (1.2 × 104 in DMEM with 10% FBS) were seeded in 96-cell plates (Costar, NY, USA) and the plates were incubated for 12 h at 37 °C in a 5% CO2 incubator. Dilutions (1:20) of overnight cultures of S. Typhimurium strain invA deletion mutant [17] and the wild type strain containing SipA-lactamase fusion cloning plasmid were grown in LB (0.3 M NaCl) for 4 h in the presence of 5 μM sanguinarine chloride. HeLa cells were washed three times with PBS and infected with S. Typhimurium at an MOI of 50. Plates were centrifuged at room temperature for 10 min at 1000 rpm and incubated at 37 °C for 20 min to allow the infection to proceed. The culture medium was discarded, and the cells were washed three times with PBS. Cells were covered with 100 µl of PBS plus 20 µl of 6×CCF4/AM (Life Technologies, Inc., Waltham, MA) and stained for 90 min at room temperature. Fluorescence was observed by fluorescence microscopy (Olympus IX-81, Japan).
For flow cytometry analysis, cells seeded in 6-cell plates were infected using the method described above and washed with PBS three times. Cells were harvested by trypsinization and centrifuged for 5 min at 1000 rpm. Supernatants were discarded, and pellets were gently resuspended in 400 µl PBS, followed by 6×CCF4/AM (Life Technologies) staining for 90 min. Cells were analyzed on a BD LSR II flow cytometer to quantify the blue and green fluorescence.
2.3. Cytotoxicity assays
HeLa cells were plated into 96-well plates at a density of 1.2 × 104 cells per well and incubated overnight at 37 °C in a 5% CO2 incubator. After washing three times, cells were treated with different concentrations of sanguinarine chloride for 24 h at 37 °C. LDH release was measured using a cytotoxicity detection kit (Roche, Mannheim, Switzerland), according to the manufacturer's instructions. Plates were read on a microplate reader (TECAN, Austria) at 490 nm. LDH released from cells lysed by a buffer included in the kit was set at 100%. The percentage of LDH release was calculated according to the formula: LDH release (%) = (Experimental LDH release - Spontaneous LDH release)/(Total LDH release - Spontaneous LDH release)x100.
2.4. Gentamicin protection assay and immunofluorescence
To determine the effect of sanguinarine chloride on S. enterica serovar Typhimurium invasion of host cells, we used gentamicin protection assay to measure bacterial invasion of cells. HeLa cells were seeded in 24-cell plates at a density of 2 × 105 per well and incubated for 16–20 h at 37 °C and 5% CO2. S. Typhimurium strain SL1344 was grown for approximately 16 h at 37 °C with shaking. The next day, cultures were diluted 20-fold in LB broth in the presence or absence of sanguinarine chloride and grown at 37 °C for 4 h with shaking. HeLa cells were infected with bacteria at an MOI of 50 followed by centrifugation for 10 min at 1000 rpm and incubated in 37 °C for 30 min. Cells were washed three times with PBS containing 10 μg/ml gentamicin. Cells were incubated in DMEM containing 100 μg/ml gentamicin at 37 °C for 1 h. Plates were washed three times with PBS and 1 ml 0.2% saponin was added per well to lyse the cells. The CFUs of bacteria were counted by plating 1:10 dilution in LB. For microscopy, a protocol was used based on a previous report. HeLa cells were seeded onto cover slips placed in the bottom of the wells of 24-well plates and allowed 24 h to adhere. Cells were infected using the protocol described above for gentamicin protection and cells were gently washed with PBS. Cells were fixed onto cover slips using 4% paraformaldehyde for 10 min at room temperature. Cover slips were washed with PBS three times and were incubated with anti-S. Typhimurium antibody diluted 1:500 in PBS for 1 h. Cells were washed three times and incubated with a 1:500 dilution of the appropriate Alexa Fluor 488-conjugated IgG secondary antibody for 30 min. The cells were permeabilized with 0.5% Triton X-100 for 10 min, washed three times and incubated with 1:500 dilution of the appropriate Alexa Fluor 594-conjugated IgG secondary antibody for 30 min. Finally, the nuclei were stained with DAPI. Cells were visualized using an Olympus microscopy.
2.5. Antibodies and immunoblotting
The HilA-specific antibody was from Dr. Yufeng Yao at Shanghai Jiaotong University. To test the production of SipA-TEM fusion plasmid expression in S. Typhimurium, SL1344 SipA*3flag or SipB*3flag mutant strain were diluted at 1:20 in 2 ml LB (0.3 M NaCl) and grown for 4 h at 37 °C with a shaker (200 rpm). Sanguinarine chloride was added to the cultures. Bacterial cells equivalent to one OD600 unit were present in each sample. Cells were centrifuged for 5 min at 10,000 rpm, the pellets were lysed in 100 µl of SDS loading buffer and 15 µl of boiled supernatant were analyzed by SDS-PAGE. After transferring to PVDF membranes, the membranes were incubated in 5% BSA supplemented with the appropriate antibodies, including anti-HilA (1:1000), anti-Flag antibody (1:2000), anti-β-lactamase (1:170) and anti-ICDH (1:5000). The results were detected by the enhanced chemiluminescence (ECL) method.
2.6. β-Galactosidase assay
β-Galactosidase activity was determined by a method described before. Briefly, bacterial strain JS749, JS751, JS752 and JS753 were grown overnight and sub-cultured in 2 ml LB with or without sanguinarine chloride. OD600 of the cultures were measured and bacteria were harvested by centrifugation for 10 min at 12,000g, followed by resuspension in Z-buffer. The mixture of 20 µl freshly prepared 0.1% SDS, 40 µl chloroform and 100 µl of the cells was vortexed for at least 10 s to permeabilize the cells. The permeabilized cell suspension was transferred to a microtiter plate and the reaction was initiated by the addition of 20 µl of ONPG (Sigma, 4 mg/ml) in Z-buffer. The plate was incubated at room temperature for 10 min before the reaction was terminated by the addition of 50 µl of 1 M Na2CO3. The microplates were read in the plate reader and the A420 was determined.
2.7. Real-time RT-PCR
S. Typhimurium strain SL1344 was grown for approximately 16 h at 37 °C with shaking. The next day, cultures were diluted 20-fold in LB broth in the presence or absence of sanguinarine chloride and grown at 37 °C for 4 h with shaking. The total RNA was extracted from bacteria, as described previously. The total RNA was reverse transcribed into cDNA using the TIANGEN Quant One Step RT-PCR kit (KR113). The PCR reactions were performed in 25 µl volumes using SYBR Pre-mix Ex Taq TM (Takara, Japan). The PCR amplification was assessed using the 7000 Sequence Detection System (Applied Biosystems, Courtaboeuf, France). All samples were analyzed in triplicate, and the Strains and plasmids are listed in Table S1, the primer pairs are listed in Table S2.
2.8. Statistical analysis
The experimental data were assessed by unpaired two-tailed t-test using GraphPad Prism6 (GraphPad software, La Jolla, CA). P value of<0.05 was considered to be statistically significant.
3. Results
3.1. Sanguinarine chloride inhibits the translocation of a SipA-lactamase fusion by Salmonella T3SS-1
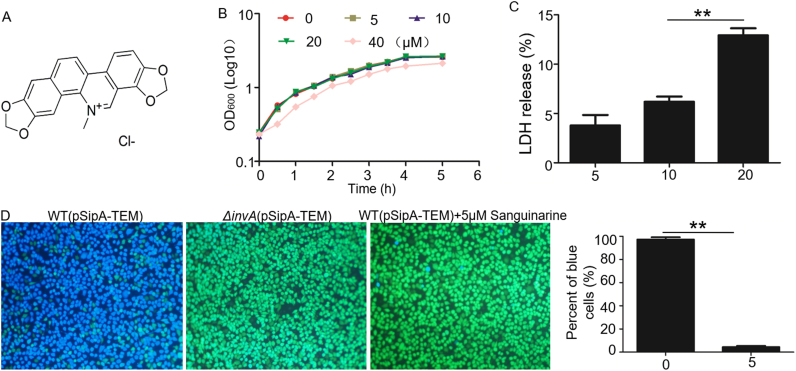
To identify natural compounds capable of inhibiting the secretion of SPI-1 T3SS effector proteins into HeLa cells, we screened some chemicals extracted from Chinese herbal medicine using a β-lactamase reporter as previously described [18]. A non-invasive S. Typhimurium invA mutant strain was used as negative control. After infection, HeLa cells were incubated with the membrane-permeant fluorescent substrate CCF4-AM. SipA secretion was measured in live cells using fluorescence microscopy, in which cells that had undergone effector translocation exhibited blue fluorescence while cells that had not been injected with β-lactamase exhibited green fluorescence. We found that sanguinarine chloride (Fig. 1A) was capable of effectively inhibiting the secretion of effector proteins into HeLa cells. As shown in Fig. 1D, an abundance of blue cells was observed when SL1344 carrying β-lactamase fusion was used to infect HeLa cells at an MOI of 50. In contrast, in the presence of sanguinarine chloride (5 μM), most of the HeLa cells displayed green fluorescence, indicating that translocation of the β-lactamase reporter was reduced dramatically.
Fig. 1.
Sanguinarine chloride inhibits T3SS-1 effector protein translocation in Salmonella without influencing bacterial growth. A. The structures of sanguinarine chloride tested in this study. B. Sanguinarine chloride (20 μM) does not significantly affect the viability of S. Typhimurium. Sanguinarine chloride was added to bacterial cultures in LB broth at the indicated concentrations, and the growth was monitored by measuring OD600 at the indicated time points. Note that 20 μM sanguinarine chloride did not detectably affect bacterial growth. The results were from three independent experiments performed in triplicate. Bar, S.E.M. (n = 3); *, p < 0.05. C. Toxicity of sanguinarine chloride to mammalian cells. Sanguinarine chloride was added to HeLa cells at the indicated concentrations and the release of LDH was measured. The results shown are from three independent experiments performed in triplicates. Bar, S.E.M. (n = 3); *, p < 0.05. D. Representative images of the translocation of SipA-lactamase fusion into HeLa cells. Wild-type or invA mutant of S. Typhimurium expressing the fusion was used to infect HeLa cells. Images were acquired with a fluorescence microscope. Blue and green cells indicate positive and negative protein translocation, respectively. Note that sanguinarine chloride treatment reduced the number of blue cells.
We also tested the growth of Salmonella in the presence of different concentrations of sanguinarine chloride. The result suggests that sanguinarine chloride less than 80 μM is capable of reducing the secretion of SPI-1 effectors into HeLa cells without affecting bacterial growth (Fig. 1B). Lactate dehydrogenase (LDH) release could serve as a potential marker of cell injury and death. Therefore, we determined the cytotoxicity of the compounds sanguinarine chloride on HeLa cells by LDH release assay. We found that 20 µM of sanguinarine chloride could damage the membranes of HeLa cells, but sanguinarine chloride at 10 µM had only slight cytotoxicity (Fig. 1C).
3.2. Sanguinarine chloride inhibits the invasion of HeLa cells by Salmonella enterica serovar Typhimurium
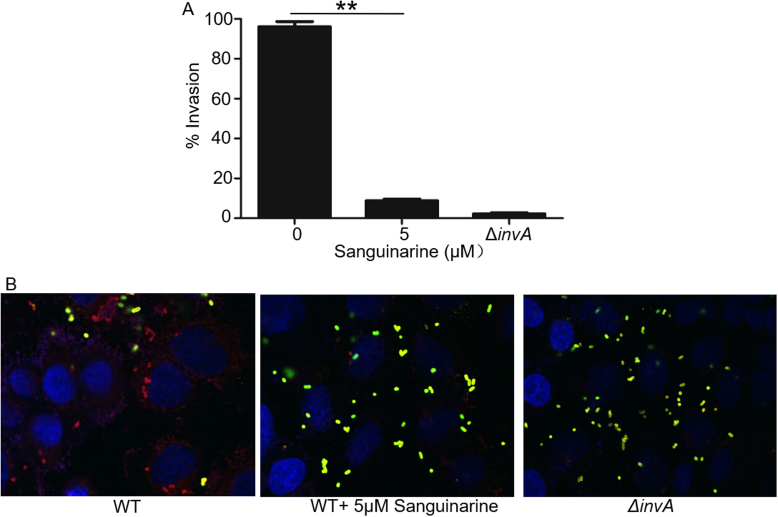
We evaluated the inhibitory effects of sanguinarine chloride on the invasion of HeLa cells by Salmonella using the gentamycin protection assay. The result showed that sanguinarine chloride inhibited the bacterial invasion of HeLa cells compared to a PBS control. The InvA mutant strain was used as a negative control, which lacks an essential component of the SPI-1 T3SS. We also observed the inhibition of HeLa cell invasion by S. Typhimurium with immunofluorescence. Our results showed that the internalized bacteria were reduced significantly in the sanguinarine chloride-treated group (Fig. 2B). When sanguinarine chloride was used at 5 µM, the rates of invasion were reduced to less than 5%, which was similar to the invA mutant strain (Fig. 2A). These results demonstrated that sanguinarine chloride was a strong inhibitor of SPI-1 T3SS-mediated invasion of host cells.
Fig. 2.
Sanguinarine chloride inhibited the invasion of HeLa cells by Salmonella enterica serovar Typhimurium. A-B. The effects of sanguinarine chloride on the invasion of HeLa cells by S. Typhimurium. Wild-type or invA mutant was used to infect HeLa cells. Internalized bacteria were enumerated after killing extracellular bacteria with gentamicin. The rates of invasion were calculated by setting the values of internalized wild type bacteria in untreated samples as 100%. Bar, S.E.M. (n = 3); **, p < 0.01. Results shown are from one representative experiment performed in triplicate. Similar results were obtained in three independent experiments (A). Representative images of the infected cells; images acquired at relevant fluorescence signals were merged. Note the reduction of internalized bacteria in infections with sanguinarine chloride-treated wild-type bacteria (B).
3.3. Sanguinarine chloride inhibits the secretion of SPI-1 virulence proteins
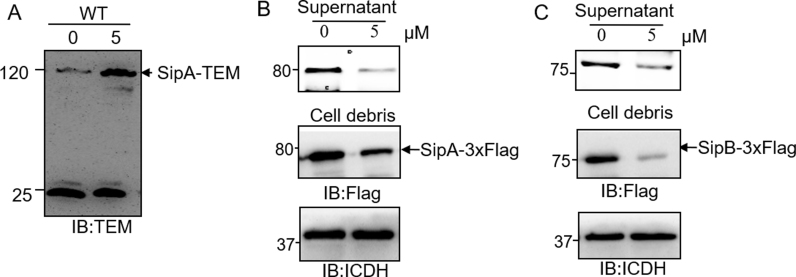
To distinguish whether sanguinarine chloride directly inhibited the secretion of the SPI-1 effector, we first examined the SipA-lactamase fusion expressed in Salmonella. Whereas untreated cells expressed readily detectable fusion protein, inclusion of 5 µM sanguinarine chloride in the bacterial cultures drastically increased the fusion protein level (Fig. 3A). A plausible explanation is that sanguinarine chloride inhibited the transcription of SPI-1 genes and that expression of SPI-1 effectors induced SipA-lactamase fusion protein accumulation in S. Typhimurium. To test this hypothesis, we constructed the S. Typhimurium SipA::3×FLAG and S. Typhimurium SipB::3×FLAG strains by placing a 3×FLAG at the C-termini of SipA and SipB, respectively. Chromosomal insertions of 3×FLAG strains were treated with or without sanguinarine chloride. As expected, the amount of expressed SipA::3×FLAG and SipB::3×FLAG proteins decreased (Fig. 3B, C). These results provided evidence that sanguinarine chloride can affect the production of multiple SPI-1 effectors.
Fig. 3.
Sanguinarine chloride inhibited the secretion of SPI-1 virulence proteins. A. Cultures of S. Typhimurium harboring the construct that directs the expression of SipA-lactamase. B-C. SipA-3xFlag and SipB-3xFlag from the chromosome were treated with the indicated concentrations of sanguinarine chloride for 4 h. Cleared cell lysates resolved by SDS-PAGE were probed for SipA and SipB by antibodies specific for the tag. The metabolic enzyme, isocitrate dehydrogenase (ICDH), was probed as a loading control. Similar results were obtained from three independent experiments.
3.4. Sanguinarine chloride inhibits SPI-1 effectors through SPI-1 transcription regulation
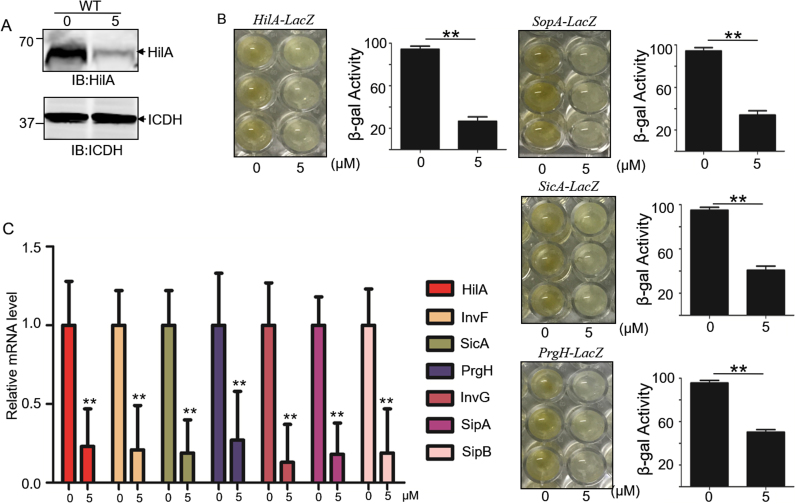
Given that expression of various SPI-1 effector proteins was reduced upon exposure to sanguinarine chloride, we tested whether some key transcription regulators of SPI-1 were influenced as well. Transcription factors help modulate the expression of invasion genes in S. Typhimurium, among which HilA acts as the key regulator by directly or indirectly regulating the expression of the secreted effectors of type III secretion system [19]. We thus investigated the expression of HilA under sanguinarine chloride treatment. The HilA protein level decreased when treated with 5 μM sanguinarine chloride (Fig. 4A). The hilA-lacZ, SopA-LacZ, SicA-LacZ and PrgH-LacZ fusion strain was treated with or without sanguinarine chloride and the relative β-galactosidase activity was measured. The level of β-galactosidase activity changed when treated with sanguinarine chloride, indicating that sanguinarine chloride influenced the expression of these proteins (Fig. 4B). To further determine the mechanism of sanguinarine chloride action, the relative mRNA levels of several genes regulating the transcription of SPI-1 were assessed by real-time qPCR (Fig. 4C). In this study, we tested HilA [20], the key regulator of SPI-1, downstream regulator protein genes invF and sicA [21], [22], SPI-1 apparatus protein genes prgH and invG [23], effector protein genes sipA and sipB [24]. In this study, sanguinarine chloride inhibited the transcription of HilA induced downstream genes.
Fig. 4.
Sanguinarine chloride inhibited SPI-1 effectors through regulation of SPI-1 transcription. A. Regulator protein HilA was expressed in S. Typhimurium with or without sanguinarine chloride for 4 h and the protein levels were evaluated by immunoblotting with a HilA-specific antibody. The metabolic enzyme isocitrate dehydrogenase (ICDH) was probed as a loading control. Note that only the protein level of HilA decreased in cultures treated with sanguinarine chloride. Similar results were obtained in two independent experiments. B. Cultures of an S. Typhimurium strain expressing a HilA-LacZ, SopA-LacZ, SicA-LacZ, PrgH-LacZ fusion from the chromosome prepared by including sanguinarine chloride was tested for β-galactosidase activity. Similar results were obtained from three independent experiments. Bar, S.E.M. (n = 3); **, P ≤ 0.01. The P value was calculated by comparing the value for the positive control. C. Effect of sanguinarine chloride on relative mRNA levels of the SPI-1 regulatory factors, effector gene, and apparatus protein gene. RNA was extracted from bacteria treated with sanguinarine chloride at a final concentration of 5 µM. The y-axis represents the relative transcriptional level of each gene (real-time qPCR) by the relative quantification method. Significant differences: **, P ≤ 0.01. The P value was calculated by comparing the value for the positive control.
4. Discussion
The type III secretion system is a highly specialized virulent protein nanoinjector, by which Salmonella enterica interacts with its hosts [1], [2]. T3SS-blocking agents can reduce the pathogenicity of Salmonella while having no inhibitory effect on bacterial growth. T3SS is increasingly being proposed and explored as an attractive drug target for developing novel antibacterial agents. Importantly, the virulence-associated SPI-1 plays an important role in the whole infectious mechanism of Salmonella. SPI-1 is the key to causing host infections, initiating systemic diseases, and sheltering Salmonella by promoting biofilm formation [11], [12]. HilA, the key regulator of SPI-1, combined with the promoters of AraC-like regulator genes invF and sicA, directly activates the transcription of its downstream genes, such as the SPI-1 apparatus genes prg and org, inv and spa operon genes, and effector genes. We demonstrated that sanguinarine chloride inhibited the activity of the T3SSs but did not affect the growth of Salmonella. Thus, this effect may allow circumventing the development of resistance. Currently, we and other groups have identified several small compounds as specific T3SS inhibitors. Akio Abe's group found that aurodox specifically inhibits the secretion of type III-secreted proteins such as EspB, EspF and Map, without affecting the expression of the housekeeping protein GroEL [25]. Cytosporone B also affects SPI-1, probably by interfering with gene expression [26]. In contrast, sanguinarine chloride targets SPI-1 regulatory protein HilA that controls the expression of a large number of virulence genes, which inhibit the activity of the T3SS-1 machinery.
In animal experiments, sanguinarine chloride was found to have animal toxicity even at a dose of 20 mg/kg. In future work, we need to modify sanguinarine chloride to reduce its drug toxicity.
Acknowledgements
This work was supported by grant from the National Natural Science Foundation of China (31620103918); Tianjin Municipal Natural Science Foundation, China (grant number 14ZCZDSY00046).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.04.011.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2018.04.011.
Contributor Information
Xuming Deng, Email: dengxm@jlu.edu.cn.
Xiao Chu, Email: 421591993@qq.com.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Transparency document. Supplementary material
Supplementary material
References
- 1.Galan J.E. Salmonella interactions with host cells: type III secretion at work. Ann. Rev. Cell Dev. Biol. 2001;17:53–86. doi: 10.1146/annurev.cellbio.17.1.53. [DOI] [PubMed] [Google Scholar]
- 2.Fass E., Groisman E.A. Control of Salmonella pathogenicity island-2 gene expression. Curr. Opin. Microbiol. 2009;12(2):199–204. doi: 10.1016/j.mib.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacFadden D.R., Bogoch I.I., Andrews J.R. Advances in diagnosis, treatment, and prevention of invasive Salmonella infections. Curr. Opin. Infect. Dis. 2016;29(5):453–458. doi: 10.1097/QCO.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 4.Raymond B., Young J.C., Pallett M. Subversion of rafficking, apoptosis, and innate immunity by type III secretion system effectors. Trends Microbiol. 2013;21(8):430–441. doi: 10.1016/j.tim.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Chousalkar K., Gole V.C. Salmonellosis acquired from poultry. Curr. Opin. Infect. Dis. 2016;29(5):514–519. doi: 10.1097/QCO.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 6.Stamm L.M., Goldberg M.B. Microbiology. Establishing the secretion hierarchy. Science. 2011;331:1147–1148. doi: 10.1126/science.1203195. [DOI] [PubMed] [Google Scholar]
- 7.Marlovits T.C., Stebbins C.E. Type III secretion systems shape up as they ship out. Curr. Opin. Microbiol. 2010;13:47–52. doi: 10.1016/j.mib.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marlovits T.C., Kubori T., Sukhan A., Thomas D.R., Galan J.E., Unger V.M. Structural insights into the assembly of the type III secretion needle complex. Science. 2004;306:1040–1042. doi: 10.1126/science.1102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yip C.K., Kimbrough T.G., Felise H.B., Vuckovic M., Thomas N.A., Pfuetzner R.A., Frey E.A., Finlay B.B., Miller S.I., Strynadka N.C. Structural characterization of the molecular platform for type III secretion system assembly. Nature. 2005;435:702–707. doi: 10.1038/nature03554. [DOI] [PubMed] [Google Scholar]
- 10.Lostroh C.P., Lee C.A. The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect. 2001;3:1281–1291. doi: 10.1016/s1286-4579(01)01488-5. [DOI] [PubMed] [Google Scholar]
- 11.Hapfelmeier S., Ehrbar K., Stecher B. Role of the Salmonella pathogenicity island 1 effector proteins SipA, SopB, SopE, and SopE2 in Salmonella enterica subspecies 1 serovar Typhimurium colitis in streptomycin-pretreated mice. Infect. Immun. 2004;72(2):795–809. doi: 10.1128/IAI.72.2.795-809.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrbar K., Mirold S., Friebel A. Characterization of effector proteins translocated via the SPI1 type III secretion system of Salmonella typhimurium. Int. J. Med. Microbiol. 2002;291(6–7):479–485. doi: 10.1078/1438-4221-00156. [DOI] [PubMed] [Google Scholar]
- 13.Cragg G.M., Newman D.J. Biodiversity: a continuing source of novel drug leads. Pure Appl. Chem. 2005;77:7–24. [Google Scholar]
- 14.Bajaj V., Hwang C., Lee C.A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol. Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 15.Gulig P.A., Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect. Immun. 1987;55(12):2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. (PubMed PMID: 3316027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin D., Rao C.V., Slauch J.M. The Salmonella SPI1 type three secretion system responds to periplasmic disulfide bond status via the flagellar apparatus and the RcsCDB system. J. Bacteriol. 2008;190(1):87–97. doi: 10.1128/JB.01323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginocchio C.C., Galan J.E. Functional conservation among members of the Salmonella typhimurium InvA family of proteins. Infect. Immun. 1995;63(2):729–732. doi: 10.1128/iai.63.2.729-732.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou D., Mooseker M.S., Galan J.E. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science. 1999;283(5410):2092–2095. doi: 10.1126/science.283.5410.2092. [DOI] [PubMed] [Google Scholar]
- 19.Ellermeier J.R., Slauch J.M. Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr. Opin. Microbiol. 2007;10(1):24–29. doi: 10.1016/j.mib.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 20.De Keersmaecker S.C., Marchal K., Verhoeven T.L., Engelen K., Vanderleyden J., Detweiler C.S. Microarray analysis and motif detection reveal new targets of the Salmonella enterica serovar Typhimurium HilA regulatory protein, including hilA itself. J. Bacteriol. 2005;187:4381–4391. doi: 10.1128/JB.187.13.4381-4391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darwin K.H., Miller V.L. The putative invasion protein chaperone SicA acts together with InvF to activate the expression of Salmonella typhimurium virulence genes. Mol. Microbiol. 2000;35:949–960. doi: 10.1046/j.1365-2958.2000.01772.x. [DOI] [PubMed] [Google Scholar]
- 22.Darwin K.H., Miller V.L. Type III secretion chaperone-dependent regulation: activation of virulence genes by SicA and InvF in Salmonella typhimurium. EMBO J. 2001;20:1850–1862. doi: 10.1093/emboj/20.8.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimbrough T.G., Miller S.I. Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc. Natl. Acad. Sci. USA. 2000;97(20):11008–11013. doi: 10.1073/pnas.200209497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou D., Galan J. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 2001;3:1293–1298. doi: 10.1016/s1286-4579(01)01489-7. [DOI] [PubMed] [Google Scholar]
- 25.Kimura K., Iwatsuki M., Nagai T., Matsumoto A., Takahashi Y., Shiomi K., Omura S. Abe A small-molecule inhibitor of the bacterial type III secretion system protects against in vivo infection with Citrobacter rodentium. J. Antibiot. 2011;64(2):197–203. doi: 10.1038/ja.2010.155. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Lv C., Sun W., Li Z., Han X., Li Y. Cytosporone B, an inhibitor of the type III secretion system of Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 2013;57(5):2191–2198. doi: 10.1128/AAC.02421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material