Abstract
To investigate possible roles of polar auxin transport in vein patterning, cotyledon and leaf vein patterns were compared for plants grown in medium containing polar auxin transport inhibitors (N-1-naphthylphthalamic acid, 9-hydroxyfluorene-9-carboxylic acid, and 2,3,5-triiodobenzoic acid) and in medium containing a less well-characterized inhibitor of auxin-mediated processes, 2-(p-chlorophynoxy)-2-methylpropionic acid. Cotyledon vein pattern was not affected by any inhibitor treatments, although vein morphology was altered. In contrast, leaf vein pattern was affected by inhibitor treatments. Growth in polar auxin transport inhibitors resulted in leaves that lacked vascular continuity through the petiole and had broad, loosely organized midveins, an increased number of secondary veins, and a dense band of misshapen tracheary elements adjacent to the leaf margin. Analysis of leaf vein pattern developmental time courses suggested that the primary vein did not develop in polar auxin transport inhibitor-grown plants, and that the broad midvein observed in these seedlings resulted from the coalescence of proximal regions of secondary veins. Possible models for leaf vein patterning that could account for these observations are discussed.
In all plants, efficient delivery of water and dissolved nutrients and transfer of fixed carbon are vital for plant survival. The challenge of this material transfer is especially acute in leaves, because this is where most fixed carbon is produced, and because the high surface area to volume ratio can result in significant water loss. Plants solve this problem through the development of an integrated network of veins (the vascular system) that interconnect all parts of the plant. The veins are composed of two tissues, xylem and phloem, which function to transport water and photosynthate, respectively. Within the leaf, some veins connect to the main vascular system in the stem, and others extend through the lamina. In dicots, it is the central midvein (or primary vein) that extends in the proximal/distal axis and connects with the vascular tissue of the stem, while secondary veins branch from the midvein, and additional smaller veins (minor veins) interconnect the larger veins and provide access to the vascular system for cells that are distant from the major veins. These basic pattern elements can appear in distinctly different patterns in different groups of plants.
Mechanisms controlling leaf vein pattern are poorly understood (Nelson and Dengler, 1997). Many studies have indicated that the hormone auxin is important for the induction of vascular tissue development (Jacobs, 1952; Fosket and Roberts, 1964; Fukuda and Komamine, 1980; Klee et al., 1987; Romano et al., 1991; Aloni, 1995). Auxin is synthesized in apical portions of the plant (leaf primordia) and is transported basipetally through the plant. Positioning of auxin efflux carriers on the basal end of specialized cells has been proposed to serve as the driving force for this basipetal movement (Goldsmith, 1977; Lomax et al., 1995). Auxin transport has been shown to occur through cells that are either within or adjacent to vascular tissue (Wangermann, 1974; Morris and Thomas, 1978). Increased concentrations of auxin in the vascular cambium due to basipetal transport has also been shown to be important for the delivery of sufficient auxin to activate mitosis in vascular cambium (Uggla et al., 1996), to influence the size of the differentiating tracheary elements (Sheldrake and Northcote, 1968), and to serve as the signal for different vascular cell type specification (Uggla et al., 1996, 1998; Tuominen et al., 1997).
A model that integrates the biology of auxin synthesis, auxin movement, and auxin's role in vascular tissue development to explain vein patterning has been proposed by Sachs (1981, 1991). This model, called the canalization of auxin flow hypothesis, proposes that one response to elevated auxin levels is the induction of cells that are specialized for auxin polar transport. These specialized cells are proposed to elongate and position auxin efflux carriers on their basal membrane. Functioning of these cells would result in the release of auxin at their basal end, which would then induce an adjacent cell to also specialize for polar auxin transport. Because the establishment of polar auxin transport paths both delivers auxin to underlying cells and removes auxin from adjacent tissues, the net result is the establishment of files of cells, or canalized paths, for auxin transport. Furthermore, these canalized paths of auxin transport are proposed to provide the positional cues that define vein positions, a suggestion supported by auxin's role in vascular cell type induction and the association of auxin polar transporting cells with veins in stems.
Data to support this model come from several sources. Detailed observations of wound repair of severed stem veins has shown that the polarity of repair is consistent with the polar transport of auxin, and that interrupting this transport prevents repair (Jacobs, 1952; Fosket and Roberts, 1964; Sachs, 1981). Observations of abnormal tissue-culture-derived carnation plants showed that decreased vascular tissue was accompanied by a loss of polar auxin transport in leaves (Gersani et al., 1986). Callus cultures, manipulated such that auxin gradients were created, were able to respond by differentiation of veins (Wetmore and Rier, 1963).
Several Arabidopsis mutants with altered veins have been described. For example, the pin-formed (pin) and lopped (lop1) mutants have vascular defects and decreased auxin transport in their stems (Okada et al., 1991; Bennett, 1995; Carland and McHale, 1996; Gälweiler et al., 1998). The auxin-resistant mutant axr1 has reduced auxin responses, and mutants show a subtle decrease in the size of vascular bundles in their stems (Lincoln et al., 1990). Finally, the MONOPTEROS (MP) gene product has similarity to protein motifs that bind auxin response elements located in promoters of some auxin-inducible genes (Hardtke and Berleth, 1998). Mutants of mp display a variety of defects ranging from the lack of a root and hypocotyl to discontinuities in both cotyledon and leaf veins (Przemeck et al., 1996). The connection between the mp vein discontinuity phenotype and the predicted gene function of MP in auxin responses provides some of the strongest evidence linking auxin and vascular pattern. Nevertheless, elements of vascular pattern still remain with all of these mutants, and a clear picture of how auxin and the auxin signaling pathway confers vascular pattern throughout the plant has yet to emerge.
Auxin inhibitors provide an additional tool for investigating the role of auxin in development. For example, a pivotal role for auxin polar transport in the establishment of bilateral symmetry in embryos has been shown by comparing embryos cultured with or without auxin transport inhibitors (Shiavone and Cooke, 1987; Liu et al., 1993; Fischer and Neuhaus, 1996). Furthermore, that embryo bilateral symmetry was blocked by culture in medium containing either synthetic auxins or 2,3,5-triiodobenzoic acid (TIBA, a polar auxin transport inhibitor), but was not blocked by culture in medium containing 2-(p-chlorophynoxy)-2-methylpropionic acid (PCIB), indicates that this developmental step specifically required polar auxin transport (Fischer and Neuhaus, 1996).
In the present study, the effects of growing plants in the presence of polar auxin transport inhibitors on leaf and cotyledon vein pattern were studied. Disruption of polar auxin transport results in abnormal vein formation in cotyledons and multiple defects in leaf vein pattern. These effects are discussed in terms of models for leaf vein patterning.
MATERIALS AND METHODS
Plant Growth
Arabidopsis ecotypes Landsberg erecta and Columbia-0 were grown in sterile growth medium that contained 0.5× Murashige and Skoog salts (Sigma, St. Louis), 1% (w/v) Suc, 0.5 g/L 2-[N-morpholino]HEPES (MES), pH 5.8 (KOH), 0.8% (w/v) Phytagar (Gibco-BRL, Gaithersburg, MD). Auxin inhibitor stocks were prepared in ethanol. TIBA, 9-hydroxyfluorene-9-carboxylic acid (HFCA), and PCIB were obtained from Sigma, and N-1-naphthylphthalamic acid (NPA) was obtained from Chemical Service (Westchester, PA). Seeds were surface-sterilized by immersion in a solution of 50% (v/v) bleach and 0.02% (v/v) Triton X-100 (Sigma) for 7 min, and rinsed three times with sterile deionized water prior to plating. Plates were sealed with gas-permeable tape and incubated for 2 d in the dark at 4°C. Plates were then incubated at 24°C under constant illumination (approximately 100 μm m−2 s−1). Seedling development was measured in terms of days post imbibition (DPI). Thus, a 3-DPI seedling is one that had been placed into the growth chamber 72 h earlier.
Tissue Fixation
Plant tissue was fixed by immersing it in a 3:1 mixture of ethanol:acetic acid overnight. Chlorophyll was then removed by passage of the tissue through 80%, 90%, 95%, and 100% (v/v) ethanol. The tissue was cleared by incubation overnight in saturated chloral hydrate (Sigma).
Microscopy
Tissue was mounted in saturated chloral hydrate and visualized with dark-field and differential interference contrast optics using a microscope (model BX-50, Olympus, Tokyo).
Gravitropism Measurements
Gravitropism measurements were obtained by counting the number of seedlings whose roots penetrated the 0.8% (w/v) agar medium of a horizontally incubated plate at 7 DPI, and expressing this number as a percentage of the total number of seedlings on the plate.
Cotyledon Vascular Tissue
Dry seeds were imbibed in 70% (v/v) ethanol overnight, and the embryos were removed and cleared in aqueous chloral hydrate as described above.
RESULTS
The goal of this study was to investigate whether auxin plays a role in patterning of leaf and cotyledon vascular tissue. To address this question, I analyzed Arabidopsis seedlings grown in the presence of two classes of auxin inhibitors. The first class, polar auxin transport inhibitors, included NPA, TIBA, and HFCA (Krelle and Libbert, 1968; Thomson et al., 1973). The second class of inhibitor, which interferes with auxin activities but is not a polar auxin transport inhibitor, was represented by a single compound, PCIB (McRae and Bonner, 1953; Foster et al., 1955; Evans and Hokanson, 1969). For each of the four inhibitors, seedlings were grown on sterile medium containing varying concentrations of each auxin inhibitor, and the effects on overall seedling development, vein development in cotyledons, and vein development in leaves was assessed.
Auxin Inhibitor Effects on Seedling Development
I first analyzed the effects of the auxin inhibitors at the whole-plant level. Table I summarizes the effects of these inhibitors on cotyledon morphology, leaf morphology, leaf fusion, inflorescence morphology, and root growth. Representative polar auxin transport-inhibitor-grown seedlings are shown in Figure 1. Cotyledon morphology, leaf morphology, and root growth were affected by both inhibitor classes. Cotyledons and leaves were reduced in size and curved downward (epinastic). Roots were stunted and club-shaped for NPA-grown tissue, and root growth occurred in a random orientation instead of being oriented in a downward direction. Because these effects occurred as a result of both inhibitor class treatments, they were probably caused by a general perturbation of auxin conditions.
Table I.
Effect of auxin inhibitors on Arabidopsis seedling development
| Inhibitor/Treatment | Cotyledon Morphology | Leaf Morphology | Leaf Fusion | Inflorescence Morphology | Root Gravitropism |
|---|---|---|---|---|---|
| μm | |||||
| HFCA | |||||
| 0 | Normal | Normal | Not obs. | Normal | 88 (50) |
| 1 | Normal | Normal | 3% (157) | Normal | 92 (50) |
| 5 | Normal | Normal | 9% (189) | Abnormal | 14 (50) |
| 10 | Normal; ep. | Normal | 16% (125) | Abnormal | 8 (50) |
| 30 | sl. st.; ep. | sl. st. | 18% (147) | Abnormal | 0 (20)# |
| 50 | v. st.; ep. | v. st. | 16% (169) | Abnormal | ** |
| 100 | v. st.; chl.; ep. | v. st.; chl. | 14% (192) | Abnormal | ** |
| TIBA | |||||
| 0 | Normal | Normal | Not obs. | Normal | 85 (69) |
| 1 | Normal | Normal | 6% (144) | Normal | 38 (86) |
| 5 | sl. st. | sl. st. | 12% (156) | Abnormal | 16 (96) |
| 10 | sl. st; ep. | sl. st.; ep. | 11% (181) | Abnormal | 5.8 (104) |
| 30 | sl. st.; ep. | sl. st.; chl.; ep. | 22% (196) | Abnormal | 0 (113) |
| 50 | v. st.; chl.; ep. | v. st; chl.; ep. | 15% (112) | Abnormal | ** |
| 100 | v. st.; | – | – | – | ** |
| NPA | |||||
| 0 | Normal | Normal | Not obs. | Normal | 90 (49) |
| 1 | Normal | sl. st. | 21% (194) | Normal | 39 (44) |
| 5 | Normal; ep. | sl. st. | 18% (170) | Abnormal | 14 (63) |
| 10 | Normal; ep. | st. | 16% (222) | Abnormal | 4 (66) |
| 30 | st.; ep. | st. | 15% (155) | Abnormal | 3 (64) |
| 50 | v. st.; ep. | v. st. | 7% (182) | Abnormal | ** |
| 100 | v. st.; ep. | v. st. | 1% (160) | Abnormal | ** |
| PCIB | |||||
| 0 | Normal | Normal | Not obs. | Normal | 85 (120) |
| 1 | Normal | Normal | Not obs. | Normal | 60 (148) |
| 5 | Normal | Normal | Not obs. | Normal | 77 (106) |
| 10 | Normal | Normal | Not obs. | Normal | 64 (115) |
| 30 | sl. st.; | sl. st. | Not obs. | Normal | 18 (89) |
| 50 | v. st.; ep. | st.a; ep. | Not obs. | Normal | 16 (100) |
| 100 | v. st., chl., ep. | v. st.a; chl.; ep. | Not obs. | Normal | ** |
Cotyledon and leaf morphology: sl. st., slightly stunted; v. st., very; stunted; st., stunted; chl., chlorotic; ep., epinastic. Leaf fusion: percent indicates proportion seedlings showing any fusion of first leaf pair, numbers in parenthesis indicate the number of seedlings scored. Abnormal inflorescence morphologies included inflorescences containing flowers with reduced numbers of stamens and increased numbers of petals and inflorescences devoid of flowers and that resembled pin-like structures. Root gravitropism was scored by assessing whether roots penetrated the agar; numbers in parenthesis indicate number scored.; **, roots too short to score; #, scoring based on a small fraction of plants with roots more than 1 cm.
Some plants grown in 50 and 100 μM PCIB produced narrow and elongate leaves, data not shown.
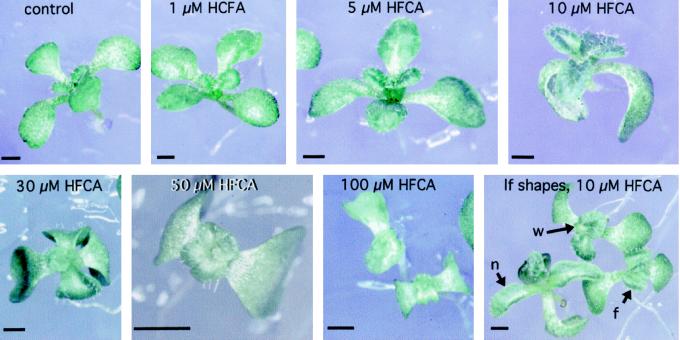
Figure 1.
Eleven-day-old seedlings grown in the presence of HFCA. The 10 μm HFCA panel on the lower right shows three extremes of leaf shape observed among Ler seedlings grown on the same plate. n, A seedling with long narrow leaves; w, a seedling with short wide leaves; and f, a seedling with fused first leaf pair. Size bars = 1 mm.
Leaf size was affected by both inhibitor classes (Table I; Fig. 1), and the polar auxin transport inhibitors also affected leaf shape (Fig. 1). Leaf size was similar among the control, 1, 5, and 10 μm treatments, but at higher concentrations was progressively reduced. I observed a diversity of leaf shapes among seedlings grown at low to moderate concentrations of the polar auxin transport inhibitors. Three seedlings with different leaf shapes, but grown on the same plate of medium containing 10 μm HFCA are shown in Figure 1. Leaf shape ranged from long and narrow to short and wide. At higher inhibitor concentrations, the long narrow class of leaf shape disappeared, and all treated plants contained short wide leaves.
Leaf fusion and inflorescence morphology were affected by only one class of auxin inhibitors, the auxin polar transport inhibitors (Table I). In contrast to normal leaves, some seedlings grown on auxin polar transport inhibitors had leaves fused between 20% and 100% of their length. These fused leaves resembled the array of fused cotyledons that are observed among embryos with compromised auxin polar transport (Liu et al., 1993; Bennett et al., 1995; Hadfi et al., 1998). These observations suggest that for leaves, as for cotyledons, transport of auxin is important for the definition of organ boundaries. Two types of abnormal inflorescences were observed. At low inhibitor concentrations, abnormal inflorescences most commonly contained flowers with reduced stamen number and increased petal number, and at higher concentrations, inflorescences devoid of flowers and resembling pin-like structures, were also present. This inflorescence morphology matched that described previously for plants grown in the presence of polar auxin transport inhibitors (Okada et al., 1991). Because the fused leaves and altered inflorescences were only observed in plants treated with polar auxin transport inhibitors, these changes probably result specifically from the loss of auxin polar transport.
Cotyledon Vein Pattern
Untreated Seedlings
I characterized the cotyledon vein pattern in untreated Arabidopsis seedlings. The typical pattern for mature cotyledons is depicted in Figure 2A and shown in Figure 3A. Two types of veins are present, a midvein (or primary vein) and several secondary veins that branch from the midvein and then unite to form areoles (a space delimited by veins). Most often, four secondary veins and four areoles were formed (59%, n = 200 L. er cotyledons). A common deviation from this pattern was the failure of one (29%) or both (12%) of the proximal secondary veins to connect with the midvein, thus forming cotyledons that had only three or two areoles, respectively (Fig. 3B). Infrequently (7%), a fifth secondary vein (Fig. 3C) or a short spur of vascular tissue (arrow in Fig. 3A) was also observed. The Col-0 ecotype showed similar cotyledon vein patterns.
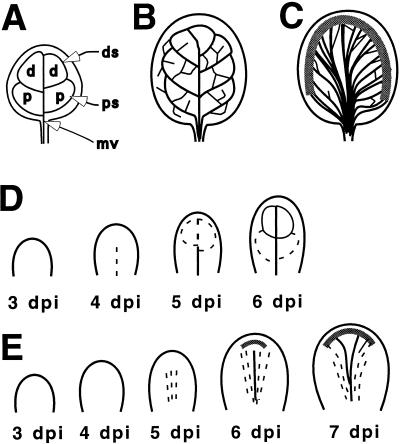
Figure 2.
Cotyledon and leaf vein patterns. A, Normal cotyledon vein pattern. mv, Midvein; ds, distal secondary vein; ps, proximal secondary vein; d, distal areole; p, proximal areole. B, Leaf vein pattern of an untreated leaf. C, Leaf vein pattern typical of a seedling treated with polar auxin transport inhibitors. The thick gray line represents a broad band of disorganized TEs. D, Early vein development in a normal leaf. Dashed lines represent provascular tissue and solid lines represent veins containing TEs. E, Early leaf vein development in a seedling grown in the presence of polar auxin transport inhibitors. Dashed and solid lines are as in the untreated leaves. The gray line represents the band of misshapen TEs that accumulate around the leaf margin.
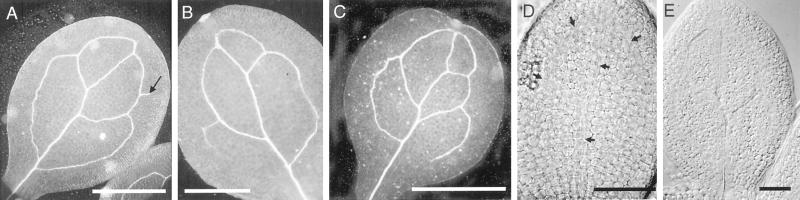
Figure 3.
Cotyledon vein pattern. A, Dark-field view of the typical cotyledon vein pattern in an 11-DPI Ler seedling; arrow indicates a spur of vascular tissue. B, Dark-field view of an 11-DPI Col-0 cotyledon, in this cotyledon only three areoles formed. C, Dark-field view of an 11-DPI Ler cotyledon in which a fifth secondary vein was formed. D, Ler cotyledon from a dry seed embryo, differential interference contrast optics. Arrows point to a network of elongated cells (provascular tissue) that is present in the typical cotyledon vein pattern. E, Cotyledon of a 3-DPI Ler seedling, TEs have differentiated in the midvein and are partially formed in the two distal secondary veins. Size bars in A through C = 1 mm; in D and E = 100 μm.
Because my experimental plan was to examine the influence of auxin inhibitors on vascular tissue development by germinating seeds on medium containing the inhibitors, it was important to know the status of cotyledon vascular tissue in dry seed embryos. Vascular tissue arises from the recruitment of undifferentiated cells in organ primordia. The recruited cells first elongate to form provascular tissue and then differentiate into cells of the xylem and phloem (Northcote, 1995). It was possible that cotyledons of dry seeds could have fully differentiated vascular tissue, provascular tissue, or neither of these. Observations of more than 20 cotyledons dissected from dry seed embryos showed that all contained provascular tissue in the typical cotyledon pattern (Fig. 3D), indicating that cotyledon vein pattern is established during embryogenesis.
Because provascular tissue is already present in the dry seed embryo, the next question was to determine when cotyledon provascular tissue differentiates. I used tracheary elements (TEs) as a marker for vascular tissue differentiation. TEs are the water-conducting cells of the xylem, and are narrow, elongated cells arranged end-to-end in linear files. These cells can be identified by their secondary cell wall, which in Arabidopsis seedlings is usually arranged either as a spiral or as coaxial rings. At 1 DPI, cotyledons contained provascular tissue, but no differentiated TEs were observed. At 2 DPI, differentiated TEs were occasionally observed (approximately 40% of the 2-DPI cotyledons examined), and these TEs were generally restricted to the midvein. By 3 DPI, files of TEs were observed in the midvein and the two distal secondary veins (Fig. 3E). By 4 DPI, I observed TEs in all of the cotyledon veins. These observations indicate that TEs in the cotyledon veins begin to be formed at approximately 2 DPI, and that the cotyledon secondary veins appear in a sequential order, with the distal veins differentiating prior to the proximal secondary veins.
Auxin-Inhibited Seedlings
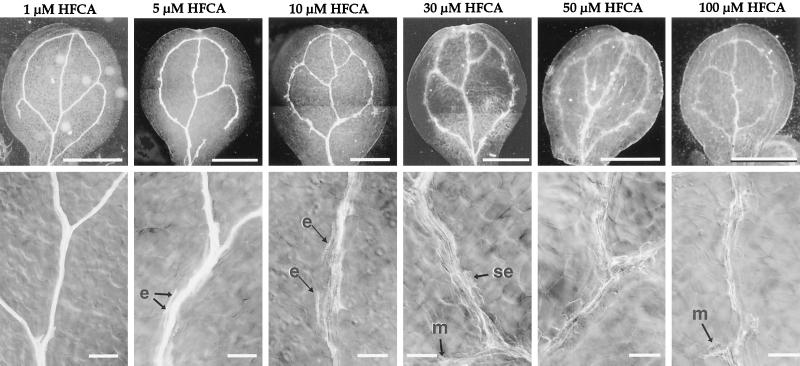
I scored cotyledon vein pattern and vein morphology in 50 inhibitor-treated cotyledons from each treatment and compared them with the untreated control. No differences in cotyledon vein pattern were observed, consistent with cotyledon vein pattern being established during embryogenesis. Cotyledon vein morphology, however, was affected by the inhibitor treatment. Vein morphology in inhibitor-treated seedlings varied from that of untreated seedlings in three ways. First, the veins appeared thicker because they contained more files of TEs (Fig. 4). Second, some TEs were observed outside of the normal vein files (ectopic TEs, Fig. 4). These ectopic TEs were typically either solitary or short files of two to four cells. Third, some TEs in cotyledons of inhibitor-treated plants were misshapen, with a variety of rounded shapes, in contrast to the narrow, elongated TEs in the untreated tissue (Fig. 4). These three changes were observed in seedlings treated with both inhibitor classes (Table II). The appearance of misshapen and ectopic TEs suggests that interfering with auxin conditions results in cells outside of normal vein positions being recruited to a TE fate.
Figure 4.
Vein pattern of cotyledon from auxin polar transport inhibitor-grown seedlings. The top row are dark-field images of Ler cotyledons from plants grown in the presence of the polar auxin transport inhibitor HFCA. The bottom row contains differential interference contrast images of the midvein between or adjacent to the branch site of the distal secondary. Arrows with an “e” point to short files of ectopic TEs, arrows with “se” point to solitary ectopic tracheary elements, and arrows with an “m” point to ectopic misshapen tracheary elements. Size bars for the top row = 1 mm; for the bottom row = 100 μm.
Table II.
Auxin inhibitor effects on cotyledon veins
| Inhibitor | 1 μM | 5 μM | 10 μM | 30 μM | 50 μM | 100 μM |
|---|---|---|---|---|---|---|
| HFCA | Normal | E, M | E, M, T | E, M, T | E, M, T | E, M, T |
| TIBA | Normal | E, M, T | E, M, T | E, M, T | E, M, T | * |
| NPA | (T, E) | E, M, T | E, M, T | E, M, T | E, M, T | E, M, T |
| PCIB | Normal | Normal | Normal | E, M | E, M, T | E, M, T |
E, Ectopic TEs; M, misshapen TEs; T, thick veins composed of more files of TEs than the same vein in the untreated control; *, lethal dose; (T, E), thick veins and ectopic TEs observed in approximately one-half of the samples examined.
Auxin Inhibitors Affect Leaf Vein Pattern
To determine whether auxin inhibitors affect leaf vein pattern, I grew Arabidopsis seedlings in the presence of auxin inhibitors and compared leaf vein pattern to that of noninhibited controls. Leaf vein pattern for Arabidopsis has been described previously (Nelson and Dengler, 1997; Kinsman and Pyke, 1998; Van Lijsebettens and Clarke, 1998; Candela et al., 1999) and is shown in Figures 5A and 2B. Like cotyledons, leaves contain a central midvein (primary vein) and secondary veins that branch from the midvein. However, in contrast to cotyledons, leaves typically contain six to eight secondary veins, as well as veins that bridge the other veins or form free-ending veinlets (minor veins) (Nelson and Dengler, 1997).
Figure 5.
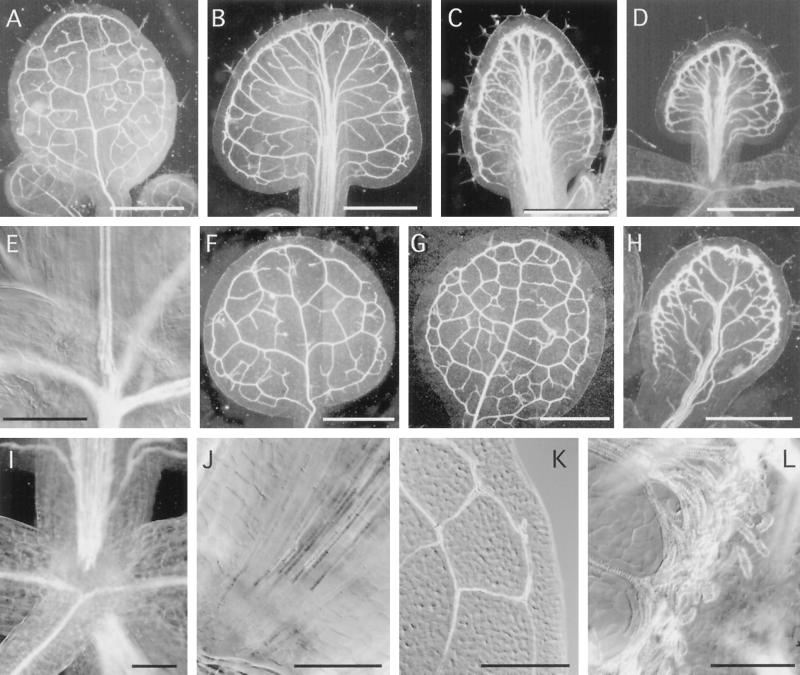
Auxin inhibitors affect leaf vein pattern. A, Normal, untreated leaf vein pattern (11 DPI); B, vein pattern of an 11-DPI seedling grown in medium containing 1 μm NPA; C, vein pattern of an 11-DPI seedling grown in medium containing 10 μm NPA; D, vein pattern of an 11-DPI seedling grown in medium containing 100 μm NPA; E, petiole/hypocotyl junction of an untreated 11-DPI seedling; F, first leaf of an 11-DPI seedling grown in medium containing 1 μm PCIB; G, first leaf of an 11-DPI seedling grown in medium containing 10 μm PCIB; H, First leaf of an 11-DPI seedling grown in medium containing 100 μm PCIB; I and J, petiole/hypocotyl junction from plant grown in 100 μm NPA; K, vein along margin of untreated leaf; and L, vein from along margin of 10 μm NPA-treated leaf. Size bars for A through D and F through H = 1 mm; for E, I, and K = 200 μm; and for J and L = 100 μm.
The leaf vein pattern of auxin inhibitor-treated seedlings differed from the untreated seedlings (Fig. 5). In addition, there were some differences in the leaf vein pattern between the two inhibitor classes. For the polar auxin transport inhibitors (NPA, HFCA, and TIBA), the most pronounced effects on leaf veins were alterations in the midvein and in the veins adjacent to the leaf margin (Figs. 5, B–D, and 2C). Leaves of polar auxin transport inhibitor-grown seedlings differed from those of untreated seedlings in five ways: (a) there was often space between some TE cell files; (b) the midvein was often bifurcated; (c) more secondary veins branched from the midvein; (d) the midvein was much thicker; and (e) the midvein was not continuous with the stem vascular tissue, but instead terminated in the petiole (compare untreated tissue, Fig. 5E, with polar auxin transport inhibitor-treated tissue in Fig. 5, I and J). The veins adjacent to the leaf margin of the polar auxin transport inhibitor-grown seedlings differed in that a thick band of TEs extended around the leaf margin (compare Fig. 5, K and L). These TEs were a combination of normal-appearing, elongated TEs and rounded, misshapen TEs (Fig. 5L). These changes were observed with all three polar auxin transport inhibitors.
The leaf vein pattern for seedlings grown in medium containing the second auxin inhibitor class (PCIB) showed a subset of the defects observed for the polar auxin transport inhibitors (Fig. 5, F–H). As in the polar auxin transport inhibitor-grown seedlings, the midvein was thicker and spaces between TE cell files were evident. However, the midvein was continuous with the stem at all concentrations tested (up to 100 μm). The veins at the leaf margin of the PCIB-grown leaves appeared the same as those of the polar auxin transport inhibitor-grown seedlings. At intermediate concentrations, leaves contained a greater number of veins, however, the overall pattern was similar to that of wild type (Fig. 5G).
Development of Leaf Vein Patterns
To understand the developmental basis for the changes in leaf vein pattern in the inhibitor-grown seedlings, I compared a developmental time course (3–19 DPI) for untreated and polar auxin transport inhibitor-grown seedlings (Fig. 6, and depicted in Fig. 2, D and E).
Figure 6.
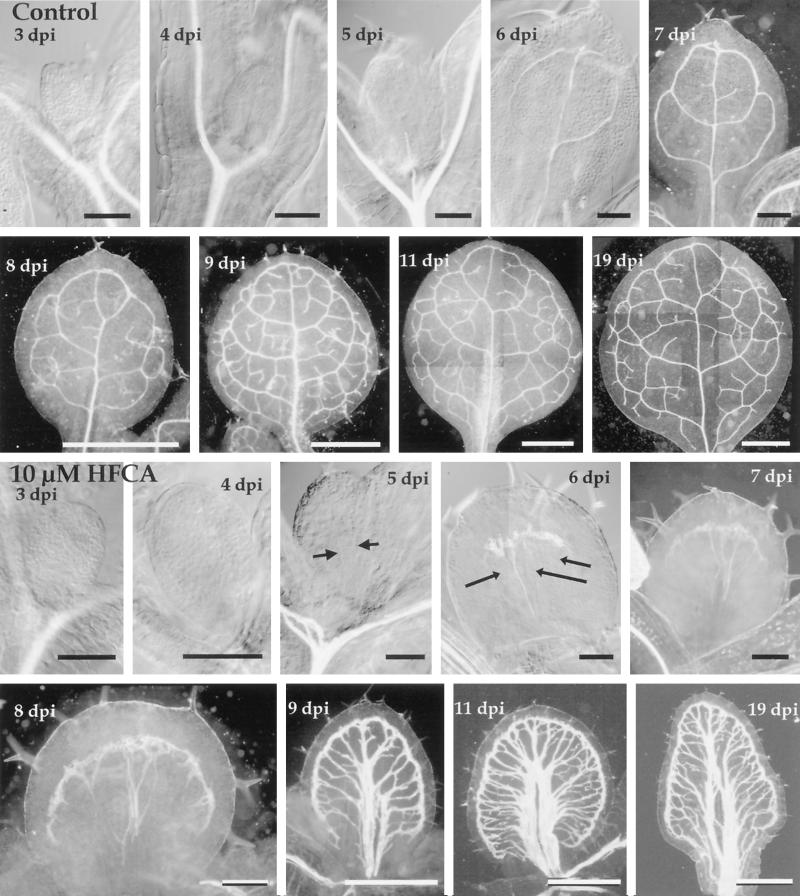
Development of leaf vein pattern in control and polar auxin transport-inhibited plants. The top two rows show the vein pattern of the first leaf from a developmental time course of control plants. The bottom two rows show the same time points for the first leaf of seedlings grown in the presence of 10 μm HFCA, a polar auxin transport inhibitor. Arrows in the 5- and 6-DPI HFCA-treated leaf primordium indicate files of provascular tissue. Time is indicated as DPI, days post imbibition. Size bars for 3- to 6-DPI leaves (control and HFCA-treated) = 100 μm; for 7-DPI leaves (control and HFCA-treated) and 8-DPI HFCA-treated = 200 μm; for 8- to 19-DPI control and 9- to 19-DPI HFCA-treated = 1 mm.
Untreated Seedlings
Development of leaf vein pattern in untreated seedlings is shown in Figure 6 and depicted in Figure 2D. At 3 DPI, there was no vascular or provascular tissue in the leaf primordia (Fig. 6). At 4 DPI, the leaf primordia contained a single strand of provascular tissue that was continuous with the stem vascular tissue and extended to approximately the center of the leaf primordia (Fig. 6). This location of the provascular tissue suggested that it was the nascent primary vein.
At 5 DPI, the primary vein provascular tissue extended to the apex of the developing leaf, and files of TEs were present in the proximal regions of the midvein (Fig. 6). In addition, provascular tissue for two secondary veins was present; each provascular strand extended from the midpoint of the midvein into the developing blade region and joined the midvein at its distal end (Fig. 6).
At 6 DPI, TEs were present all the way to the distal end of the primary vein, and along the entire length of the distal-most secondary veins. Proximal to these secondary veins were provascular strands for the next set of secondary veins (Fig. 6). At 7 DPI, this second pair of secondary veins contained files of TEs (Fig. 6). Additional secondary veins were present as provascular strands in more proximal positions. In addition, the 7-DPI leaves also contained many strands of provascular tissue that bridged either established veins or other provascular tissue. Because of their positions, these provascular strands were likely to be the precursors of minor veins.
At 8 DPI, the leaves displayed a diverse array of vein patterns; in general, I observed TEs in three to five additional veins (relative to the 7-DPI leaves) (Fig. 6), and many more strands of provascular tissue. At 9 DPI, leaves contained an essentially complete leaf vein pattern (Fig. 6). Most of the veins contained TEs, the only exceptions being three to five veins in proximal regions of the leaf that contained some provascular segments devoid of TEs. At 10 DPI, leaf provascular segments were not detected, and the leaf vein pattern was the same as that in 10- to 19-DPI leaves (Fig. 6 shows 11- and 19-DPI leaves).
These observations reveal several steps in leaf vein patterning. First, the timing of appearance of the midvein and secondary veins differ; the primary vein can be observed in 4 DPI leaves, whereas no secondary veins were observed until 5 DPI or later time points. Second, the primary vein develops in an acropetal direction; both provascular tissue and TEs are first observed in proximal regions, and later in the distal regions of the midvein. Third, the secondary veins appear sequentially; the first secondary veins to develop appear at the distal end of the leaf, and subsequent secondary veins appear in more proximal regions. Finally, under these growth conditions, vein pattern is complete by 8 DPI.
Polar Auxin Transport Inhibitor-Treated Seedlings
The development of leaf vein pattern in inhibitor-treated seedlings is shown in Figure 6 and is depicted in Figure 2E. At both 3 and 4 DPI, there was no vascular or provascular tissue in leaf primordia of polar transport inhibitor-treated seedlings (Fig. 6). At 5 DPI, between two and four files of provascular tissue were detected (Fig. 6). These cell files extended through the center of the blade, between the distal end of the leaf and proximal regions of the blade, and were not connected to the hypocotyl vascular tissue. The direction of differentiation of these veins could not be determined.
At 6 DPI, at least one of the proximal/distal extending files of provascular tissue contained differentiated TEs, and at least four strands of provascular tissue extended the length of the leaf primordium. None of the provascular or TE cell files connected to the hypocotyl. A band of TEs was also present at the distal end of the developing leaf (Fig. 6). This band contained mostly misshapen TEs, although some elongated TEs were present along the proximal side (data not shown).
At 7 DPI, at least three developing veins that extended along the proximal/distal axis of the leaf contained differentiated TEs (Fig. 6). In contrast to the control, in which early-differentiating veins were distributed through much of the developing leaf, these veins were located close together, in the central region of the developing leaf. The band of TEs at the distal end of the leaf extended across one-half of the leaf, and a small number of elongated cells extended from the proximal regions at each end.
Inhibitor-grown leaves at 8 DPI showed a diverse array of vein patterns. In general, two or three additional provascular strands that extended along the proximal/distal axis contained TEs, and many provascular strands extended between the distal band of TEs and the center of the developing leaf. Some of the cell files that contained differentiated TEs were closely aligned in the center of the developing leaf. The distal band of misshapen TEs now extended along more than 80% of the leaf margin.
At 9 DPI, many veins with TEs were present in the leaf, although many provascular strands devoid of differentiated TEs were also present. By 10 DPI, however, no veins containing only provascular strands were present. The veins that extended between the band of TEs adjacent to the leaf margin and the center of the leaf were loosely aligned and defined a broad central midvein. Between 11 and 19 DPI, no additional veins appeared.
These observations suggest that the polar auxin transport inhibitors affected many different aspects of leaf vein development. First, the timing of midvein appearance differed; the first provascular tissue was observed in 5-DPI seedlings of the treated plants, in contrast to the 4-DPI provascular tissue observed for the untreated control.
Second, this provascular tissue of inhibitor-treated seedlings differed in conformation relative to the untreated control. In inhibitor-grown seedlings, three or more separate files of provascular tissue was present, as opposed to the single file of the control, and it terminated in proximal regions of the leaf primordium, instead of being continuous with the stem vascular tissue as in the control. Third, the primary and secondary veins were difficult to distinguish in inhibitor-treated leaves. The multiple files of provascular tissue in the center of the leaf differentiated into veins that were encompassed in the midvein in proximal regions of the leaf, and usually branched toward the leaf margin in distal portions of the leaf. Fourth, a novel pattern element appeared in the inhibitor-grown seedlings; a band of mostly misshapen TEs arose at the distal end of the leaf, and expanded proximally along the leaf margin.
DISCUSSION
The leaves of dicots contain three major pattern elements: a midvein that extends from the stem through the petiole and into the leaf blade; secondary veins that branch from the midvein; and minor veins that further subdivide the main leaf area. The patterns in which these veins appear vary widely across different groups of dicots, yet the mechanisms controlling vein pattern are poorly understood. Auxin has been proposed to function at many different steps of vascular tissue development, ranging from specification of pattern to later stages of vascular cell type differentiation (Aloni, 1995). To examine possible roles of auxin and polar auxin transport in establishing vein pattern, I compared cotyledon and leaf vein pattern development in Arabidopsis seedlings grown in medium containing polar auxin transport inhibitors. Growth in these inhibitors caused many changes in the leaf vein pattern, and my observations are in agreement with a recent publication by Mattsson et al. (1999) describing a similar study.
Polar Auxin Transport Is Required for Leaf Midvein Development
Growth of seedlings in media containing polar auxin transport inhibitors resulted in leaves containing a central, broad, loosely organized vein from which a large number of secondary veins branched (Fig. 5). The origin of this vein was explored in a developmental time course (Fig. 6). In the control, the first provascular tissue corresponded to the primary vein, and it appeared as a single file of cells that extended from the stem along a proximal/distal axis in the center of the leaf of 4-DPI seedlings. In the treated plants, the first provascular tissue that appeared in the leaves differed from the control in several respects. One difference was temporal; provascular tissue first appeared in 5-DPI leaf primordia of inhibitor-grown tissue rather than the 4-DPI leaf primordia of the control. Another difference was the conformation of these provascular strands. Several dispersed cell files of provascular tissue appeared within the same time interval. These cell files extended along the proximal/distal axis, but were not connected to the stem (Fig. 6, and depicted in Fig. 2).
One interpretation of these data is that inhibition of auxin transport delays leaf development. Thus, the multiple provascular cell files observed in the 5-DPI inhibitor-grown seedlings might all correspond to primary vein provascular tissue. This interpretation would suggest that polar auxin transport inhibition results in a disorganization of the primary vein provascular tissue such that more than one cell file was able to form. Although I cannot discount this possibility, I would expect a delay in leaf development to also be detectable as smaller leaf primordia relative to the controls. However, at concentrations of polar auxin transport inhibitors that produced these changes in leaf venation, leaf size was unaffected (Table I; Fig. 6).
An alternative interpretation of the time course is that treatment with polar auxin transport inhibitors results in a loss of the primary vein, and the provascular tissue in the 5-DPI inhibitor-grown seedlings gives rise to secondary veins. In support of this idea, the timing of appearance of the provascular strands in the 5-DPI inhibitor-grown seedlings is the same as secondary veins in the control, and the positions of the provascular strands in the 5-DPI inhibitor-grown seedlings is appropriate for secondary veins (proximal regions located at the leaf midline, and distal regions extended toward the leaf margin). If this interpretation is correct, the broad and loosely spaced vein in the center of mature inhibitor-grown leaves may in fact be the confluence of proximal portions of secondary veins. Distinguishing between these alternatives will require molecular markers that allow distinction between primary and secondary veins.
Vascular Tissue Accumulation at the Leaf Margin
One of the predicted outcomes of inhibiting polar auxin transport is its accumulation adjacent to its site of synthesis. Leaf primordia have been shown to be important sites of auxin biosynthesis. Within the leaf, immature tobacco leaves contain a gradient of the auxin indole-3-acetic acid, with higher concentrations in proximal regions than distal regions, while in mature tobacco leaves, indole-3-acetic acid is present uniformly across the proximal/distal axis (Edlund et al., 1995). However, a site(s) within the leaf where auxin synthesis occurs is not known. One of the striking features of the inhibitor-grown leaves was the broad band of TEs that accumulated adjacent to the leaf margin (Fig. 5). Observations from the developmental time course suggested that some of these TEs derived from the differentiation of provascular strands, but that most of the TEs were misshapen cells that transdifferentiated directly from mesophyll cells (Fig. 6 and data not shown). One explanation for this broad band of TEs might be that high local concentrations of auxin are resulting in TE differentiation. If this explanation is correct, then these observations suggest that within the leaf, the primary site of auxin synthesis is adjacent to the margin.
Models for Leaf Vein Patterning
Although little is known about the mechanisms used for leaf vein patterning, auxin has been proposed to play a central role (Sachs, 1991; Aloni, 1995; Nelson and Dengler, 1997). The many changes in leaf vein pattern observed for polar auxin transport in inhibitor-grown seedlings is consistent with a role for auxin in vascular tissue development. Interpretation of these results in terms of models for leaf vein patterning must account for at least two different types of changes to the leaf vein pattern: the loss of the primary vein and the increase in the number of secondary veins.
One model is that vein positions are specified by polar auxin transport paths, as has been proposed by Sachs (1991), but that not all leaf tissues responded to polar auxin transport inhibitors. Polar auxin transport inhibitors are believed to target the auxin efflux carrier. Recently, a gene proposed to encode one of the components of the auxin efflux carrier was cloned (Chen et al., 1998; Gälweier et al., 1998; Luschnig et al., 1998). This gene has been shown to be a part of a gene family, and at least one member shows tissue-specific expression (Chen et al., 1998; Luschnig et al., 1998). It is possible that proximal and distal portions of the leaf contain molecularly distinct auxin efflux carriers that differ in their sensitivity to polar auxin transport inhibitors. The polar auxin transport inhibitors might disrupt polar auxin transport in the petiole, preventing specification of the primary vein, but might not disrupt polar auxin transport in the leaf blade (and thus veins form). However, because all three polar auxin transport inhibitors produced similar changes in leaf vein pattern, and at least two of these inhibitors target different components of the auxin efflux transporter (Thomson et al., 1973), this explanation seems unlikely.
A second model is that the primary vein and secondary veins are patterned using distinct developmental mechanisms. The specific disruption of the primary vein in the inhibitor-grown seedlings provides strong evidence supporting a role for polar auxin transport in specification of this vein. However, because secondary veins are present, their position could be specified by a developmental mechanism that is independent of polar auxin transport. Precedence for a developmental process that only requires polar auxin transport under specific circumstances is provided by hypocotyl elongation. For Arabidopsis seedlings grown in the dark, the developmental program controlling hypocotyl elongation does not use polar auxin transport; however, for light-grown seedlings, polar auxin transport is required for normal hypocotyl elongation (Jensen et al., 1998).
A third model is that the polar auxin transport inhibitors had a specific effect on the primary leaf vein, and that the increase in secondary veins is a secondary consequence of the loss of vascular continuity through the petiole. One possible secondary consequence is that polar auxin transport inhibitors failed to enter the developing leaf. However, inhibitor concentrations exceeding that which resulted in the loss of the primary vein caused further diminution of leaf size (Table I; Fig. 1), suggesting that the elevated levels of inhibitors were continuing to affect the leaf. Another likely secondary effect is the accumulation of high auxin levels in the developing leaf. Auxin is synthesized in leaf primordia, and under normal circumstances would be exported from the leaf through cells associated with the vascular tissue. The interruption of vascular tissue in the leaf petiole is thus likely to provide an anatomical block to auxin transport, on top of the biochemical block provided by the polar auxin transport inhibitors. If polar auxin transport is blocked within the blades of these leaves, then the elevated number of secondary veins might indicate that the pathway for secondary vein patterning (such as proposed above) is responsive to auxin levels.
A screen for mutants with defects in cotyledon and leaf vein patterning was conducted recently, and allowed the recovery more than 12 mutants with vein patterning defects. Five of these mutants have been mapped to regions that differ from other reported vein patterning or auxin mutants (M.K. Deyholos, G. Corder, M. Szego, and L.E. Sieburth, unpublished data). In this paper, I explored the influence of polar auxin transport inhibitors on leaf vein patterning in wild-type plants; analysis of the results suggested that more than one mechanism may be available to pattern leaf veins. Delineation of these putative pathways awaits further genetic analyses of vein patterning mutants and analysis of the interaction of vein patterning genes with the auxin pathway.
ACKNOWLEDGMENTS
I would like to thank Gary Drews for his useful comments on this manuscript and Candace Waddell and Michael K. Deyholos for useful discussions during the course of this work.
Footnotes
This work was supported by the National Science and Engineering Research Council of Canada (NSERC).
LITERATURE CITED
- Aloni R. The induction of vascular tissues by auxin and cytokinin. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Amsterdam: Kluwer Academic Publishers; 1995. pp. 531–546. [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR. Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 1995;8:505–520. [Google Scholar]
- Candela H, Martínez-Laborda A, Micol JL. Venation pattern formation in Arabidopsis thaliana vegetative leaves. Dev Biol. 1999;205:205–216. doi: 10.1006/dbio.1998.9111. [DOI] [PubMed] [Google Scholar]
- Carland FM, McHale NA. LOP1: a gene involved in auxin transport and vascular patterning in Arabidopsis. Development. 1996;122:1811–1819. doi: 10.1242/dev.122.6.1811. [DOI] [PubMed] [Google Scholar]
- Chen R, Hilson P, Sedbrook J, Rosen E, Caspar T, Masson PH. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc Natl Acad Sci USA. 1998;95:15112–15117. doi: 10.1073/pnas.95.25.15112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund A, Eklöf S, Sundberg B, Moritz T, Sandberg G. A microscale technique for gas chromatography-mass spectrometry measurements of picogram amounts of indole-3-acetic acid in plant tissues. Plant Physiol. 1995;108:1043–1047. doi: 10.1104/pp.108.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ML, Hokanson R. Timing of the response of coleoptiles to the application and withdrawal of various auxins. Planta. 1969;85:85–95. doi: 10.1007/BF00387663. [DOI] [PubMed] [Google Scholar]
- Fischer C, Neuhaus G. Influence of auxin on the establishment of bilateral symmetry in monocots. Plant J. 1996;9:659–669. [Google Scholar]
- Fosket DE, Roberts LW. Induction of wound-vessel differentiation in isolated coleus stem segments in vitro. Am J Bot. 1964;51:19–25. [Google Scholar]
- Foster RJ, Harold D, McRae J, Bonner J. Auxin-antiauxin interactions at high auxin concentrations. Plant Physiol. 1955;30:323–327. doi: 10.1104/pp.30.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Komamine A. Establishment of an experimental system for the tracheary elements differentiation from single cells isolated from the mesophyll of Zinnia elegans. Plant Physiol. 1980;65:57–60. doi: 10.1104/pp.65.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälweiler L, Juan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Gersani M, Leshem B, Sachs T. Impaired polarity in abnormal plant development. J Plant Physiol. 1986;123:91–95. [Google Scholar]
- Goldsmith MHM. The polar transport of auxin. Annu Rev Plant Physiol. 1977;28:349–378. [Google Scholar]
- Hadfi K, Speth V, Neuhaus G. Auxin-induced developmental patterns in Brassica juncea embryos. Development. 1998;125:879–887. doi: 10.1242/dev.125.5.879. [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs WP. The role of auxin in differentiation of xylem around a wound. Am J Bot. 1952;39:245–300. [Google Scholar]
- Jensen PJ, Hangarter RP, Estelle M. Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 1998;116:455–462. doi: 10.1104/pp.116.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsman EA, Pyke KA. Bundle sheath cells and cell-specific plastid development in Arabidopsis leaves. Development. 1998;125:1815–1822. doi: 10.1242/dev.125.10.1815. [DOI] [PubMed] [Google Scholar]
- Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffmann NL. The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev. 1987;1:86–96. [Google Scholar]
- Krelle E, Libbert E. Inhibition of the polar auxin transport by a morphactin. Planta. 1968;80:317–320. [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Xu Z-H, Chua N-H. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell. 1993;5:621–630. doi: 10.1105/tpc.5.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Amsterdam: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. Responses of plant vascular systems to auxin transport inhibition. Development. 1999;126:2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- McRae HD, Bonner J. Chemical structure and antiauxin activity. Physiol Plant. 1953;6:485–510. [Google Scholar]
- Morris DA, Thomas AG. A microautoradiographic study of auxin transport in the stem of intact pea seedlings. J Exp Bot. 1978;29:148–157. [Google Scholar]
- Nelson T, Dengler N. Leaf vascular pattern formation. Plant Cell. 1997;9:1121–1135. doi: 10.1105/tpc.9.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcotte DH. Aspects of vascular tissue differentiation in plants: parameters that may be used to monitor the process. Int J Plant Sci. 1995;156:245–256. [Google Scholar]
- Okada K, Udea J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemeck GKH, Mattsson J, Hardtke CS, Sung AR, Berleth T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta. 1996;200:229–237. doi: 10.1007/BF00208313. [DOI] [PubMed] [Google Scholar]
- Romano C, Hein M, Klee H. Inactivation of auxin in tobacco transformed with the indoleacetic acid-lysine synthetase gene of Pseudomonas savastonoi. Genes Dev. 1991;5:438–446. doi: 10.1101/gad.5.3.438. [DOI] [PubMed] [Google Scholar]
- Sachs T. The control of the patterned differentiation of vascular tissues. Adv Bot Res. 1981;9:152–262. [Google Scholar]
- Sachs T. Cell polarity and tissue patterning in plants. Dev Suppl. 1991;1:83–93. [Google Scholar]
- Sheldrake AR, Northcote DH. The production of auxin by tobacco internode tissues. New Phytol. 1968;67:1–13. [Google Scholar]
- Shiavone FM, Cooke TJ. Unusual patterns of somatic embryogenesis in the domesticated carrot: developmental effects of exogenous auxins and auxin transport inhibitors. Differentiation. 1987;21:53–62. doi: 10.1016/0045-6039(87)90448-9. [DOI] [PubMed] [Google Scholar]
- Thomson K-S, Hertel R, Müller S, Tavares JE. 1-N-Naphthylphthalamic acid and 2,3,5-triiodobenzoic acid: in-vitro binding to particulate cell fractions and action on auxin transport in corn coleoptiles. Planta. 1973;109:337–352. doi: 10.1007/BF00387102. [DOI] [PubMed] [Google Scholar]
- Tuominen H, Puech L, Fink S, Sundberg B. A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiol. 1997;115:577–585. doi: 10.1104/pp.115.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Mellerowicz EF, Sundberg B. Indole-3-acetic acid controls cambial growth in Scots pine by positional signaling. Plant Physiol. 1998;117:113–121. doi: 10.1104/pp.117.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B. Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci USA. 1996;93:9282–9286. doi: 10.1073/pnas.93.17.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lijsebettens M, Clarke J. Leaf development in Arabidopsis. Plant Physiol Biochem. 1998;36:47–60. [Google Scholar]
- Wangermann The pathway of transport of applied IAA through internode segments. New Phytol. 1974;73:623–636. [Google Scholar]
- Wetmore RH, Rier JP. Experimental induction of vascular tissues in callus of angiosperms. Am J Bot. 1963;50:418–430. [Google Scholar]