Abstract
Background
The activity of cerebrospinal fluid (CSF) β-site APP cleaving enzyme (BACE) is a potential diagnostic biomarker for Alzheimer disease (AD).
Methods
A total of 340 non-Hispanic Caucasian participants from the Alzheimer’s Disease Neuroimaging Initiative cohort (ADNI) database were included in this study with quality-controlled CSF BACE and genotype data. Association of CSF BACE with the genetic variants of single nucleotide polymorphisms (SNPs) was assessed using PLINK under the additive genetic model. The P values of all SNPs for CSF BACE were adjusted for multiple comparisons.
Results
One SNP (rs1481950) in the ATP6V1H gene reached genome-wide significance for associations with CSF BACE (P = 4.88 × 10− 9). The minor allele (G) of rs1481950 was associated with higher CSF BACE activity. Although seven SNPs in SNX31, RORA, CDH23, RGS20, LRRC4C, MAPK6PS1 and LOC105378355 did not reach genome-wide significance (P < 10− 8), they were identified as suggestive loci (P < 10− 5).
Conclusion
This study identified rs1481950 within ATP6V1H influencing human CSF BACE activity, which indicated that ATP6V1H gene may play some roles in the pathogenesis of neurodegenerative diseases such as AD.
Electronic supplementary material
The online version of this article (10.1186/s12881-018-0603-z) contains supplementary material, which is available to authorized users.
Keywords: ADNI, Alzheimer’s disease, BACE, ATP6V1H
Background
Amyloid plaque which is composed primarily of amyloid-beta (Aβ) has been reported as an important pathological change of some neurodegenerative diseases including Alzheimer’s disease (AD) [1]. Aβ is generated from the transmembrane polypeptide called amyloid precursor protein (APP) by β- and γ-secretase enzymes [1, 2]. β-secretase or β-site APP cleaving enzyme (BACE) is the important rate-limiting enzyme and its increased activity may lead to the elevation of Aβ in the brain [1, 2]. Previous studies discovered that AD subjects have increased cerebrospinal fluid (CSF) BACE enzymatic activity compared with the controls [3–12]. So researchers hypothesized that BACE enzymatic activities in CSF may become potential biomarkers for AD.
The recent development of a sensitive assay for BACE in CSF makes it feasible to study the association between CSF BACE enzymatic activity and AD [13]. Moreover, the use of quantitative traits in genome-wide association study (GWAS) has been shown to increase statistical power over case-control designs [14]. In this study we regard CSF BACE enzymatic activity as an endophenotype for a separate GWAS in the ADNI (Alzheimer’s Disease Neuroimaging Initiative database, adni.loni.usc.edu) cohort in order to discover genetic factors involved in BACE protein.
Methods
Alzheimer’s Disease Neuroimaging Initiative
Data used in this study were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu/ADNI). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD, VA Medical Center and University of California–San Francisco. ADNI includes more than 800 participants ranging in age from 55 to 90. All these individuals were recruited from over 50 sites across the United States and Canada, including approximately 200 healthy controls (HC), 400 patients diagnosed with mild cognitive impairment (MCI) and 200 patients diagnosed with early AD. The AD patients were followed for 2 years and others were followed for 3 years. Structural 1.5-T magnetic resonance imaging (MRI) collected the full sample. PIB and FDG positron emission tomography (PET) imaging of a subset, some other biological markers, and performance on neuropsychological or clinical assessments were collected at baseline and at follow-up visits in 6- to 12-month intervals. The CSF BACE and genome-wide genotyping in this study were respectively available on approximately half of the cohort and the full ADNI sample. Further information about ADNI can be found in previous publications and at www.adni-info.org [15].
Standard protocol approvals, registrations, and patient consents
This study was approved by institutional review boards of all participating institutions and written informed consent was obtained from all participants or authorized representatives.
Participants
Our ADNI cohort included all healthy controls (HC), MCI group and AD group participants with available baseline CSF BACE samples and genotype data. To reduce the likelihood of population stratification effects in the GWAS, all the participants were restricted to non-Hispanic Caucasians. We also tested a multidimensional scaling (MDS) plot and found some genetic outliers (Additional file 1). After quality control (QC) of the CSF BACE data and removal of the outliers, there were 340 participants (AD = 86, MCI = 163, HC = 91) with CSF BACE data left. Detailed QC steps for CSF and genotype data have been previously reported and are briefly described below.
CSF BACE measurement and quality control
Samples were obtained from 382 ADNI subjects, enrolled at 56 participating centers using previously reported methods for CSF measurements [13]. For most samples, the time from collection to freezing was within 60 min. Samples were processed, aliquoted, and stored at − 80 °C according to the ADNI Biomarker Core Laboratory Standard Operating Procedures [16].
CSF BACE proteins of all the samples were tested by a solution-based BACE enzymatic assay which has been regarded as best assay format for BACE enzymatic activity in previous studies [13]. This assay format uses a biotin labeled 15 amino acid peptide biotin-KTEEISEVNFEVEFR (NFEV) as the BACE substrate and uses a baculovirus expressed c-terminally truncated BACE (bBACE) as the BACE enzyme standard. A source of BACE using either purified recombinant truncated BACE, human or rhesus monkey CSF was co-incubated with this BACE substrate. The BACE cleavage product was then detected using an “anti-NF” neo-epitope specific rabbit polyclonal antibody and an indirect anti-rabbit horseradish peroxidase (HRP) or alkaline phosphatase (AP) development of the reaction [13]. The BACE activity assay includes two steps (1 Enzyme+substrate, 2 ELISA to measure product) [13]. Luminescence from assay plates was read on EnVision (PerkinElmer, model 2104). The counts from individual CSF samples were converted to BACE enzymatic activity using coefficients determined by a quadratic fit to the bBACE standard curve [13].
Mean and standard deviations (SD) baseline of CSF BACE measures were calculated by observers blind to diagnostic information and subjects who had a value greater or smaller than 3-fold SD from the mean value were regarded as extreme outliers and removed from the analysis.
Genotyping and quality control
Single nucleotide polymorphism (SNP) genotyping for more than 620,000 target SNPs was completed on all ADNI participants using the following protocol. A total of 7 mL blood of each participant was taken in EDTA containing Vacutainer tubes and genomic DNA was extracted using the QIAamp DNA Blood Maxi Kit (Qiagen, Inc., Valencia, CA) following the manufacturer’s protocol. EBV-transformed B lymphoblastoid cell lines were established. Genomic DNA samples were analyzed using the Human 610-Quad BeadChip (Illumina, Inc., San Diego, CA) according to the manufacturer’s protocols (Infinium HD Assay; Super Protocol Guide; rev. A, May 2008).
Stringent QC assessment was performed using the PLINK software package (http://pngu.mgh.harvard.edu/purcell/plink/), release v 1.07,15 as described previously. Stringent QC assessment followed these criteria: minimum call rate for SNPs and individuals > 95%, minimum minor allele frequencies (MAF) > 0.05, Hardy-Weinberg equilibrium test P > 0.001. The restriction to SNPs with a MAF greater than 5% served to reduce the likelihood of false-positive results in the context of modest sample size to enhance statistical power [14]. What’s more, elimination of relatively rare markers reduced the severity of the multiple comparison correction which in turn enhanced statistical power. After the QC procedure, all 340 participants remained in the analysis and only 519,442 out of 620,901 SNPs remained in the analysis. The overall genotyping rate for the remaining dataset was 99.5%.
Statistical analyses
To find the association of CSF BACE with the genetic polymorphism, a separate GWAS was performed using PLINK software under an additive genetic model. The thresholds of P < 10− 5 and P < 10− 8 were used for suggestive and genome-wide significant associations respectively [17]. The analysis included a total of 519,442 genotyped variants. Age, gender and APOEε4 status were included as covariates. One-way analysis of variance (ANOVA) and Tukey’s multiple comparisons test were used to determine the difference of CSF BACE enzymatic activity in different diagnostic groups. The effects of genotypes on CSF BACE were examined with a multiple linear regression model. The relations between top SNP and AD-related phenotypes were also performed using PLINK software. Genome-wide associations were visualized by a software program (R, version 3.4.0; The R Foundation). Regional associations were visualized with the LocusZoom web tool (http://locuszoom.org/).
Results
Demographic, clinical, and cognitive characteristics
The demographic information on the final set of 340 non-Hispanic Caucasian participants and summary statistics on the CSF BACE are showed in Table 1. This study includes 86 AD subjects, 163 MCI subjects and 91 HC subjects. As shown in the Additional file 2, there was no significant difference in gender (AD P = 0.32470, MCI P = 0.26220, HC P = 0.82720, Total P = 0.81770) among the three diagnostic groups. For age (Additional file 3), though we did not find the linear correlation in the AD, MCI and total samples (AD P = 0.9007, MCI P = 0.4659, Total P = 0.1447), the results showed a linear correlation between age and CSF BACE activity in HC groups (P = 0.0063). As expected, the ε4 allele of APOE gene substantially increased the risk of AD with dose effects. Furthermore, CSF BACE enzymatic activity did not have statistical difference (P = 0.2762, after ANOVA and Tukey’s multiple comparisons test) among three diagnostic groups.
Table 1.
Demographic information
| Baseline diagnosis | AD | MCI | HC | Total |
|---|---|---|---|---|
| n | 86 | 163 | 91 | 340 |
| Age (years), mean ± SD (range) | 75.1 ± 7.8 (56.4–89.1) | 74.2 ± 7.7 (54.4–88.8) | 75.5 ± 5.2 (62.0–89.6) | 74.8 ± 7.1 (54.4–89.6) |
| Gender, male/female | 47/39 | 109/54 | 49/42 | 205/135 |
| APOE ε4 carrier (%) | 45.3 | 33.1 | 13.2 | 30.9 |
| CSF BACE (pM), mean ± SD | 44.5 ± 17.5 | 48.8 ± 20.1 | 46.1 ± 18.0 | 47.0 ± 18.9 |
AD Alzheimer’s disease, MCI mild cognitive impairment, HC healthy control, SD standard deviation
Characteristics of SNPs associated with CSF BACE activity
After adjusting for age, gender and APOEε4 status, one SNP (rs1481950, NM_015941.3:c.871-5558G > T) was identified associated with CSF BACE enzymatic activity at genome-wide significance levels of P < 10− 8. As shown in the Fig. 1, one SNP (rs1481950) in the region of ATP6V1H gene reached genome-wide significance (P = 4.88 × 10− 9). Moreover, all SNPs whose P values reached the level of P < 10− 5 for CSF BACE enzymatic activity and their annotation information are listed in Table 2. Although these SNPs did not reach the genome-wide significance level in this limited sample, they may still have the potential significance in other larger samples. So we regarded these SNPs whose P values reached the level of P < 10− 5 as suggestive SNPs. Seven independent potential candidates (uncorrected P < 10− 5) were included (rs2507780, rs1866007, rs7903757, rs6473895, rs4075903, rs9693226, rs1900511). The Quantile-Quantile plot (QQ plot) is shown in Fig. 2 and the inflation factor is 1.The linkage disequilibrium (LD) pattern between rs1481950 and nearby SNPs is shown in Fig. 3a. These nearby SNPs showed association with CSF BACE levels with the P < 0.01. As the Fig. 3b shows, when the top SNP (rs1481950) was controlled, these nearby SNPs also disappeared, which indicated that these nearby SNPs were driven by the top SNP.
Fig. 1.
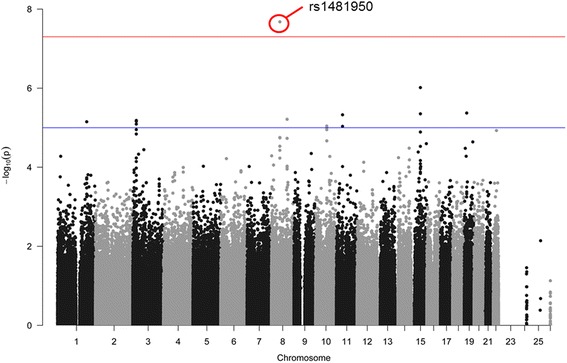
Manhattan plots for associations with CSF BACE. In the Manhattan plot, the blue and red lines represent the -log10 (10− 5) and -log10 (3.10 × 10− 8) threshold levels
Table 2.
Suggestive SNPs
| SNP | Chr | Position | SNP Type | Closest RefSeq gene | Gene name | P value |
|---|---|---|---|---|---|---|
| rs2507780 | 8 | 100588738 | intron | SNX31 | sorting nexin 31 | 3.15 × 10−6 |
| rs1866007 | 15 | 60526051 | intron | RORA-AS1 RORA |
RAR related orphan receptor A | 6.02 × 10−6 |
| rs7903757 | 10 | 71552620 | intron | CDH23 LOC105378355 |
cadherin related 23 | 9.21 × 10−6 |
| rs6473895 | 8 | 53850344 | upstream variant 2 KB | RGS20 | regulator of G-protein signaling 20 | 8.42 × 10−6 |
| rs4075903 | 11 | 40186373 | intron | LRRC4C | leucine rich repeat containing 4C | 9.34 × 10−6 |
| rs9693226 | 8 | 53540570 | MAPK6PS1 | mitogen-activated protein kinase 6 pseudogene 1 | 7.38 × 10−6 | |
| rs1900511 | 10 | 71559814 | intron | LOC105378355 | 9.32 × 10−6 |
SNP single nucleotide polymorphism, Chr chromosome
Fig. 2.

Quantile-Quantile plot
Fig. 3.
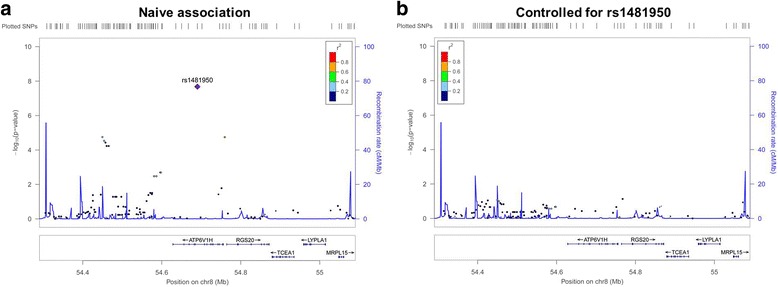
Regional plots for associations with CSF BACE. a Regional association results for the ATP6V1H region of chromosome 8. b Association results for chromosome 8:54290285–55090285 controlling for rs1481950
CSF BACE activity and top SNP
All these eight SNPs were analyzed further to examine possible interactions among baseline diagnosis and genotypes on associated CSF BACE enzymatic activity by a multiple linear regression test considering age, gender and APOEε4 status as covariates. For the top SNP (rs1481950), as indicated in Fig. 4, CSF BACE activity in the whole samples showed positive linear association with the G allele in a multiple linear regression model after adjusting for age, gender, and APOEε4 status (GG + GT: 63.86 ± 22.64pM, TT: 45.49 ± 17.99pM, P = 4.88 × 10− 9, Beta: 22.48, 95% CI: 15.22 ~ 29.75). Further analysis found this linear association between genotype and CSF BACE activity in both AD (GG + GT: 60.14 ± 19.13pM, TT: 43 ± 16.85pM, P = 0.02720 Beta: 0.23820, 95% CI: 0.02137 ~ 0.43360) and MCI groups (GG + GT: 69.36 ± 23.99pM, TT: 46.99 ± 18.72pM, P = 0.00030, Beta: 0.28280, 95% CI: 0.13040 ~ 0.42210). However, in HC group, results did not show significant differences in CSF BACE activity among three genotype subgroups in both ANOVA (P = 0.06370) and multiple linear regression analysis (P = 0.16480, Beta: 0.14690, 95%CI: -0.06708~ 0.34790).
Fig. 4.
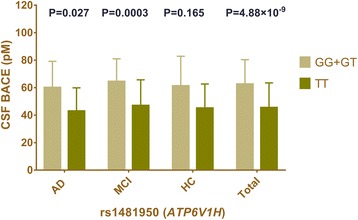
Mean CSF BACE activity as a function of baseline diagnosis and genotype. Mean and standard errors of CSF BACE activity are shown for groups defined by baseline diagnosis. The CSF BACE activity in the total samples showed positive linear association with the G allele in a multiple linear regression model after adjusting for age, gender, and APOEε4 status (GG+ GT: 63.86 ± 22.64pM, TT: 45.49 ± 17.99pM, P = 4.88 × 10− 9). The P value of a multiple linear regression model in each diagnosis group was displayed in the fig. (AD: P = 0.027 MCI: P = 0.0003 HC: P = 0.165)
Top SNP and AD-related phenotypes
This study also tested relations between rs1481950 and AD-related phenotypes. In all samples, results showed that mutations of rs1481950 had significant correlations with the atrophy of middle temporal gyrus (Beta: -0.01425, 95% CI: -0.02803 ~ − 0.00046, P = 0.04389) and parahippocampal gyrus (Beta: -0.12170, 95% CI: -0.22010 ~ − 0.02327, P = 0.01570). Similarly to this result, in AD patients, results also showed that mutations of rs1481950 had significant correlations with the atrophy of middle temporal gyrus (Beta: -0.13140, 95% CI: -0.19900 ~ − 0.06380, P = 0.01894) and parahippocampal gyrus (Beta: -0.15100, 95% CI: -0.25480 ~ − 0.04708, P = 0.04648). In MCI patients, results showed that mutations of rs1481950 had correlations with the atrophy of hippocampus (Beta: -0.04772, 95% CI: -0.09203 ~ − 0.00342, P = 0.03619), entorhinal cortex (Beta:-0.19020, 95% CI: -0.36850 ~ − 0.01194, P = 0.03728) and parahippocampal gyrus(Beta: -0.15180, 95% CI: -0.28830 ~ − 0.01528, P = 0.03002) (Fig. 5). For HC groups, though we did not find some correlations between rs1481950 and brain atrophy, the results showed that mutations of rs1481950 had significant correlations with the CSF phosphorylated tau protein (p-tau) (Beta: 8.52600, 95% CI: 2.74700 ~ 14.31000, P = 0.00426).
Fig. 5.

Forest plot of rs1481950 and AD-related encephalic regions in ADNI. 24 M: The follow-up period is 24 months; BL: Baseline; CI: credibility interval
Discussion
In this multicenter study, we identified a genome-wide significant association of a SNP (rs1481950) in the gene ATP6V1H region with CSF BACE activity and found seven additional suggestive association loci. Interestingly, this study is the first to show that the rs1481950 risk variant in ATP6V1H significantly affects CSF BACE activity. We found statistically significant differences in CSF BACE activity between the two genotype groups (GG + GT and TT). It is worth noting that this statistical differences between gene polymorphism of ATP6V1H and CSF BACE activity existed in both AD (P = 0.0027) and MCI groups (P = 0.0003). These results indicated that the rs1481950 risk variant (G) in ATP6V1H might significantly increase the CSF BACE activity especially in the AD and MCI individuals.
Rs1481950 is in an intronic region. Current studies haven’t found the exact mechanisms of impairing the expression of ATP6V1H. However, the roles of intronic regions which have been ignored all the time have attracted the attention of scientists. And more and more studies have proved that intronic region may play some important roles in controling the initiation and termination of transcription. Moreover, common molecular mechanisms for an intronic SNP to alter mRNA levels are to affect transcription, RNA elongation, splicing, or maturation [18–22].
The ATPase, H+ transporting, lysosomal 50/57 kDa, V1 subunit H gene (ATP6V1H) at Chr8q11.2 encodes for the V1H subunit of vacuolar ATPase (V-ATPase) [23, 24]. ATP6V1H gene was mainly studied and discussed about its roles in diabetes in previous researches and the data showed that the down-regulation of its gene expression correlates with the presence of type-2 diabetes [25]. Though there is still no research point out a direct relation between ATP6V1H gene and AD, some studies about encoded protein and metabolic process of BACE indicate that mutations of ATP6V1H gene may contribute to the increased BACE activity. V-ATPase belongs to the rotary ATPase family and is a multiprotein membrane complex. The most important function of V-ATPase is to acidify intracellular compartments by using the energy gathered from ATP hydrolysis to pump protons [23]. One of the most important influence factors of BACE activity is PH. Acidic Intracellular environment (PH = 4.5) is optimal for BACE activity. So the dysfunction of V-ATPase may lead to the change of the acidic Intracellular environment and then influence the BACE activity. BACE activity is also associated with mature processing. The mature process of BACE requires the formation of disulfide bonds, glycosylation and some other modification processes [26]. These steps occurs in the endoplasmic reticulum and golgi body and may be influenced by the changes of the intracellular environment. Moreover, V-ATPase also plays an important role in lysosomal acidification. Lysosomal pathway is an important degradation pathway of BACE protein [27, 28]. To sum up, V-ATPase may play some important roles in both BACE protein levels and activity.
The results also showed that mutations of rs1481950 had significant correlations with the atrophy of AD-related encephalic regions including middle temporal gyrus, parahippocampal gyrus, hippocampus and entorhinal cortex. Moreover, the results showed that mutations of rs1481950 had significant correlations with the CSF p-tau. These results indicated that mutations of rs1481950 may relate to AD and may influence the volume of AD-related encephalic regions by changing the metabolism of some AD-related proteins such as tau.
In this study, we did not find any significant differences in CSF BACE activity among the three diagnostic groups. This aspect of our research is in line with two previous studies including an ADNI cohort study [29, 30]. Some previous studies show different results including increased activity in MCI and AD [31], and increased BACE1 activity in MCI but not in AD [13, 32]. These inconsistent results may be explained by the characteristics of the study samples, the wide range of BACE1 activity measurements, and the large overlap between the groups.
There are several potential limitations of this study. First, the ADNI-1 sample was limited in sample size when CSF BACE data, different genotypes and diagnosis subgroups were taken into consideration, which makes effective sample size small for some tests. Thus, it will still be necessary to replicate these findings in a larger dataset. Second, recent studies also indicated that except CSF BACE, plasmatic BACE was also associated with AD [33]. The associations among gene polymorphism, CSF BACE, plasmatic BACE and AD still need further study. Third, our sample was restricted to Caucasians to avoid genetics stratification across ethnicities, while all the genes may show different frequencies and polymorphisms in different populations. The relationships between these genes and AD need to be tested in more populations. Fourth, the ADNI database did not cover the detailed classification of the AD patients (sporadic and familial; early onset and late onset). So further studies with a better profile of AD patients should be tested for comparative purposes with other studies.
Conclusions
In summary, after a separate GWAS of CSF BACE, we found a top SNP (rs1481950) in ATP6V1H gene with the P value reaching genome-wide significance and seven suggestive SNPs with the P value lower than 10− 5. Rs1481950 risk variant (G) in ATP6V1H may increase the CSF BACE activity. Seven genes (SNX31, RORA, CDH23, RGS20, LRRC4C, MAPK6PS1, LOC105378355) were regarded as candidate genes. These results provide clues to some novel pathogenic genes associated with some BACE related diseases, such as AD. The in-depth discussion and study of these associations can help us to find the exact mechanisms of AD, which may indicate some new diagnostic methods and therapeutic directions.
Additional files
The MDS plot of samples. (TIF 8989 kb)
The correlation between gender and CSF BACE activity. (TIF 2480 kb)
The correlation between age and CSF BACE activity. (TIF 2436 kb)
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Funding
This work was supported by grants from the Taishan Scholars Program of Shandong Province (ts201511109 and tsqn20161079), Qingdao Key Health Discipline Development Fund, Qingdao Outstanding Health Professional Development Fund, and Qingdao Innovation and Entrepreneurship Leading Talent Program.
Availability of data and materials
Data are available to researchers by applying to the ADNI consortia. Application is required to protect participant confidentiality. The ADNI data are available at (http://adni.loni.usc.edu/).
Abbreviations
- AD
Alzheimer disease
- ADNI
Alzheimer’s Disease Neuroimaging Initiative
- ANOVA
One-way analysis of variance
- AP
Alkaline phosphatase
- APP
Amyloid precursor protein
- Aβ
Amyloid-beta
- BACE
β-site APP cleaving enzyme
- bBACE
Baculovirus expressed cterminally truncated BACE
- CSF
Cerebrospinal fluid
- GWAS
Genome-wide association study
- HC
Healthy controls
- HRP
Horseradish peroxidase
- LD
Linkage disequilibrium
- MAF
Minor allele frequencies
- MCI
Mild cognitive impairment
- MRI
Magnetic resonance imaging
- PET
Positron emission tomography
- QC
Quality control
- SD
Standard deviations
- SNPs
Single nucleotide polymorphisms
Authors’ contributions
LT and JTY led and supervised the research. JTY designed the research. HH, HYL and JQL prepared the genotyping data, performed the gene analysis, and performed quality control and GWAS. HH drafted the manuscript. All the authors reviewed, commented and approved the manuscript.
Ethics approval and consent to participate
The study procedures were approved by the institutional review boards of all participating centers (https://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ ADNI_Acknowledgement_List.pdf), and written informed consent was obtained from all participants or their authorized representatives. Ethics approval was obtained from the institutional review boards of each institution involved: Oregon Health and Science University; University of Southern California; University of California—San Diego; University of Michigan; Mayo Clinic, Rochester; Baylor College of Medicine; Columbia University Medical Center; Washington University, St. Louis; University of Alabama at Birmingham; Mount Sinai School of Medicine; Rush University Medical Center; Wien Center; Johns Hopkins University; New York University; Duke University Medical Center; University of Pennsylvania; University of Kentucky; University of Pittsburgh; University of Rochester Medical Center; University of California, Irvine; University of Texas Southwestern Medical School; Emory University; University of Kansas, Medical Center; University of California, Los Angeles; Mayo Clinic, Jacksonville; Indiana University; Yale University School of Medicine; McGill University, Montreal-Jewish General Hospital; Sunnybrook Health Sciences, Ontario; U.B.C.Clinic for AD & Related Disorders; Cognitive Neurology—St. Joseph’s, Ontario; Cleveland Clinic Lou Ruvo Center for Brain Health; Northwestern University; Premiere Research Inst (Palm Beach Neurology); Georgetown University Medical Center; Brigham and Women’s Hospital; Stanford University; Banner Sun Health Research Institute; Boston University; Howard University; Case Western Reserve University; University of California, Davis—Sacramento; Neurological Care of CNY; Parkwood Hospital; University of Wisconsin; University of California, Irvine—BIC; Banner Alzheimer’s Institute; Dent Neurologic Institute; Ohio State University; Albany Medical College; Hartford Hospital, Olin Neuropsychiatry Research Center; Dartmouth-Hitchcock Medical Center; Wake Forest University Health Sciences; Rhode Island Hospital; Butler Hospital; UC San Francisco; Medical University South Carolina; St. Joseph’s Health Care Nathan Kline Institute; University of Iowa College of Medicine; Cornell University; and University of South Florida: USF Health Byrd Alzheimer’s Institute.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12881-018-0603-z) contains supplementary material, which is available to authorized users.
Contributor Information
Hao Hu, Email: qingdaoshennei@163.com.
Haiyan Li, Email: yudahai007@163.com.
Jieqiong Li, Email: qdljq891124@163.com.
Jintai Yu, Phone: +86-532-8890-5659, Email: yu-jintai@163.com.
Lan Tan, Phone: +86-532-8890-5659, Email: dr.tanlan@163.com.
References
- 1.Scheltens P, et al. Alzheimer’s disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Vassar R, Citron M. Abeta-generating enzymes: recent advances in beta- and gamma-secretase research. Neuron. 2000;27(3):419–422. doi: 10.1016/S0896-6273(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhong Z, et al. Levels of beta-secretase (BACE1) in cerebrospinal fluid as a predictor of risk in mild cognitive impairment. Arch Gen Psychiatry. 2007;64(6):718–726. doi: 10.1001/archpsyc.64.6.718. [DOI] [PubMed] [Google Scholar]
- 4.Fukumoto H, et al. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59(9):1381–9. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 5.Yang LB, et al. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9(1):3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 6.Li R, et al. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2004;101(10):3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston JA, et al. Expression and activity of beta-site amyloid precursor protein cleaving enzyme in Alzheimer’s disease. Biochem Soc Trans. 2005;33(Pt 5):1096–1100. doi: 10.1042/BST0331096. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed RR, et al. BACE1 and BACE2 enzymatic activities in Alzheimer’s disease. J Neurochem. 2010;112(4):1045–1053. doi: 10.1111/j.1471-4159.2009.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holsinger RM, et al. Increased beta-secretase activity in cerebrospinal fluid of Alzheimer’s disease subjects. Ann Neurol. 2004;55(6):898–899. doi: 10.1002/ana.20144. [DOI] [PubMed] [Google Scholar]
- 10.Verheijen JH, et al. Detection of a soluble form of BACE-1 in human cerebrospinal fluid by a sensitive activity assay. Clin Chem. 2006;52(6):1168–1174. doi: 10.1373/clinchem.2006.066720. [DOI] [PubMed] [Google Scholar]
- 11.Zetterberg H, et al. Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol. 2008;65(8):1102–1107. doi: 10.1001/archneur.65.8.1102. [DOI] [PubMed] [Google Scholar]
- 12.Ewers M, et al. Increased CSF-BACE 1 activity is associated with ApoE-epsilon 4 genotype in subjects with mild cognitive impairment and Alzheimer’s disease. Brain. 2008;131(Pt 5):1252–1258. doi: 10.1093/brain/awn034. [DOI] [PubMed] [Google Scholar]
- 13.Wu G, et al. Decrease in age-adjusted cerebrospinal fluid beta-secretase activity in Alzheimer’s subjects. Clin Biochem. 2008;41(12):986–996. doi: 10.1016/j.clinbiochem.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, et al. Genome-wide association study identifies MAPT locus influencing human plasma tau levels. Neurology. 2017;88(7):669–676. doi: 10.1212/WNL.0000000000003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen RC, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw LM, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, et al. Genome-wide association study of CSF biomarkers Abeta1-42, t-tau, and p-tau181p in the ADNI cohort. Neurology. 2011;76(1):69–79. doi: 10.1212/WNL.0b013e318204a397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacopelli F, et al. The first intron of the human osteopontin gene contains a C/EBP-beta-responsive enhancer. Gene Expr. 2003;11(2):95–104. doi: 10.3727/000000003108748991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugo H, et al. Mutations in the MYB intron I regulatory sequence increase transcription in colon cancers. Genes Chromosomes Cancer. 2006;45(12):1143–1154. doi: 10.1002/gcc.20378. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, et al. Regulatory polymorphism in vitamin K epoxide reductase complex subunit 1 (VKORC1) affects gene expression and warfarin dose requirement. Blood. 2008;112(4):1013–1021. doi: 10.1182/blood-2008-03-144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baralle M, et al. Influence of Friedreich ataxia GAA noncoding repeat expansions on pre-mRNA processing. Am J Hum Genet. 2008;83(1):77–88. doi: 10.1016/j.ajhg.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buratti E, et al. Nuclear factor TDP-43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: a functional link with disease penetrance. Am J Hum Genet. 2004;74(6):1322–1325. doi: 10.1086/420978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshansky V, Rubinstein JL, Gruber G. Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta. 2014;1837(6):857–879. doi: 10.1016/j.bbabio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Colacurcio DJ, Nixon RA. Disorders of lysosomal acidification-the emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res Rev. 2016;32:75–88. doi: 10.1016/j.arr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molina MF, et al. Decreased expression of ATP6V1H in type 2 diabetes: a pilot report on the diabetes risk study in Mexican Americans. Biochem Biophys Res Commun. 2011;412(4):728–731. doi: 10.1016/j.bbrc.2011.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hogl S, et al. Determination of the proteolytic cleavage sites of the amyloid precursor-like protein 2 by the proteases ADAM10, BACE1 and gamma-secretase. PLoS One. 2011;6(6):e21337. doi: 10.1371/journal.pone.0021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qing H, et al. Degradation of BACE by the ubiquitin-proteasome pathway. FASEB J. 2004;18(13):1571–1573. doi: 10.1096/fj.04-1994fje. [DOI] [PubMed] [Google Scholar]
- 28.Koh YH, et al. BACE is degraded via the lysosomal pathway. J Biol Chem. 2005;280(37):32499–32504. doi: 10.1074/jbc.M506199200. [DOI] [PubMed] [Google Scholar]
- 29.Perneczky R, Alexopoulos P, Alzheimer's Disease euroimaging Initiative Cerebrospinal fluid BACE1 activity and markers of amyloid precursor protein metabolism and axonal degeneration in Alzheimer’s disease. Alzheimers Dement. 2014;10(5 Suppl):S425–S429. doi: 10.1016/j.jalz.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savage MJ, et al. Soluble BACE-1 activity and sAbetaPPbeta concentrations in Alzheimer’s disease and age-matched healthy control cerebrospinal fluid from the Alzheimer’s Disease Neuroimaging Initiative-1 baseline cohort. J Alzheimers Dis. 2015;46(2):431–440. doi: 10.3233/JAD-142778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosen C, et al. Cerebrospinal fluid profiles of amyloid beta-related biomarkers in Alzheimer’s disease. NeuroMolecular Med. 2012;14(1):65–73. doi: 10.1007/s12017-012-8171-4. [DOI] [PubMed] [Google Scholar]
- 32.Wu G, et al. Decrease in brain soluble amyloid precursor protein beta (sAPPbeta) in Alzheimer’s disease cortex. J Neurosci Res. 2011;89(6):822–832. doi: 10.1002/jnr.22618. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, et al. Increased Plasma Beta-Secretase 1 May Predict Conversion to Alzheimer's Disease Dementia in Individuals With Mild Cognitive Impairment. Biol Psychiatry. 2018;83(5):447-55. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The MDS plot of samples. (TIF 8989 kb)
The correlation between gender and CSF BACE activity. (TIF 2480 kb)
The correlation between age and CSF BACE activity. (TIF 2436 kb)
Data Availability Statement
Data are available to researchers by applying to the ADNI consortia. Application is required to protect participant confidentiality. The ADNI data are available at (http://adni.loni.usc.edu/).


