Abstract
The role of ethylene in regulating growth in tomato (Lycopersicon esculentum Mill.) during compaction stress was examined using wild-type (cv Ailsa Craig) and transgenic (ACO1AS) genotypes; the latter has a reduced capacity to produce ethylene. Ethephon or silver ions were applied to increase ethylene production or block its action. Shoot growth in both genotypes was comparable in uncompacted (1.1 g cm−3) and uniformly compacted soil (1.5 g cm−3). However, a 1.1/1.5-g cm−3 split-pot treatment invoked marked genotypic differences: growth was reduced in cv Ailsa Craig but was comparable to uncompacted control plants in ACO1AS. As xylem sap abscisic acid levels were similar, abscisic acid was not responsible for inhibiting growth in cv Ailsa Craig. These genotypic differences in growth were accompanied by increased ethylene evolution in cv Ailsa Craig, suggesting that the ability of ACO1AS to maintain growth in the split-pot treatment reflected its lower ethylene levels, a view supported by the observation that excising the roots in the compacted compartment reduced ethylene evolution and restored shoot growth in cv Ailsa Craig. Treatment with silver restored shoot growth in cv Ailsa Craig, whereas treatment with ethephon reduced growth in ACO1AS. Thus, ethylene apparently has a key role in determining growth when tomato plants encounter differential soil compaction.
Plants grown in compacted soil frequently exhibit reductions in root and shoot growth and stomatal conductance in the absence of significant effects on foliar water status (Masle, 1990, 1992; Tardieu et al., 1992; Andrade et al., 1993; Beemster and Masle, 1996; Mulholland et al., 1996a; Hussain et al., 1999). These responses have been attributed to the accumulation of abscisic acid (ABA) in impeded roots and its subsequent transport to the shoot via the transpiration stream (Hartung and Davies, 1991; Tardieu et al., 1992), such as occurs in water-stressed plants (Davies and Zhang, 1991). Moreover, applications of ABA have been reported to promote the growth of short, thick roots and to reduce leaf expansion and stomatal conductance in a manner analogous to plants experiencing compacted soil conditions (Davies and Zhang, 1991; Hartung and Davies, 1991; Mulholland et al., 1996b). Davies et al. (1994) proposed that the observed increases in xylem sap ABA concentration and the associated reductions in leaf expansion and stomatal conductance in compaction-stressed plants resulted from local reductions in root water potential and turgor. Several studies have attempted to preclude such effects by using pressurized columns of sand supplied with aerated nutrient solution or soil columns with a uniformly high moisture content (Tardieu et al., 1991, 1992; Cook et al., 1996; Mulholland et al., 1996a; Young et al., 1997). However, as these studies all produced similar growth and stomatal responses, it may be argued that localized soil drying adjacent to roots growing in compacted soil was not a significant factor, and that changes in the pressure exerted upon root cells resulting from differences in soil strength may have been responsible for inducing ABA accumulation (Davies and Zhang, 1991).
The existence of an inverse correlation between stomatal conductance and xylem sap ABA concentration in plants experiencing soil compaction has been demonstrated using wild-type and ABA-deficient mutants of barley (Hordeum vulgare L. cv Steptoe and cv Az34; Mulholland et al., 1996a). However, the link between root-sourced ABA and compaction-induced reductions in leaf expansion proved more complex, as both genotypes exhibited reduced growth in severely compacted soil (1.7-g cm−3 bulk density), whereas significant genotypic differences were apparent at a subcritical bulk density of 1.6 g cm−3 (Mulholland et al., 1996a). In the latter treatment, the wild-type plants maintained uncompacted (1.1 g cm−3) rates of leaf expansion, while the ABA-deficient mutant exhibited reductions in growth comparable to plants grown in severely compacted soil (Mulholland et al., 1996a, 1996b). These results demonstrate that elevated xylem sap ABA concentrations were not responsible for the observed inhibition of shoot growth in the ABA-deficient mutant. Nor was any relationship between shoot growth and xylem sap ABA detected when the same genotypes were grown in columns containing horizons of differing bulk density, although stomatal conductance was again inversely correlated with xylem sap ABA concentration (Hussain et al., 1999). Munns (1992) previously questioned whether ABA is the only potential inhibitor of leaf expansion present in the xylem sap of wheat and barley plants grown in drying soil.
Increased ethylene production rates in impeded roots have been correlated with their characteristic short, thick root morphology and concurrent reductions in shoot growth (Kays et al., 1974; Sarquis et al., 1991; Morgan et al., 1993). As ethylene may act as a root-sourced chemical signal transported as its soluble precursor, 1-aminocyclopropane-1-carboxylic acid (ACC), under anaerobic soil conditions (Jackson, 1997), it is possible that this hormone may also be involved in mediating shoot responses to soil compaction (He et al., 1996). The present study examined the role of ethylene in mediating shoot growth in tomato (Lycopersicon esculentum Mill.) plants experiencing compacted soil conditions. A split-pot approach enabled the root system to be divided between uncompacted (1.1 g cm−3) and compacted (1.5 g cm−3) soil; the majority of the roots grew unhindered in uncompacted soil, while the remainder experienced impeded growing conditions. This system enables responses to be examined in species such as tomato, which are particularly sensitive to compaction (Mulholland et al., 1999).
Isogenic ABA-deficient mutant and wild-type genotypes represent a powerful tool for studies of the role of ABA as a root-sourced signal regulating plant responses to compaction (Mulholland et al., 1996a, 1996b, 1999; Hussain et al., 1999). This approach was extended in the present study to examine the role of ethylene by using a genetically modified, low-ethylene genotype, ACO1AS, and an isogenic wild-type cultivar (Ailsa Craig). Applications of ethephon were used to promote ethylene production, while the action of this gaseous plant growth regulator was blocked using silver ions applied as silver thiosulfate (STS). The objective was to test the hypothesis that ethylene has a central role in mediating plant responses to soil compaction.
MATERIALS AND METHODS
Plant Material
Isogenic wild-type (cv Ailsa Craig) and transgenic ACC oxidase antisense genotypes (ACO1AS) of tomato (Lycopersicon esculentum Mill.) were used. The cv ACO1AS plants were generated by Hamilton et al. (1990) using a chimeric antisense pTOM13 construct. ACO1AS plants exhibit a greatly reduced synthesis of ACC oxidase (ACO) and a decreased capacity to convert ACC to ethylene.
Soil Preparation, Compaction, and Seedling Establishment
Plants were grown in an Arrow series brown earth (Thomasson, 1971), whose characteristics were reported by Mulholland et al. (1996a); the procedures used to create homogeneous bulk-density soil columns and split-pot systems were described by Hussain et al. (1999). Soil was air-dried, sieved (<0.2 mm), and oven-dried (105°C) for 24 h before incorporating nutrients at the following rates (in grams per kilogram): N, 0.75; P, 0.275; K, 1.675; and Mg, 1.075. Homogeneous soil columns (75 mm in diameter and 90 mm deep) with bulk densities of 1.1 g cm−3 (uncompacted) or 1.5 g cm−3 (compacted) were prepared as described by Mulholland (1996a). The columns were then divided vertically, and half-columns of each bulk density were taped together to form vertically divided soil columns (Hussain et al., 1999). Uniform 1.1-g cm−3 uncompacted control and 1.5-g cm−3 compacted treatments were also prepared by combining half columns of the appropriate bulk density. In all treatments, the two compartments within the split-pot system were separated by vertical polythene membranes to prevent the exchange of gases, water, nutrients, and roots. A 30-mm layer of loose soil was placed over the top of the split-pot system to facilitate seedling establishment. Fifty columns were prepared for each genotype and treatment, each containing one plant.
Seeds of both genotypes were germinated on moist filter paper in foil-covered Petri dishes under the following experimental conditions: 25°C/20°C day/night temperature, 16-h photo- and thermoperiod, approximately 150 μmol m−2 s−1 photosynthetically active radiation, and 80% relative humidity). When the radicle was a few millimeters in length, the seedlings were transplanted into the loose soil covering the split-pot system; as they developed, their root systems became divided between the two underlying compartments. The columns were weighed and rewatered twice daily until 10 d after emergence (DAE) and three times daily thereafter as transpiration increased to maintain the soil close to its initial water content. This procedure has been shown to prevent significant soil drying (Mulholland et al., 1996a). The roots in the compacted compartment of the 1.1/1.5-g cm−3 treatment were severed at 15 DAE for 20 plants of each genotype.
Growth Analysis
Plants were grown for 30 DAE under the conditions described above and harvested for growth analysis at 5- or 10-d intervals depending on the experiment involved. Leaf area was determined immediately using a leaf area meter (model 3100, LI-COR, Lincoln, NE), while shoot dry weight was measured after oven-drying (85°C for 24 h). Root dry weight was determined separately for each compartment after washing the soil from the roots and oven-drying them as described above.
Stomatal Conductance, CO2 Uptake, and Leaf Water Potential
Stomatal conductance and net photosynthesis were determined using a combined IR gas analyzer (CIRAS-1) and a Parkinson leaf cuvette with a 2.5-cm2 chamber (both from PP Systems, Hitchin, Hertshire, UK). Measurements were made at 2-d intervals commencing at 10 DAE starting 4 h after the beginning of the photoperiod, and were completed within 60 to 90 min. On each occasion, 20 measurements were made for the youngest expanded leaf of each genotype and treatment; both variables were expressed on a unit leaf area basis. Leaf water potential was determined for the same leaves 3 h after the beginning of the photoperiod using a pressure chamber (PMS Instruments, Corvallis, OR).
Xylem Sap ABA Analysis
Shoots were excised 20 mm above the soil surface and silicone rubber tubing (3–8 mm i.d. depending on stem size) was attached to the detopped root system to collect xylem sap exuded under naturally developing root pressure. The samples were stored at −20°C prior to ABA analysis by radioimmunoassay using the monoclonal antibody AFRC MAC252 (Quarrie et al., 1988), as described by Mulholland et al. (1996a), and validated by parallel gas chromatography-mass spectrometry analysis (Mulholland, 1994).
Ethylene Analysis and Applications of Ethylene Sources and Inhibitors
Ethylene evolution was measured by gas chromatography. Small pre-weighed leaf samples were placed in 7.6-mL glass vials containing moist filter paper to minimize evaporation from the tissue and immediately sealed. One-milliliter air samples were extracted after 1 and 2 h and injected into a gas chromatograph (610Ati Unicam, Perkin-Elmer Applied Biosystems, Foster City, CA) for analysis; ethylene evolution was expressed as a function of tissue fresh weight. Preliminary measurements showed that ethylene evolution from control leaf samples peaked after approximately 30 min of incubation as a result of wound-induced ethylene production; as expected, evolution rates were much lower in cv ACO1AS. Although some wound-induced ethylene was still produced after 1 h of incubation, this was assumed to occur at similar rates in all treatments of each genotype for the purposes of the present study, thereby enabling genotypic differences and treatment effects on ethylene evolution to be quantified.
Ethephon, an ethylene-releasing agent, was supplied as a 400-μL L−1 aqueous solution; this compound is hydrolyzed following delivery to the shoots, releasing ethylene (Maynard and Swain, 1963). A 1 mm STS solution was used to block the action of ethylene, as silver ions have been proposed to reduce the capacity of ethylene to interact with its receptors (Beyer, 1976); STS is readily absorbed and transported by plants (Morgan et al., 1993). Both solutions were supplied in place of water to the surface of the compartment containing compacted soil in the 1.1/1.5-g cm−3 treatment from 5 DAE; the volumes of solution required were determined gravimetrically as described above.
Statistical Analysis
The data were analyzed by ANOVA using Genstat 5 (Lawes Agricultural Trust, IACR Rothamsted, UK).
RESULTS
Compaction Treatments
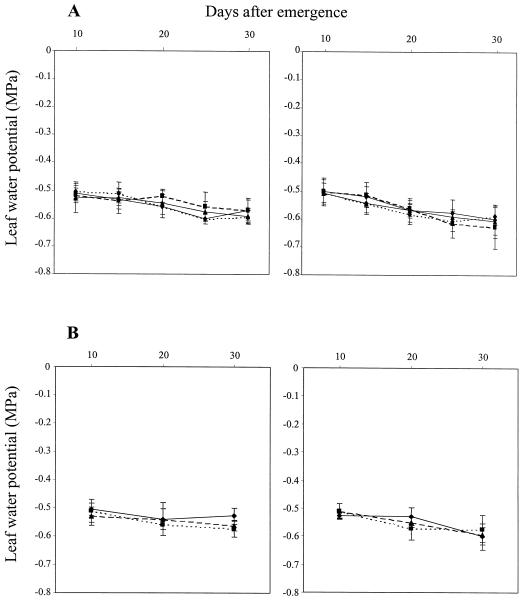
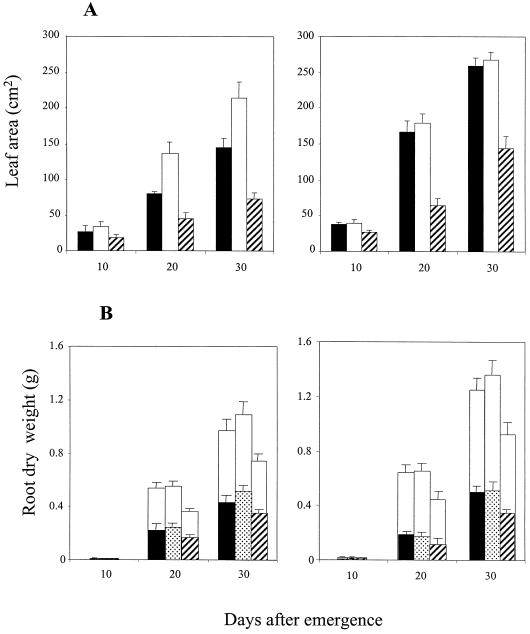
Leaf water potential showed no detectable treatment effect (Fig. 1A) and was closely comparable in both genotypes, reflecting their similar stomatal conductances (Figs. 3C and 4B); water potential decreased slightly with time in all treatments. However, despite the absence of significant treatment effects on foliar water status, leaf area and shoot dry weight were significantly reduced (P < 0.001) from 15 DAE on in the 1.1/1.5-g cm−3 split-pot and in the uniform 1.5-g cm−3 treatments of cv Ailsa Craig relative to uncompacted control plants (Fig. 2, A and B). Leaf area in the 1.1/1.5-g cm−3 treatment varied from 53% to 72% of the uncompacted control values, while plants in the 1.5-g cm−3 treatment exhibited up to 5-fold reductions relative to control plants. Shoot dry weight followed a similar trend, although the effect of compaction increased with time; shoot dry weight decreased from 62% of the uncompacted control values at 15 DAE to 26% at 30 DAE in the 1.1/1.5-g cm−3 treatment and from 64% to 17% in the 1.5-g cm−3 treatment.
Figure 1.
Influence of compaction treatment and excision of the roots in the compacted compartment of the 1.1/1.5-g cm−3 split-pot treatment (A) and treatment with STS and ethephon (eth) (B) on the water potential of the youngest expanded leaf in wild-type (cv Ailsa Craig) and transgenic (ACO1AS) genotypes of tomato. Double ses are shown. ▴, 1.1 g cm−3; ▪, 1.5 g cm−3; −−♦−−, split-pot; - - -♦- - -, split-cut.
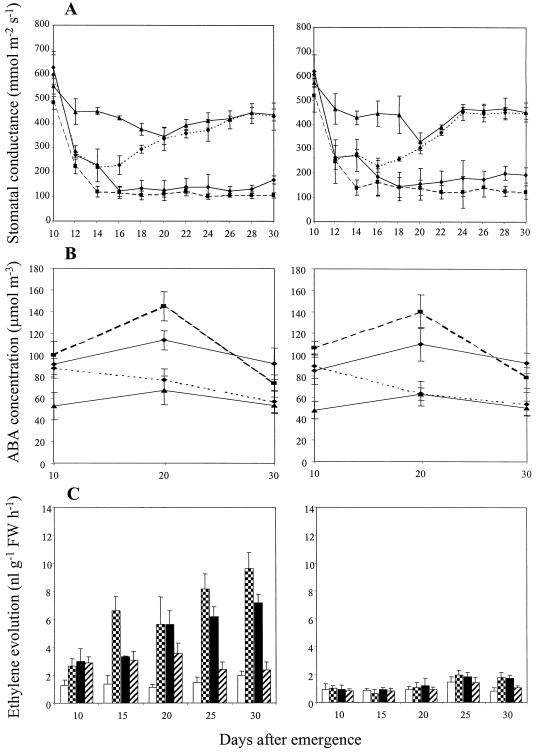
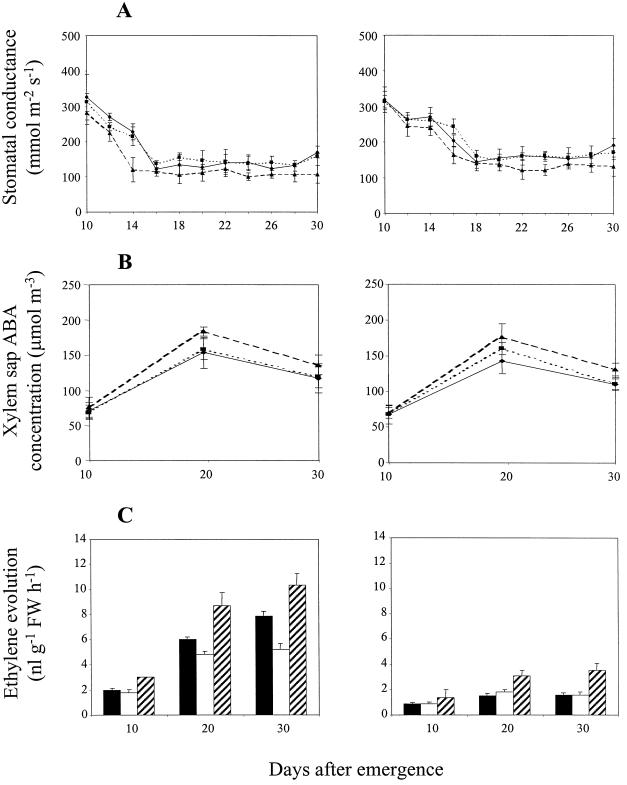
Figure 3.
Influence of compaction treatment and excision of the roots in the compacted compartment of the 1.1/1.5-g cm−3 split-pot treatment on stomatal conductance (A), xylem sap ABA concentration (B), and ethylene evolution (C) from leaf tissue in wild-type (cv Ailsa Craig) and transgenic (ACO1AS) genotypes of tomato. Double (A and B) or single ses (C) are shown. FW, Fresh weight. A and B, ▴, 1.1 g cm−3; ▪, 1.5 g cm−3; −−♦−−, split-pot; - - -♦- - -, split-cut. C, White bars, 1.1 g cm−3; checked bars, 1.5 g cm−3; black bars, split-pot; hatched bars, split-cut.
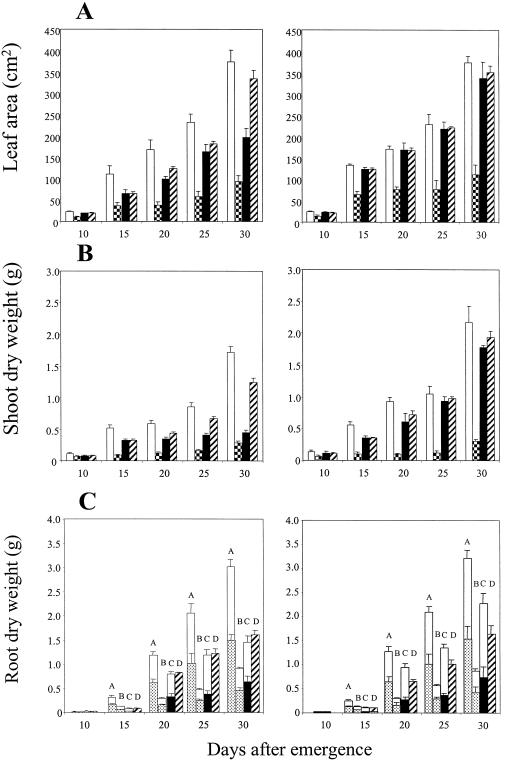
Figure 4.
Influence of STS and ethephon (eth) on leaf area (A) and root dry weight (B) in wild-type (cv Ailsa Craig) and transgenic (ACO1AS) genotypes of tomato grown in 1.1/1.5-g cm−3 split-pot soil columns. A, Black columns, split-pot; white columns, STS; hatched columns, ethephon. B, Total root dry weights and their distribution between the 1.1- and 1.5-g cm−3 compartments; the shaded areas denote roots in the 1.5-g cm−3 compartment. Single ses are shown. White/black bars, Split-pot; white/stippled bars, STS; white/hatched bars, ethephon.
Figure 2.
Influence of compaction treatment and excision of the roots in the compacted compartment of the 1.1/1.5 g cm−3 split-pot treatment on leaf area (A), shoot dry weight (B), and root dry weight (C) in wild-type (cv Ailsa Craig) and transgenic (ACO1AS) genotypes of tomato. A and B, White bars, 1.1 g cm−3; checked bars, 1.5 g cm−3; black bars, split-pot; hatched bars, split-cut. C, Letters (A, B, C, and D) denote the uniform 1.1- and 1.5-g cm−3 treatments, the 1.1/1.5-g cm−3 split-pot treatment, and 1.1/1.5-g cm−3 split-pot plants in which the roots in the 1.5-g cm−3 compartment were severed at 15 DAE, respectively. Overall column height shows total root dry weight for both compartments, while the white and filled areas within columns show root dry weights within each compartment. In C, the white and black areas show root dry weights in the 1.1- and 1.5-g cm−3 compartments; the hatched area in D shows root dry weight in the 1.1-g cm−3 compartment following excision of the roots in the 1.5-g cm−3 compartment at 15 DAE. Single ses are shown.
Excision of the roots at 15 DAE in the compacted compartment of the 1.1/1.5-g cm−3 treatment of cv Ailsa Craig (split-cut treatment) promoted a recovery of shoot growth (Fig. 2, A and B). Leaf area and shoot dry weight were, respectively, 58% and 62% of the uncompacted control values at 15 DAE, but increased to approximately 75% by 20 DAE, when the values for both variables were significantly greater than in plants with intact root systems (split-pot treatment). During the ensuing 10-d period, leaf area and shoot dry weight in split-cut plants reached approximately 90% of the uncompacted control values, whereas equivalent plants with intact root systems showed no recovery.
Shoot growth in 1.1-g cm−3 control plants of ACO1AS was comparable to the wild-type genotype, cv Ailsa Craig, and was again greatly reduced in the uniformly compacted 1.5-g cm−3 treatment, as in cv Ailsa Craig (P < 0.001; Fig. 2, A and B); thus, leaf area and shoot dry weight were 58% and 49%, respectively, of the uncompacted control values at 10 DAE, but declined to 30% and 14%, respectively, over the ensuing 20-d period. However, the response of shoot growth in the 1.1/1.5-g cm−3 treatment contrasted sharply with that of wild-type plants, as leaf area in ACO1AS was unaffected relative to 1.1-g cm−3 control plants at all harvests. Excision of the roots in the compacted compartment of the 1.1/1.5-g cm−3 treatment at 15 DAE had no effect on leaf area (Fig. 2A). The responses of the ACO1AS therefore clearly differed from the wild-type control plants.
Root dry weight was evenly distributed between the two compartments in the uniform 1.1- and 1.5-g cm−3 treatments, although the values were consistently much lower in the 1.5-g cm−3 treatment of both genotypes (P < 0.001; Fig. 2C). In the 1.1/1.5-g cm−3 treatment, root growth was lower in the compacted compartment than in the uncompacted compartment for both genotypes, particularly in ACO1AS. Excision of the roots in the 1.5-g cm−3 compartment also promoted genotypic differences in root growth; thus, total root dry weight in split-cut plants of cv Ailsa Craig was comparable to plants in the split-pot treatment, indicating that compensatory growth occurred in the uncompacted compartment (Fig. 2C). In contrast, total root dry weights for split-cut ACO1AS plants were 69% to 75% of the equivalent values for the split-pot treatment between 20 and 30 DAE (P < 0.001), suggesting that they were unable to compensate fully for the loss of the roots in the 1.5-g cm−3 compartment by increasing growth in the 1.1-g cm−3 compartment.
Both genotypes exhibited comparable trends for stomatal conductance (Fig. 3A), which declined slightly between 10 and 12 DAE in the uniform 1.1-g cm−3 treatment and then remained relatively constant for the remainder of the experiment. In the 1.5-g cm−3 treatment, stomatal conductance decreased sharply in both genotypes between 10 and 12 DAE (P < 0.001), then declined more gradually until 14 DAE, before remaining almost constant for the rest of the observation period. The values for the 1.5-g cm−3 treatment were significantly lower than for the 1.1-g cm−3 treatment after 12 DAE in both genotypes (P < 0.001), while those for ACO1AS were consistently, but not significantly, higher than in cv Ailsa Craig.
Stomatal conductance in the 1.1/1.5-g cm−3 treatment was generally similar to that in the uniform 1.5-g cm−3 treatment of both genotypes, although there was some evidence that conductance decreased more slowly between 12 and 16 DAE (Fig. 3A); conductance remained low for the remainder of the experiment. Conductance began to increase shortly after the roots in the compacted compartment were excised at 15 DAE, and was significantly greater (P < 0.001) than in equivalent split-pot plants of cv Ailsa Craig and ACO1AS by 16 and 18 DAE, respectively. Both genotypes exhibited full recovery to the uncompacted control values by 20 DAE, and these were then maintained for the remainder of the observation period (Fig. 3A). Thus, conductances typical of uncompacted plants were restored in the 1.1/1.5-g cm−3 treatment by severing the roots growing in compacted soil, thereby preventing the export of root-sourced signal(s) to the shoot.
The reduced stomatal conductances in the 1.5-g cm−3 and 1.1/1.5-g cm−3 treatments of both genotypes were reflected by higher xylem sap ABA concentrations (P < 0.001; Fig. 3B). ABA concentrations in both genotypes were approximately double those for uncompacted control plants at 10 DAE, increased by 45% and 24%, respectively, at 20 DAE, and declined again by 30 DAE, although this decrease was significant only in the 1.5-g cm−3 treatment (P < 0.001). Severing the roots in the compacted compartment of the 1.1/1.5-g cm−3 treatment at 15 DAE promoted a significant decrease in xylem sap ABA (P < 0.001) during the ensuing 5-d period relative to intact split-pot plants in both genotypes; by 30 DAE, the values for split-cut plants were identical to those for uncompacted control plants. The values for control plants were closely comparable in both genotypes.
As expected, ethylene evolution was lower in ACO1AS than in cv Ailsa Craig (Fig. 3C), particularly in plants which encountered compacted soil; ethylene evolution rates for the 1.5-g cm−3 treatment of cv Ailsa Craig were 3- to 5-fold greater than in uncompacted control plants (P < 0.001). The values for the 1.1/1.5-g cm−3 treatment of cv Ailsa Craig were significantly lower than in the 1.5-g cm−3 treatment at 15, 25, and 30 DAE (P < 0.01), but were consistently 2- to 5-fold greater than in uncompacted control plants. Excision of the roots in the compacted compartment greatly reduced ethylene evolution in cv Ailsa Craig, with the values at 20, 25, and 30 DAE being 64%, 39%, and 33%, respectively, of those for equivalent plants with intact root systems (P < 0.01).
Impact of Ethylene Releasing Agents and Inhibitors of Ethylene Action
Genotypic differences in shoot growth were again apparent when cv Ailsa Craig and ACO1AS were grown in the 1.1/1.5-g cm−3 split-pot system in the absence of detectable effects on leaf water status (Fig. 1B); leaf area was 48% to 71% smaller in cv Ailsa Craig than in ACO1AS (P < 0.001; Fig. 4A). Treatment with STS restored leaf area in cv Ailsa Craig to values approaching ACO1AS; thus, leaf area was increased by 47% in STS-treated plants of cv Ailsa Craig at 30 DAE, whereas the same treatment had no detectable effect on ACO1AS at all harvests. Treatment with ethephon significantly reduced leaf area in both genotypes (P < 0.001; Fig. 4A); thus, leaf area was 71%, 56%, and 51% of equivalent untreated plants at 10, 20, and 30 DAE, respectively, in cv Ailsa Craig and 71%, 39%, and 56%, respectively, in ACO1AS. Root growth was unaffected by applications of STS in both cv Ailsa Craig and ACO1AS, whereas treatment with ethephon reduced root biomass relative to control plants supplied with water (P < 0.001; Fig. 4B).
Stomatal conductance followed a generally similar pattern in both genotypes (Fig. 5A), decreasing significantly (P < 0.001) by 16 DAE in the split-pot and STS treatments of cv Ailsa Craig to reach minimum values of 121 and 136 mmol m−2 s−1, respectively. Treatment of cv Ailsa Craig with ethephon promoted a more rapid decline in conductance, with a significant decrease being apparent by 14 DAE (P < 0.001). Conductance then remained almost constant in all treatments, although the values for ethephon-treated plants were generally slightly lower than in the 1.1/1.5-g cm−3 and STS treatments. ACO1AS plants exhibited similar responses except that the decline in conductance was slightly delayed relative to wild-type plants in all treatments. As in cv Ailsa Craig, conductance was slightly lower in ethephon-treated plants than in the other treatments.
Figure 5.
Influence of STS and ethephon (eth) on stomatal conductance (A), xylem sap ABA concentration (B), and ethylene evolution (C) from leaf tissue in wild-type (cv Ailsa Craig) and transgenic (ACO1AS) genotypes of tomato grown in 1.1/1.5-g cm−3 split-pot soil columns. Double (A and B) or single ses (C) are shown. FW, Fresh weight. A and B, ♦, Split-pot; ▪, STS; ▴, ethephon. C, Black bars, Split-pot; white bars, STS; hatched bars, etephon.
Xylem sap ABA concentrations again exhibited similar trends in both genotypes, increasing between 10 and 20 DAE (P < 0.001; Fig. 5B), when maximum values ranged from 154 to 184 μmol m−3 in cv Ailsa Craig and 143 to 176 μmol m−3 in ACO1AS and declining by 30 DAE in all treatments of both genotypes; the maximum values therefore coincided with the observed reductions in stomatal conductance. The values for the 1.1/1.5-g cm−3 and STS treatments were comparable in both genotypes, and were generally slightly greater in ethephon-treated plants, reflecting their lower stomatal conductances.
Ethylene evolution was invariably much lower in ACO1AS than in cv Ailsa Craig and increased with time in all treatments of cv Ailsa Craig and ethephon-treated plants of ACO1AS (P < 0.001; Fig. 5C). Treatment with ethephon significantly increased ethylene evolution in both genotypes (P < 0.001), although the values for ACO1AS were consistently lower than in cv Ailsa Craig (P < 0.001). Treatment with STS reduced ethylene evolution in cv Ailsa Craig (P < 0.001), particularly during the latter stages of the experiment, but had no detectable effect on ACO1AS.
DISCUSSION
The split-pot system used here permitted investigation of the role of ethylene in mediating the impact of soil compaction on shoot and root growth in tomato, as described previously for ABA in barley (Hussain et al., 1999) and tomato (Mulholland et al., 1999). This system enables the effects of compaction to be examined in species particularly sensitive to impeded soil conditions by enabling most of the root system to be grown in uncompacted soil, while a smaller proportion penetrates the compartment containing compacted soil. The quantity of root produced in the compacted compartment was clearly sufficient to elicit significant growth and stomatal responses.
As leaf water potential was not affected in either genotype (Fig. 1), the observed growth and stomatal responses to compaction and treatment with ethephon or STS were apparently initiated by root-sourced chemical signals, as opposed to hydraulic signals. Stomatal conductance was inversely correlated with xylem sap ABA concentration (Figs. 3 and 5), in agreement with previous studies of the impact of water stress (Griffiths et al., 1996), suggesting that ABA transported from the impeded roots was responsible for inducing the observed stomatal responses in both genotypes. The ABA-deficient tomato mutant flacca has previously been shown to be incapable of regulating stomatal aperture effectively in a similar split-pot system, emphasizing the role of root-sourced ABA in coordinating stomatal responses to compacted soil conditions (Hussain et al., 1999; Mulholland et al., 1999). It therefore appears that a key role of root-sourced ABA in plants experiencing compacted soil conditions is to reduce stomatal conductance, thus limiting transpiration and permitting leaf water potential to be maintained at unstressed levels (Davies and Zhang, 1991).
The use of the transgenic low-ethylene ACO1AS and isogenic wild-type genotypes proved invaluable in establishing a role for ethylene in mediating growth responses to compaction. Thus, although shoot growth was similar in both genotypes in the uniform 1.1- and 1.5-g cm−3 treatments, significant genotypic differences were apparent in the 1.1/1.5-g cm−3 treatment, which provided a subcritical level of compaction stress comparable to that reported previously for barley grown in uniform 1.6-g cm−3 soil columns (Mulholland et al., 1996a). Leaf area and shoot dry weight increased almost linearly between harvests in both genotypes, but were reduced to a greater extent in the 1.1/1.5-g cm−3 treatment of cv Ailsa Craig than in ACO1AS relative to equivalent uncompacted control plants (Fig. 2, A and B). These effects occurred in the absence of detectable effects on leaf water potential both in the present (Fig. 1) and previous compaction studies (Mulholland et al., 1996a, 1999; Hussain et al., 1999).
The 1.1/1.5-g cm−3 treatment therefore promoted intriguing genotypic differences in which the wild-type, cv Ailsa Craig, exhibited greatly reduced shoot growth, while the low-ethylene genotype, ACO1AS, was able to maintain uncompacted control growth rates (Fig. 2, A and B). Excision of the roots in the 1.5-g cm−3 compartment at 15 DAE promoted a recovery of shoot growth in cv Ailsa Craig from 20 DAE, whereas shoot growth in ACO1AS was unaffected. These results demonstrate that root-sourced signal(s) other than ABA were responsible for the growth responses induced when cv Ailsa Craig encountered compacted soil, as xylem sap ABA concentrations were closely comparable in both genotypes yet differing genotypic responses were observed (Figs. 2 and 3). To our knowledge, this is the first reported comparison of ABA levels in the wild-type cv Ailsa Craig and ACO1AS genotypes of tomato, and the results clearly demonstrate the potency of the antisense strategy for modifying the synthesis of specific hormones without affecting the levels or stress responses of others. Such approaches offer a powerful tool for studies of the importance of specific hormones in regulating the stress physiology of plants.
Our results support the hypothesis that the impaired capacity of ACO1AS to convert ACC to ethylene permitted shoot growth to continue at uncompacted rates in the 1.1/1.5-g cm−3 treatment because endogenous ethylene levels remained below a critical threshold. Thus, the genotypic differences in growth observed in the 1.1/1.5-g cm−3 treatment were reflected by the significantly greater ethylene evolution in cv Ailsa Craig compared with ACO1AS (Figs. 3 and 5), even though evolution rates were comparable when both genotypes were grown in uncompacted soil. Leaf area and shoot dry weight were inversely correlated with ethylene evolution, suggesting that the greater ethylene production of wild-type plants in the 1.1/1.5-g cm−3 treatment contributed to the observed reduction in shoot growth. Comparisons may be drawn with the subcritical compaction stress imposed on barley by uniformly compacted 1.6-g cm−3 soil (Mulholland et al., 1996a, 1996b). These studies demonstrated that the inability of the ABA-deficient mutant, Az34, to synthesize and export ABA to the shoot at wild-type rates restricted its capacity to maintain leaf expansion at uncompacted levels during subcritical compaction stress (Mulholland et al., 1996a), and that treatment with synthetic ABA induced phenotypic reversion to wild-type growth rates (Mulholland et al., 1996b). Subsequent studies with barley (Hussain et al., 1999) provided evidence that an additional root-sourced signal, possibly ethylene, was involved in reducing shoot growth in the ABA-deficient mutant at the subcritical bulk density and in both genotypes during severe compaction stress; it was concluded that the growth responses induced may depend on the balance between endogenous ABA and ethylene levels.
In sharp contrast to the 1.1/1.5-g cm−3 split-pot system, the uniform 1.5-g cm−3 treatment provided a critical level of stress that greatly reduced shoot and root growth in both genotypes of tomato (Fig. 2), possibly by a mechanism that does not directly involve either ethylene or ABA. Thus, although ethylene evolution was significantly greater in the uniform 1.5-g cm−3 treatment of ACO1AS than in uncompacted control plants at 30 DAE (P < 0.001; Fig. 3C), this effect became apparent only after significant reductions in root and shoot growth had occurred. The hypothesis that increased delivery of root-sourced ABA was responsible for the inhibition of shoot growth in plants growing on severely compacted soil may be discounted, as previous studies have shown that comparable reductions in shoot growth are induced in ABA-deficient mutants of both tomato and barley (Mulholland et al., 1996a, 1999; Hussain et al., 1999). Another, as-yet-unidentified mechanism, perhaps involving changes in nutrient uptake or imbalances in hormones other than ethylene and ABA, may therefore act to inhibit shoot growth when the entire root system encounters severely compacted soil.
The possibility that ABA is the only root-sourced signal produced in response to soil compaction, but has the secondary effect of invoking increased ACC synthesis within the shoot, may also be discounted. Under this scenario, ethylene production and consequent reductions in leaf expansion and shoot growth would be greater in wild-type plants, whereas the limited ability of ACO1AS to convert ACC to ethylene would result in the maintenance of shoot growth. However, previous studies have shown that ethylene production is greater in ABA-deficient mutants of tomato in than in wild-type plants, even though xylem sap ABA concentrations are much lower (Hussain, 1998). Furthermore, similar studies of wild-type and ABA-deficient mutants of barley revealed that the increases in xylem sap ABA concentration induced by subcritical compaction stress had the contrary effect of reducing ethylene evolution from leaf tissue (Hussain, 1998).
The original hypothesis that genotypic differences in ethylene production were responsible for the observed reduction of growth in the 1.1/1.5-g cm−3 split-pot treatment of cv Ailsa Craig was substantiated by the experiments in which ethephon and STS were supplied. Treatment with ethephon increased ethylene evolution and reduced leaf area in both genotypes, whereas limiting ethylene action by blocking its binding sites with silver ions partially restored leaf expansion in cv Ailsa Craig (Figs. 4A and 5C). Thus, a partial restoration of uncompacted growth rates was achieved simply by blocking the physiological activity of the increased ethylene levels induced by compaction in the absence of any change in the soil conditions experienced by the plants. Treatment with STS significantly reduced ethylene evolution in cv Ailsa Craig, perhaps by eliminating the induction of autocatalytic ethylene production by stress (Sfakiotakis et al., 1996); this may have further contributed to the impact of silver ions in alleviating the symptoms of compaction. As STS-treated plants grown under a range of compaction treatments exhibited no significant effects on root growth relative to control plants treated with water (Fig. 4B; Hussain, 1998), the possibility that the restoration of shoot growth in STS-treated plants of cv Ailsa Craig resulted from an inhibition of root growth and subsequent reallocation of assimilates to support shoot growth may be discounted. Furthermore, a similar stimulation of shoot growth would have been expected in STS-treated ACO1AS plants if reallocation of available assimilates was responsible for the observed growth responses; however, no detectable effects were observed relative to control plants treated with water.
Genotypic differences were also apparent for root biomass, which was evenly distributed between both compartments in the uniform 1.1- and 1.5-g cm−3 treatments, but reduced in the compacted compartment of the 1.1/1.5-g cm−3 system in both genotypes (Fig. 2). Root dry weight was also reduced in the uncompacted compartment of the split-pot treatment of cv Ailsa Craig relative to 1.1-g cm−3 control plants, suggesting that root growth may have been limited by reductions in assimilate availability resulting from the greatly reduced leaf area. Thus, the significantly greater ethylene levels in the wild-type genotype may have been responsible for reducing root growth in the uncompacted compartment in addition to shoot growth. In contrast, the absence of any reduction of root growth in the 1.1-g cm−3 compartment of the 1.1/1.5-g cm−3 split-pot treatment of ACO1AS relative to uncompacted control plants reflected its ability to maintain leaf growth, and hence assimilate supplies, at near control levels. Wild-type plants showed a marked increase in root biomass in the uncompacted compartment following excision of the roots in the compacted compartment at 15 DAE, with the result that total root biomass was slightly greater in split-cut than in split-pot plants at 25 and 30 DAE (Fig. 2C); this increase in root biomass reflects the concurrent recovery of leaf area and hence assimilate production. In contrast, root biomass in the uncompacted compartment was comparable for split-pot and split-cut plants of ACO1AS, reflecting the absence of significant compensatory root growth or any promotion of shoot growth following excision of the roots in the compacted compartment. These results suggest that the observed root growth responses are intimately linked to effects on shoot growth and assimilate availability.
CONCLUSIONS
Leaf expansion and shoot growth in plants growing on compacted soil were more closely correlated with endogenous ethylene levels than with xylem sap ABA concentration, whereas root-sourced ABA appeared to function as a signal regulating stomatal conductance, as reported previously (Davies et al., 1994; Mulholland et al., 1996a, 1999; Hussain et al., 1999). The use of the ethylene-deficient transgenic ACO1AS clearly demonstrated the constraining influence of ethylene on shoot growth when roots encounter compacted soil. Excision of roots in the compacted compartment of the 1.1/.1.5-g cm−3 treatment confirmed that a root-sourced signal was responsible for limiting leaf area and shoot growth, while artificially increasing ethylene production by applying ethephon or limiting its perception using silver ions induced phenotypic reversion in ACO1AS and cv Ailsa Craig, respectively. Although its concentrations and fluxes have yet to be measured, it appears likely that ACC may have a role as a potential root-sourced signal, as this ethylene precursor is readily transported in the transpiration stream and ACO1AS would be insensitive to changes in its concentration (English et al., 1995). The uniformly high bulk density in the 1.5-g cm−3 treatment provided a critical compaction stress in both genotypes, which greatly reduced root and shoot growth, demonstrating that the ability of genotypic differences in endogenous ethylene levels to ameliorate the adverse effects of compaction is lost when the entire root system encounters severely compacted soil.
ACKNOWLEDGMENT
Financial support from the UK Natural Environment Research Council and the Biotechnology and Biological Science Research Council is gratefully acknowledged.
Footnotes
The work reported here was funded by the UK Natural Environment Research Council and the Biotechnology and Biological Sciences Research Council.
LITERATURE CITED
- Andrade A, Wolfe DW, Ferres E. Leaf expansion, photosynthesis and water relations of sunflower plants grown on compacted soil. Plant Soil. 1993;149:175–184. [Google Scholar]
- Beemster GTS, Masle J. Effects of soil resistance to root penetration on leaf expansion in wheat (Triticum aestivum L.): composition, number and size of epidermal cells in mature blades. J Exp Bot. 1996;47:1651–1662. [Google Scholar]
- Beyer EM. Silver ion: a potent inhibitor of ethylene action in plants. Plant Physiol. 1976;56:268–271. doi: 10.1104/pp.58.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A, Marriott CA, Seel W, Mullins CE. Effects of soil mechanical impedance on root and shoot growth of Lolium perenne L., Agrostis capillaris L. and Trifolium repens L. J Exp Bot. 1996;47:1075–1084. [Google Scholar]
- Davies WJ, Tardieu F, Trejo CL. How do chemical signals work in plants that grow in drying soil? Plant Physiol. 1994;104:309–314. doi: 10.1104/pp.104.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WJ, Zhang J. Root signals and the regulation of growth and development of plants in drying soil. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:55–76. [Google Scholar]
- English PJ, Lycett GW, Roberts JA, Jackson MB. Increased 1-aminocyclopropane-1-carboxylic acid oxidase activity in shoots of flooded tomato plants raises ethylene production to physiologically active levels. Plant Physiol. 1995;109:1435–1440. doi: 10.1104/pp.109.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths A, Parry AD, Jones HG, Tomos AD. Abscisic acid and turgor pressure regulation in tomato roots. J Plant Physiol. 1996;149:372–376. [Google Scholar]
- Hamilton AJ, Lycett GW, Grierson D. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants. Nature. 1990;346:284–287. [Google Scholar]
- Hartung W, Davies WJ. Drought induced changes in physiology and ABA. In: Davies WJ, Jones HG, editors. Abscisic Acid: Physiology and Biochemistry. Oxford: Bios Scientific Publishers; 1991. pp. 63–79. [Google Scholar]
- He C, Finlayson SA, Drew MC, Jordan WR, Morgan PW. Ethylene biosynthesis during aerenchyma formation in roots of maize subjected to mechanical impedance and hypoxia. Plant Physiol. 1996;112:1679–1685. doi: 10.1104/pp.112.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A. Soil compaction: mediation of plant responses by root-sourced ABA and ethylene. PhD thesis. UK: University of Nottingham; 1998. [Google Scholar]
- Hussain A, Mulholland BJ, Black CR, Taylor IB, Roberts JA (1999) Novel approaches for examining the effects of differential soil compaction on xylem sap ABA concentration, stomatal conductance and growth in barley (Hordeum vulgare L.). Plant Cell Environ 22 (in press)
- Jackson MB. Hormones from roots as signals for the shoots of stressed plants. Trends Plant Sci. 1997;2:22–28. [Google Scholar]
- Kays SJ, Nicklow CW, Simons DH. Ethylene in relation to the response of roots to physical impedance. Plant Soil. 1974;40:565–571. [Google Scholar]
- Masle J. Growth and stomatal behaviour: response to soil resistance to root penetration. In: Davies WJ, Jeffcoat B, editors. Importance of Root to Shoot Communication in the Responses to Environmental Stress, Monograph 21. Bristol, UK: British Society for Plant Growth Regulation; 1990. pp. 95–106. [Google Scholar]
- Masle J. Genetic variation in the effects of root impedance on growth and transpiration rates of wheat and barley. Aust J Plant Physiol. 1992;19:109–125. [Google Scholar]
- Maynard JA, Swain JM. Organophosphorus compounds. I. 2-chloroalkylphosphonic acids as phosphorylating agents. Aust J Chem. 1963;16:596–608. [Google Scholar]
- Morgan PW, Sarquis JL, He C, Jordan WR, Drew MC. Regulation of ethylene biosynthesis in maize root responses to stress. In: Pech JC, Latche A, Balague C, editors. Cellular and Molecular Aspects of the Plant Hormone Ethylene. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1993. pp. 232–237. [Google Scholar]
- Mulholland BJ. ) Soil compaction and plant growth: the role of root-sourced chemical signals. PhD thesis. UK: University of Nottingham; 1994. [Google Scholar]
- Mulholland BJ, Black CR, Taylor IB, Roberts JA, Lenton JR. Effect of soil compaction on barley (Hordeum vulgare L.) growth. I. Possible role for ABA as a root-sourced chemical signal. J Exp Bot. 1996a;47:539–549. [Google Scholar]
- Mulholland BJ, Hussain A, Black CR, Taylor IB, Roberts JA (1999) Does root-sourced ABA have a role in mediating growth and stomatal responses to soil compaction in tomato (Lycopersicon esculentum Mill.)? Physiol Plant 107 (in press)
- Mulholland BJ, Taylor IB, Black CR, Roberts JA. Effect of soil compaction on barley (Hordeum vulgare L.) growth. II. Are increased xylem sap ABA concentrations involved in maintaining leaf expansion in compacted soils? J Exp Bot. 1996b;47:551–556. [Google Scholar]
- Munns R. A leaf elongation assay detects an unknown growth inhibitor in xylem sap from wheat and barley. Aust J Plant Physiol. 1992;19:127–135. [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE, Loveys BR. A monoclonal antibody to (S) abscisic acid: its characterization and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta. 1988;173:330–339. doi: 10.1007/BF00401020. [DOI] [PubMed] [Google Scholar]
- Sarquis JI, Jordan WR, Morgan PW. Ethylene evolution from maize (Zea mays L.) seedling roots and shoots in response to mechanical impedance. Plant Physiol. 1991;96:1171–1177. doi: 10.1104/pp.96.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfakiotakis E, Antunes MD, Stavroulakis G, Niklis N, Ververidis P, Gerasopoulos D. Ethylene biosynthesis and its regulation in ripening “Hayward” kiwifruit. In: Kanellis AK, Chang C, Kende H, Grierson D, editors. Biology and Biotechnology of the Plant Hormone Ethylene. London: Kluwer Academic Publishers; 1996. pp. 47–57. [Google Scholar]
- Tardieu F, Katerji N, Bethonod O, Zhang J, Davies WJ. Maize stomatal conductance in the field: its relationship with soil and leaf water potentials, mechanical constraints and ABA concentration in the xylem sap. Plant Cell Environ. 1991;14:121–126. [Google Scholar]
- Tardieu F, Zhang J, Katerji N, Bethonod O, Davies WJ. Xylem ABA controls the stomatal conductance of field-grown maize subjected to soil compaction or soil drying. Plant Cell Environ. 1992;15:193–197. [Google Scholar]
- Thomasson AJ. Soils of the Melton Mowbray District. London: Her Majesty's Stationary Office; 1971. [Google Scholar]
- Young IM, Montagu D, Conroy J, Bengough AG. Mechanical impedance of root growth directly reduces leaf elongation rates in cereals. New Phytol. 1997;135:613–621. [Google Scholar]