Significance
The frequency of the human-specific EDAR V370A isoform is highly elevated in North and East Asian populations. The gene is known to have several pleiotropic effects, among which are sweat gland density and ductal branching in the mammary gland. The former has led some geneticists to argue that the near-fixation of this allele was caused by selection for modulation of thermoregulatory sweating. We provide an alternative hypothesis, that selection instead acted on the allele’s effect of increasing ductal branching in the mammary gland, thereby amplifying the transfer of critical nutrients to infants via mother’s milk. This is likely to have occurred during the Last Glacial Maximum when a human population was genetically isolated in the high-latitude environment of the Beringia.
Keywords: mammary epithelium, dental anthropology, Beringia, adaptation, UV radiation
Abstract
Because of the ubiquitous adaptability of our material culture, some human populations have occupied extreme environments that intensified selection on existing genomic variation. By 32,000 years ago, people were living in Arctic Beringia, and during the Last Glacial Maximum (LGM; 28,000–18,000 y ago), they likely persisted in the Beringian refugium. Such high latitudes provide only very low levels of UV radiation, and can thereby lead to dangerously low levels of biosynthesized vitamin D. The physiological effects of vitamin D deficiency range from reduced dietary absorption of calcium to a compromised immune system and modified adipose tissue function. The ectodysplasin A receptor (EDAR) gene has a range of pleiotropic effects, including sweat gland density, incisor shoveling, and mammary gland ductal branching. The frequency of the human-specific EDAR V370A allele appears to be uniquely elevated in North and East Asian and New World populations due to a bout of positive selection likely to have occurred circa 20,000 y ago. The dental pleiotropic effects of this allele suggest an even higher occurrence among indigenous people in the Western Hemisphere before European colonization. We hypothesize that selection on EDAR V370A occurred in the Beringian refugium because it increases mammary ductal branching, and thereby may amplify the transfer of critical nutrients in vitamin D-deficient conditions to infants via mothers’ milk. This hypothesized selective context for EDAR V370A was likely intertwined with selection on the fatty acid desaturase (FADS) gene cluster because it is known to modulate lipid profiles transmitted to milk from a vitamin D-rich diet high in omega-3 fatty acids.
From Thomas Jefferson’s archaeological excavations (1) to modern genomics (2–4), scientists have been fascinated by the first migration of humans into the Americas. A myriad of new evidence reveals that the earliest people in the Western Hemisphere dispersed from a population that lived in genetic isolation for thousands of years on the exposed Beringian platform in the Arctic during the Last Glacial Maximum [LGM; 28,000–18,000 y ago (2–7)]. The Arctic is an extreme environment because of the very low UV radiation (UV) reaching the earth’s surface at such high latitude. UV is essential to almost all life forms because it catalyzes biochemical processes, especially the synthesis of vitamin D (8).
Extreme environments can impact genetic variation and provide opportunities to elucidate relationships between genotype and phenotype (9). The classic human example is the range of our physiological adaptations to the hypoxic conditions of high altitude, such as are experienced in the highlands of Ethiopia, the Tibetan Plateau, and the Andean Mountains (10–12). Evidence shows that the populations which have long-occupied these high-elevation regions have an increased frequency of red blood cell polymorphisms that likely underlie these physiologies (10–12).
Here, we investigate whether the population occupying Beringia during the LGM represents another example of human adaptation to an extreme environment, this time adapting to very low UV exposure (Fig. 1). There are two lines of genetic evidence for this: variation in the fatty acid desaturase (FADS) gene cluster that modulates the manufacture of polyunsaturated fatty acids and variation in the ectodysplasin A receptor (EDAR) gene that influences ectodermally derived structures, such as teeth, hair, and mammary gland ductal branching. A study on selection on the FADS gene cluster in the ancestral population of Native Americans has been published previously (13), but, here, we shift the emphasis from phenotypic effects on older adults to focus on those that influence fertility via breast milk. We then present evidence that EDAR may have undergone an episode of selection in the same population, likely due to its influence on mammary ductal branching. We hypothesize that the genetically isolated population which occupied Beringia during the LGM experienced selection for an increase in vitamin D in breast milk in response to the low UV environment. Traces of this previously intense selection appear to still be present in the genetic variation of Native American and North and East Asian populations today.
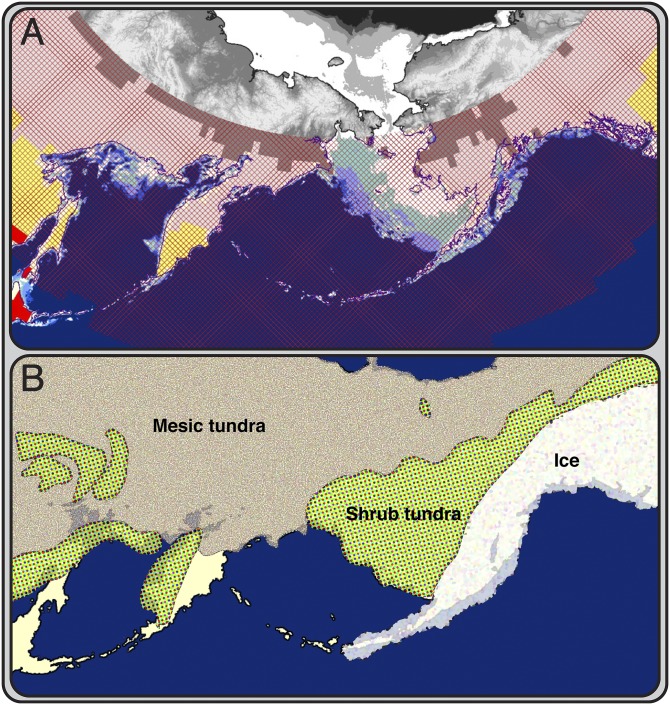
Fig. 1.
Geography of Beringia and levels of UV radiation. (A) Map of Beringia today. Cross-hatching indicates the region in which levels of UVMED (defined as the amount of UV radiation that will produce minimal erythema) that reach the Earth’s surface are too low to promote cutaneous synthesis of vitamin D in humans on a year-by-year basis, requiring dietary supplementation (modified from ref. 84; projected to show an equal distance map of Beringia). The black and white region marks the Arctic Circle, for which there are no Total Ozone Mapping Satellite Data version 7. Other data show that this region has even less UV-B exposure, as would be expected from the increased latitude. The areas below the Arctic Circle in white and light blue are shallow seas as discerned from modern bathymetry using Etopo2 data, indicating land that would have been exposed during the LGM. (B) Map of Beringia during the LGM showing the exposure of land at 117 m below current sea level and the reconstructed terrestrial environments. The shrub tundra is the only area biologically productive enough to support a human population of the size estimated by molecular data. This population was genetically isolated for ∼2,500–9,000 y during the LGM because of the ice to the east and extensive mesic tundra to the west.
The FADS Gene Cluster
Allelic variation in the FADS gene complex corresponds strongly to geographic ancestry (14, 15), and is the most pronounced allele frequency difference between the Greenlandic Inuit and other human populations (16). Because modern Western cultures place much attention on the cardiovascular disease risks associated with diets rich in omega-6 versus omega-3 long-chain polyunsaturated fatty acids (LC-PUFAs) (17, 18), the high frequencies of these alleles in the Inuit have been primarily interpreted as an adaptation to their traditionally meat-rich (and omega-3–rich) diet (16, 19). However, and surprisingly, 95% of native Central and South Americans also show evidence of selection on the same FADS polymorphisms, yet these cultural groups do not traditionally consume diets nearly as rich in omega-3 fatty acids as do the Inuit, suggesting that their common ancestral population is characterized by one which did (13).
From an evolutionary perspective, the possibility that the selective force favoring these FADS alleles was a healthier ratio of omega-3 to omega-6 fatty acids in adults would indicate that the effect must have been very strong, as selective pressure is relatively low on genetic variation that influences later and post-reproductive years of life (20). In contrast, even very small positive effects on fertility and childhood survival have far greater selective power (20). Considering this, the selective benefits of these FADS polymorphisms are more likely connected to their significant role in modulating the relative levels of omega-3 and omega-6 fatty acids during gestation and postnatal growth of infants.
To elaborate, the fatty acids arachidonic acid, docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA) are essential to cognitive and visual development (21). Maternal polymorphisms in the FADS gene cluster have strong effects on the levels of EPA and DHA in breast milk [in humans (22–27) and in other mammals (27, 28)]. This suggests that lipid levels in a mother’s diet are optimized by specific polymorphisms in her FADS gene cluster during milk synthesis (Fig. 2A). These polymorphisms are therefore a potentially strong target of selection because fetuses and infants have a very limited ability to synthesize LC-PUFAs on their own and are dependent on their mother’s genotype to modulate their relative proportions (22–27). We propose that the phenotypic effects of FADS polymorphisms on the LC-PUFA content of breast milk was the primary target of selection in past human populations that consumed diets with compromised proportions of omega-3 and omega-6 fatty acids, such as is seen in the traditional diets of people living in the Arctic.
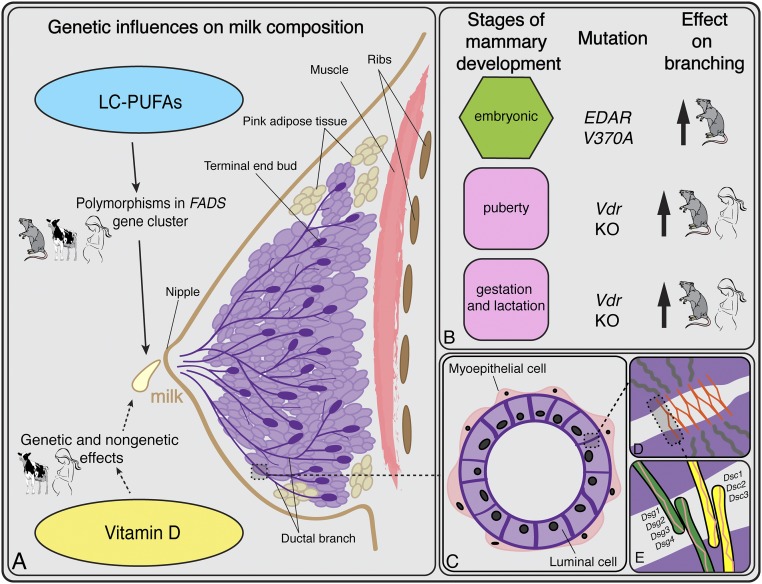
Fig. 2.
Overview of breast anatomy, development, milk production, and histology. (A) Genetic influences on milk production summarized from the main text. The rodent, human, and cow figures indicate the systems from which the results are known. (B) Three stages of mammary development alongside the genetic mutations known to increase ductal branching. Hormones induce the pubertal and gestation/lactation stages (denoted in pink). In vitamin D-deficient adipose tissue, ductal branching increases during these two stages of development. The embryonic stage (in green) is not hormonally induced; branching density is influenced by the ectodysplasin pathway (108) and, specifically, increased by EDAR V370A. (C) Cross-section of a ductal lumen showing the bilayer of luminal and myoepithelial cells. (D) Schematic of a desmosome, one of the main adhesive structures between mammary luminal cells. The orange lines represent desmosomal cadherins in the extracellular space. The gray lines in the purple area represent the intracellular filaments. (E) Close-up view of the desmocolin proteins that comprise the desmo-adhesome. An increase in the activity of the ectodyplasin pathway alters the relative proportions of Dsc2 and Dsc3, leading to a reduction in the adhesive strength.
EDAR.
The human EDAR V370A variant encodes a change in amino acid sequence in the highly conserved death domain of EDAR (29–32). EDAR functions as a protein receptor on a cell’s surface that activates the transcription factor NF-ĸB in the ectodysplasin pathway (29). Comparative studies show this pathway is functionally conserved across virtually all vertebrates, playing essential roles in the development of ectodermal structures from bird feathers to fish scales (33, 34).
EDAR is one of four genes implicated in hypohidrotic ectodermal dysplasia (HED), a set of ∼150 syndromes characterized by mild to severe defects in ectodermally derived structures, such as hair, teeth, breasts, and sweat glands (33, 35–37). In contrast to HED, the EDAR V370A allele has the opposite effect. Genome-wide association studies (GWASs) of multiple Asian populations show that EDAR V370A is correlated with hair shaft caliber (38, 39), earlobe and chin shape (40), and a suite of morphological variants on teeth (40–43). Knock-in mouse studies reveal that the V370A allele leads to a twofold increase in NF-ĸB activation (30, 31, 44). Just like humans, the EDAR V370A mouse has thicker hair shafts, an increase in the branching density of mammary gland ducts, and (inconsistently) an increase in the number of eccrine glands on the footpads (30, 31).
Several studies conclude that EDAR V370A experienced a bout of intense positive selection ∼20,000 y ago in northern China (29, 31, 45). Previous interpretations as to why this selection occurred focused on the associated increase in eccrine sweat gland density on the fingertips, concluding that the selection was for improved thermoregulatory sweating during a warm spell during the LGM (31) or that the increase in sebaceous glands would offer protection from the cold, dry air of the LGM (30). These interpretations rely primarily on the current distribution of the allele in living populations (Fig. 3A), which shows high frequencies of EDAR V370A in North and East Asian and Native American populations but virtual absence in other populations around the world (29–31, 45).
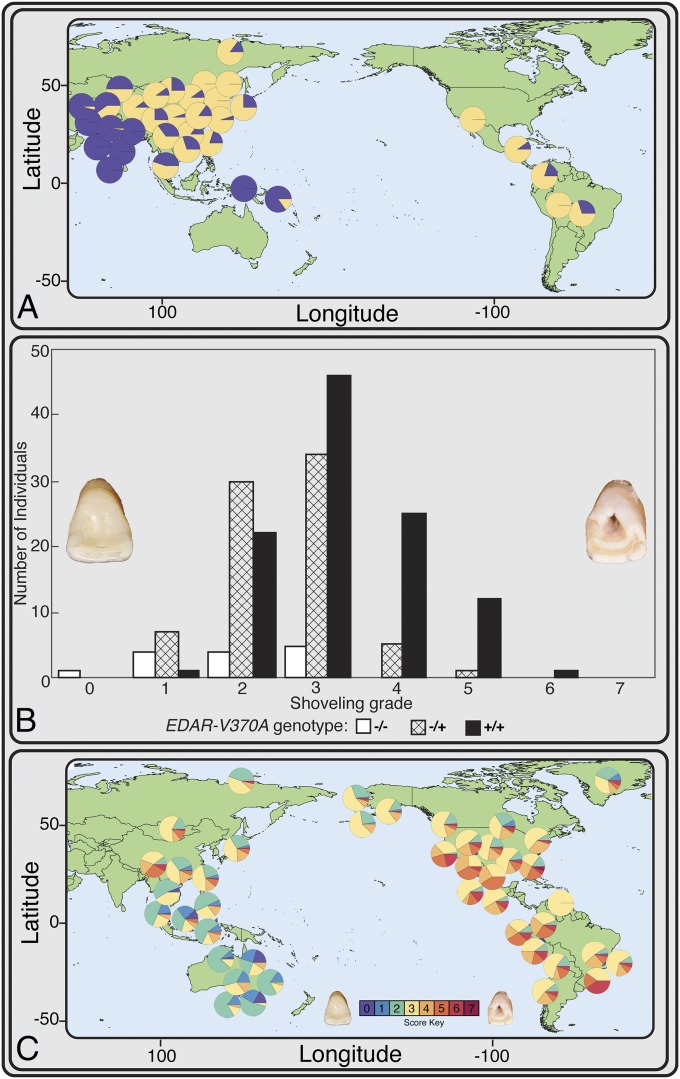
Fig. 3.
Relationship between EDAR V370A and degree of incisor shoveling. (A) Current world-wide allele frequencies of EDAR V370A (in yellow) and other EDAR haplotypes (in blue) (data from ref. 29). Because these data are from living people, the Native American data include significant admixture from European colonization, a population with essentially no occurrence of EDAR V370A. These modern data likely vastly underrepresent the occurrence of EDAR V370A among indigenous people before European contact. Current Asian allele frequencies may be higher than pre-LGM frequencies due to back-migration from the Beringian population after the end of the LGM, a migration supported by linguistic data (46). (B) Histogram showing EDAR V370A genotype and degree of incisor shoveling, demonstrating the imperfect but strongly additive nature of EDAR V370A’s influence on incisor shoveling (adapted from ref. 43). (C) Frequencies of incisor shoveling scores observed in archaeological populations from Africa, Europe, South Asia, East Asia, North America, and South America (Table S1). Purple and blue represent a lack of incisor shoveling, and as such, an individual who is EDAR V370A−/−. Note the lack of shoveling scores of 0 and 1, and very low occurrence of 2, in the indigenous people of the Western Hemisphere, indicating a very high frequency of EDAR V370A before European contact.
The post-LGM diaspora into the Americas and the subsequent centuries of European colonization (and genetic admixture) have dramatically overwritten the allelic variation of ∼20,000 y ago when EDAR V370A experienced a bout of selective pressure. To best interpret the evolutionary history of EDAR V370A, we need information on past allele frequencies. This task is difficult from a genome sequence approach because of the paucity of ancient DNA available. However, the pleiotropic effects of EDAR V370A provide a unique opportunity to more precisely reconstruct its evolutionary history without DNA sequence data.
Temporal and Geographic Variation of EDAR V370A
As noted previously, EDAR V370A is correlated with a suite of morphological variants on teeth, the most notable of which is incisor shoveling (40–43). GWASs report a close relationship between genotype (EDAR V370A) and this dental phenotype in living Chinese, Japanese, and Koreans (40–43). In these populations, incisor shoveling of any degree correlates with the presence of one or two copies of the EDAR V370A allele in an imperfect but clearly additive manner (41–43) (Fig. 3B).
We scored the degree of expression in maxillary incisor shoveling for 5,333 people from >54 archaeological populations from across Europe, Asia, and North and South America (Supporting Information and Tables S1 and S2). Incisor shoveling is absent or expressed only to a slight degree outside of North and East Asian populations (where EDAR V370A frequencies are close to zero) but close to ubiquity in Native American populations, a pattern long interpreted as the result of an unknown selective pressure (47, 48) (Fig. 3C).
Considering the GWAS results that indicate an additive effect of EDAR V370A’s contribution to incisor shoveling, we interpreted a score of 1 or greater as evidence of an individual likely carrying at least one copy of EDAR V370A. There are no reported instances of a score of 0 among the 3,183 individuals assessed from North and South America, and as such, no evidence of any Native Americans before European contact without at least one copy of the EDAR V370A allele (Fig. 3C and Supporting Information). A series of ANOVAs comparing the frequency of each shoveling grade (range: 0–7) across geographic regions demonstrates that a score of 1 has the greatest variation between geographic groups, further suggesting that a score of 1 or higher is reflective of the presence of at least one copy of EDAR V370A (Supporting Information and Table S3).
These phenotypic data strongly suggest that incisor shoveling (and, concomitantly, the EDAR V370A allele) reached near-fixation in the population ancestral to all indigenous people of the Western Hemisphere. Given the timing of the dispersal into the Americas ∼17,000 y ago (3, 49), these dental data support the conclusion that EDAR V370A underwent positive selection in the ancestral Native American population during the LGM, similar to what we see with the FADS gene cluster (13), rather than in a population in what is now China (our evolutionary quantitative genetic analysis further supports this; Supporting Information).
The Ancestral Population of Native Americans and the LGM
Indigenous people in the Western Hemisphere derive from people who occupied latitudes above 55°N in Asia before 40,000 y ago (50). Well-dated archaeological sites near the mouth of the Yana River indicate a year-round adaptation to the Beringian Arctic Zone (latitude of 70°N) by 32,000 calibrated radiocarbon years (cal) B.P. (51).
Between 28,000 and 18,000 y ago, during the LGM, as aridity increased and biological productivity reduced dramatically (52), plants and animals in many parts of the world retreated to refugia, leaving signals of genetic isolation and hybridization that are documented widely by molecular ecologists (53, 54). Human populations similarly abandoned arid regions in Africa, Eurasia, and Australia, as indicated by genetic bottlenecks and evidence of local settlement hiatus (e.g., refs. 55, 56).
Various lines of evidence suggest that the Native American lineages during the LGM were genetically isolated in one or more refugia within Beringia (2, 5–7), and at least one archaeological site appears to confirm a human presence in northeastern Beringia (latitude of 67°N) during that time frame (57). Analyses of Native American genetic variation indicate that the ancestral population was isolated for up to 9,000 y before dispersal in North and South America (after 15,000 cal B.P.), with an effective population size of at least a few thousand individuals (3, 49, 58).
The “Beringian Standstill” model places the ancestral Native American population in the Beringian refugium during much or all of the LGM (2, 5–7), where it was geographically isolated from the Siberian population by uninhabitable areas in northeastern Asia and unable to expand into the Americas because of the coalesced Laurentide and Cordilleran ice sheets (Fig. 1B). The genetic isolation is a key point, as evolution occurs more readily in the absence of gene flow (59). The FADS polymorphisms and EDAR V370A likely existed in the pre-LGM ancestral populations [although the allele is not present in Denisovans (60)]. However, there was a significant opportunity for selection to shift the frequencies of functional alleles dramatically when this population became genetically isolated in the extreme UV environment of Beringia, including Arctic Beringia (above 66°N).
Biological Consequences of Low UV
Most vitamin D necessary for human health and reproductive success is produced through biosynthesis initiated by skin exposure to UV-B photons (61, 62). The lower rates of cutaneous synthesis of vitamin D that result from low UV environments have a wide range of health consequences. Vitamin D is among a large family of fat-soluble micronutrients that accumulate in adipose tissue proportional to circulating serum levels, and is released slowly as serum levels reduce (63). The bioactive form of vitamin D plays a secosteroid hormonal role in permitting absorption of dietary calcium through the lining of the gut (64). Through the Vitamin D receptor (Vdr), vitamin D also regulates expression of more than 220 genes in the human genome, with significant involvement in immune function and autoimmune disorders (65, 66). It is therefore not surprising that variation in immune function and disease risk correlates with latitude and UV-B exposure (67). Vitamin D also plays an important immunomodulatory role at the maternal/fetal interface (the placenta) (68) and is associated with sex-specific variation in birth weight (69).
However important the immunological role of vitamin D, adipose tissue appears to be the main phenotypic target of Vdr (63, 65, 66, 70). Vitamin D deficiency significantly compromises the metabolic function of adipose tissue, with a wide range of deleterious health effects (63). Body fat has a remarkable degree of phenotypic plasticity that enables a wide range of essential physiological functions, from maintaining body heat, to responding to cold, to forming the pink adipocytes in the mammary glands during pregnancy in anticipation of lactation (71–73) (Fig. 2A).
Nutritional studies have long shown interrelationships between vitamin D and LC-PUFAs (74–76). For example, the vitamin D synthesized through skin exposure to UV-B (vitamin D3) has positive effects on lipid profiles that cannot be replicated with dietary vitamin D2 supplementation (77). These interrelationships result from the effects of vitamin D on adipose tissue and immunological function, which are modulated by environmental exposure to UV-B.
Human populations that dispersed into geographic regions above 30° latitude must have experienced selection for reduced cutaneous eumelanin pigmentation to facilitate vitamin D biosynthesis (78, 79). Selection for depigmented skin phenotypes has occurred at least twice in modern human evolution and once in Neanderthals (78, 80), with selective pressure readily detected in genomic analyses (81–83).
At latitudes above 48° (e.g., 450 km north of Hokkaido, Japan; Fig. 1A), sunlight reaching the Earth’s surface contains almost no UV-B except for low levels at or near the summer solstice (84, 85). Year-round human habitation of even more extreme high latitudes leads to vitamin D deficiency that cannot be counterbalanced by depigmentation alone (84) (Fig. 1A). In these geographic regions, diets centered on vitamin D-rich foods, such as marine mammals, oily fish, reindeer, or caribou, have been adopted across a range of traditional Arctic cultures (85). Archaeological evidence shows that these dietary innovations correlate with the earliest occupation of these latitudes (55, 86, 87) and shifts in allele frequencies of the genes involved in fatty acid synthesis (16).
Dietary practices can mitigate vitamin D deficiency for older children and adults, but pregnant and nursing mothers and their breast-feeding infants are still at significant risk because they need particularly high levels of vitamin D to avoid the litany of deleterious effects of vitamin D insufficiency (88, 89).
Fat and Vitamin D Content in Milk
Human breast milk varies greatly in fat content, with the relative proportions of omega-3 and omega-6 fatty acids varying by ethnicity independent of maternal diet (90), most likely because of polymorphisms in the FADS gene cluster as discussed earlier (22–27). The LC-PUFAs in milk are drawn from the specialized pink adipocytes found only in mammary glands, which are characterized by a higher number of intracellular lipid droplets (71–73) and are dependent on healthy vitamin D levels to function properly (63).
Vitamin D content in human milk also varies between individuals and across populations (90–93). Although the effects of dietary supplementation, sun exposure, and genetic variation affect the vitamin’s concentration within an individual, there is nevertheless a strong correlation between maternal and infant circulating vitamin D levels (94, 95), and both mother and infant levels are related to each other’s sun exposure (93, 96). Importantly, vitamin D levels in milk are about threefold lower than the circulating levels in the mother (97). Endogenously produced vitamin D3 from UV-B exposure appears to be preferentially transferred into milk compared with the vitamin D2 consumed through diet (93). It is thus reasonable to suggest that infants in extremely low UV environments would be particularly dependent on the mother’s potential capacity to transfer vitamin D from her adipose stores across the placenta and also into her breast milk.
Fat, Vitamin D, and the Mammary Gland
Given that vitamin D is intricately intertwined with adipose physiology, it is not surprising that vitamin D plays a role in virtually all aspects of mammary gland function, as evinced by the presence and activation of the Vdr in mesenchymal and epithelial breast tissue (98, 99). Vitamin D also appears to influence ductal branching and, with it, milk content.
There are three distinct phases of breast development (Fig. 2B): (i) hormone-independent embryonic development during which buds form, sprout, and start to branch into the precursor of the fatty stroma to form a ductal tree; (ii) the onset of pubertal hormonal signaling when terminal end buds form at the tips of the mammary ducts and start to invade the fat pad from which they will extract milk fats, vitamins, and other nutrients (100–102); and (iii) the repeatable hormone-induced cycles of pregnancy, lactation, and weaning (101, 102).
Vitamin D-deficient adipose tissue has been modeled by an adipose-specific Vdr knockout (Vdr KO) mouse, revealing significant and, from an evolutionary perspective, fascinating sex-by-diet effects (99). Female mice that are unable to bioactivate vitamin D in adipocytes exhibit increased visceral adipose tissue overall, but they have a normal mammary fat pad mass. Although the mass of the mammary fat pad is unaffected by the loss of Vdr, there is an increase in the ductal branching density within it (99). Surprisingly, however, this only occurred when the mice are fed a high-fat diet (reminiscent of traditional human diets in Artic cultures) (99). These results suggest that ductal branching is stimulated by adjacent vitamin D-deficient adipose tissue in response to high-fat diets during the hormonally induced stages of mammary development (98, 99, 103). In sharp contrast to the results for female mice, there are no effects in males with the adipose-specific Vdr deletion in either the normal or high-fat diet (99).
The results of the Vdr KO study, bolstered by additional research (reviewed in ref. 73), reveal a sex-specific relationship between vitamin D deficiency and adipose tissue that includes an increase in mammary ductal branching during the hormone-induced stages of breast development (Fig. 2B). This physiological increase in ductal branching in response to very low levels of vitamin D indicates that other genetic mechanisms that increase ductal branching may be positively selected for in populations experiencing chronic vitamin D deficiency, such as in UV-poor environments at high latitudes.
A completely independent line of evidence suggests that variation in ductal branching influences milk content (Fig. 2A). Variation in milk vitamin D levels is heritable in cows and shows evidence of positive selection over the short time frames associated with animal domestication, demonstrating that the underlying genetic mechanisms are highly responsive to selective pressure (104, 105). Milk vitamin D content varies by cattle breed, with some producing notably higher concentrations irrespective of UV exposure (reviewed in ref. 106). Functional associations of 31 genes expressed in cows producing different milk protein and fat concentrations include transcriptomes of genes involved in mammary gland bud elongation (107), suggesting that mammary duct branching contributes to the genetic underpinnings of variation in milk composition.
EDAR V370A’s Influence on Ductal Branching
The relationship between vitamin D and ductal branching calls for a renewed consideration of EDAR V370A’s influence on mammary duct branching density as the phenotypic target of selection. As with other ectodermally derived structures (e.g., teeth), the earliest phase of mammary development results from epithelial–mesenchymal tissue interactions at embryonic day 11 in the mouse (100–102). Ductal branching is established via NF-ĸB signaling, a downstream target of the ectodysplasin pathway (108). The increase in branching observed in mouse models of EDAR V370A (30, 31, 44) is likely the result of the EDA/EDAR/NF-ĸB pathway’s influence on the proteins that modulate the strength of the junctions that adhere the myoepithelial cells of the mammary ducts (109–111) (Fig. 2 C–E), facilitating or hindering branching during morphogenesis (112). The more elaborate ductal branching induced by EDAR V370A during the embryonic stage of mammary development likely enhances the physiological effect of the increased branching induced by vitamin D deficiency in the later stages of breast development (during puberty and gestation/lactation).
Conclusions
The Arctic is an extreme environment for humans because of the almost complete lack of UV-B exposure that is required for the biosynthesis of vitamin D. This lack of vitamin D would have reduced immunological function and bone development and hindered the healthy function of adipose tissue. Here, we have presented a diverse array of evidence supporting the hypothesis that a genetically isolated population living in this environment during the LGM ∼20,000 y ago experienced selection for polymorphisms in the FADS gene cluster and for EDAR V370A because of the advantage these genetic variants likely confer in transmitting nutrients from mother to infant through breast milk under conditions of extremely low UV.
Supplementary Material
Acknowledgments
The synthesis presented here is a product of the American Association for the Advancement of Science’s 2017 annual meeting, as the ideas came together in the session entitled “Beringia and the Dispersal of Modern Humans to the Americas.” We thank Leslie Aiello, Marianne Brasil, Sarah Greenlee, Ophir Klein, Peter Kloess, Owen Lovejoy, Kunxin Luo, Whitney Reiner, Cat Taylor, Tim White, and three anonymous reviewers for critical feedback on earlier versions of this manuscript. Development of the ideas presented herein also benefited from conversations with Christopher Barrett, Michael Bell, Angelique Corthals, Liliana Davalos-Alvarez, Robert Dudley, Jennifer Raff, Ellen Simms, and Justin Tackney. L.J.H. also thanks the Department of Ecology and Evolution at the State University of New York at Stony Brook for feedback when she shared this research in their departmental colloquium series. Many thanks also to Christy G. Turner II, as his extensive study of incisor shoveling variation provided the foundation for the dental anthropology presented herein.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1711788115/-/DCSupplemental.
References
- 1.Trigger BG. A History of Archaeological Thought. Cambridge Univ Press; New York: 1989. [Google Scholar]
- 2.Tamm E, et al. Beringian standstill and spread of Native American founders. PLoS One. 2007;2:e829. doi: 10.1371/journal.pone.0000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghavan M, et al. POPULATION GENETICS. Genomic evidence for the Pleistocene and recent population history of Native Americans. Science. 2015;349:aab3884. doi: 10.1126/science.aab3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reich D, et al. Reconstructing Native American population history. Nature. 2012;488:370–374. doi: 10.1038/nature11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tackney JC, et al. Two contemporaneous mitogenomes from terminal Pleistocene burials in eastern Beringia. Proc Natl Acad Sci USA. 2015;112:13833–13838. doi: 10.1073/pnas.1511903112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno-Mayar JV, et al. Terminal Pleistocene Alaskan genome reveals first founding population of Native Americans. Nature. 2018;553:203–207. doi: 10.1038/nature25173. [DOI] [PubMed] [Google Scholar]
- 7.Hoffecker JF, Elias SA, O’Rourke DH, Scott GR, Bigelow NH. Beringia and the global dispersal of modern humans. Evol Anthropol. 2016;25:64–78. doi: 10.1002/evan.21478. [DOI] [PubMed] [Google Scholar]
- 8.Björn LO. Photobiology: The Science of Light and Life. Springer; New York: 2015. [Google Scholar]
- 9.Pibernat RA, Ellis-Evans C, Hinghofer-Szalkay HG. Life in Extreme Environments. Springer Science & Business Media, Dordrecht; The Netherlands: 2007. [Google Scholar]
- 10.Beall CM. Human adaptability studies at high altitude: Research designs and major concepts during fifty years of discovery. Am J Hum Biol. 2013;25:141–147. doi: 10.1002/ajhb.22355. [DOI] [PubMed] [Google Scholar]
- 11.Azad P, et al. High-altitude adaptation in humans: From genomics to integrative physiology. J Mol Med (Berl) 2017;95:1269–1282. doi: 10.1007/s00109-017-1584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho JI, et al. Ethnically Tibetan women in Nepal with low hemoglobin concentration have better reproductive outcomes. Evol Med Public Health. 2017;2017:82–96. doi: 10.1093/emph/eox008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amorim CEG, et al. Genetic signature of natural selection in first Americans. Proc Natl Acad Sci USA. 2017;114:2195–2199. doi: 10.1073/pnas.1620541114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathias RA, Pani V, Chilton FH. Genetic variants in the FADS gene: Implications for dietary recommendations for fatty acid intake. Curr Nutr Rep. 2014;3:139–148. doi: 10.1007/s13668-014-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley MT, et al. Selection in Europeans on fatty acid desaturases associated with dietary changes. Mol Biol Evol. 2017;34:1307–1318. doi: 10.1093/molbev/msx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fumagalli M, et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343–1347. doi: 10.1126/science.aab2319. [DOI] [PubMed] [Google Scholar]
- 17.Chilton FH, et al. Diet-gene interactions and PUFA metabolism: A potential contributor to health disparities and human diseases. Nutrients. 2014;6:1993–2022. doi: 10.3390/nu6051993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oscarsson J, Hurt-Camejo E. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and their mechanisms of action on apolipoprotein B-containing lipoproteins in humans: A review. Lipids Health Dis. 2017;16:149. doi: 10.1186/s12944-017-0541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JY, Kothapalli KSD, Brenna JT. Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis. Curr Opin Clin Nutr Metab Care. 2016;19:103–110. doi: 10.1097/MCO.0000000000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- 21.Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr. 2010;30:237–255. doi: 10.1146/annurev.nutr.012809.104742. [DOI] [PubMed] [Google Scholar]
- 22.Moltó-Puigmartí C, et al. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. Am J Clin Nutr. 2010;91:1368–1376. doi: 10.3945/ajcn.2009.28789. [DOI] [PubMed] [Google Scholar]
- 23.Lattka E, et al. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am J Clin Nutr. 2011;93:382–391. doi: 10.3945/ajcn.110.004515. [DOI] [PubMed] [Google Scholar]
- 24.Moltó-Puigmartí C, et al. Maternal but not fetal FADS gene variants modify the association between maternal long-chain PUFA intake in pregnancy and birth weight. J Nutr. 2014;144:1430–1437. doi: 10.3945/jn.114.194035. [DOI] [PubMed] [Google Scholar]
- 25.Mennitti LV, et al. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J Nutr Biochem. 2015;26:99–111. doi: 10.1016/j.jnutbio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Ding Z, et al. Association of polyunsaturated fatty acids in breast milk with fatty acid desaturase gene polymorphisms among Chinese lactating mothers. Prostaglandins Leukot Essent Fatty Acids. 2016;109:66–71. doi: 10.1016/j.plefa.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Sosa-Castillo E, Rodríguez-Cruz M, Moltó-Puigmartí C. Genomics of lactation: Role of nutrigenomics and nutrigenetics in the fatty acid composition of human milk. Br J Nutr. 2017;118:161–168. doi: 10.1017/S0007114517001854. [DOI] [PubMed] [Google Scholar]
- 28.Ibeagha-Awemu EM, Akwanji KA, Beaudoin F, Zhao X. Associations between variants of FADS genes and omega-3 and omega-6 milk fatty acids of Canadian Holstein cows. BMC Genet. 2014;15:25. doi: 10.1186/1471-2156-15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryk J, et al. Positive selection in East Asians for an EDAR allele that enhances NF-kappaB activation. PLoS One. 2008;3:e2209. doi: 10.1371/journal.pone.0002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang SH, Jobling S, Brennan K, Headon DJ. Enhanced Edar signalling has pleiotropic effects on craniofacial and cutaneous glands. PLoS One. 2009;4:e7591. doi: 10.1371/journal.pone.0007591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamberov YG, et al. Modeling recent human evolution in mice by expression of a selected EDAR variant. Cell. 2013;152:691–702. doi: 10.1016/j.cell.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kowalczyk-Quintas C, Schneider P. Ectodysplasin A (EDA) - EDA receptor signalling and its pharmacological modulation. Cytokine Growth Factor Rev. 2014;25:195–203. doi: 10.1016/j.cytogfr.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Sadier A, Viriot L, Pantalacci S, Laudet V. The ectodysplasin pathway: From diseases to adaptations. Trends Genet. 2014;30:24–31. doi: 10.1016/j.tig.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Glazer AM, Cleves PA, Erickson PA, Lam AY, Miller CT. Parallel developmental genetic features underlie stickleback gill raker evolution. Evodevo. 2014;5:19. doi: 10.1186/2041-9139-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefebvre S, Mikkola ML. Ectodysplasin research–Where to next? Semin Immunol. 2014;26:220–228. doi: 10.1016/j.smim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin AF, et al. Craniofacial morphometric analysis of individuals with X-linked hypohidrotic ectodermal dysplasia. Mol Genet Genomic Med. 2014;2:422–429. doi: 10.1002/mgg3.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindfors PH, Voutilainen M, Mikkola ML. Ectodysplasin/NF-κB signaling in embryonic mammary gland development. J Mammary Gland Biol Neoplasia. 2013;18:165–169. doi: 10.1007/s10911-013-9277-5. [DOI] [PubMed] [Google Scholar]
- 38.Fujimoto A, et al. A replication study confirmed the EDAR gene to be a major contributor to population differentiation regarding head hair thickness in Asia. Hum Genet. 2008;124:179–185. doi: 10.1007/s00439-008-0537-1. [DOI] [PubMed] [Google Scholar]
- 39.Tan J, et al. The adaptive variant EDARV370A is associated with straight hair in East Asians. Hum Genet. 2013;132:1187–1191. doi: 10.1007/s00439-013-1324-1. [DOI] [PubMed] [Google Scholar]
- 40.Peng Q, et al. EDARV370A associated facial characteristics in Uyghur population revealing further pleiotropic effects. Hum Genet. 2016;135:99–108. doi: 10.1007/s00439-015-1618-6. [DOI] [PubMed] [Google Scholar]
- 41.Park JH, et al. Effects of an Asian-specific nonsynonymous EDAR variant on multiple dental traits. J Hum Genet. 2012;57:508–514. doi: 10.1038/jhg.2012.60. [DOI] [PubMed] [Google Scholar]
- 42.Tan J, et al. Characteristics of dental morphology in the Xinjiang Uyghurs and correlation with the EDARV370A variant. Sci China Life Sci. 2014;57:510–518. doi: 10.1007/s11427-014-4654-x. [DOI] [PubMed] [Google Scholar]
- 43.Kimura R, et al. A common variation in EDAR is a genetic determinant of shovel-shaped incisors. Am J Hum Genet. 2009;85:528–535. doi: 10.1016/j.ajhg.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mou C, et al. Enhanced ectodysplasin-A receptor (EDAR) signaling alters multiple fiber characteristics to produce the East Asian hair form. Hum Mutat. 2008;29:1405–1411. doi: 10.1002/humu.20795. [DOI] [PubMed] [Google Scholar]
- 45.Sabeti PC, et al. International HapMap Consortium Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449:913–918. doi: 10.1038/nature06250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sicoli MA, Holton G. Linguistic phylogenies support back-migration from Beringia to Asia. PLoS One. 2014;9:e91722. doi: 10.1371/journal.pone.0091722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizoguchi Y. Shovelling: A statistical analysis of its morphology. Univ Tokyo Bull. 1985;26:1–176. [Google Scholar]
- 48.Scott GR, Turner CG., II . The Anthropology of Modern Human Teeth: Dental Morphology and Its Variation in Recent Human Populations. Cambridge Univ Press; New York: 1997. [Google Scholar]
- 49.Llamas B, et al. Ancient mitochondrial DNA provides high-resolution time scale of the peopling of the Americas. Sci Adv. 2016;2:e1501385. doi: 10.1126/sciadv.1501385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu Q, et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–449. doi: 10.1038/nature13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitulko V, et al. Human habitation in arctic western Beringia prior to the LGM. In: Graf KE, Ketron CV, Waters MR, editors. Paleoamerican Odyssey. Texas A&M Univ Press; College Station, TX: 2013. pp. 13–44. [Google Scholar]
- 52.Claussen M, Selent K, Brovkin V, Raddatz T, Gayler V. Impact of CO2 and climate on Last Glacial Maximum vegetation–A factor separation. Biogeosciences. 2013;10:3593–3604. [Google Scholar]
- 53.Hewitt GM. Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond B Biol Sci. 2004;359:183–195, discussion 195. doi: 10.1098/rstb.2003.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Provan J, Bennett KD. Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol. 2008;23:564–571. doi: 10.1016/j.tree.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Cai X, et al. Genographic Consortium Human migration through bottlenecks from Southeast Asia into East Asia during Last Glacial Maximum revealed by Y chromosomes. PLoS One. 2011;6:e24282. doi: 10.1371/journal.pone.0024282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams AN, Ulm S, Cook AR, Langley MC, Collard M. Human refugia in Australia during the Last Glacial Maximum and terminal Pleistocene: A geospatial analysis of the 25–12 ka Australian archaeological record. J Archaeol Sci. 2013;40:4612–4625. [Google Scholar]
- 57.Hoffecker JF, Elias SA. Human Ecology of Beringia. Columbia Univ Press; New York: 2007. [Google Scholar]
- 58.Mulligan CJ, Kitchen A. Three-stage colonization model for the peopling of the Americas. In: Graf KE, Ketron CV, Waters MR, editors. Paleoamerican Odyssey. Texas A&M Univ Press; College Station, TX: 2013. pp. 171–181. [Google Scholar]
- 59.Hartl DL. A Primer of Population Genetics. 2nd Ed Sinauer; Sunderland, MA: 1988. [Google Scholar]
- 60.Zanolli C, Hourset M, Esclassan R, Mollereau C. Neanderthal and Denisova tooth protein variants in present-day humans. PLoS One. 2017;12:e0183802. doi: 10.1371/journal.pone.0183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holick MF, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 62.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 63.Abbas MA. Physiological functions of vitamin D in adipose tissue. J Steroid Biochem Mol Biol. 2017;165:369–381. doi: 10.1016/j.jsbmb.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 64.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Carlberg C, Seuter S, Heikkinen S. The first genome-wide view of vitamin D receptor locations and their mechanistic implications. Anticancer Res. 2012;32:271–282. [PubMed] [Google Scholar]
- 66.Ramagopalan SV, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: More than just the effects of vitamin D? Nat Rev Immunol. 2011;11:584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 68.Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Arch Biochem Biophys. 2012;523:37–47. doi: 10.1016/j.abb.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 69.Workalemahu T, et al. Placental genetic variations in vitamin D metabolism and birthweight. Placenta. 2017;50:78–83. doi: 10.1016/j.placenta.2016.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mutt SJ, Hyppönen E, Saarnio J, Järvelin MR, Herzig KH. Vitamin D and adipose tissue-more than storage. Front Physiol. 2014;5:228. doi: 10.3389/fphys.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cinti S. White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur J Endocrinol. 2014;170:R159–R171. doi: 10.1530/EJE-13-0945. [DOI] [PubMed] [Google Scholar]
- 72.Cinti S. UCP1 protein: The molecular hub of adipose organ plasticity. Biochimie. 2017;134:71–76. doi: 10.1016/j.biochi.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 73.Valencak TG, Osterrieder A, Schulz TJ. Sex matters: The effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 2017;12:806–813. doi: 10.1016/j.redox.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harris RS, Bunker JW. Vitamin D potency of human breast milk. Am J Public Health Nations Health. 1939;29:744–747. doi: 10.2105/ajph.29.7.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee JH, O’Keefe JH, Lavie CJ, Harris WS. Omega-3 fatty acids: Cardiovascular benefits, sources and sustainability. Nat Rev Cardiol. 2009;6:753–758. doi: 10.1038/nrcardio.2009.188. [DOI] [PubMed] [Google Scholar]
- 76.Linday LA. Cod liver oil, young children, and upper respiratory tract infections. J Am Coll Nutr. 2010;29:559–562. doi: 10.1080/07315724.2010.10719894. [DOI] [PubMed] [Google Scholar]
- 77.Challoumas D. Vitamin D supplementation and lipid profile: What does the best available evidence show? Atherosclerosis. 2014;235:130–139. doi: 10.1016/j.atherosclerosis.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 78.Norton HL, et al. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol Biol Evol. 2007;24:710–722. doi: 10.1093/molbev/msl203. [DOI] [PubMed] [Google Scholar]
- 79.Lamason RL, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005;310:1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 80.Lalueza-Fox C, et al. A melanocortin 1 receptor allele suggests varying pigmentation among Neanderthals. Science. 2007;318:1453–1455. doi: 10.1126/science.1147417. [DOI] [PubMed] [Google Scholar]
- 81.Nielsen R, et al. Tracing the peopling of the world through genomics. Nature. 2017;541:302–310. doi: 10.1038/nature21347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fan S, Hansen ME, Lo Y, Tishkoff SA. Going global by adapting local: A review of recent human adaptation. Science. 2016;354:54–59. doi: 10.1126/science.aaf5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lachance J, Tishkoff SA. Population genomics of human adaptation. Annu Rev Ecol Evol Syst. 2013;44:123–143. doi: 10.1146/annurev-ecolsys-110512-135833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jablonski NG, Chaplin G. Colloquium paper: Human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci USA. 2010;107:8962–8968. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chaplin G, Jablonski NG. The human environment and the vitamin D compromise: Scotland as a case study in human biocultural adaptation and disease susceptibility. Hum Biol. 2013;85:529–552. doi: 10.3378/027.085.0402. [DOI] [PubMed] [Google Scholar]
- 86.Chen TC, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richards MP, Pettitt PB, Stiner MC, Trinkaus E. Stable isotope evidence for increasing dietary breadth in the European mid-Upper Paleolithic. Proc Natl Acad Sci USA. 2001;98:6528–6532. doi: 10.1073/pnas.111155298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: Causes and future directions. Ann Trop Paediatr. 2006;26:1–16. doi: 10.1179/146532806X90556. [DOI] [PubMed] [Google Scholar]
- 89.Valentine CJ, Wagner CL. Nutritional management of the breastfeeding dyad. Pediatr Clin North Am. 2013;60:261–274. doi: 10.1016/j.pcl.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 90.Picciano MF. Nutrient composition of human milk. Pediatr Clin North Am. 2001;48:53–67. doi: 10.1016/s0031-3955(05)70285-6. [DOI] [PubMed] [Google Scholar]
- 91.Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: A review on its composition and bioactivity. Early Hum Dev. 2015;91:629–635. doi: 10.1016/j.earlhumdev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 92.Kasalová E, Aufartová J, Krčmová LK, Solichová D, Solich P. Recent trends in the analysis of vitamin D and its metabolites in milk–A review. Food Chem. 2015;171:177–190. doi: 10.1016/j.foodchem.2014.08.102. [DOI] [PubMed] [Google Scholar]
- 93.Specker BL, Tsang RC, Hollis BW. Effect of race and diet on human-milk vitamin D and 25-hydroxyvitamin D. Am J Dis Child. 1985;139:1134–1137. doi: 10.1001/archpedi.1985.02140130072032. [DOI] [PubMed] [Google Scholar]
- 94.Lee JM, et al. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46:42–44. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 95.Saraf R, Morton SM, Camargo CA, Jr, Grant CC. Global summary of maternal and newborn vitamin D status–A systematic review. Matern Child Nutr. 2016;12:647–668. doi: 10.1111/mcn.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dawodu A, et al. Heightened attention to supplementation is needed to improve the vitamin D status of breastfeeding mothers and infants when sunshine exposure is restricted. Matern Child Nutr. 2014;10:383–397. doi: 10.1111/j.1740-8709.2012.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Greer FR, Hollis BW, Napoli JL. High concentrations of vitamin D2 in human milk associated with pharmacologic doses of vitamin D2. J Pediatr. 1984;105:61–64. doi: 10.1016/s0022-3476(84)80361-3. [DOI] [PubMed] [Google Scholar]
- 98.Ching S, Kashinkunti S, Niehaus MD, Zinser GM. Mammary adipocytes bioactivate 25-hydroxyvitamin D3 and signal via vitamin D3 receptor, modulating mammary epithelial cell growth. J Cell Biochem. 2011;112:3393–3405. doi: 10.1002/jcb.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matthews DG, D’Angelo J, Drelich J, Welsh J. Adipose-specific Vdr deletion alters body fat and enhances mammary epithelial density. J Steroid Biochem Mol Biol. 2016;164:299–308. doi: 10.1016/j.jsbmb.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paine IS, Lewis MT. The terminal end bud: The little engine that could. J Mammary Gland Biol Neoplasia. 2017;22:93–108. doi: 10.1007/s10911-017-9372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: Cell fate specification, stem cells and the microenvironment. Development. 2015;142:1028–1042. doi: 10.1242/dev.087643. [DOI] [PubMed] [Google Scholar]
- 102.Robinson GW. Identification of signaling pathways in early mammary gland development by mouse genetics. Breast Cancer Res. 2004;6:105–108. doi: 10.1186/bcr776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zinser G, Packman K, Welsh J. Vitamin D(3) receptor ablation alters mammary gland morphogenesis. Development. 2002;129:3067–3076. doi: 10.1242/dev.129.13.3067. [DOI] [PubMed] [Google Scholar]
- 104.Qanbari S, et al. A genome-wide scan for signatures of recent selection in Holstein cattle. Anim Genet. 2010;41:377–389. doi: 10.1111/j.1365-2052.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- 105.Pan D, et al. Genome-wide detection of selective signature in Chinese Holstein. PLoS One. 2013;8:e60440. doi: 10.1371/journal.pone.0060440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weir RR, et al. Environmental and genetic factors influence the vitamin D content of cows’ milk. Proc Nutr Soc. 2017;76:76–82. doi: 10.1017/S0029665116000811. [DOI] [PubMed] [Google Scholar]
- 107.Cui X, et al. Transcriptional profiling of mammary gland in Holstein cows with extremely different milk protein and fat percentage using RNA sequencing. BMC Genomics. 2014;15:226. doi: 10.1186/1471-2164-15-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Voutilainen M, et al. Ectodysplasin regulates hormone-independent mammary ductal morphogenesis via NF-κB. Proc Natl Acad Sci USA. 2012;109:5744–5749. doi: 10.1073/pnas.1110627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Basham KJ, et al. Chemical genetic screen reveals a role for desmosomal adhesion in mammary branching morphogenesis. J Biol Chem. 2013;288:2261–2270. doi: 10.1074/jbc.M112.411033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tokonzaba E, et al. Plakoglobin as a regulator of desmocollin gene expression. J Invest Dermatol. 2013;133:2732–2740. doi: 10.1038/jid.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Celentano A, Mignogna MD, McCullough M, Cirillo N. Pathophysiology of the desmo-adhesome. J Cell Physiol. 2017;232:496–505. doi: 10.1002/jcp.25515. [DOI] [PubMed] [Google Scholar]
- 112.Owens MB, Hill AD, Hopkins AM. Ductal barriers in mammary epithelium. Tissue Barriers. 2013;1:e25933. doi: 10.4161/tisb.25933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.