Significance
N-ethylmaleimide sensitive factor (NSF) and α-soluble NSF attachment protein (α-SNAP) are key components of vesicle trafficking systems and are conserved across eukaryotes. This study shows that these two essential housekeeping proteins have coevolved toward atypical forms in soybean to confer resistance to a highly damaging nematode pathogen while balancing plant fitness. We report discovery of a naturally occurring NSF variant carrying unusual polymorphisms that enhance interaction with and assuage the cytotoxicity of the Rhg1 resistance-associated α-SNAPs. Pathogen selection pressure has apparently driven this rewiring of multiple components of the conserved SNARE recycling machinery. Useful introduction of the agriculturally valuable Rhg1 resistance source into other plants is likely to require a cofunctional NSF protein partner.
Keywords: plant disease resistance, α-SNAP, NSF, soybean cyst nematode, Rhg1
Abstract
N-ethylmaleimide sensitive factor (NSF) and α-soluble NSF attachment protein (α-SNAP) are essential eukaryotic housekeeping proteins that cooperatively function to sustain vesicular trafficking. The “resistance to Heterodera glycines 1” (Rhg1) locus of soybean (Glycine max) confers resistance to soybean cyst nematode, a highly damaging soybean pest. Rhg1 loci encode repeat copies of atypical α-SNAP proteins that are defective in promoting NSF function and are cytotoxic in certain contexts. Here, we discovered an unusual NSF allele (Rhg1-associated NSF on chromosome 07; NSFRAN07) in Rhg1+ germplasm. NSFRAN07 protein modeling to mammalian NSF/α-SNAP complex structures indicated that at least three of the five NSFRAN07 polymorphisms reside adjacent to the α-SNAP binding interface. NSFRAN07 exhibited stronger in vitro binding with Rhg1 resistance-type α-SNAPs. NSFRAN07 coexpression in planta was more protective against Rhg1 α-SNAP cytotoxicity, relative to WT NSFCh07. Investigation of a previously reported segregation distortion between chromosome 18 Rhg1 and a chromosome 07 interval now known to contain the Glyma.07G195900 NSF gene revealed 100% coinheritance of the NSFRAN07 allele with disease resistance Rhg1 alleles, across 855 soybean accessions and in all examined Rhg1+ progeny from biparental crosses. Additionally, we show that some Rhg1-mediated resistance is associated with depletion of WT α-SNAP abundance via selective loss of WT α-SNAP loci. Hence atypical coevolution of the soybean SNARE-recycling machinery has balanced the acquisition of an otherwise disruptive housekeeping protein, enabling a valuable disease resistance trait. Our findings further indicate that successful engineering of Rhg1-related resistance in plants will require a compatible NSF partner for the resistance-conferring α-SNAP.
Cyst nematodes infest the roots of many valuable crops and establish elaborate feeding structures (1). Soybean cyst nematode (Heterodera glycines; SCN) is a highly damaging soybean pest and causes annual US yield losses of over $1 billion US dollars (2–5). SCN parasitizes host roots by secreting a complex arsenal of effector molecules that reprogram host root cells and trigger fusion with adjacent host cells, forming a large unicellular feeding site termed a syncytium (6–8). The soybean “resistance to Heterodera glycines 1” (Rhg1) locus is very widely used by soybean growers to restrict SCN feeding site formation, thereby reducing yield loss (4, 9). The genes at Rhg1 do not encode proteins normally associated with disease resistance (4, 10–12). Instead, resistance is mediated by copy number variation of multiple genes at the Rhg1 locus, one of which encodes an α-soluble N-ethylmaleimide sensitive factor (NSF) attachment protein (α-SNAP) with unusual C-terminal polymorphisms (10, 11, 13).
α-SNAP (Sec17 in yeast) is a functionally conserved eukaryotic housekeeping protein that works in concert with NSF (Sec18 in yeast). α-SNAP and NSF promote cellular vesicular trafficking by mediating the disassembly and reuse of soluble NSF attachment protein receptor (SNARE) protein complexes that form when t-SNARE and v-SNARE proteins associate during vesicle docking and fusion (14–16). We recently discovered that the soybean resistance-associated α-SNAPs encoded by Rhg1 are unusual α-SNAP proteins that bind less well to wild-type (WT) NSF and, when expressed in Nicotiana benthamiana, disrupt vesicle trafficking and eventually cause cell death (17). The relative abundance of Rhg1-encoded defective α-SNAP variants increases substantially within developing host syncytial cells, apparently disrupting syncytium viability and thereby restricting nematode growth and reproduction (17). SCN-resistant soybeans carry WT α-SNAP genes at other loci that can functionally complement the Rhg1 resistance-type α-SNAPs in a dosage-dependent manner (17). However, the capacity of soybean varieties to yield well despite expression of cytotoxic Rhg1 resistance-type α-SNAPs throughout the plant is not fully explained.
The complex Rhg1 locus on soybean chromosome 18 is a tandemly repeated block of four genes: Glyma.18G022400, Glyma.18G022500, Glyma.18G022600, and Glyma.18G022700. SCN-susceptible soybeans carry only a single copy of the above four genes, including a Glyma.18G022500 α-SNAP gene whose product matches the WT α-SNAP consensus and maintains normal NSF interactions (10, 13, 17). Resistance-conferring Rhg1 loci group into two structural classes based on the type of α-SNAP polymorphisms they encode, which also correlates with the copy number of Rhg1 repeats that are present (11, 13) (SI Appendix, Table S1). Rhg1HC (high-copy) loci carry four or more and frequently 9 or 10 Rhg1 repeats, and Rhg1LC (low-copy) loci carry three or fewer Rhg1 repeats. Rhg1HC (also known as rhg1-b) and Rhg1LC (also known as rhg1-a) encode distinct α-SNAP variants that are impaired in normal α-SNAP−NSF interactions (17) (Fig. 1A). All Rhg1HC loci examined to date also carry a single Rhg1 repeat that encodes a WT α-SNAP adjacent to multiple repeats that encode resistance-type α-SNAPs, while Rhg1LC loci encode only resistance-type α-SNAPs and no WT α-SNAP (10, 11, 13) (Fig. 1A). Plants carrying Rhg1HC or Rhg1LC loci exhibit elevated transcript abundance for the repeat genes that correlates approximately with copy number, including for the Rhg1 α-SNAP gene (10, 13). Collectively, the above findings suggest that modulation of vesicle trafficking and cell health at the SCN feeding site is at least one core mechanism of Rhg1-mediated SCN resistance.
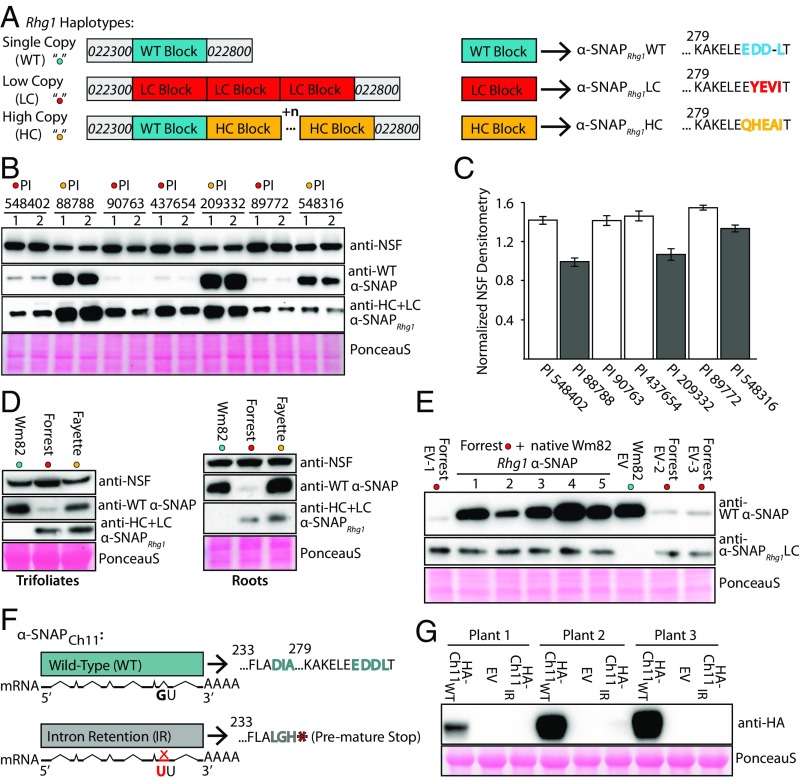
Fig. 1.
WT α-SNAP proteins are much less abundant while NSF is more abundant in Rhg1LowCopy soybeans. (A) Schematic of Rhg1 haplotype classes. (Left) Rhg1 WT (shown blue), Rhg1 LC (shown red), Rhg1 HC (shown orange; n = variable HC-type repeat numbers); not drawn to scale. The C-terminal amino acid polymorphisms encoded by the Rhg1 α-SNAPs are shown at Right. HC Rhg1 haplotypes retain a single WT-like Rhg1 repeat. (B) Immunoblot of WT α-SNAPs, Rhg1 resistance-type α-SNAPs and NSF in roots of soybean HG test varieties (two samples for each genotype). Rhg1LC varieties (red dot; 3 Rhg1 copies): PI 548402 (Peking), PI 89772, PI 437654, PI 90763; Rhg1HC varieties (orange dot): PI 88788 (9 copies), PI 209332 (10 copies), PI 548316 (7 copies). PonceauS staining shows similar loading of total protein. (C) Densitometry indicating total NSF expression in HG type test lines. (D) Like B, but immunoblots for trifoliate leaves or roots of Wm82 and modern Rhg1LC and Rhg1HC varieties Forrest and Fayette. (E) Immunoblots for total WT α-SNAPs and α-SNAPRhg1LC in Forrest (Rhg1LC) transgenic roots transformed with an empty vector (EV; three transgenic lines) or with the native Wm82 α-SNAPRhg1WT locus (five transgenic lines), or in WT Wm82 roots transformed with EV. (F) Schematic of chromosome 11 α-SNAP alleles with exon/intron models, and nucleotide and amino acid polymorphisms. (G) The encoded α-SNAPCh11 intron retention protein, unlike the WT α-SNAPCh11, does not accumulate. Anti-HA immunoblot of total protein from N. benthamiana leaves is agroinfiltrated to express empty vector, N-HA-α-SNAPCh11, or N-HA-α-SNAPCh11-IR (intron retention). PonceauS staining shows similar loading of total protein.
Two other genes within the Rhg1 repeat were reported by Cook et al. (10) to contribute to Rhg1HC-mediated SCN resistance. Glyma.18G022400 encodes an amino acid permease-like protein and Glyma.18G022700 encodes a wound-inducible protein otherwise lacking annotated domains or predicted functions; their molecular function in SCN resistance remains unknown. Liu et al. (18) recently provided evidence that the Rhg1LC α-SNAP may function differently than the Rhg1HC α-SNAP.
The eukaryotic endomembrane network is an intricate sorting and secretion system that ferries cargoes between cellular compartments using transport vesicles. Cognate SNARE proteins on the surface of vesicle and target membranes drive membrane fusion by “zippering” into stable bundles (SNARE complexes), which pull the membranes together (14, 19). The role of α-SNAP and NSF as dedicated SNARE-recycling chaperones has been studied extensively (14, 19–22). NSF is an “ATPases associated with various cellular activities” (AAA+) family protein with three domains: the N domain that binds and interacts with the C terminus of the α-SNAP cochaperone, the D1 ATPase domain that couples ATP hydrolysis to SNARE complex remodeling, and the D2 ATPase domain that mediates NSF hexamerization (23–25). The α-SNAP proteins are required by NSF to cochaperone SNARE remodeling. The α-SNAP serves both as an adaptor for NSF binding to SNARE complexes and as a stimulator of the NSF D1 domain ATPase activity that powers SNARE remodeling/recycling (15). Beyond disassembling SNARE complexes, additional roles of α-SNAP and NSF have been reported, including binding to trans-SNARE complexes to accelerate fusion (26), as well as binding of channels and other receptors and regulation of apoptosis (20, 27–30). The structure and function of α-SNAP, NSF, and SNARE proteins has been elucidated in substantial detail, including cryo-EM structures for 20S complexes that consist of a four-helix SNARE bundle, four α-SNAPs, and six NSFs in various conformational states (15, 21).
Although most animal genomes carry a single NSF and a single α-SNAP gene, polyploidization and other events have caused most plant genomes to encode multiple NSF and α-SNAP genes (31). The reference Williams 82 (Wm82) soybean genome (32) encodes seven SNAP family members: five putative α-SNAPs and two putative γ-SNAPs. Soybean also encodes two unlinked NSF genes, Glyma.07G195900 and Glyma.13G180100. As in animals, plants contain >100 genes encoding diverse SNARE and SNARE-like proteins (14, 33). Unlike plant SNARE proteins [including SNAREs with potentially confusing names such as synaptosomal-associated protein 25 (SNAP-25) and soluble N-ethylmaleimide−sensitive factor adaptor protein 33 (SNAP33)], there are very few published studies of plant NSF, α-SNAP, or γ-SNAP proteins (10, 13, 17, 18, 34–37). However, close analysis of recombinant-inbred lines has recently shown that a gene at or linked to the soybean chromosome 11 locus encoding an α-SNAP makes a minor contribution to SCN resistance in the Peking (Rhg1LC + Rhg4) genetic background (38). Other previous work (37) had identified an allele encoding a splice-variant α-SNAP in this genetic background, although that work misidentified it as an allele of the chromosome 18 Rhg1 locus despite it now being known to be a chromosome 11 α-SNAP allele (13, 38).
In the present study, we demonstrate that evolution/selection of both Rhg1LC and the chromosome 11 α-SNAP gene Glyma.11G234500 has had major impacts on the relative abundance of WT α-SNAP proteins in the Rhg1LC genetic background. We also examined soybean NSF proteins. We discovered an unusual NSF protein in Rhg1-containing lines that is unlike that encoded in the soybean Wm82 reference genome or any publicly available plant reference genomes. We found that this variant NSFRAN07 (Rhg1-associated NSF on chromosome 07; NSFRAN07) protein contains unique N-domain polymorphisms that mitigate the cytotoxicity and poor NSF binding activity of the SCN resistance-conferring Rhg1 α-SNAPs. We then noted that the genetic region containing this NSF and neighboring genes has been identified in previous SCN resistance mapping studies, including a 1995 study by Webb showing strong cosegregation with resistance-conferring Rhg1 alleles (39, 40). More recently, a high-resolution 80-kb candidate gene interval was identified (41) but this segregation distortion at the chromosome 07 locus had remained unexplained. We therefore investigated soybean germplasm genotype data and recombinant inbred lines from Rhg1+ x rhg1− parental crosses. We discovered strict coinheritance of NSFRAN07 alleles in plants homozygous for resistance-associated Rhg1 haplotypes, demonstrating the functional necessity of NSFRAN07 for viable occurrence of SCN resistance-conferring Rhg1.
Results
WT α-SNAP Proteins Are Much Less Abundant While NSF Is More Abundant in Rhg1LC Soybeans.
We previously reported that the PI 88788-type high-copy (HC) Rhg1 (Rhg1HC) locus in soybean line “Fayette” drives a localized increase of resistance-type α-SNAPRhg1HC protein to disrupt the developing SCN-induced syncytium (17). We also observed that endogenous NSF levels increased when resistance-associated Rhg1 α-SNAP proteins were overexpressed in N. benthamiana (17). However, for lines carrying LC-type Rhg1 (Rhg1LC, “Peking-type”), the cellular balance of WT α-SNAP to α-SNAPRhg1LC or NSF proteins was unknown. To investigate the relative abundances of WT and resistance-associated α-SNAPs, we used previously described anti−α-SNAP antibodies and performed immunoblots on the Rhg1HC and Rhg1LC soybean varieties commonly used to phenotype SCN resistance (the HG Type Test varieties; see SI Appendix, Table S1) (17, 42). We also examined the abundance of the α-SNAP cochaperone NSF in these samples, using an antibody raised to a conserved NSF region (17). Fig. 1A presents a schematic of the various Rhg1 haplotypes as well as the C-terminal polymorphisms of Rhg1 α-SNAPs encoded by the Rhg1 repeat types. As shown in Fig. 1B, immunoblots from root tissue indicated that WT α-SNAP protein levels in all tested Rhg1LC lines (PI 548402/Peking, PI 90763, PI 437654, PI 89772) are dramatically reduced compared with the Rhg1HC lines (PI 88788, PI 209332, PI 548316). As mentioned in the Introduction, the Wm82 soybean genome encodes five putative α-SNAPs, and the anti−WT-α-SNAP antibody was raised against the conserved C terminus shared by all of those predicted WT α-SNAP gene products but not the resistance-associated Rhg1 α-SNAPs (17). In addition, one Rhg1 repeat in Rhg1HC haplotypes encodes a WT Glyma.18G022500 α-SNAP protein and all other repeats encode a resistance-type Rhg1 α-SNAP protein, while the Rhg1LC repeats encode only resistance-type α-SNAPRhg1LC proteins (Fig. 1A) (11, 13). The results of Fig. 1B did not match initial predictions; the tested Rhg1LC soybean lines exhibit very low WT α-SNAP protein levels despite the presence of multiple α-SNAP genes at other loci.
We further discovered that total NSF protein abundance in the Rhg1LC lines is increased compared with the Rhg1HC lines PI 88788 and PI 209332 (Fig. 1B and SI Appendix, Fig. S1A). These differences in NSF abundance, across two independent experiments, were quantified using densitometry (Fig. 1C).
We then explored whether WT α-SNAP protein abundance is similarly reduced in a more recent agriculturally utilized Rhg1LC soybean variety, “Forrest.” Immunoblots on both total leaf and root proteins from Wm82 (Rhg1 single copy), Forrest (Rhg1LC), and Fayette (Rhg1HC) again revealed sharp decreases in total WT α-SNAP abundance in the Rhg1LC source (Fig. 1D). Altogether, diminished WT α-SNAP protein levels were observed to be a shared trait of Rhg1LC but not Rhg1HC soybean varieties. In at least two previously studied Rhg1LC varieties, as well as PI 548316, the chromosome 11 α-SNAP allele (Glyma.11G234500) carries a SNP at an intronic splice donor site (Fig. 1F and SI Appendix, Table S1), leading to intron retention and early translational termination, presumably truncating the protein (13, 37, 38). Hence a likely hypothesis for this strikingly low abundance is the absence of a WT-α-SNAP−encoding allele at Rhg1LC, low or no product from the α-SNAPCh11 allele whose transcript retains a translation-terminating intron, and a relatively minor contribution of protein from the other three putative α-SNAP−encoding loci.
Contributions to WT α-SNAP abundance were investigated further. First, we examined overall WT α-SNAP protein abundance when a locus encoding α-SNAPRhg1WT is ectopically placed into Rhg1LC soybean lines. We cloned from Wm82 the genomic chromosome 18 (Ch18) Glyma.18G022500 α-SNAPRhg1WT locus with its native promoter and terminator sequences, generated transgenic Forrest (Rhg1LC) roots carrying this native α-SNAPRhg1WT locus, and assessed total WT α-SNAP protein levels using immunoblots (Fig. 1E). Transgenic addition of the Wm82 α-SNAPRhg1WT locus increased total WT α-SNAP protein expression in Forrest to levels similar to Wm82 empty vector controls (Fig. 1E). This result indicates that, if an appropriate gene is present, normal WT α-SNAP protein levels can develop in the Rhg1LC genetic background.
Next, we examined α-SNAP protein production from the chromosome 11 (Ch11) WT locus from Wm82 vs. the Ch11 intron retention allele (α-SNAPCh11-IR) that is present in many soybean lines that carry Rhg1LC on Ch18. The transcript from the intron retention allele encodes a premature stop codon (13, 37, 38) (Fig. 1F), but the abundance/stability of this putative α-SNAP protein was not known. As such, we cloned ORFs of both the WT α-SNAPCh11 and the intron retention (α-SNAPCh11-IR) alleles, added an N-terminal HA tag, and examined transient protein expression in N. benthamiana. We observed that the HA-α-SNAPCh11 WT protein, but not the truncated HA-α-SNAPCh11-IR protein, was readily detectable (Fig. 1G). The apparent instability of this truncated Ch11 α-SNAP was consistent with a homology model of WT α-SNAPCh11 we generated using the yeast α-SNAP (Sec17) crystal structure (43), which predicted that the α-SNAPCh11-IR protein would terminate several residues into alpha-helix 12 (SI Appendix, Fig. S1B). We also noted a ∼300-bp deletion occurring within the promoter of this allele. The presence and absence of this promoter deletion was verified using PCR on genomic DNA from Forrest (α-SNAPCh11-IR) and Wm82 (WT α-SNAPCh11), respectively (SI Appendix, Fig. S1D). Finally, as for the Ch18 locus tested in Fig. 1E, we cloned the Ch11 genomic WT locus of Glyma.11G234500 (α-SNAPCh11) from Wm82 with native promoter and terminator and noted that presence of this native locus in transgenic roots of Forrest elevated total WT α-SNAP protein expression compared with empty vector controls (SI Appendix, Fig. S1C). Together, the findings of Fig. 1 and SI Appendix, Fig. S1, implicate the Ch18 and Ch11 WT α-SNAP loci as the major sources of total WT α-SNAP proteins in soybean and indicate that their combined absence from the examined Rhg1LC varieties is responsible for the low levels of WT α-SNAP observed in Fig. 1 B and D. The low abundance of WT α-SNAPs in lines carrying Rhg1LC may improve SCN resistance but may also incur costs with respect to plant health and yield if other compensatory mechanisms for tolerance of Rhg1LC are not also present.
A Unique NSFCh07 Allele (NSFRAN07) Is Present in Commonly Used Rhg1-Containing Accessions.
NSF and α-SNAP are essential eukaryotic housekeeping proteins, and null mutations in either partner are lethal in animals, which typically encode only single copies of NSF or α-SNAP (31, 44–46). Because Rhg1 resistance-type α-SNAPs (α-SNAPRhg1LC or α-SNAPRhg1HC) exhibit compromised binding to WT NSFs and are toxic at high doses in N. benthamiana (17), it was unclear how Rhg1LC lines are viable given the diminished WT α-SNAP levels observed in Fig. 1. Since soybean is an ancestrally polyploid organism encoding multiple α-SNAP and NSF loci, we searched for alterations in the other α-SNAP or NSF loci by examining our previously generated whole-genome sequence (WGS) data from multiple Rhg1-containing varieties (13). For all five putative α-SNAP loci from Rhg1LC varieties, we detected no obvious polymorphisms other than the previously mentioned Glyma.11G234500 intron retention allele (SI Appendix, Tables S1 and S2) (13, 38).
Intriguingly, a novel NSF allele was present at Glyma.07G195900 (NSFCh07) among all six of the Rhg1LC and Rhg1HC lines examined, encoding five N-domain amino acid polymorphisms (R4Q, N21Y, S25N, ^116F, and M181I; ^ = insertion) (Fig. 2A and SI Appendix, Fig. S2A and Table S1). Using cDNA from Forrest (Rhg1LC), we cloned and sequenced this unique NSFCh07 transcript and confirmed the five N-domain polymorphisms. Additionally, we designed two different PCR primer pairs at the encoded NSF polymorphisms and verified the presence of this unique NSFCh07 allele, and the absence of the WT NSFCh07 allele, in all Rhg1 test lines (SI Appendix, Fig. S2B). Furthermore, using WGS data from the Soybean Nested Association Mapping (SoyNAM) project (47), we determined that this unique NSFCh07 allele was also present in every Rhg1-containing NAM parent, while SCN-susceptible NAM parents carried the WT NSFCh07 allele (SI Appendix, Table S2). We therefore named the protein from this Rhg1-associated allele of Glyma.07G195900 “NSFRAN07” for “Rhg1-associated NSF on chromosome 07.”
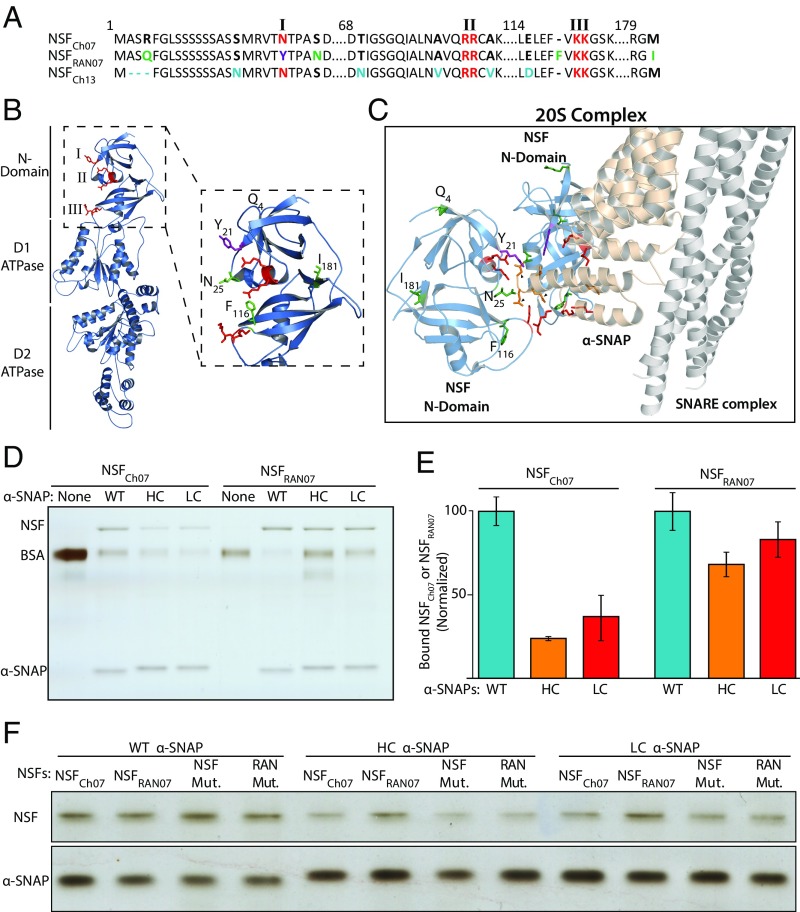
Fig. 2.
Rhg1-containing lines carry an NSFCh07 allele (RAN07) with N-domain polymorphisms at the α-SNAP binding interface that enhance binding with polymorphic Rhg1 resistance-type α-SNAPs. (A) Alignment of N-terminal domains of soybean NSFCh07, NSFCh13, and NSFRAN07. Large identical regions are omitted. N-domain residues corresponding to those that bind α-SNAP are colored red (N21, RR82–83, KK117–118). NSFRAN07 polymorphisms R4Q, S25N, 116F, and M181I are colored green or purple (N21Y); unique NSFCh13 residues are colored light blue. (B) NSFRAN07 modeled to NSFCHO cryo-EM structure (3J97A, State II). NSF residue patches implicated in α-SNAP binding are colored red and labeled I, II, or III. Zoomed-in view shows NSFRAN07 N-domain polymorphisms colored green or purple (N21Y). (C) Cryo-EM structure of mammalian 20S supercomplex, masked to show only SNARE bundle (white), one α-SNAP (yellow), and two NSF N domains (light blue). Shown are the mammalian residues; conserved NSF N-domain patches (I, R10; II, RK67-68; III, KK104–105) are shown in red, and α-SNAP C-terminal contacts (D217DEED290–293) are shown in orange. Black arrowheads point to three orange α-SNAP residues EED291–293 corresponding to sites of C-terminal polymorphisms in α-SNAPRhg1HC and α-SNAPRhg1LC. NSFRAN07 polymorphism sites are colored green, except N21Y is in purple. (D) Silver-stained SDS/PAGE showing amount of recombinant NSFCh07 or NSFRAN07 bound in vitro by a fixed quantity of the recombinant α-SNAP protein indicated on second line: no-α-SNAP control (None) or WT, LC or HC Rhg1 α-SNAP. (E) Densitometric quantification of NSFCh07 or NSFRAN07 bound as in D by the Rhg1 α-SNAPs denoted at bottom; data are from three independent experiments, and error bars show SEM. (F) Like D, but showing recombinant NSFCh07, NSFRAN07, or mutants of either, bound in vitro by Rhg1 α-SNAPs. NSF Mut. and RAN Mut. refer to NSFCh07 N21A F115A and NSFRAN07 Y21N F116^, respectively.
In addition to NSFRAN07, an allele of the chromosome 13 Glyma.13G180100 gene encoding an NSFCh13 V555I protein was found in some varieties, including SCN-susceptible soybeans, but it was not present in every Rhg1LC or Rhg1HC line (SI Appendix, Table S2). Normalized RNA sequencing reads from Wm82 indicate that both Glyma.07G195900 and Glyma.13G180100 are expressed similarly across examined plant tissues (SI Appendix, Fig. S2C) (48). SI Appendix, Fig. S2A provides the complete NSFRAN07 amino acid alignment to NSFCh07 from the Wm82 genome.
The NSFRAN07 and Rhg1 α-SNAP Polymorphisms Lie at the NSF/α−SNAP Binding Interface.
The NSF/α−SNAP interface consists of complementary electrostatic patches located at the NSF N domain and α-SNAP C terminus (15, 21). The Rhg1 polymorphisms of both α-SNAPRhg1HC and α-SNAPRhg1LC are located at conserved C-terminal residues shown in other α-SNAPs to bind and stimulate NSF (13, 17, 49). These binding patches are conserved in yeast, animals, and plants, and interkingdom interactions between α-SNAP and NSF have been reported between mammals and yeast and plants, including soybean WT α-SNAP and Chinese hamster NSF (NSFCHO) (17, 35, 36, 50). We performed homology modeling of NSFRAN07 to the NSFCHO cryo-EM structure (21) [Protein Data Bank (PDB) ID code 3j97.1] that placed three of the NSFRAN07 polymorphisms, N21Y, S25N, and the ^116F insertion, adjacent to the NSFCHO α-SNAP-binding residues R10 and RK104–105 (Fig. 2B and SI Appendix, Fig. S3A). NSFRAN07 polymorphism R4Q was outside of the model, and the final NSFRAN07 polymorphism M181I was not located near the α-SNAP binding patches. Further homology modeling was conducted using the mammalian 20S cryo-EM structure (PDB ID code 3j97). In Fig. 2C and SI Appendix, Fig. S4 A and B, the complementary NSF and α-SNAP binding residues, and the NSFRAN07 and Rhg1 α-SNAP polymorphisms, are colored. These results suggest that, upon α-SNAP binding, NSFRAN07 N21Y, S25N, and ^116F are close to the WT α-SNAP amino acid residues that are polymorphic in α-SNAPRhg1HC and α-SNAPRhg1LC. In separate bioinformatics work, we examined the NSF N-domain consensus in plants and determined that residues corresponding to N21 and F115 of WT soybean NSF are present in a majority of plant species, while neither the N21Y nor the ^116F insertion of NSFRAN07 were detected in any available plant reference genome sequences (SI Appendix, Fig. S3B). Altogether, this modeling suggested that NSFRAN07 carries rare alterations at the α-SNAP binding interface that potentially influence interactions with the unusual C termini of Rhg1 resistance-type α-SNAPs.
NSFRAN07 Polymorphisms Enhance Binding with Rhg1 Resistance-Type α-SNAPs.
In light of the above results, NSFRAN07 binding with Rhg1 resistance-type α-SNAPs and α-SNAPRhg1WT was investigated. As in refs. 17 and 51, we produced recombinant NSFRAN07, NSFCh07, and Rhg1 α-SNAP proteins and performed in vitro binding assays. NSFRAN07 and NSFCh07 binding was quantified using densitometry across three independent experiments (Fig. 2E). As previously reported (17), diminished NSFCh07 binding was observed for α-SNAPRhg1HC and α-SNAPRhg1LC, compared with α-SNAPRhg1WT (Fig. 2D). The α-SNAPRhg1HC or α-SNAPRhg1LC binding of NSFRAN07, on the other hand, was more similar to α-SNAPRhg1WT binding of NSFRAN07 and was increased ∼30% relative to the binding of NSFCh07 (Fig. 2 D and E).
To investigate the contribution of the α-SNAP C terminus to NSFRAN07 binding, we tested NSFRAN07 binding to an otherwise WT α-SNAP that lacked the final 10 C-terminal residues (α-SNAPRhg1WT1–279). Similar to the “no α-SNAP” binding controls, essentially no binding of either NSFCh07 or NSFRAN07 with α-SNAPRhg1WT1–279 was observed (SI Appendix, Fig. S4C). To more specifically investigate the NSF binding contribution of just the C-terminal residues polymorphic in α-SNAPRhg1LC (Fig. 1A), we mutagenized α-SNAPRhg1LC from 286YEVI289 to 286AAAA289. Binding of either NSFCh07 or NSFRAN07 to α-SNAPRhg1LC 286AAAA289 was similar to “no α-SNAP” controls (SI Appendix, Fig. S4 D and E). Hence NSFRAN07 binding is sensitive to the α-SNAP C-terminal residues that are polymorphic in the Rhg1 resistance-type α-SNAPs.
We then examined whether binding to Rhg1 α-SNAPs is influenced by two of the key NSFRAN07 polymorphisms (Y21 and F116) that are near predicted α-SNAP binding patches in the 3D model. We restored these two residues back to the identities in WT NSFCh07, while retaining the other three NSFRAN07 polymorphisms (Q4, N25, and I181). Performing in vitro binding assays as above, we observed a reduced ability of NSFRAN07 Y21N F116^, compared with unaltered NSFRAN07, to bind resistance-type α-SNAPs (Fig. 2F and SI Appendix, Fig. S4E). Mutating these two positions to alanine in an otherwise WT NSFCh07 (NSFCh07 N21A F115A) did not restrict binding with WT α-SNAP, and binding of this NSFCh07 N21A F115A with either α-SNAPRhg1HC or α-SNAPRhg1LC was still impaired (Fig. 2F and SI Appendix, Fig. S4D). Combined, these in vitro binding results suggest that NSFRAN07 not only maintains normal binding with WT α-SNAPs but can also accommodate the unusual C-terminal polymorphisms of the Rhg1 resistance-type α-SNAPs.
The NSFRAN07 Polymorphisms Guard Against the Cell Death Induced by Rhg1 Resistance-Type α-SNAPs.
We previously observed that transient expression of either α-SNAPRhg1HC or α-SNAPRhg1LC in N. benthamiana leaves, via Agrobacterium infiltration, is cytotoxic and elicits a hyperaccumulation of the endogenous NSF protein (17). Coexpression of a WT α-SNAP with the Rhg1 resistance-type α-SNAP diminishes this toxicity in a dose-dependent manner, and also relieves negative impacts on sec-GFP secretion (17). The penultimate amino acid (conserved leucine) of α-SNAP, which has been implicated in stimulation of NSF ATPase, is needed for rescue of this N. benthamiana cytotoxicity (17, 20, 51). We subsequently conducted site-directed mutagenesis experiments which provided further evidence that the N. benthamiana assay closely correlates with known α-SNAP/NSF behaviors. In a first set of replicated studies, the toxicity of Rhg1 α-SNAP expression and the capacity of coexpressed WT α-SNAP to protect against Rhg1 α-SNAP toxicity were both observed to be dependent on intact SNARE-binding sites within the respective α-SNAPs (SI Appendix, Fig. S5).
We then examined whether, like WT α-SNAP, coexpression of soybean NSF might alleviate the toxicity of Rhg1 resistance-type α-SNAPs in N. benthamiana. Similar to ref. 17, mixed Agrobacterium inocula were used, with ratios varying from 1:4 (one part NSF-expressing strain to four parts α-SNAPRhg1LC-expressing strain) all the way down to 1:19. NSF coexpression strongly reduced Rhg1 α-SNAP cytotoxicity (Fig. 3 and SI Appendix, Fig. S6). No macroscopic phenotypes indicative of stress were observed upon expressing NSFRAN07 or NSFCh07 alone (SI Appendix, Fig. S6A). Titration of the dose–response for NSF-expressing Agrobacterium strains identified a range of effective strain ratios (Fig. 3B). We observed that coexpressing soybean NSFCh07, NSFCh13, or NSFRAN07 reduced cell death caused by α-SNAPRhg1LC compared with empty vector controls (Fig. 3 A and B). However, NSFRAN07 coexpression consistently conferred greater protection than either NSFCh07 or NSFCh13 (Fig. 3 A and B). Across multiple independent sets of leaves tested at a variety of ratios, we observed that leaf patches coinfiltrated with NSFRAN07 exhibited less cell death and/or slower death. Both NSFRAN07 and NSFCh07 were more effective than NSFCh13 at rescuing cell death (Fig. 3 A and B and SI Appendix, Fig. S6B). Protection against α-SNAPRhg1HC−induced cell death with NSFRAN07 vs. NSF Ch07 produced similar results (SI Appendix, Fig. S6B).
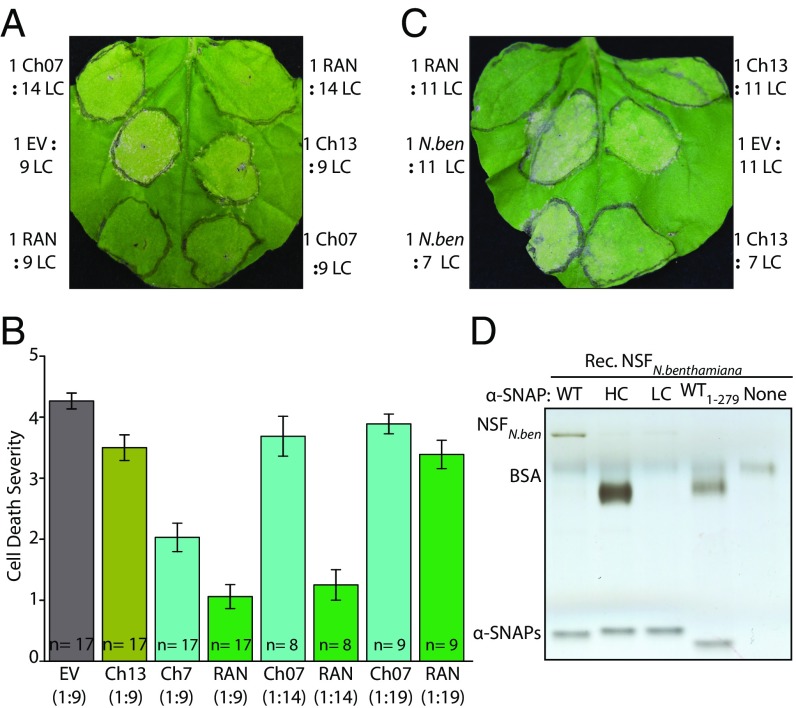
Fig. 3.
Coexpression of soybean NSFs reduces cell death symptoms caused by α-SNAPRhg1LC; NSFRAN07 gives strongest protection. (A) N. benthamiana leaves ∼6 d after agroinfiltration with 9:1 or 14:1 strain mixture (9 or 14 parts Agrobacterium that delivers LC [α-SNAPRhg1LC] to one part Agrobacterium that delivers the indicated soybean NSF [NSFCh07, NSFRAN07, NSFCh13] or EV control). (B) Scoring of cell death severity, across multiple independent experiments, in N. benthamiana leaf patches coexpressing NSFCh07, NSFRAN07, or NSFCh13; n is number of leaves scored; error bars show SEM. (C) Like A, but 7:1 or 11:1 mixed cultures expressing α-SNAPRhg1LC with NSFRAN07, NSFN.benth, NSFCh13, or empty vector. (D) Silver-stained SDS/PAGE of recombinant NSFN.benth bound in vitro by recombinant WT, LC, or HC Rhg1 α-SNAP proteins or WT α-SNAP lacking the final 10 C-terminal residues (WT1–279).
As noted above, we have consistently observed elevated abundance of the endogenous N. benthamiana NSF (NSFN.benth) upon expression of Rhg1 resistance-type α-SNAPs, yet this does not prevent cell death (17) (Fig. 1). However, it was unclear whether immediate coexpression of NSFN.benth (81% identity to soybean NSFCh07; see SI Appendix, Fig. S7 for alignment) might lessen the cytotoxicity. Therefore, we agroinfiltrated mixed cultures expressing NSFN.benth and α-SNAPRhg1LC, as well as empty vector, NSFCh13, and NSFRAN07 as controls. As in Fig. 3A, NSFCh13 gave partial protection while NSFRAN07 coexpression gave strong protection (Fig. 3C). NSFN.benth coexpression, on the other hand, was similar to empty vector controls and did not guard against α-SNAPRhg1LC−induced cell death (Fig. 3C). Because no obvious cell death rescue from coexpressing NSFN.benth was apparent, we also examined NSFN.benth physical binding with Rhg1 resistance-type α-SNAPs, using recombinant NSFN.benth protein. NSFN.benth readily bound α-SNAPRhg1WT, but binding to either Rhg1 resistance-type α-SNAP was much lower, only slightly over negative controls (Fig. 3D). These experiments suggest that NSFN.benth exhibits little or no functional interaction with SCN resistance-associated soybean Rhg1 α-SNAPs, which likely accounts for the high toxicity of Rhg1 α-SNAPs in N. benthamiana.
We then used the N. benthamiana assay to examine NSFRAN07 function predictions. One set of experiments tested whether cell death caused by α-SNAPRhg1LC1–279, which lacks the final 10 C-terminal residues, could be rescued by NSFRAN07 or NSFCh07. Neither NSFRAN07 nor NSFCh07 prevented the cell death caused by α-SNAPRhg1LC1–279, despite guarding against cell death in the positive control treatments involving full-length α-SNAPRhg1LC (SI Appendix, Fig. S8A). Likewise, we tested whether cell death caused by α-SNAPRhg1LC 286AAAA289—which also did not exhibit in vitro binding of NSF—could be rescued by either NSFRAN07 or NSFCh07. The α-SNAPRhg1LC 286AAAA289, like α-SNAPRhg1LC, elicited increased expression of the endogenous N. benthamiana NSF (SI Appendix, Fig. S8 B and C). However, compared with α-SNAPRhg1LC, which does bind the tested soybean NSF to some extent, we observed that α-SNAPRhg1LC 286AAAA289-induced cell death was not strongly protected by NSFRAN07 or NSFCh07 coexpression (SI Appendix, Fig. S8B). These experiments provide further evidence that C-terminally mutagenized α-SNAPs can disrupt the function of N. benthamiana 20S complexes, and that NSF rescue of the cell death induced by toxic α-SNAPs requires an intact C terminus of α-SNAPs to mediate successful α-SNAP−NSF interaction.
Turning to the NSFs mutagenized at the inferred α-SNAP binding interface, α-SNAPRhg1LC cell death rescue via coexpression of mutated NSFCh07 or NSFRAN07 (NSFCh07 N21A F115A or NSFRAN07 Y21A F116^) was not as robust as rescue by the normal NSFCh07 or NSFRAN07 (SI Appendix, Fig. S8 F and G). Anti-NSF immunoblots confirmed the expression of NSFCh07, NSFRAN07, and their respective mutants (SI Appendix, Fig. S8E). This supports the contribution of the mutated NSF residues to optimal NSF/α-SNAP interaction.
Finally, we made and used an α-SNAPRhg1LC I289A to examine how the penultimate α-SNAP residue, which has been shown in other α-SNAPs to help stimulate NSF ATPase, affected rescue by NSFRAN07 or NSFCh07 (20, 49). Protection against α-SNAPRhg1LC I289A was evident but was much less than that observed for α-SNAPRhg1LC (SI Appendix, Fig. S8D), suggesting that although NSFRAN07 may bind Rhg1 resistance-type α-SNAPs more effectively, ATPase stimulation is likely an additional factor in relieving cytotoxicity. Overall, the findings of Fig. 3 extend the Fig. 2 finding that NSFRAN07 binds Rhg1 α-SNAPs better, demonstrating in vivo that the NSFRAN07 polymorphisms more effectively guard against the disruptive effects of the polymorphic Rhg1 α-SNAPs, and demonstrating that, among site-directed mutants, the extent of this in planta protection correlates with observed in vitro α-SNAP−NSF binding differences.
One Hundred Percent of Predicted Rhg1+ Glycine max Accessions in the US Department of Agriculture Soybean Collection Contain the NSFRAN07 R4Q Amino Acid Polymorphism.
NSFRAN07 was present in all Rhg1-containing -type and NAM lines (SI Appendix, Tables S1 and S2), but we sought to test whether this Rhg1/NSFRAN07 association was universal rather than “frequent.” In 2015, Song and coworkers (52–54) reported genotyping the US Department of Agriculture (USDA) soybean germplasm collection of ∼20,000 accessions—collected from over 80 countries—using a 50,000 SNP DNA microarray chip (SoySNP50K iSelect BeadChip). The data are available in a searchable SNP database at Soybase (https://soybase.org/snps/). Using this Soybase SNP browser, we found that a C/T SNP (ss715597431, Gm07:36,449,014) causes the NSFRAN07 R4Q polymorphism. Analyzing all 19,645 USDA Glycine max accessions for ss715597431, we estimated the NSFRAN07 allele frequency in the USDA collection at 11.0% (2,165 NSFRAN07+/NSFRAN07+, 33 NSFRAN07+/NSFRAN07−) (Fig. 4A). Q4 was not found in the predicted NSF protein sequences of any plant species available for query at Phytozome.org (55) (SI Appendix, Fig. S9).
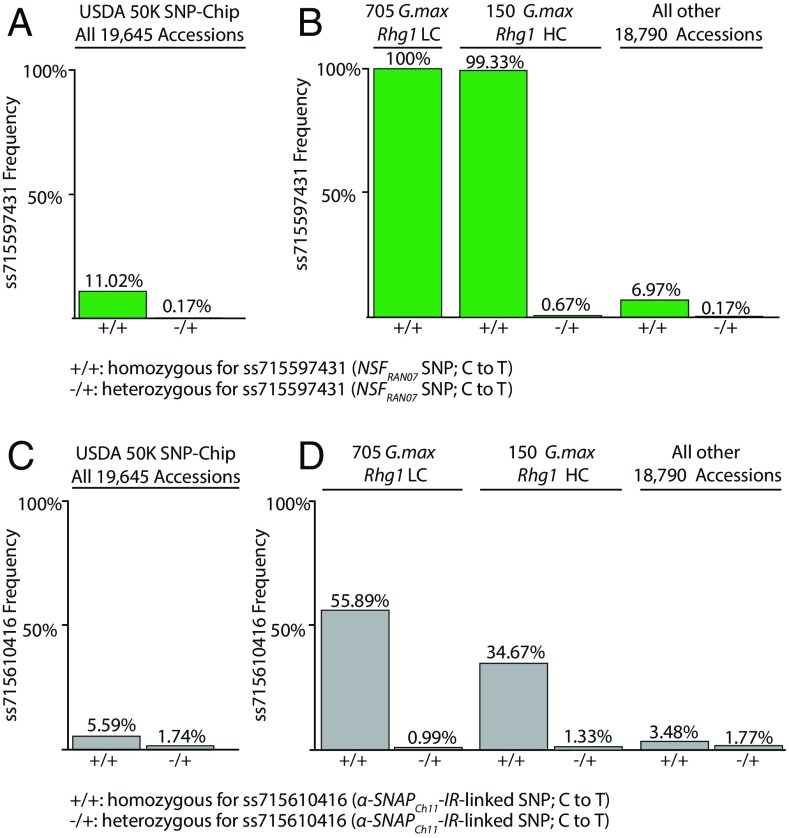
Fig. 4.
All soybeans in the USDA germplasm collection that carry an Rhg1+ SNP signature also carry the R4Q NSFRAN07 polymorphism. (A) Frequency of SoySNP50K SNP ss715597431 (corresponding to NSFRAN07 R4Q) in all 19,645 SoySNP50K-genotyped Glycine max accessions. (B) Frequency of ss715597431 in all USDA collection G. max with Rhg1LC or Rhg1HC haplotype signatures, or in the remainder of SoySNP50K-genotyped G. max. (C) Frequency of SoySNP50K SNP ss715610416 that is closest marker for α-SNAPCh11-IR allele, across all 19,645 genotyped USDA G. max accessions. (D) Frequency of ss715610416 in all USDA collection G. max with Rhg1LC or Rhg1HC haplotype signatures, or in remainder of SoySNP50K-genotyped G. max.
Rhg1-mediated SCN resistance is uncommon among soybean accessions, and less than 5% of the USDA soybean collection carries a multicopy Rhg1 haplotype. Previously, Lee et al. (11) identified SoySNP50K signatures for Rhg1HC, Rhg1LC, and single-copy (SCN-susceptible) haplotypes, and estimated that 705 Rhg1LC and 150 Rhg1HC accessions were present in the USDA Glycine max collection. Among these 855 Rhg1-signature Glycine max accessions, we determined a 100% incidence of the ss715597431 NSFRAN07 signature (Fig. 4B).
To better define the Rhg1-cosegregating locus within the Ch07 interval, we examined amino acid changes within candidate loci adjacent to NSFRAN07 from Rhg1-carrying and NAM lines, between markers ss715597415 and ss715597431. We observed that the NSFRAN07 SNPs, especially those causing the five polymorphisms in the N domain, were 100% maintained across all Rhg1-containing varieties. On the other hand, SNPs causing amino acid changes within candidate loci adjacent to NSFRAN07 were not 100% conserved across all Rhg1-containing varieties (SI Appendix, Table S3). The predicted amino acid sequence of most candidate loci matched the Wm82 (SCN-susceptible) sequence. Among candidate loci with amino acid substitutions, including Glyma.07g196000 and Glyma.07g196200 flanking NSFCh07/Glyma.07g195900 on the side not described in SI Appendix, Table S3, only NSFRAN07 encoded the same consistent amino acid changes across all examined Rhg1-containing germplasm.
An SNP Associated with the Ch11 α-SNAP Intron Retention Allele—a Predicted SCN Resistance Quantitative Trait Locus—Is also Enriched Among Predicted Rhg1+ Accessions in the USDA Collection.
A recent study implicated the interval carrying the intron retention allele of α-SNAPCh11 (α-SNAPCh11-IR) in SCN resistance, but the responsible gene(s) within this quantitative trait locus (QTL) interval were not defined (38). The α-SNAPCh11-IR allele may have emerged randomly or it may confer some selective advantage, for example, by reducing available levels of WT α-SNAP proteins and shifting the balance toward the disruptive Rhg1 α-SNAP proteins. This could be particularly relevant in Rhg1LC soybean lines that typically carry only three copies of α-SNAPRhg1LC with correspondingly lower mRNA abundance, in contrast to the 9- or 10-copy Rhg1HC lines (10, 13). We therefore used SoySNP50K data to analyze the frequency of the α-SNAPCh11-IR allele in the whole USDA collection and in the 855 Rhg1+ Glycine max accessions noted in the preceding section of Results. We found a C/T SNP (ss715610416, Gm11:32951515) located ∼17,000 bp downstream of the α-SNAPCh11 locus that was associated with the α-SNAPCh11-IR allele, as indicated by our WGS data. Using immunoblots, we tested for total levels of WT α-SNAP protein among several Rhg1LC accessions that had either WT or α-SNAPCh11-IR−associated SNPs (ss715610416). The Rhg1LC accessions possessing the WT-linked SNP had higher WT α-SNAP abundance relative to the Rhg1LC accessions with the ss715610416 SNP (SI Appendix, Fig. S10). Across the USDA soybean collection, we then found that the α-SNAPCh11-IR−associated ss715610416 genotype was present in 5.6% of accessions (Fig. 4C). Perhaps surprisingly, we observed the α-SNAPCh11-IR−associated ss715610416 genotype in only half (55.9%) of the Rhg1LC soybean lines and in about a third (34.7%) of the Rhg1HC lines (Fig. 4D). However, this enrichment of the α-SNAPCh11-IR–linked SNP within Rhg1+ germplasm provides further evidence that this allele beneficially contributes to Rhg1+ soybean varieties.
All Rhg1+ Recombinant Inbred Lines Derived from Rhg1+ x rhg1− Crosses Inherit NSFRAN07.
Our findings regarding NSFRAN07 cooccurrence with Rhg1 in the USDA soybean germplasm collection are an indication of strong segregation distortion. However, recalling that Webb et al. (39) reported that only 91 of 96 lines with a resistant parent marker type linked to Rhg1 also had a resistant parent marker type near the QTL now known to encode NSFRAN07, we explored whether the Rhg1+ progeny of more recent biparental crosses strictly inherited NSFRAN07. From the SoyNAM project (47), we examined F5 genotypic data for populations of derived recombinant inbred lines (RILs) developed from crosses of the IA3023 (SCN-susceptible) hub parent to eight different soybean accessions carrying either Rhg1HC (seven accessions) or Rhg1LC (one accession). There were 122 to 139 RILs in each population. The segregation for NSFRAN07:NSFCh07WT in soybean lines lacking Rhg1 did not deviate from the null hypothesis of 1:1 segregation in six of the eight populations. The segregation distortion for NSFRAN07 was obvious among RILs that carried a resistance-associated Rhg1 allele, but, out of a total of 309 Rhg1+ RILs, 8 appeared to have inherited Rhg1HC or Rhg1LC and a WT NSFCh07 allele (SI Appendix, Table S4). This was based upon the low-density SoySNP6K mapping data that used linked rather than perfect genetic markers for Rhg1 and NSF (47). We therefore genotyped all eight of these RILs via sequencing and/or primers detecting the Rhg1 repeat junction and a WT NSFCh07 vs. NSFRAN07 allele and found that all eight reexamined RILs containing Rhg1HC or Rhg1LC also carried the NSFRAN07 ^116F and M181I mutations. Thus, all Rhg1+ RILs also inherited NSFRAN07 (SI Appendix, Table S4). We analogously infer that the five lines of the Webb et al. (39) study that appeared to break coinheritance between Rhg1HC and NSFRAN07 likely underwent a cross-over between the gene in question and the genetic markers linked to either Rhg1 or NSF. Taken together with the described biochemical and in planta impacts of Rhg1 α-SNAPs and NSFRAN07, the SoySNP50K and NAM data indicate that NSFRAN07 coinheritance is a necessary balance that confers viability to soybeans carrying a resistance-type Rhg1 haplotype.
Discussion
Across eukaryotes, NSF and α-SNAP interact through conserved electrostatic contacts to disassemble SNARE complexes, thereby maintaining cellular vesicle trafficking (14, 15). Our findings indicate that Rhg1-mediated SCN resistance in soybean encompasses not just unusual changes in Rhg1 α-SNAP sequence and abundance in syncytium cells, as previously published, but also changes in other housekeeping α-SNAP and NSF genes whose products comprise the SNARE recycling machinery. These results showcase how a functionally related set of multiple conserved housekeeping genes has coevolved toward atypical forms, apparently to confer resistance to a highly damaging nematode pathogen while balancing plant fitness. The findings suggest that the two common resistance-conferring Rhg1 haplotypes employ similar yet distinct strategies to combat SCN: They decrease WT α-SNAP availability via greater Rhg1 copy number expansion and/or through loss of WT α-SNAP loci. We also found that presence of the unusual Rhg1 α-SNAP proteins requires copresence of a novel NSF protein for plant viability. This explains a well-documented segregation distortion occurring between Rhg1 and a chromosome 07 region (39–41). Perhaps more importantly, this study and other recent work on Rhg1 offer a molecular framework in which to understand the interactions of multiple QTLs associated with SCN resistance (13, 17, 18, 38, 56, 57): Many of these loci modify the host vesicle fusion SNARE recycling machinery as a means of controlling SCN infection.
An understanding of the necessity of NSFRAN07 to balance Rhg1 germplasm should become a central consideration in any planned transgenic addition of Rhg1 into SCN-susceptible soybeans. Beyond soybean, this report suggests strategies to engineer Rhg1-like resistance into other cyst nematode-susceptible crop species, through introduction of sequence-edited α-SNAP alleles together with modulation of WT α-SNAP abundance and/or introduction of a compatible NSF.
It is biologically fascinating that complementary α-SNAP and NSF polymorphisms, located at the conserved binding interfaces of both members of the core SNARE recycling machinery, were apparently selected due to disease pressure from SCN. It highlights this pathway’s importance during the pathogen−host interaction. The previous finding that Rhg1 resistance-type α-SNAPs are impaired in normal NSF interactions (17) is supported by the present finding that a unique NSF allele—NSFRAN07—is a requisite balance for Rhg1 resistance-type α-SNAPs. While ref. 17 proposed the functional redundancy of multiple WT α-SNAP loci (available due to polyploidy) as the balance that allows the viability of Rhg1-containing lines, this model must be modified with the observation that Rhg1-containing lines that lack NSFRAN07 are apparently nonviable. Presence of WT α-SNAPs may still, in the presence of NSFRAN07, contribute to the vigor and normal soybean yield of lines carrying the PI 88788 source of Rhg1 (Rhg1HC), but they are not sufficient to do so in the absence of NSFRAN07.
Housekeeping genes have been reported to evolve particularly slowly due to selective constraints (58), which raises interest in the coevolution between NSFRAN07 and Rhg1 α-SNAP. It is unclear whether existing natural variation at Ch07 NSF among soybean populations enabled the development of the Ch18 Rhg1 resistance-type α-SNAPs or vice versa, or if the Rhg1 α-SNAP duplication event occurred first, followed by subsequent coevolution of NSF and resistance-type α-SNAP polymorphisms. Currently, reports of natural NSF variation appear to be limited to humans. The 1,000 Human Genomes Project showed that, in certain human ethnicities, NSF copy number expansions of up to three repeats are not uncommon (59). The original NSF locus is full length, while the subsequent NSF copy number repeats truncate near exon 13 and do not encode full-length NSF transcripts (59, 60). A recent study reported a correlation between this human NSF copy number variation and drug dependency (60). Notably, no residue substitutions were reported among human NSF alleles, and, to the best of our knowledge, no naturally occurring NSF protein variants from any organism have previously been reported.
As noted above, our findings about NSFRAN07 provide a mechanistic explanation for the previously observed segregation distortion, in SCN-resistant plants, between Rhg1 and the chromosome 07 genetic interval that encodes NSFRAN07 (39–41). An observation that remains less firmly explained, however, is why transgenic expression of α-SNAPRhg1HC or α-SNAPRhg1LC protein, in Agrobacterium rhizogenes-transformed root systems of SCN-susceptible Wm82 (which lacks NSFRAN07), elicited no apparent sensitivities such as cytotoxicity or endogenous NSF expression increases (10, 17). These sensitivities were observed with N. benthamiana expressing Rhg1 α-SNAPs (17). Notably, coexpression of NSFN.benth did not relieve the cell death in N. benthamiana leaves caused by Rhg1 α-SNAPs, while WT soybean NSFCh07 did, albeit not as effectively as NSFRAN07. Consistent with this, recombinant NSFN.benth essentially could not bind with Rhg1 resistance-type α-SNAPs in vitro, but those α-SNAPs could bind soybean WT NSFCh07. This may explain why soybean root cells do exhibit some tolerance of Rhg1 α-SNAP expression even in the absence of NSFRAN07. Nevertheless, the finding that all soybeans in the USDA collection that bear the signature of resistance-conferring Rhg1 alleles also contain the NSFRAN07 R4Q signature, coupled with the universal copresence of the NSFRAN07 allele with Rhg1 in the segregating progeny of NAM crosses, provides compelling evidence that, at the organismal level, NSFRAN07 is essential for viability at some stage of growth for all Rhg1-containing germplasm.
Rhg1LC and Rhg4 contribute together to the SCN resistance of Rhg1LC soybean lines (4, 61), and it remains unclear why Rhg1LC confers only partial SCN resistance when Rhg4, which encodes a putative serine hydroxymethyltransferase, is absent (61–63). Whether or not the Rhg4 product directly impacts Rhg1-associated α-SNAP/NSF/SNARE interactions, consideration of the present findings may be influenced by published evidence that Rhg1HC soybean lines are substantially more effective than Rhg1LC+ rhg4− lines at conferring SCN resistance against HG type 0 SCN populations (62, 63).
The present findings add to what was already known or inferred about loss of some WT α-SNAPs in Peking-type Rhg1LC soybean lines (11, 13, 37, 38). Rhg1LC varieties without or with the α-SNAPCh11-IR allele exhibit reduced or sharply reduced WT α-SNAP expression, respectively. This further supports the idea that, in addition to the unusual Rhg1 α-SNAP proteins, WT α-SNAP levels and the [WT α-SNAP:Rhg1 α-SNAP] ratio can be important determinants of successful Rhg1-mediated SCN resistance. Models for resistance involving evasion of nematode effectors should also be considered. NSFRAN07 may have allowed the nontoxic presence of Rhg1-type α-SNAPs, and Rhg1 α-SNAPs may confer SCN resistance by failing to cooperate with nematode manipulation of the host. This model could explain why divergence of Rhg1 α-SNAP types has occurred: Different SCN populations may carry effectors that manipulate or interact with the host SNARE recycling machinery, but to varying degrees depending on the α-SNAP protein that is present.
The α-SNAPCh11-IR−associated SNP, which correlated with modest changes in WT α-SNAP abundance, was present in only about half of the Rhg1LC soybean lines and a third of the Rhg1HC lines. Only a subtle positive impact on SCN resistance was reported for the broader QTL locus carrying the α-SNAPCh11-IR allele (38). However, because not all Rhg1+ soybean lines carry the α-SNAPCh11-IR−associated genotype, its intentional use or exclusion may, in the future, translate to subtle but economically useful shifts in SCN resistance, in the HG type specificity of that resistance, or in soybean yield potential.
Discovery of the need for NSFRAN07 in Rhg1-containing soybeans may reveal a protective mechanism that reduces the toxic effects of Rhg1 α-SNAPs in some cell types/conditions by facilitating participation of Rhg1 α-SNAPs in productive 20S complexes that disassemble SNARE bundles, while the toxicity of Rhg1 α-SNAPs remains predominant in syncytium cells. Such conditionally functional NSF mutants are known in the laboratory-derived Drosophila NSF comatose mutants, whereby the NSF-1 protein encoded by the comatose allele supports SNARE complex disassembly at room temperature but is nonfunctional at elevated temperatures, leading to failure of synaptic vesicle transport and fly paralysis (31, 64). However, other mechanistic hypotheses are viable. Future studies could examine the dynamics of NSFRAN07 abundance and function over time in developing SCN syncytia. For example, increased NSF levels were detected in syncytia-containing root segments in Rhg1HC varieties, and we had associated this with WT α-SNAP deficiency (17), but whether it is NSFRAN07 or NSFCh13 that increases is of interest and might suggest whether α-SNAP and NSF functionality is being promoted or disrupted by the host. We did observe that NSFRAN07 apparently can work with WT α-SNAPs, or at least is not toxic in the way that resistance-associated Rhg1 α-SNAPs can be toxic. Expression of NSFRAN07 in N. benthamiana caused no macroscopically detectable leaf phenotypes, and NSFRAN07 is expressed in Rhg1HC soybeans that also express high levels of WT α-SNAPs. The random segregation of the alleles encoding NSFCh07WT and NSFRAN07 in soybean progeny that lack Rhg1, and the presence of NSFRAN07 in over 1,300 USDA soybean accessions that lack Rhg1, also suggests that NSFRAN07 likely functions effectively with WT α-SNAPs.
A summarizing model can be constructed. We hypothesize that coexpression of WT α-SNAPs or soybean NSFs can compete away the toxicity of Rhg1 α-SNAPs by restoring functionally compatible partners to the 20S complex. The α-SNAPs bind bundles of three or four SNARE proteins and provide a key portion of the platform for binding of NSF proteins and the stimulation of ATP hydrolysis to disassemble those SNARE bundles. The success of the α-SNAP N. benthamiana toxicity assay apparently derives from the inability of NSFN.benth to function on SNARE bundles that carry Rhg1 α-SNAPs. The phenotype caused by Rhg1 α-SNAP expression is extreme in N. benthamiana but mild in most soybean cells because of the partial compatibility of Rhg1 α-SNAPs with WT soybean NSFs. Our data indicate that even greater compatibility with Rhg1 α-SNAPs is restored by presence of NSFRAN07. Nevertheless, the findings of the present work and ref. 17 indicate that Rhg1 α-SNAPs are a less compatible partner than WT α-SNAPs. When the relative level of Rhg1 α-SNAPs goes up, as has been documented for syncytium cells (17), we hypothesize that the suboptimal function of Rhg1 α-SNAPs poisons syncytia. Alternative models for SCN resistance are possible; for example, the Rhg1 α-SNAPs may be less sensitive to SCN effectors that manipulate WT α-SNAPs to the advantage of the nematode. In either case, we propose that NSFRAN07 is sufficiently compatible with Rhg1 α-SNAPs to confer viability and productivity to Rhg1+ soybean lines, especially when WT α-SNAPs are also abundant. NSFRAN07 may not be sufficient to overcome the toxicity of Rhg1 α-SNAPs in syncytia. The lower abundance of WT α-SNAPs in many Rhg1LC lines may be important to enhancing the SCN resistance of those lines, where there are only 3 rather than ∼10 tandem repeat copies of Rhg1, but it may also be a primary reason why Rhg1LC lines have been widely observed to exhibit minor reductions in grain yield.
The amassing evidence for the importance of altered α-SNAP/NSF/SNARE interactions in SCN−soybean interactions also suggests that these proteins may be attractive targets for cyst nematode effectors (13, 17, 18, 38, 56, 57, 65). Preliminary evidence for one such effector is already in place (57), and extensive variation is present in the SCN genes that encode putative SNARE-like protein effectors (66). The gradual evolution of SCN populations toward an increasing number of individuals that can overcome the widely used Rhg1HC SCN resistance is a major issue for global soybean production (67). Future work to discover and understand relevant nematode effectors in these SCN populations, and a means of reestablishing resistance against such nematodes, may benefit from assays that directly test for effectors that impact the soybean α-SNAP and NSF protein variants characterized in the present study.
Materials and Methods
Antibodies and Immunoblotting.
Affinity-purified polyclonal rabbit antibodies raised against peptides from soybean NSF, α-SNAPRhg1HC, α-SNAPRhg1LC, and WT α-SNAPs were previously generated and validated using recombinant proteins as described in ref. 17. Tissue preparation and immunoblots were performed essentially as in refs. 17 and 68.
Transgenic Soybean Root Generation.
Binary expression constructs were transformed into A. rhizogenes strain “ARqua1,” and transgenic soybean roots were produced from cotyledons of the noted genetic background as described in ref. 10.
Recombinant Protein Production.
Soluble, native recombinant His-SUMO-α-SNAP or His-SUMO-NSF proteins were expressed and purified by similar procedures as described in ref. 17.
In Vitro α-SNAP NSF Binding Assays.
In vitro binding assays were performed with recombinant α-SNAP and NSF proteins essentially as described in refs. 17 and 49. Briefly, 20 µg of recombinant α-SNAP was adhered to the bottom of a polypropylene tube at room temperature, and then washed. Subsequently, 20 µg of recombinant NSF was added to each tube containing immobilized α-SNAP and allowed to bind on ice for 10 min, followed by two washes; α-SNAP and bound NSF were then collected, separated by SDS/PAGE, and visualized by silver staining.
Transient Agrobacterium Expression in N. benthamiana.
Agrobacterium tumefaciens strain GV3101 was used for transient protein expression in N. benthamiana. Plant growth, culture induction, and infiltration were performed essentially as in ref. 17. N. benthamiana toxicity results were quantified using a standardized 0 to 5 lesion severity scoring system with blinded treatments (raters unaware of which treatments they are scoring). Consistency of scoring among independent raters was confirmed.
Segregating NAM Crosses.
Soybean parental crosses and 6K SNP genotyping mapping are described in ref. 47.
Protein Structure Modeling.
NSFRAN07, α-SNAPCh11, and α-SNAPCh11IR structural homology models were generated using SWISS-MODEL (Biozentrum), and the resulting PDB files were analyzed with PyMOL (The PyMOL Molecular Graphics System, Version 1.8; Schrödinger, LLC).
DNA Sequence and SNP Analysis.
WGS data of 12 soybean varieties were obtained from previously published studies and analyzed as in Cook et al. (13, 47).
Detailed information regarding the procedures used is provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Shaojie Han for experiment contributions and discussion, and Drs. Sebastian Bednarek, Barry Ganetzky, Ann MacGuidwin, John M. Smith, and Matthew Hudson for additional guidance and suggestions. Thanks to Jaret Schroeder, Keilaa-Demi De La Cruz, and Ryan Kessens for assistance in growing and caring for plants and other resources. This work was funded by United Soybean Board, Wisconsin Soybean Board, and Wisconsin Experiment Station Hatch awards and by USDA–National Institute of Food and Agriculture–Agriculture and Food Research Initiative Award 2014-67013-21775 (to A.F.B.). The findings build on work supported by the National Science Foundation Graduate Research Fellowship under Grant DGE-1256259 (to A.M.B.).
Footnotes
Conflict of interest statement: A patent application covering the presently described work has been filed by the Wisconsin Alumni Research Foundation.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. MH136642).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717070115/-/DCSupplemental.
References
- 1.Kyndt T, Vieira P, Gheysen G, de Almeida-Engler J. Nematode feeding sites: Unique organs in plant roots. Planta. 2013;238:807–818. doi: 10.1007/s00425-013-1923-z. [DOI] [PubMed] [Google Scholar]
- 2.Niblack TL, Lambert KN, Tylka GL. A model plant pathogen from the kingdom Animalia: Heterodera glycines, the soybean cyst nematode. Annu Rev Phytopathol. 2006;44:283–303. doi: 10.1146/annurev.phyto.43.040204.140218. [DOI] [PubMed] [Google Scholar]
- 3.Jones JT, et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol. 2013;14:946–961. doi: 10.1111/mpp.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchum MG. Soybean resistance to the soybean cyst nematode Heterodera glycines: An update. Phytopathology. 2016;106:1444–1450. doi: 10.1094/PHYTO-06-16-0227-RVW. [DOI] [PubMed] [Google Scholar]
- 5.Allen TW, et al. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 2017;18:19–27. [Google Scholar]
- 6.Gheysen G, Mitchum MG. How nematodes manipulate plant development pathways for infection. Curr Opin Plant Biol. 2011;14:415–421. doi: 10.1016/j.pbi.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Mitchum MG, et al. Nematode effector proteins: An emerging paradigm of parasitism. New Phytol. 2013;199:879–894. doi: 10.1111/nph.12323. [DOI] [PubMed] [Google Scholar]
- 8.Hewezi T, Baum TJ. Manipulation of plant cells by cyst and root-knot nematode effectors. Mol Plant Microbe Interact. 2013;26:9–16. doi: 10.1094/MPMI-05-12-0106-FI. [DOI] [PubMed] [Google Scholar]
- 9.Concibido VC, Diers BW, Arelli PR. A decade of QTL mapping for cyst nematode resistance in soybean. Crop Sci. 2004;44:1121–1131. [Google Scholar]
- 10.Cook DE, et al. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science. 2012;338:1206–1209. doi: 10.1126/science.1228746. [DOI] [PubMed] [Google Scholar]
- 11.Lee TG, Kumar I, Diers BW, Hudson ME. Evolution and selection of Rhg1, a copy-number variant nematode-resistance locus. Mol Ecol. 2015;24:1774–1791. doi: 10.1111/mec.13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodds PN, Rathjen JP. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 13.Cook DE, et al. Distinct copy number, coding sequence, and locus methylation patterns underlie Rhg1-mediated soybean resistance to soybean cyst nematode. Plant Physiol. 2014;165:630–647. doi: 10.1104/pp.114.235952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahn R, Scheller RH. SNAREs–Engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Brunger AT. Recent advances in deciphering the structure and molecular mechanism of the AAA+ ATPase N-ethylmaleimide-sensitive factor (NSF) J Mol Biol. 2016;428:1912–1926. doi: 10.1016/j.jmb.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker RW, Hughson FM. Chaperoning SNARE assembly and disassembly. Nat Rev Mol Cell Biol. 2016;17:465–479. doi: 10.1038/nrm.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bayless AM, et al. Disease resistance through impairment of α-SNAP-NSF interaction and vesicular trafficking by soybean Rhg1. Proc Natl Acad Sci USA. 2016;113:E7375–E7382. doi: 10.1073/pnas.1610150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, et al. The soybean GmSNAP18 gene underlies two types of resistance to soybean cyst nematode. Nat Commun. 2017;8:14822. doi: 10.1038/ncomms14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wickner W, Schekman R. Membrane fusion. Nat Struct Mol Biol. 2008;15:658–664. doi: 10.1038/nsmb.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zick M, Orr A, Schwartz ML, Merz AJ, Wickner WT. Sec17 can trigger fusion of trans-SNARE paired membranes without Sec18. Proc Natl Acad Sci USA. 2015;112:E2290–E2297. doi: 10.1073/pnas.1506409112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao M, et al. Mechanistic insights into the recycling machine of the SNARE complex. Nature. 2015;518:61–67. doi: 10.1038/nature14148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickner W. Membrane fusion: Five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol. 2010;26:115–136. doi: 10.1146/annurev-cellbio-100109-104131. [DOI] [PubMed] [Google Scholar]
- 23.Hanson PI, Whiteheart SW. AAA+ proteins: Have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 24.Whiteheart SW, Schraw T, Matveeva EA. N-ethylmaleimide sensitive factor (NSF) structure and function. Int Rev Cytol. 2001;207:71–112. doi: 10.1016/s0074-7696(01)07003-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Matveeva EA, Ren Q, Whiteheart SW. Dissecting the N-ethylmaleimide-sensitive factor: Required elements of the N and D1 domains. J Biol Chem. 2010;285:761–772. doi: 10.1074/jbc.M109.056739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song H, Orr A, Duan M, Merz AJ, Wickner W. Sec17/Sec18 act twice, enhancing membrane fusion and then disassemblingcis-SNARE complexes. eLife. 2017;6:e26646. doi: 10.7554/eLife.26646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteheart SW, Matveeva EA. Multiple binding proteins suggest diverse functions for the N-ethylmaleimide sensitive factor. J Struct Biol. 2004;146:32–43. doi: 10.1016/j.jsb.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Zhao C, Slevin JT, Whiteheart SW. Cellular functions of NSF: Not just SNAPs and SNAREs. FEBS Lett. 2007;581:2140–2149. doi: 10.1016/j.febslet.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naydenov NG, et al. Loss of soluble N-ethylmaleimide-sensitive factor attachment protein α (αSNAP) induces epithelial cell apoptosis via down-regulation of Bcl-2 expression and disruption of the Golgi. J Biol Chem. 2012;287:5928–5941. doi: 10.1074/jbc.M111.278358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao Y, et al. An essential and NSF independent role for α-SNAP in store-operated calcium entry. eLife. 2013;2:e00802. doi: 10.7554/eLife.00802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Littleton JT, et al. SNARE-complex disassembly by NSF follows synaptic-vesicle fusion. Proc Natl Acad Sci USA. 2001;98:12233–12238. doi: 10.1073/pnas.221450198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmutz J, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463:178–183. doi: 10.1038/nature08670. [DOI] [PubMed] [Google Scholar]
- 33.Sanderfoot AA, Assaad FF, Raikhel NV. The Arabidopsis genome. An abundance of soluble N-ethylmaleimide-sensitive factor adaptor protein receptors. Plant Physiol. 2000;124:1558–1569. doi: 10.1104/pp.124.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachem CW, et al. Oomen RJF Antisense suppression of a potato alpha-SNAP homologue leads to alterations in cellular development and assimilate distribution. Plant Mol Biol. 2000;43:473–482. doi: 10.1023/a:1006492205788. [DOI] [PubMed] [Google Scholar]
- 35.Bassham DC, Raikhel NV. The pre-vacuolar t-SNARE AtPEP12p forms a 20S complex that dissociates in the presence of ATP. Plant J. 1999;19:599–603. doi: 10.1046/j.1365-313x.1999.00552.x. [DOI] [PubMed] [Google Scholar]
- 36.Rancour DM, Dickey CE, Park S, Bednarek SY. Characterization of AtCDC48. Evidence for multiple membrane fusion mechanisms at the plane of cell division in plants. Plant Physiol. 2002;130:1241–1253. doi: 10.1104/pp.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsye PD, et al. The expression of a naturally occurring, truncated allele of an α-SNAP gene suppresses plant parasitic nematode infection. Plant Mol Biol. 2012;80:131–155. doi: 10.1007/s11103-012-9932-z. [DOI] [PubMed] [Google Scholar]
- 38.Lakhssassi N, et al. Characterization of the soluble NSF attachment protein gene family identifies two members involved in additive resistance to a plant pathogen. Sci Rep. 2017;7:45226. doi: 10.1038/srep45226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb DM, et al. Genetic mapping of soybean cyst nematode race-3 resistance loci in the soybean PI 437.654. Theor Appl Genet. 1995;91:574–581. doi: 10.1007/BF00223282. [DOI] [PubMed] [Google Scholar]
- 40.Kopisch-Obuch FJ, Diers BW. Segregation at the SCN resistance locus rhg1 in soybean is distorted by an association between the resistance allele and reduced field emergence. Theor Appl Genet. 2006;112:199–207. doi: 10.1007/s00122-005-0104-2. [DOI] [PubMed] [Google Scholar]
- 41.Vuong TD, et al. Genetic architecture of cyst nematode resistance revealed by genome-wide association study in soybean. BMC Genomics. 2015;16:593. doi: 10.1186/s12864-015-1811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niblack TL, et al. A revised classification scheme for genetically diverse populations of Heterodera glycines. J Nematol. 2002;34:279–288. [PMC free article] [PubMed] [Google Scholar]
- 43.Rice LM, Brunger AT. Crystal structure of the vesicular transport protein Sec17: Implications for SNAP function in SNARE complex disassembly. Mol Cell. 1999;4:85–95. doi: 10.1016/s1097-2765(00)80190-2. [DOI] [PubMed] [Google Scholar]
- 44.Chae TH, Kim S, Marz KE, Hanson PI, Walsh CA. The hyh mutation uncovers roles for alpha Snap in apical protein localization and control of neural cell fate. Nat Genet. 2004;36:264–270. doi: 10.1038/ng1302. [DOI] [PubMed] [Google Scholar]
- 45.Horsnell WG, Steel GJ, Morgan A. Analysis of NSF mutants reveals residues involved in SNAP binding and ATPase stimulation. Biochemistry. 2002;41:5230–5235. doi: 10.1021/bi0160359. [DOI] [PubMed] [Google Scholar]
- 46.Sanyal S, Krishnan KS. Lethal comatose mutation in Drosophila reveals possible role for NSF in neurogenesis. Neuroreport. 2001;12:1363–1366. doi: 10.1097/00001756-200105250-00015. [DOI] [PubMed] [Google Scholar]
- 47.Song Q, et al. Genetic characterization of the soybean nested association mapping population. Plant Genome. 2017;10 doi: 10.3835/plantgenome2016.10.0109. [DOI] [PubMed] [Google Scholar]
- 48.Severin AJ, et al. RNA-seq atlas of Glycine max: A guide to the soybean transcriptome. BMC Plant Biol. 2010;10:160. doi: 10.1186/1471-2229-10-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnard RJ, Morgan A, Burgoyne RD. Domains of alpha-SNAP required for the stimulation of exocytosis and for N-ethylmalemide-sensitive fusion protein (NSF) binding and activation. Mol Biol Cell. 1996;7:693–701. doi: 10.1091/mbc.7.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griff IC, Schekman R, Rothman JE, Kaiser CA. The yeast SEC17 gene product is functionally equivalent to mammalian alpha-SNAP protein. J Biol Chem. 1992;267:12106–12115. [PubMed] [Google Scholar]
- 51.Barnard RJ, Morgan A, Burgoyne RD. Stimulation of NSF ATPase activity by alpha-SNAP is required for SNARE complex disassembly and exocytosis. J Cell Biol. 1997;139:875–883. doi: 10.1083/jcb.139.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song Q, et al. Development and evaluation of SoySNP50K, a high-density genotyping array for soybean. PLoS One. 2013;8:e54985. doi: 10.1371/journal.pone.0054985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Q, et al. Fingerprinting soybean germplasm and its utility in genomic research. G3 (Bethesda) 2015;5:1999–2006. doi: 10.1534/g3.115.019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant D, Nelson RT, Cannon SB, Shoemaker RC. SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res. 2010;38:D843–D846. doi: 10.1093/nar/gkp798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goodstein DM, et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:D1178–D1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pant SR, et al. Syntaxin 31 functions in Glycine max resistance to the plant parasitic nematode Heterodera glycines. Plant Mol Biol. 2014;85:107–121. doi: 10.1007/s11103-014-0172-2. [DOI] [PubMed] [Google Scholar]
- 57.Bekal S, et al. A SNARE-like protein and biotin are implicated in soybean cyst nematode virulence. PLoS One. 2015;10:e0145601. doi: 10.1371/journal.pone.0145601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang L, Li WH. Mammalian housekeeping genes evolve more slowly than tissue-specific genes. Mol Biol Evol. 2004;21:236–239. doi: 10.1093/molbev/msh010. [DOI] [PubMed] [Google Scholar]
- 59.Sudmant PH, et al. 1000 Genomes Project Diversity of human copy number variation and multicopy genes. Science. 2010;330:641–646. doi: 10.1126/science.1197005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cabana-Domínguez J, et al. A highly polymorphic copy number variant in the NSF gene is associated with cocaine dependence. Sci Rep. 2016;6:31033. doi: 10.1038/srep31033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu S, et al. A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature. 2012;492:256–260. doi: 10.1038/nature11651. [DOI] [PubMed] [Google Scholar]
- 62.Brucker E, Carlson S, Wright E, Niblack T, Diers B. Rhg1 alleles from soybean PI 437654 and PI 88788 respond differentially to isolates of Heterodera glycines in the greenhouse. Theor Appl Genet. 2005;111:44–49. doi: 10.1007/s00122-005-1970-3. [DOI] [PubMed] [Google Scholar]
- 63.Yu N, Lee TG, Rosa DP, Hudson M, Diers BW. Impact of Rhg1 copy number, type, and interaction with Rhg4 on resistance to Heterodera glycines in soybean. Theor Appl Genet. 2016;129:2403–2412. doi: 10.1007/s00122-016-2779-y. [DOI] [PubMed] [Google Scholar]
- 64.Littleton JT, et al. Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron. 1998;21:401–413. doi: 10.1016/s0896-6273(00)80549-8. [DOI] [PubMed] [Google Scholar]
- 65.Grunewald W, Cannoot B, Friml J, Gheysen G. Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog. 2009;5:e1000266. doi: 10.1371/journal.ppat.1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gardner M, et al. Novel global effector mining from the transcriptome of early life stages of the soybean cyst nematode Heterodera glycines. Sci Rep. 2018;8:2505. doi: 10.1038/s41598-018-20536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niblack T, Colgrove A, Colgrove K, Bond J. Shift in virulence of soybean cyst nematode is associated with use of resistance from PI 88788. Plant Health Prog. 2008 doi: 10.1094/PHP-2008-0118-01-RS. [DOI] [Google Scholar]
- 68.Song J, Keppler BD, Wise RR, Bent AF. PARP2 is the predominant poly(ADP-ribose) polymerase in Arabidopsis DNA damage and immune responses. PLoS Genet. 2015;11:e1005200. doi: 10.1371/journal.pgen.1005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.