Significance
Helicoverpa armigera is a major agricultural and horticultural pest that recently spread from its historical distribution throughout much of the Old World to the Americas, where it is already causing hundreds of millions of dollars in damage every year. The species is notoriously quick to generate and disseminate pesticide resistance throughout its range and has a wider host range than the native Helicoverpa zea. Hybridization between the two species increases the opportunity for novel, agriculturally problematic ecotypes to emerge and spread through the Americas.
Keywords: hybridization, gene flow, population genomics, pest, selective sweep
Abstract
Within the mega-pest lineage of heliothine moths are a number of polyphagous, highly mobile species for which the exchange of adaptive traits through hybridization would affect their properties as pests. The recent invasion of South America by one of the most significant agricultural pests, Helicoverpa armigera, raises concerns for the formation of novel combinations of adaptive genes following hybridization with the closely related Helicoverpa zea. To investigate the propensity for hybridization within the genus Helicoverpa, we carried out whole-genome resequencing of samples from six species, focusing in particular upon H. armigera population structure and its relationship with H. zea. We show that both H. armigera subspecies have greater genetic diversity and effective population sizes than do the other species. We find no signals for gene flow among the six species, other than between H. armigera and H. zea, with nine Brazilian individuals proving to be hybrids of those two species. Eight had largely H. armigera genomes with some introgressed DNA from H. zea scattered throughout. The ninth resembled an F1 hybrid but with stretches of homozygosity for each parental species that reflect previous hybridization. Regions homozygous for H. armigera-derived DNA in this individual included one containing a gustatory receptor and esterase genes previously associated with host range, while another encoded a cytochrome P450 that confers insecticide resistance. Our data point toward the emergence of novel hybrid ecotypes and highlight the importance of monitoring H. armigera genotypes as they spread through the Americas.
Longstanding assumptions regarding the integrity of species are increasingly coming under challenge as we begin to comprehend the extent of gene flow between species (1, 2). Several cases have now been reported where large portions of the genome have been exchanged between closely related species (1, 3) and, on occasion, with more distantly related taxa (4). Lepidoptera have been useful in exploring the dynamics of hybridization (5), with invasive species in particular offering a rare opportunity to follow the formation of novel ecotypes following secondary contact. Some lepidopteran species are major agricultural pests among which lateral gene transfer could have significant implications for adaptive traits such as pesticide resistance and host range, and therefore, for agricultural production. The recent spread of the cotton bollworm, Helicoverpa armigera, into South America presents such a threat (6, 7).
H. armigera is a significant pest of diverse agricultural and horticultural crops throughout temperate and tropical regions of Asia, Europe, and Africa (subspecies H. armigera armigera), as well as Australasia (subspecies H. armigera conferta). Chemical pesticides and genetically modified crops expressing insecticidal proteins have been widely used to control outbreaks, but the moth has shown a remarkable propensity to develop resistance to many pesticide classes (8); it is claimed to be responsible for more reports of resistance than any other agricultural pest (9). Adult H. armigera are also adept at long-range, facultative migration (10–12), and so far the species shows little evidence for population structure below the subspecies level (13), enabling the rapid spread of resistance. In 2013, it was discovered that H. armigera had become established in Brazil (6, 7, 14), and while the origin of the incursion is still debated, previous analyses have implicated several source populations that were likely facilitated by international trade (13, 15). Since then, H. armigera is estimated to have caused annual crop losses averaging $1 billion in Brazil alone (16, 17), with losses set to increase as the species spreads through South, Central, and potentially North America (18).
It is estimated that H. armigera previously entered the Americas ∼1.5 Mya, leading to the formation of the closely related sister species Helicoverpa zea (19, 20). While the two species remain similar in many respects, H. zea has a narrower host range and lower propensity to develop pesticide resistance. Recent work by the Helicoverpa genome consortium (20) has suggested that these differences result, in part, from H. zea possessing fewer gustatory receptor and detoxification genes. Thus, one of the major concerns following the recent invasion of H. armigera is that it will hybridize with H. zea, introducing genes associated with pesticide resistance and host range expansion. Evidence in the field has thus far fallen short of unequivocal identification of hybridization (13, 21), although high levels of synteny between the two species’ genomes suggest that genomic compatibility is likely (20). Hybridization in the laboratory has been demonstrated previously (22, 23); Laster and Sheng (24) specifically demonstrated that there were no instances of sterility in reciprocal crosses inbred for two generations or in lines backcrossed for four.
Other Helicoverpa species in the heliothine mega-pest lineage that have been classified as pests include the early diverging Helicoverpa punctigera in Australia (12), Helicoverpa assulta in much of the Old World (12), and Helicoverpa gelotopoeon in South America (25). These three species vary considerably in their host range and resistance to pesticides (26), while the closely related Helicoverpa hardwicki persists on a relatively specialized diet and is restricted to northern Australia (22). The natural capacity for gene flow among these species and with H. armigera or H. zea is not well understood, but hybridization has also been suggested between H. armigera and H. assulta under laboratory conditions (23).
Here we use whole-genome resequencing to screen for hybridization and introgression among the six Helicoverpa species named above. We find little population structure within H. armigera or H. zea, other than a separation between the two subspecies of H. armigera, and no evidence of hybridization involving the other four species. Importantly, we find unequivocal evidence of hybridization between H. armigera and H. zea in South America. This involves all 31 chromosomes, although the chromosomal regions involved vary widely among individuals. Most cases involve introgression of large genomic regions from H. zea into a substantially H. armigera genetic background. However, one individual that more closely resembles H. zea is nevertheless homozygous for significant genomic regions from H. armigera, including ones previously associated with host use and insecticide resistance (20). The data highlight the potential for novel Helicoverpa ecotypes to form and spread in South America, presenting new challenges to agriculture in the region.
Results and Discussion
Relationships Among Taxa and Evidence for Hybrids in Brazil.
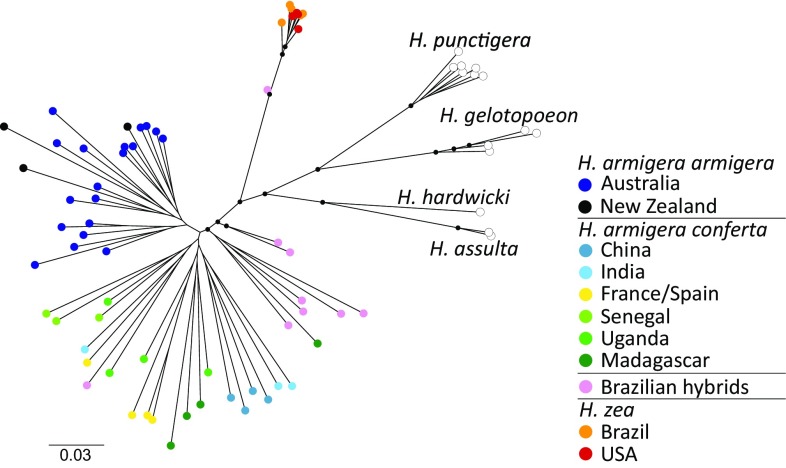
A phylogeny constructed from fourfold degenerate (4D) autosomal sites in 76 individuals from across the six Helicoverpa species named above recapitulates the relationships previously demonstrated by Cho et al. (Fig. 1) (26). Additionally, this phylogeny and a more targeted analysis of only H. armigera and H. zea (Fig. S1) show that nine individuals from Brazil are scattered between the major H. armigera and H. zea lineages, with eight closer to the former and the ninth closer to H. zea. The first eight of these individuals had previously been classified as H. armigera and the ninth as H. zea on the basis of mitochondrial sequence data (15). Although the two subspecies of H. armigera are generally discrete, any further distinction among the diverse geographic samples of H. armigera is poorly supported and likely reflects a complex history of migration and gene flow within subspecies. The data suggest that these nine Brazilian individuals may be hybrids, albeit with varying degrees of genetic admixture, and that H. armigera armigera was the source of the Brazilian incursion (13, 15). This interpretation is also borne out in the corresponding phylogenies for Z chromosome sites (Fig. S2).
Fig. 1.
Maximum likelihood phylogenies for all six Helicoverpa species, calculated using 4D SNPs across autosomes. Interior nodes marked with a black circle represent bootstrap support greater than 50%.
Application of the F3-test to estimate signals of contemporary gene flow among the six species found evidence for genetic exchange only in a single comparison (Dataset S1). This instance, where the nine Brazilian individuals in question were the target population and H. armigera armigera and H. zea from the United States (which H. armigera had not penetrated at the time) were sources, yielded a negative f3 (−0.009, Z = −7.775), indicative of gene flow. Henceforth, we refer to these individuals as the “Brazilian hybrids.”
We then examined the relationships between H. zea and the H. armigera subspecies using the variance in allele frequencies among subpopulations relative to the global variance (FST) (Fig. S3 and Table S1) as a prelude to principal component analysis (PCA) (Fig. S4) and admixture analysis (Fig. S5 and ref. 27) to further investigate the affinities of the Brazilian hybrids. The FST analysis, which excluded the Brazilian hybrids, showed significantly lower FST values between H. armigera armigera and H. armigera conferta than between either subspecies and H. zea (P < 0.001). Interestingly, the values in all three of those comparisons are slightly, but significantly, higher (P < 0.001) for the Z chromosome than for autosomes, indicating increased evolutionary rates in the former (28). The PCA separated H. zea from H. armigera on principal component 1 (PC1) and clearly distinguished clusters representing both H. armigera subspecies on principal component 2 (PC2). Consistent with the phylogenies above, most of the Brazilian hybrids fall between H. armigera armigera and H. zea but lie closer to the former, while a single individual has closer affinity with H. zea. As is consistent with both those analyses, admixture analyses show strong differentiation between the two species, which is supported by the lowest cross-validation (CV) error of any assessed, when the number of populations (K) is two. When K = 3, it is clear that the Brazilian hybrids are derived from H. armigera armigera and H. zea, while Australian H. armigera conferta forms a discrete cluster.
We then employed an outgroup F3-test to examine which of the sequences within H. armigera armigera yielded the highest scores, indicative of the most shared genetic history with Brazilian hybrids. We found that the Brazilian hybrids most resemble the four European individuals, with several African samples presenting broadly comparable but somewhat smaller values for f3 and followed by Asian individuals that yielded lower values still (Fig. 2).
Fig. 2.
Outgroup f3 statistic for the model f3 (H. armigera conferta; Brazilian hybrids, x), demonstrating shared genetic drift between Brazilian hybrids and individual H. armigera armigera (x). Error bars represent SEs.
Genetic Diversity and Effective Population Size.
Several recent studies have found unusually high levels of genetic variation both within native populations of H. armigera and within the H. armigera that have been introduced into South America (13, 15, 29). Our own calculations of average genome-wide nucleotide diversity (Table S2) concur that H. armigera armigera and H. armigera conferta are unusually polymorphic in autosomal regions (0.008 and 0.010, respectively). Their values are in excess of those of the other species (0.004–0.005) even when randomly subsampled for equivalency (n = 2) (Table S2). As is consistent with the findings of Tay et al. (15), who documented several mitochondrial haplotypes from H. armigera armigera among Brazilian individuals, our autosomal value for the Brazilian hybrids is also high (0.009). This indicates that the incursion of H. armigera into Brazil had the potential to introduce considerable genetic variation via the generation of highly polymorphic hybrids.
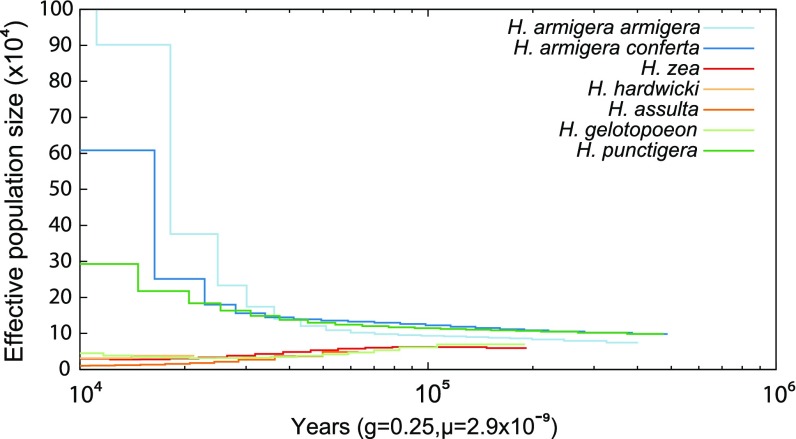
We then inferred the historical population dynamics for each of our taxa by applying PSMC software (30) to consensus genotype calls representing all samples for each of the H. armigera subspecies (excluding the Brazilian hybrids) and the five other species. We calculated that effective population size (Ne) was around 7,000–15,000 for both H. armigera subspecies and all four of the other species 100 kya (Fig. 3). Ne then appears to have grown substantially for both H. armigera subspecies and H. punctigera, with H. armigera armigera peaking at ∼2,800,000 (Fig. S6). By contrast, Ne for all other species, including H. zea, declined over the same period, with all estimates for more recent populations having fallen below 5,000. It is possible that these latter estimates are biased downward because of their smaller sample sizes (31); therefore we subsampled the two H. armigera subspecies. While this did indeed reduce the Ne estimates for the most recent populations of the subspecies [∼74,000 and 43,000 for H. armigera armigera (n = 6) and H. armigera conferta (n = 5), respectively], they remained considerably higher than those of the other species.
Fig. 3.
Averages of 100 PSMC bootstrap estimates of effective population size for all species. The hybrids were not included in this analysis.
We conclude that H. armigera has had a large population capable of harboring considerable genetic diversity for many generations and that the size of the hybrid population subsequently formed has been sufficient to retain much of that variation.
Evidence of Selection Within H. armigera.
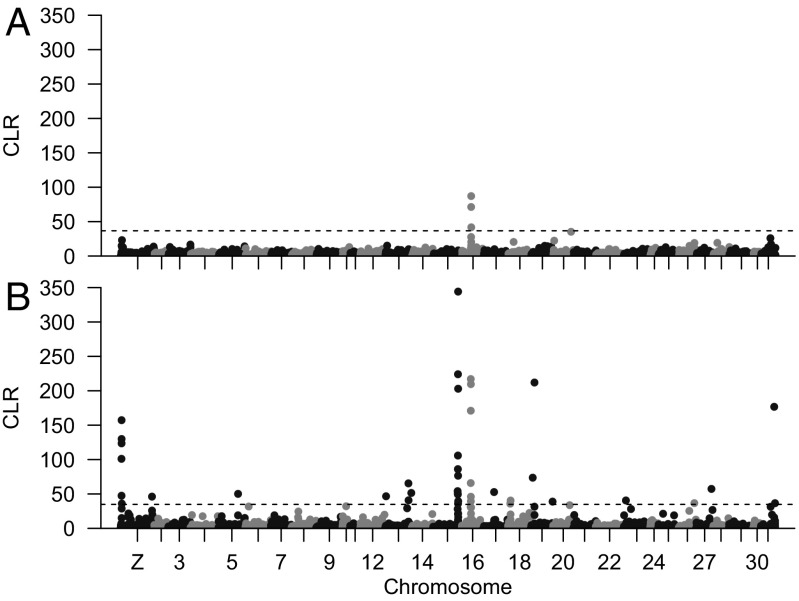
Given the species’ spectacular success in exploiting diverse agricultural systems, genes subject to recent selective sweeps in H. armigera would also be good candidates for variants of adaptive value in hybrids (32). We therefore used SweeD (33) to calculate the composite likelihood ratio (CLR) indicative of regions undergoing selective sweeps for all H. armigera samples (excluding the hybrids) (Fig. 4 and Table S3). Only a single sweep was observed in H. armigera armigera that was perhaps due to the geographically diverse nature of our samples for that subspecies, meaning only selection common to all the various geographies represented would likely be detected. Importantly, the region found to be swept, a 90-kb stretch containing seven genes half-way along chromosome 16, was also identified as having experienced a selective sweep in H. armigera conferta, suggesting it was of very general adaptive value for the species. Other regions found to be subject to selective sweeps in H. armigera conferta (Table S3) (which has a significantly smaller geographic range) include one containing a cytochrome P450 gene on chromosome 15 (CYP337B3) that is associated with resistance to the insecticide fenvalerate (9). This is not the first evidence for selection at the CYP337B3 locus in H. armigera, with previous analyses demonstrating independently derived alleles that have risen to high frequencies in Asian and African populations of H. armigera armigera following historical exposure to fenvalerate (13). European H. armigera armigera contain both the African and Asian CYP337B3 variants, and, notably in the current context, the Brazilian hybrids all possess the Asian variant of that gene (Table S4).
Fig. 4.
CLR plots screening for selective sweeps in (A) H. armigera armigera and (B) H. armigera conferta, where the significance thresholds are 36.6 and 34.9, respectively, as shown by dashed lines.
The Genetic Composition of the Hybrids.
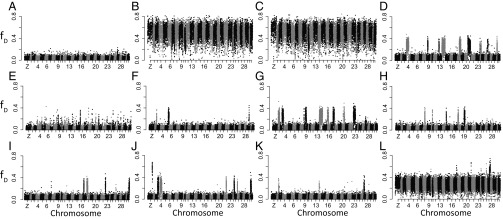
We used a version of the D statistic, fD (34), to identify genomic regions that appear most H. zea-like in the Brazilian hybrids. Values of fD calculated for 10-kb windows were plotted for each hybrid and unadmixed representatives of H. armigera armigera and H. zea. The data derived from each individual are plotted in Fig. 5, and data for some additional unadmixed individuals are shown in Fig. S7). Unadmixed individuals have fD values around zero for H. armigera armigera (e.g., individual A in Fig. 5) or tending toward 0.8 for H. zea (e.g., individuals B and C, from Brazil and the United States, respectively). By contrast, the hybrids (individuals D–L) present highly variable patterns.
Fig. 5.
Profiles of fD values calculated for 10-kb nonoverlapping windows across the genome for individuals A–L. Individual A is a European H. armigera. Individuals B and C are H. zea from the United States and Brazil, respectively. Individuals D–L are Brazilian hybrids, and their profiles provide evidence for hybridization between H. armigera and H. zea. Individual-specific metadata, including mitochondrial genotypes, are listed in Table S4.
Eight of the nine hybrids (D–K) appear to be the products of initial hybridization between H. armigera and H. zea followed by varying degrees of backcrossing into predominantly H. armigera backgrounds. This results in fD profiles across the genome that generally resemble that of H. armigera but are interspersed with more H. zea-like spikes. Although we find evidence of hybridization on all chromosomes, those bearing fD spikes vary widely among the different hybrids, suggesting different histories of recombination and introgression following the initial hybridization event(s). All individuals except E have several relatively broad regions derived from H. zea (between three and nine, each peaking around fD values of ∼0.4), with some extending across hundreds of kilobases and even entire chromosomes (e.g., chromosome 14, 9.9 Mb in individual D of Fig. 5). The fD profile across the genome of individual E differs, in which we observe more numerous but generally smaller spikes that are likely representative of a longer hybrid ancestry, providing more opportunity for recombination to break up blocks of H. zea DNA.
As each of these eight individuals appears to have undergone hybridization followed by several generations of backcrossing with H. armigera, we sought to determine the proportion of each of the eight genomes that was ancestrally derived from H. zea. We used 127,880 SNPs that strongly segregated between the two parent taxa to calculate the proportion of alleles derived from H. zea, which we found ranged from 2.0% in individual K to 8.9% in individual D. Multistratum permutation analysis of average chromosomal fD demonstrated no significant difference (P > 0.05) among chromosomes in the proportions of H. zea-derived DNA; although the small sample size limits the sensitivity of this analysis, our results suggest there are no major differences among the chromosomes in the persistence of introgressed material since hybridization.
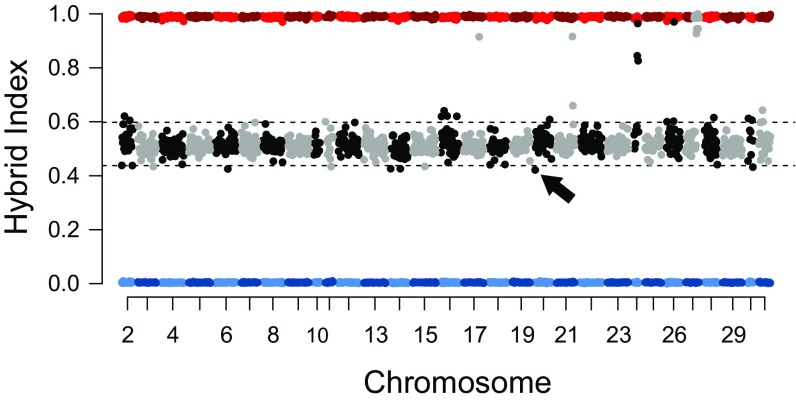
The genome of the ninth hybrid, individual L (Fig. 5), has significantly more H. zea DNA than would be expected in an F1 hybrid of H. armigera and H. zea, with 51.4% (P < 0.001) of its genome derived from the latter. Values of fD generally fell approximately midway between the reference values for the two parent species; however, a few regions approached values seen in reference H. zea. A sliding-window plot of a hybrid index (HI) (35) confirmed that most of the genome comprised alleles from both parent taxa, with stretches clearly homozygous for H. zea DNA as well as some small but significant signals of H. armigera homozygosity (Fig. 6). A parsimonious interpretation of the pedigree of this individual would involve parents which were both hybrids, one with a majority of H. zea DNA and the other with a smaller majority of H. armigera DNA.
Fig. 6.
HI across 250-kb windows for individual L, highlighting homozygosity indicating previous hybridization. SNPs segregating strongly between H. armigera armigera and H. zea were used to infer ancestry of individual L (black and gray), while calculations using all H. armigera armigera and H. zea (red and blue, respectively) are plotted for reference. Windows are considered significantly different from 0.5 where HI is above 0.598 or below 0.4375 (P < 5 × 10−7). The arrow highlights the lowest value of HI for individual L.
The regions of homozygosity in individual L (listed in Table S5) include tracts of H. zea DNA with significantly high HI values totaling about 7.75 Mb over several chromosomes, with the largest stretch occurring over 2.75 Mb of chromosome 27. A few other windows demonstrate significantly low HI, with the lowest occurring at the beginning of chromosome 20 (HI = 0.42), highlighting a short (∼11 kb) stretch of homozygosity derived from H. armigera. This region is notable for containing genes encoding a clade 16 detoxifying esterase and two gustatory receptors that have been implicated in host use (20). HI was also low (0.45) where we observed the selective sweep on chromosome 16 in H. armigera armigera, although this was not considered statistically significant. The HI analysis also failed to identify significant levels of H. armigera homozygosity in the region encompassing the CYP337B3 fenvalerate-resistance gene (HI = 0.48) which had been subject to a selective sweep in H. armigera conferta, but targeted analysis using Sanger sequencing of PCR products demonstrated that this individual was homozygous for this gene (Table S4).
As noted, eight of the nine hybrids were previously classified as H. armigera and the ninth as H. zea by Tay et al. (15), who sequenced mitochondrial DNA. Five other individuals also collected from Brazil in the same year, which Tay et al. (15) classified as H. zea from their mitochondrial sequences, all proved to have pure H. zea nuclear genomes in our analysis. By contrast, no Brazilian individuals that Tay et al. (15) had classified as H. armigera were found to have pure H. armigera nuclear genomes in our analysis. Overall, these data indicate that females of both parent species have given rise to fertile hybrid lineages, but they leave open the question of whether the mitochondria from the two parent species differ in their effects on the fitness of the hybrids.
Conclusions
The repeated introduction of one of the most significant agricultural pests in the Old World into South America is clearly cause for concern. H. armigera appears to be unlike the other Helicoverpa species in that it possesses relatively high genetic diversity, likely contributing to its very high adaptive and invasive capacities. A population with European origins is clearly part of an ongoing, multigenerational hybridization with H. zea in a region of the New World that contributes a significant proportion of the world’s crops. It is too early to reliably detect signals of selection in the resulting hybrids, but clear evidence for the transfer of a wide range of H. zea genes into H. armigera backgrounds and H. armigera genes related to host use and pesticide resistance into an H. zea background is already apparent. Ongoing evolution in hybrid populations could generate new ecotypes in which features of the H. zea genome that may be important to adaptation in the New World are retained alongside genes from H. armigera that are capable of augmenting its properties as a pest.
Future analyses of South American samples could provide insight into the progression of hybridization and potential for displacement of H. zea as a discrete species. Given the variation in introgressed regions observed, monitoring populations using genes that are likely under selection, such as CYP337B3, offer the highest throughput and most relevant way to follow the spread of H. armigera genotypes across the Americas. Given increased trade-associated movements of Helicoverpa around the world, we should also be prepared to see genetic variation originating from H. zea appearing in Old World H. armigera.
Methods
Sample Collection and DNA Extraction.
Heliothine moths were collected between 2004 and 2014 from 16 countries and included 52 that had previously been classified as H. armigera, 10 previously classified as H. zea, seven H. punctigera, four H. gelotopoeon, two H. assulta, and one H. hardwicki. Those classified as H. armigera included 22 from Australia and New Zealand [conventionally designated as H. armigera conferta (22)], 22 from seven countries in Asia, Europe, and Africa [designated as H. armigera armigera (22)], plus eight collected from Brazil in 2013, which had been identified previously using mitochondrial markers (15) but which we show herein are actually hybrids with H. zea. The 10 H. zea moths included six collected from Brazil in 2013 identified as such from mitochondrial markers (15), one of which we show here is a hybrid with H. armigera armigera, plus four from the United States in 2004 that are considered to be geographically and temporally isolated from the current H. armigera invasion (15). There was no heteroplasmy in mitochondrial DNA among Brazilian hybrids, thereby eliminating the possibility that contamination contributed to any potential signals of hybridization (15). Sample collection data are listed in Table S4; all samples were preserved in ethanol/RNAlater or stored at −20 °C following collection. DNA was extracted using DNeasy blood and tissue kits (Qiagen) before quantification with a Qubit 2.0 fluorometer (Thermo Fisher Scientific).
Genome Resequencing and SNP Genotyping.
Nextera (Illumina) libraries were produced following the manufacturer’s instructions, and 100-bp paired-end reads were generated (Illumina HiSeq. 2000, Biological Resources Facility, Australian National University, Australia). Sample and sequencing data are included in Table S4. Raw sequence reads were aligned to the H. armigera genome using BBMap v. 33.43 (sourceforge.net/projects/bbmap/). Reads were trimmed when quality in at least two bases fell below Q10, and only uniquely aligning reads were included in the analysis. Resulting BAM files were sorted before duplicate reads were removed, and files were annotated using Picard v. 1.138 (broadinstitute.github.io/picard) before indexing with SAMtools v. 1.1.0 (36). UnifiedGenotyper in gatk v. 3.3-0 (37) was used to estimate genotypes across all individuals simultaneously, implementing a heterozygosity value of 0.01. VCFtools (38) was used to calculate mean coverage statistics for each sample.
Phylogenetic Analyses.
A reduced SNP file consisting of 4D sites, which are essentially neutral (39), was used to derive phylogenetic and population structure end points. We annotated SNPs using SnpEff v. 4.0 (40) with default parameters to create a database before transferring annotations from the genome (20). Bases were then selected as in Martin et al. (41), where the first and second codon positions were invariable, providing us with 1,015,455 SNPs.
RAxML v. 8.2.10 (42) was used to plot phylogenetic trees for both the Z-chromosome and autosomes of all individuals, implementing a generalized time-reversible model of sequence evolution. The best topology was selected from 20 maximum likelihood searches, and its statistical support was assessed with 100 bootstrap pseudoreplicates. When only H. armigera and H. zea were used to construct a phylogeny, RAxML (42) was invoked using the -N autoMRE -f a -x options for autosomes and the Z chromosome, resulting in 600 and 804 rapid bootstrap searches, respectively, before a thorough maximum likelihood search.
Population Structure.
We used markers from all chromosomes simultaneously for analysis of population structure in H. armigera and H. zea. EIGENSOFT v. 6.0.1 (43) was used to perform PCA. A Tracy–Widom distribution was used to infer statistical significance for principal components, with a threshold of 1 × 10−12. The admixture software (27) was implemented using default parameters over K-values ranging from 1 to 10, with cross-validation enabled. Results were plotted using Distruct2.pl (www.crypticlineage.net/pages/distruct.html).
A more complete dataset was used for subsequent analyses, whereby phasing and imputation of missing bases was performed on all SNPs from all samples using default parameters in Beagle (44). Linkage disequilibrium (LD)-based pruning conducted using PLINK v. 1.07 (45) was used to filter one of a pair of SNPs using a pairwise LD threshold (r2 = 0.5) within windows of 50 SNPs, moving forward five SNPs per iteration, resulting in 22,790,059 SNPs.
Selective sweeps in each H. armigera subspecies were detected by calculating CLRs from allele frequency data using SweeD (33). SweeD was run for each chromosome with a grid size of 1,000 blocks, and analyses were continued only where CLRs over 30 were observed. A significance threshold was calculated for both subspecies of H. armigera (13) using the inferred effective population size histories as detailed by the pairwise sequentially Markovian coalescent (PSMC) analyses as the basis of MSMS (v. 1.3) simulations (46), opting for 100 simulated datasets over 1 Mb. In addition, nonnegative, weighted FST was calculated across 10-kb windows (≥50 SNPs) in autosomes and the Z chromosome for H. armigera and H. zea using VCFtools v. 0.1.14 (38). H. armigera subspecies were randomly downsampled to n = 9. Average nucleotide diversity was also calculated for each species using VCFtools v. 0.1.14 (38), using both the full data and following random downsampling, where n = 2. The extent of variation between species was examined with the t test function in R v. 3.4.3 (47).
Demographic Inference.
We used the PSMC software (30) to gain insights into historical differences in effective population size. The diploid consensus required by PSMC was generated from the mpileup command (-Q 20 -q 25) of SAMtools (36), followed by the bcftools call command for all samples from each species as well as subspecies of H. armigera (excluding the Brazilian hybrids). Vcfutils.pl (d 5 -D 34 -Q 25) was employed to prepare the data for PSMC. We used fq2psmcfa from the PSMC package on whole-genome consensus diploid sequences to create the input file before running PSMC (-N25 -t15 -r5) with the partition 6 + 2 × 4 + 3 + 13 × 2 + 3 + 2 × 4 + 6. Raw PSMC outputs were scaled to time and population sizes assuming a generation time of 0.25 y and a mutation rate of 2.9e−09 (48), plotting the average over 100 bootstraps.
Tests for Admixture.
To evaluate the possibility of admixture across the Helicoverpa phylogeny, we calculated the f3 statistic for all possible three-species combinations using AdmixTools v. 3.0 (49). Only trios where f3 is negative are indicative of admixture between two source populations against a resulting target population. To populate a tree-like scenario for the estimation of gene flow between sister taxa, populations with more than two individuals were split randomly into two groups, and f3 was calculated for all sister taxa and also for species that shared geographic distributions. All trios used in the analysis are listed in Dataset S1.
As our analyses suggest that the Brazilian hybrids are ancestrally derived from H. armigera armigera, an outgroup F3-test was conducted to determine which individual H. armigera armigera most resembled the hybrids. Under a specific tree-like scenario (outgroup; A, B), the expected value of f3 will represent the genetic drift shared between populations A and B following divergence from the outgroup (50). In this instance, we used H. armigera conferta from Australia as the outgroup in tests for a shared history between the hybrids (A) and various H. armigera armigera individuals (B). The H. armigera armigera individuals generating the highest positive values of f3 will therefore be those most related to the hybrids.
To identify introgressed genomic regions resulting from hybridization between H. armigera armigera and H. zea in the hybrid individuals, we used a version of Patterson’s D. The D-statistic is a measure of admixture between populations that is robust to biases associated with SNP ascertainment and demographic history (49). Recent work by Martin et al. (34) improved the utility of the statistic for calculation across small, kilobase-level regions, terming this variant statistic “fD.” Our calculation of fD implements a model of [H. punctigera, North American H. zea; x, H. armigera armigera] where x are hybrid individuals or H. armigera conferta and H. zea not otherwise included in the analysis. The model assumes there is no gene flow between the outgroup and H. armigera armigera, but gene flow is permitted between x and either H. zea or H. armigera armigera, which results in positive or negative fD, respectively.
We used fD to measure gene flow between individual moths and H. zea from United States. This statistic was calculated for 10-kb nonoverlapping windows using functions of the genomics.py script as used by Martin et al. (34) and plotted using a modified version of qqman (51). Only windows where the average ABBA and BABA counts were equal to or greater than two were considered. Significant differences between chromosomal averages for fD were investigated using multistratum permutation testing in the R package lmPerm (52), invoking default settings.
We determined the proportion of the genome in each hybrid individual that was ancestrally derived from H. zea using SNPs that strongly segregated between that species and H. armigera armigera. These were determined via an association test in PLINK v1.90b (45), taking only SNPs where P < 3.83 × 10−14, thereby limiting the maximum number of alleles shared between the two populations to the equivalent of one full homozygote or two heterozygotes. This resulted in a SNP set of 127,880 SNPs. We calculated the proportion of alleles from H. zea for each hybrid using a custom python script, considering the sum of H. zea alleles divided by all alleles in the analysis. For individual L, we then tested whether the estimated proportion of its genome derived from H. zea was significantly higher than that expected in an F1 hybrid using the variance of a binomial distribution, where n = 127,880.
We used a version of the HI (35) calculated using a custom python script to test for regions of homozygosity indicative of additional historical admixture events across the genome of individual L. HI calculations incorporated the panel of 127,880 segregating SNPs above, with average values for each SNP being inferred for H. armigera armigera and H. zea, which therefore achieved HI values of ∼0 and 1, respectively. For windows consisting entirely of heterozygous SNPs, synonymous with an F1 hybrid, HI = 0.5. HI was calculated across 250-kb nonoverlapping sliding windows, considering only those windows where there were more than 20 SNPs. Sliding-window averages were plotted using a modified version of qqman (51). To infer regions that varied significantly beyond neutral expectations, simulations were conducted using 112 SNPs (the average number contributing to a 250-kb window) randomly sampled from individual L. After 100,000 iterations, scores ranged from 0.598 to 0.438, and significance was calculated by dividing alpha (0.05) by the number of simulations with scores above or below these thresholds, respectively.
Hybrid individuals and H. armigera controls were screened for the presence of the CYP337B3 gene as in Joussen et al. (9). Individual L was screened in triplicate, and heterozygote/homozygote status was determined through relevant band detection on 1.5–2% agarose gel followed by confirmation with Sanger sequencing.
Supplementary Material
Acknowledgments
We thank Yidong Wu, Ibrahim Atokple, Cecilia Czepak, Gajanan Behere, Miguel Soria, and Lisa Bird for providing samples and three anonymous reviewers, whose inputs greatly enhanced our paper. C.J.A. was supported by Commonwealth Scientific and Industrial Research Organisation Office of the Chief Executive Postdoctoral Fellowship R-03255-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Raw sequencing and genotype data are available at https://data.csiro.au/dap/landingpage?pid=csiro:29053, as are the names of 127,880 segregating SNPs, the reference genome, and annotations implemented. Custom analytical scripts are available at https://github.com/CraigJAnderson/heliothine_reseq_paper.
See Commentary on page 4819.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1718831115/-/DCSupplemental.
References
- 1.Martin SH, et al. Genome-wide evidence for speciation with gene flow in Heliconius butterflies. Genome Res. 2013;23:1817–1828. doi: 10.1101/gr.159426.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sankararaman S, et al. The genomic landscape of Neanderthal ancestry in present-day humans. Nature. 2014;507:354–357. doi: 10.1038/nature12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin SH, Jiggins CD. Interpreting the genomic landscape of introgression. Curr Opin Genet Dev. 2017;47:69–74. doi: 10.1016/j.gde.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Dasmahapatra KK, Mallet J, Moreira GRP, Kronforst MR. Genome-wide introgression among distantly related Heliconius butterfly species. Genome Biol. 2016;17:25. doi: 10.1186/s13059-016-0889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heliconius Genome Consortium Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature. 2012;487:94–98. doi: 10.1038/nature11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czepak C, Albernaz KC, Vivan LM, Guimarães HO, Carvalhais T. First reported occurrence of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) in Brazil. Pesqui Agropecu Trop. 2013;43:110–113. [Google Scholar]
- 7.Tay WT, et al. A brave new world for an old world pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS One. 2013;8:e80134. doi: 10.1371/journal.pone.0080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downes S, et al. A perspective on management of Helicoverpa armigera: Transgenic Bt cotton, IPM, and landscapes. Pest Manag Sci. 2017;73:485–492. doi: 10.1002/ps.4461. [DOI] [PubMed] [Google Scholar]
- 9.Joußen N, et al. Resistance of Australian Helicoverpa armigera to fenvalerate is due to the chimeric P450 enzyme CYP337B3. Proc Natl Acad Sci USA. 2012;109:15206–15211. doi: 10.1073/pnas.1202047109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng H, Wu X, Wu B, Wu K. Seasonal migration of Helicoverpa armigera (Lepidoptera: Noctuidae) over the Bohai Sea. J Econ Entomol. 2009;102:95–104. doi: 10.1603/029.102.0114. [DOI] [PubMed] [Google Scholar]
- 11.Jones CM, et al. Genomewide transcriptional signatures of migratory flight activity in a globally invasive insect pest. Mol Ecol. 2015;24:4901–4911. doi: 10.1111/mec.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitt GP. The ecology of Heliothis species in relation to agroecosystems. Annu Rev Entomol. 1989;34:17–52. [Google Scholar]
- 13.Anderson CJ, Tay WT, McGaughran A, Gordon K, Walsh TK. Population structure and gene flow in the global pest, Helicoverpa armigera. Mol Ecol. 2016;25:5296–5311. doi: 10.1111/mec.13841. [DOI] [PubMed] [Google Scholar]
- 14.Sosa-Gómez DR, et al. Timeline and geographical distribution of Helicoverpa armigera (Hübner) (Lepidoptera, Noctuidae: Heliothinae) in Brazil. Rev Bras Entomol. 2016;60:101–104. [Google Scholar]
- 15.Tay WT, et al. Mitochondrial DNA and trade data support multiple origins of Helicoverpa armigera (Lepidoptera, Noctuidae) in Brazil. Sci Rep. 2017;7:45302. doi: 10.1038/srep45302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mastrangelo T, et al. Detection and genetic diversity of a heliothine invader (Lepidoptera: Noctuidae) from north and northeast of Brazil. J Econ Entomol. 2014;107:970–980. doi: 10.1603/ec13403. [DOI] [PubMed] [Google Scholar]
- 17.Lopes-da-Silva M, Sanches MM, Stancioli AR, Alves G, Sugayama R. The role of natural and human-mediated pathways for invasive agricultural pests: A historical analysis of cases from Brazil. Agric Sci. 2014;5:634–646. [Google Scholar]
- 18.Kriticos DJ, et al. The potential distribution of invading Helicoverpa armigera in North America: Is it just a matter of time? PLoS One. 2015;10:e0119618. doi: 10.1371/journal.pone.0119618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallet J, Korman A, Heckel DG, King P. Biochemical genetics of Heliothis and Helicoverpa (Lepidoptera: Noctuidae) and evidence for a founder event in Helicoverpa zea. Ann Entomol Soc Am. 1993;86:189–197. [Google Scholar]
- 20.Pearce S, et al. Genomic innovations, transcriptional plasticity and gene loss underlying the evolution and divergence of two highly polyphagous and invasive Helicoverpa pest species. BMC Biol. 2017;15:63, and erratum (2017) 15:69. doi: 10.1186/s12915-017-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leite NA, et al. Pan-American similarities in genetic structures of helicoverpa armigera and helicoverpa zea (lepidoptera: Noctuidae) with implications for hybridization. Environ Entomol. 2017;46:1024–1034. doi: 10.1093/ee/nvx088. [DOI] [PubMed] [Google Scholar]
- 22.Matthews M. Heliothine Moths of Australia. A Guide to Pest Bollworms and Related Noctuid Groups. CSIRO Publishing; Collingwood, Australia: 1999. [Google Scholar]
- 23.Hardwick DF. The corn earworm complex. Mem Entomol Soc Can. 1965;97:5–247. [Google Scholar]
- 24.Laster M, Sheng C. Search for hybrid sterility for Helicoverpa zea in crosses between the North-American Heliothis zea and Helicoverpa armigera (Lepidoptera, Noctuidae) from China. J Econ Entomol. 1995;88:1288–1291. [Google Scholar]
- 25.Murúa MG, et al. Species from the Heliothinae complex (Lepidoptera: Noctuidae) in Tucumán, Argentina, an update of geographical distribution of Helicoverpa armigera. J Insect Sci. 2016;16:61. doi: 10.1093/jisesa/iew052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho S, et al. Molecular phylogenetics of heliothine moths (Lepidoptera: Noctuidae: Heliothinae), with comments on the evolution of host range and pest status. Syst Entomol. 2008;33:581–594. [Google Scholar]
- 27.Alexander DH, Lange K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinformatics. 2011;12:246. doi: 10.1186/1471-2105-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kronforst MR, et al. Hybridization reveals the evolving genomic architecture of speciation. Cell Rep. 2013;5:666–677. doi: 10.1016/j.celrep.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leite NA, Alves-Pereira A, Corrêa AS, Zucchi MI, Omoto C. Demographics and genetic variability of the new world bollworm (Helicoverpa zea) and the old world bollworm (Helicoverpa armigera) in Brazil. PLoS One. 2014;9:e113286. doi: 10.1371/journal.pone.0113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Durbin R. Inference of human population history from individual whole-genome sequences. Nature. 2011;475:493–496. doi: 10.1038/nature10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gattepaille L, Günther T, Jakobsson M. Inferring past effective population size from distributions of coalescent times. Genetics. 2016;204:1191–1206. doi: 10.1534/genetics.115.185058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norris LC, et al. Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proc Natl Acad Sci USA. 2015;112:815–820. doi: 10.1073/pnas.1418892112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavlidis P, Živkovic D, Stamatakis A, Alachiotis N. SweeD: Likelihood-based detection of selective sweeps in thousands of genomes. Mol Biol Evol. 2013;30:2224–2234. doi: 10.1093/molbev/mst112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin SH, Davey JW, Jiggins CD. Evaluating the use of ABBA-BABA statistics to locate introgressed loci. Mol Biol Evol. 2015;32:244–257. doi: 10.1093/molbev/msu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goulet BE, Roda F, Hopkins R. Hybridization in plants: Old ideas, new techniques. Plant Physiol. 2017;173:65–78. doi: 10.1104/pp.16.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, et al. 1000 Genome Project Data Processing Subgroup The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenna A, et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danecek P, et al. 1000 Genomes Project Analysis Group The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Künstner A, Nabholz B, Ellegren H. Significant selective constraint at 4-fold degenerate sites in the avian genome and its consequence for detection of positive selection. Genome Biol Evol. 2011;3:1381–1389. doi: 10.1093/gbe/evr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin SH, et al. Natural selection and genetic diversity in the butterfly Heliconius melpomene. Genetics. 2016;203:525–541. doi: 10.1534/genetics.115.183285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:e190. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Browning SR, Browning BL. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet. 2007;81:1084–1097. doi: 10.1086/521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Purcell S, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ewing G, Hermisson J. MSMS: A coalescent simulation program including recombination, demographic structure and selection at a single locus. Bioinformatics. 2010;26:2064–2065. doi: 10.1093/bioinformatics/btq322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R Core Team 2014. R: A Language and Environment for Statistical Computing (R Found Stat Comput, Vienna)
- 48.Keightley PD, et al. Estimation of the spontaneous mutation rate in Heliconius melpomene. Mol Biol Evol. 2015;32:239–243. doi: 10.1093/molbev/msu302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson N, et al. Ancient admixture in human history. Genetics. 2012;192:1065–1093. doi: 10.1534/genetics.112.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sankararaman S, Patterson N, Li H, Pääbo S, Reich D. The date of interbreeding between Neandertals and modern humans. PLoS Genet. 2012;8:e1002947. doi: 10.1371/journal.pgen.1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner S. 2017 qqman: Q-Q and Manhattan Plots for GWAS Data, R Package Version 0.1.4. Available at https://cran.r-project.org/web/packages/qqman/. Accessed March 21, 2018.
- 52.Wheeler B, Torchiano M. 2016 lmPerm: Permutation Tests for Linear Models, R Package Version 2.1.0. Available at https://cran.r-project.org/web/packages/lmPerm/index.html. Accessed March 21, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.