Significance
The dendritic cell (DC) is the master regulator of host immunity. The results of our study bring significant technical improvements in DC methodology. First, a method was developed to expand primary blood DCs at unlimited amounts. Second, the established DCs are constitutively activated and readily available to prime naïve T cells. Third, the DCs can be genetically modified to deliver given tumor antigens in high efficiency and to express activating molecules in driving simultaneous production of antigen-specific T cells and natural killer (NK) cells. Fourth, introducing two allogeneic DRB1 molecules into the DCs improves generation of tumor antigen-specific T cells. Further, the DC-activated cytotoxic T lymphocytes and NK cells potently suppress tumor growth and metastasis in human lung cancer mouse models.
Keywords: human blood dendritic cells, cytotoxic T lymphocytes, NK cells, cancer/testis antigen, cancer immunotherapy
Abstract
Dendritic cell (DC)-based cancer immunotherapy has achieved modest clinical benefits, but several technical hurdles in DC preparation, activation, and cancer/testis antigen (CTA) delivery limit its broad applications. Here, we report the development of immortalized and constitutively activated human primary blood dendritic cell lines (ihv-DCs). The ihv-DCs are a subset of CD11c+/CD205+ DCs that constitutively display costimulatory molecules. The ihv-DCs can be genetically modified to express human telomerase reverse transcriptase (hTERT) or the testis antigen MAGEA3 in generating CTA-specific cytotoxic T lymphocytes (CTLs). In an autologous setting, the HLA-A2+ ihv-DCs that present hTERT antigen prime autologous T cells to generate hTERT-specific CTLs, inducing cytolysis of hTERT-expressing target cells in an HLA-A2–restricted manner. Remarkably, ihv-DCs that carry two allogeneic HLA-DRB1 alleles are able to prime autologous T cells to proliferate robustly in generating HLA-A2–restricted, hTERT-specific CTLs. The ihv-DCs, which are engineered to express MAGEA3 and high levels of 4-1BBL and MICA, induce simultaneous production of both HLA-A2–restricted, MAGEA3-specific CTLs and NK cells from HLA-A2+ donor peripheral blood mononuclear cells. These cytotoxic lymphocytes suppress lung metastasis of A549/A2.1 lung cancer cells in NSG mice. Both CTLs and NK cells are found to infiltrate lung as well as lymphoid tissues, mimicking the in vivo trafficking patterns of cytotoxic lymphocytes. This approach should facilitate the development of cell-based immunotherapy for human lung cancer.
Among professional antigen-presenting cells, dendritic cells (DCs) hold unique abilities to prime naïve T lymphocytes in mediating antigen-specific, adaptive immune response (1). DCs also possess the capacity to induce activation and proliferation of γσ T cells and natural killer (NK) cells, bridging innate immunity to adaptive immune response (2, 3). DCs evolve from the bone marrow and migrate to lymphoid and nonlymphoid tissues via blood circulation. In blood, a vast majority of DCs are immature but capable of engulfing foreign antigens from invading pathogens. Upon uptake of foreign antigens, immature DCs undergo a complex maturation and activation process through activation of pathogen recognition receptors (PRRs), such as Toll-like receptors (TLRs) (4–6). Mature and activated DCs characteristically express cell surface costimulatory molecules such as CD80 and CD86, assemble the antigen peptide–MHC complex, and migrate to secondary lymphoid tissues to promote antigen-specific T cell expansion.
DCs constitute 1% of immune cells in blood. There are two major types of blood DCs known: CD11c+ myeloid DCs (mDCs) and CD303+ plasmacytoid DCs (pDCs) (7). While pDCs are crucial in mediating antiinflammatory and antiviral response by secreting large amounts of IFN-α upon encountering pathogens, mDCs play a major role in antigen presentation to prime naïve T cells (8, 9). The CD1c+ subset of mDCs is relatively abundant, and a minor subset, the CD141+ mDCs, exhibit a potent ability to cross-present extracellular antigens on MHC class I to CD8 T cells, generating cytotoxic T lymphocytes (CTLs) (10, 11). By analyzing the transcriptional profiles of DC subsets, it is proposed that human CD141+ DCs are closely related to the counterparts of murine CD8+ DCs, the major subset of DCs in mediating antigen cross-presentation (10, 11). Since DCs can engulf foreign antigens from invading pathogens, DC-mediated cross-presentation and induction of terminally differentiated cytotoxic effector T cells are essential steps in developing adaptive immunity against pathogenic viruses and cancer cells.
Because of the paucity of blood DCs, the current DC method frequently exploits antigen-loaded monocyte-derived DCs (MoDCs) through a complex maturation and activation process with a mixture of cytokines and PRR stimulating factors (12, 13). This method has been widely utilized in clinical cancer vaccine trials; however, the clinical benefit of such an approach is rather limited. There are several drawbacks with this approach. Compared with primary blood DCs, MoDCs are more closely related to in vitro-derived macrophages (14). Additionally, MoDCs have limited growth potential and are difficult to maintain in culture for a prolonged time. Therefore, repeated preparations of MoDCs are necessary for several rounds of vaccine delivery. To address these limitations, several human DC models have been developed to evaluate their potential application for cancer therapeutic vaccines (15). For instance, MUTZ-3, a myeloid leukemia cell line, can mature and differentiate into DC-like cells in vitro (16), be stably maintained at the pre-DC stage in culture, and provide an adequate amount of inducible DC-like cells for the development of cancer therapeutic vaccines (17, 18).
We have developed a method to establish immortalized human primary dendritic cell lines using the viral protein Tax from human T cell leukemia virus type 2 (HTLV-2), a virus related to its pathogenic counterpart, HTLV-1 (19). HTLV-1 primarily infects CD4+ T cells to cause their malignant transformation and development of adult T cell leukemia-lymphoma among 3 to 5% of infected individuals (20). HTLV-1 also infects DCs, allowing cell-free virus to be transmissible to CD4+ T cells (21, 22). The viral protein Tax from HTLV-1 is known to activate various cellular signaling molecules, including NF-κB, TRAF6, Stat3, and PI3-kinase, and these molecules are also crucial in promoting DC maturation and activation (23, 24). We hypothesized that Tax might harness DCs, leading to their maturation/activation and subsequent induction of an antigen-specific T cell response. We used the Tax protein from HTLV-2, a virus that is not etiologically linked to human disease, as a molecular tool to establish human primary blood dendritic cell lines. Here, we report that these established dendritic cell lines enable the generation of highly potent anticancer cytotoxic lymphocytes.
Results
Development of Human Primary Blood Dendritic Cell Lines.
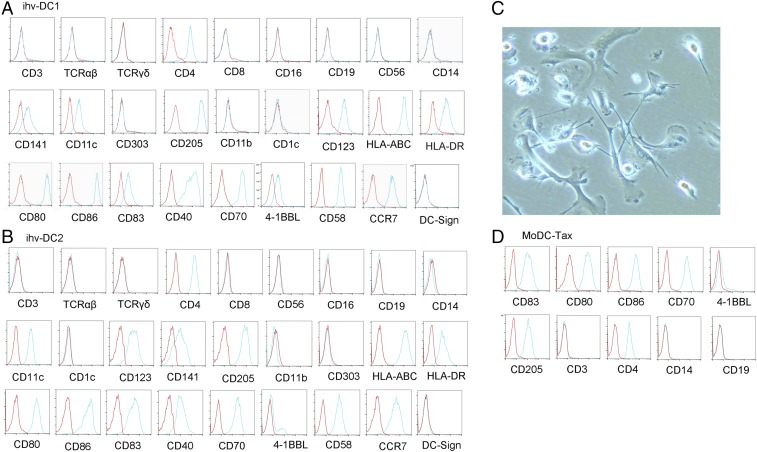
We exploited HTLV-2 Tax as a molecular tool for its role in modulating DC function and providing greater growth of these cells. To prevent potential loss of DCs during culture of the Tax-transduced peripheral blood mononuclear cells (PBMCs), we negatively selected the transduced cells using anti-CD3 magnetic beads to deplete T cells. Two CD3-negative Tax-GFP+ cell cultures from the transduced PBMCs of 10 blood donors grew continuously over 6 mo. These two CD3-negative cell lines, which were established from two different blood donors, displayed DC-related markers. These cells were lineage negative and expressed CD11c, CD141, and CD205, as well as DC maturation and activation molecules, including CD83, CD80, CD86, CD70, CCR7, and HLA-DR (Fig. 1 A and B). We named these two cell lines “ihv-DC1” and “ihv-DC2”, which represented a subset of human CD11c+/CD205+ blood DCs that maintained a maturation and activation phenotype.
Fig. 1.
Immunophenotyping analysis of the established ihv-DC cell lines. (A) ihv-DC1. (B) ihv-DC2. (C) Image of GM-CSF/IL-4–stimulated and differentiated DCs from adherent monocytes of healthy blood donors. (D) Immunophenotype of MoDC-Tax cells generated from the MoDCs that were transduced with the Tax-GFP lentivirus. In A, B, and D, red line indicates isotype antibody staining control; blue line indicates specific antibody staining.
The ihv-DCs grew in suspension with conventional culture conditions, and a few cells were adherent and showed typical dendrites. To validate the ability of Tax in promoting DC growth, we first stimulated adherent monocytes from PBMCs with GM-CSF/IL-4 to generate MoDCs (Fig. 1C). Some of these MoDCs presented dendrites, and they were subsequently transduced with the Tax lentivirus. The transduced MoDC-Tax cells were detached during culture and capable of proliferating for up to 3 mo. The MoDC-Tax cells also presented a mature and activation phenotype (Fig. 1D). These results suggested that Tax was able to promote the growth of both blood DCs and MoDCs that lost cell adherence in the standard culture conditions.
Expression of TLRs, Cytokines, and Cellular Signaling Molecules in ihv-DCs.
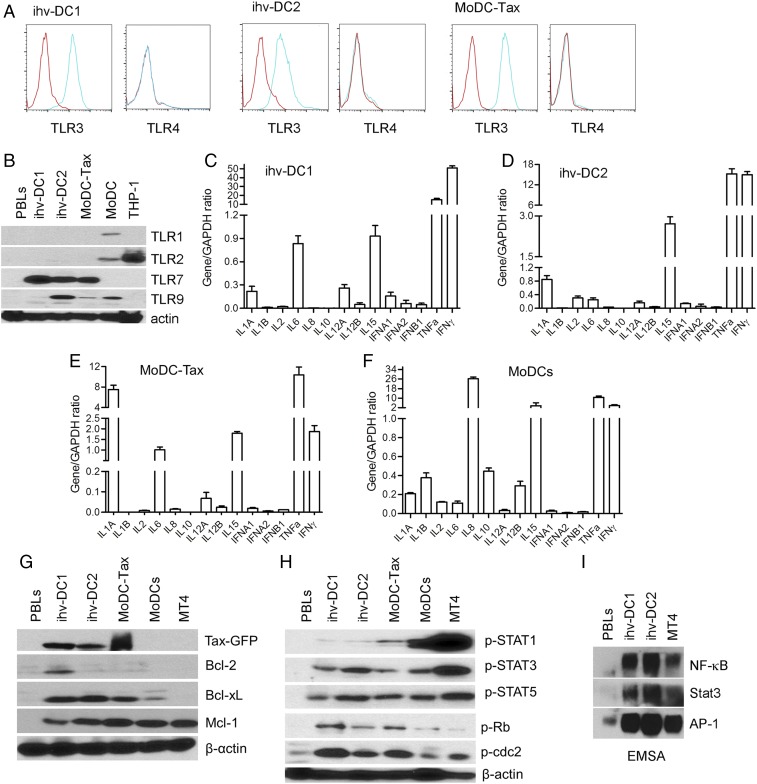
The intracellular dsRNA-sensing TLR3 receptor was expressed, while TLR4 was negative in both ihv-DCs and MoDC-Tax cells (Fig. 2A). The cleaved form of TLR7 was detected in both ihv-DCs and MoDC-Tax, but not in MoDCs (Fig. 2B), suggesting that Tax up-regulated TLR7 in DCs. TLR9 was expressed highly in ihv-DC2, and slightly in MoDCs (Fig. 2B). Regardless of the TLR expression status, these receptors were no longer required for inducing DC maturation and activation, since these established dendritic cell lines persistently expressed high levels of costimulatory molecules such as CD80, CD86, CD70, and CD83.
Fig. 2.
The expression profiles of TLRs, cytokines, and cellular signaling molecules in ihv-DCs. (A) Expression of TLR3 and TLR4 in ihv-DCs and MoDC-Tax cells examined by FACS. (B) Immunoblot analysis of TLR1, TLR2, TLR7, and TLR9 in the established DCs using relevant antibodies. THP-1 cells are monocytic leukemia cells used as control. (C–F) Cytokine expression profiles were determined by qRT-PCR in ihv-DC1 cells (C), ihv-DC2 cells (D), MoDC-Tax cells (E), and TNF-α/LPS–activated MoDCs (F). (G) Immunoblot analysis of the expressions of Tax-GFP, Bcl-2, Bcl-xL, and Mcl-1 in the established DCs. PBLs (peripheral blood lymphocytes from normal donors) and MT4 were used for controls. (H) The phosphorylation status of Stat1, Stat3, Stat5, Rb, or cdc2 was examined using specific anti-phospho-specific antibodies. (I) EMSA assay for detection of the transcriptional activities of NF-κB, Stat3, and AP-1 using the nuclear extracts prepared from ihv-DCs, PBLs, and MT4 cells.
The inflammatory cytokines IL-1A and TNF-α were produced in all ihv-DCs and activated MoDCs, while the immune regulatory factor IL-10 was expressed at a negligible level (Fig. 2 C–F). IL-15, which favors the development of CD8+ cytotoxic lymphocytes, was produced at high levels in both established dendritic cell lines and the activated MoDCs (Fig. 2 C–F). Together, these results indicated that the expression profiles of TLRs and cytokines in ihv-DCs correlated well with the phenotypes of mature and activated blood DCs that were generated by the conventional method. The prosurvival Bcl-2 protein, Bcl-xL, was notably up-regulated only in the DCs that expressed Tax (Fig. 2G). The cell cycle regulators pRb and cdc2 were phosphorylated in ihv-DCs (Fig. 2H). The phosphorylated forms of Stat1, Stat3, and Stat5 were detected in all DCs (Fig. 2H), and the activities of the transcriptional factors, including NF-κB, Stat3, and AP-1, were also observed in ihv-DCs (Fig. 2I). Thus, it appeared that Tax immortalized blood DCs by a similar mechanism to that of Tax-mediated immortalization of CD4+ T cells through activation of growth-promoting signaling and induction of cell cycle progression.
Induction of hTERT-Specific CTLs by ihv-DCs in an Autologous Setting.
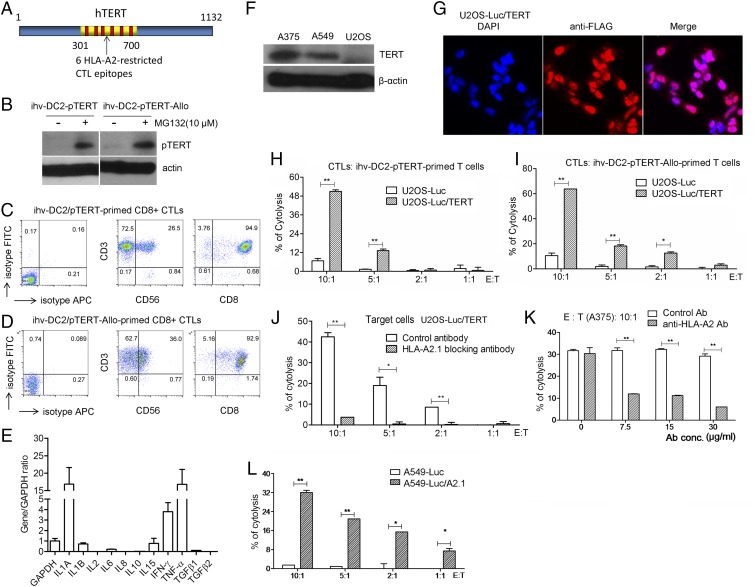
One of the significant advantages of the established dendritic cell lines is that unlike primary DCs, the dendritic cell lines can be genetically modified to enhance their functions in antigen presentation. We then selected a known universal tumor antigen, human telomerase reverse transcriptase (hTERT), and introduced it into HLA-A2+ ihv-DC2 cells with an HLA type (A2, B13/B40, Cw12/Cw16), with HLA-A2 being one of the most frequent HLA alleles. The activity of hTERT is detected in roughly 85% of human cancers and constitutes several recognized HLA-A2–restricted CTL epitopes (25). To facilitate antigen processing, we designed a fusion construct, pTERT, which consisted of the proteasomal target sequence of IκBα and a fragment from hTERT (amino acids 301 to 700) that covered six reported HLA-A2–restricted CTL epitopes (Fig. 3A). This design was based on the mechanism that the ihv-DCs exhibited constitutive activities of IκB kinase/NF-κB due to Tax expression. The activated IκB kinase induced serine phosphorylation and rapid turnover of IκBα. pTERT is to be degraded in these cells, potentially allowing production of abundant amounts of the TERT peptides that form complexes with HLA molecules. pTERT was introduced into ihv-DC2 cells via lentiviral transduction to generate ihv-DC2-pTERT cells. We found that pTERT was barely detected in DMSO-treated ihv-DC2-pTERT cells, and the pretreatment of these DCs with MG132, a chemical inhibitor of the proteasome, induced accumulation of pTERT (Fig. 3B).
Fig. 3.
Induction of hTERT-specific CTLs by ihv-DCs in an autologous setting. (A) The hTERT (amino acids 301 to 700) fragment that covers six HLA-A2–restricted epitopes is depicted. (B) Detection of the proteasome-targeted TERT (amino acids 301 to 700) or pTERT in ihv-DC2-pTERT cells or ihv-DC2-pTERT-Allo cells that were pretreated with DMSO or MG132 for 1 h. ihv-DC2-pTERT-Allo cells were generated by lentiviral transduction of DRB1*0701/DRB1*1301. (C) Immunophenotype of ihv-DC2-pTERT–primed, CD3/CD8-enriched T cells. (D) Immunophenotype of ihv-DC2-pTERT-Allo–primed, CD3/CD8-enriched T cells. (E) Cytokine expression profiles of the ihv-DC2-pTERT-Allo–primed, CD3/CD8-enriched T cells as examined by qRT-PCT. (F) Anti-hTERT immunoblot analysis for A375, A549, and U2OS cancer cells. (G) Fluorescence imaging analysis of U2OS cells transduced with FLAG-TERT(aa301–700) using anti-FLAG antibody. Luc, luciferase. (H) Cytotoxicity assay using U2OS-Luc or U2OS-Luc/TERT cells as target cells, with ihv-DC2-pTERT–primed, CD3/CD8-enriched T cells as killer cells. (I) Cytotoxicity assay using U2OS-Luc or U2OS-Luc/TERT cells as target cells, with ihv-DC2-pTERT-Allo–primed, CD3/CD8-enriched T cells as killer cells. (J) Cytotoxicity assay using U2OS-Luc or U2OS-Luc/TERT cells as target cells, with ihv-DC2-pTERT-Allo–primed, CD3/CD8-enriched T cells as killer cells, in the presence of control antibody or anti-HLA-A2 antibody (30 μg/mL). (K) Cytotoxicity assay using the melanoma cells A375 as target cells, with ihv-DC2-pTERT-Allo–primed, CD3/CD8-enriched T cells as killer cells, in the presence of increasing amounts of the control antibody or anti-HLA-A2 antibody. (L) Cytotoxicity assay using A549-Luc or A549-Luc/A2.1 cells as target cells, with ihv-DC2-pTERT-Allo–primed, CD3/CD8-enriched T cells as killer cells. *P < 0.05, **P < 0.01, as determined by two-tailed Student’s t test.
We next mixed engineered ihv-DC2-pTERT cells with naïve PBMCs directly isolated from the autologous healthy blood donor at the ratio of 1:100. We observed that ihv-DC2-pTERT DCs induced expansion of autologous T lymphocytes for 4 to 6 wk. Two weeks after cell mixing, the ihv-DC2-pTERT–primed CD8+ lymphocytes produced large amounts of IFN-γ and TNF-α (Fig. 3 C and E). Importantly, the ihv-DC2-pTERT cells completely disappeared in the mixed cell culture, as evidenced by lack of the Tax mRNA examined with RT-PCR.
As expected, the ihv-DC2-pTERT cells were found to promote modest expansion of autologous T cells, considering that antigen-specific T cells were typically fewer than 1 in 100,000 in healthy individuals. To enhance immune response to hTERT, we modified ihv-DC2-pTERT cells by introducing two allogeneic HLA-DRB1 molecules (DRB1*0701/DRB1*1301) to generate ihv-DC2-pTERT-Allo DCs. We hypothesized that the autologous CD4 T cells could react to two allo-DRB1 molecules robustly, thereby enhancing the development of hTERT-specific, CD8+ CTL response. Indeed, we observed that the autologous T cells vigorously responded to ihv-DC2-pTERT-Allo DCs, mediating a predominant CD8 CTL response (Fig. 3D). The ihv-DC2-pTERT-Allo–primed T cells proliferated far better than ihv-DC2-pTERT–primed T cells.
ihv-DC2-pTERT–primed or ihv-DC2-pTERT-Allo–primed CD3+/CD8+ T cells were tested for their tumor antigen-specific killing activity on cancer cells. U2OS cells (HLA type: A*0201/A*3201, B*4402, Cw*0501/Cw*0704) were first chosen because these cells are known to be hTERT negative (Fig. 3F). The parental U2OS cells were transduced with FLAG-TERT(aa301–700), and the resulting U2OS/TERT cells showed a nuclear localization of FLAG-TERT(aa301–700) (Fig. 3G). It was shown that ihv-DC2-pTERT–primed autologous CTLs constituted a T cell population that induced cytolysis only in U2OS cells that were reconstituted with TERT (Fig. 3H). Similarly, ihv-DC2-pTERT-Allo–primed CTLs exhibited a potent cytotoxicity on the TERT-expressing U2OS cells (Fig. 3I). Notably, ihv-DC2-pTERT-Allo DCs were able to prime autologous T cells to generate abundant hTERT-specific CTLs that proliferate healthily in a longer-lasting manner ex vivo. Because only HLA-A2 was matched between ihv-DC2-pTERT DCs and U2OS cells in MHC class I molecules, it was likely that the killing on the TERT-expressing U2OS cells was mediated through HLA-A2 restriction. To validate this notion, we applied HLA-A2–blocking antibody, which effectively inhibited ihv-DC2-pTERT-Allo–primed CTL-mediated killing on U2OS/TERT cells (Fig. 3J). Similarly, ihv-DC2-pTERT-Allo–primed CTLs induced cytolysis on the HLA-A2+ melanoma cells A375 (HLA type: A1/A2, B57/B44, C*16/C*6), and this killing effect was inhibited by increasing doses of anti-HLA-A2 antibody (Fig. 3K). Further, we tested HLA-2–negative, hTERT+ A549 lung cancer cells (HLA type: A30, B44, Cw12/16). ihv-DC2-pTERT-Allo–primed CTLs had no significant cytotoxicity on the parental A549 cells but were able to kill the A549 cells that were engineered to express the HLA-A2 molecule (Fig. 3L). Collectively, these results indicated that the ihv-DC2-pTERT–primed or ihv-DC2-pTERT-Allo–primed CTLs recognized hTERT antigen and had the capacity to mediate HLA-2–restricted killing on cancer cells.
Simultaneous Induction of MAGEA3-Specific CTLs and NK Cells Using Engineered ihv-DCs.
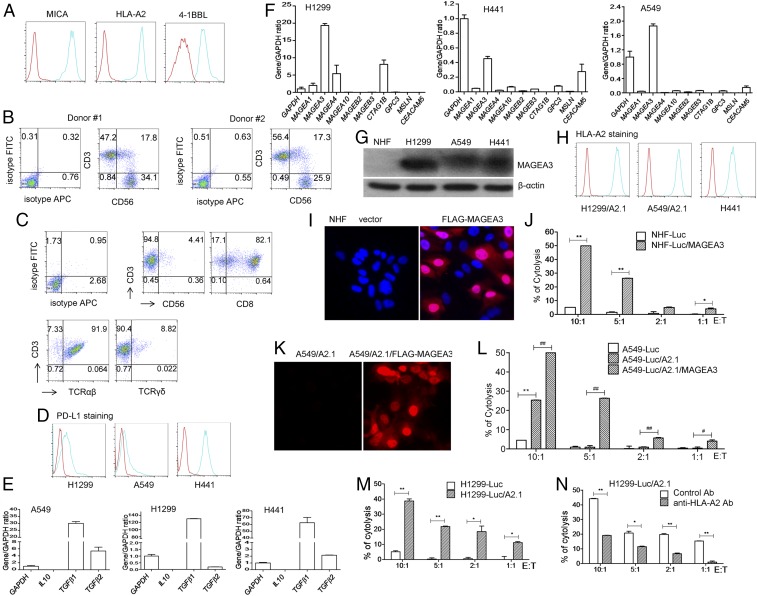
In cancer patients, cancer cells are typically heterogeneous, with expression of different levels of HLA molecules. CTL-mediated killing mandates the presence of an HLA molecule/CTA (cancer/testis antigen) on the surface of cancer cells, while NK cells recognize target cells with down-regulated HLA molecules. It is desirable that engineered DCs would have the capacity to induce simultaneous production of both CTA-specific CTLs and NK cells. To test this idea, ihv-DC1 cells (HLA type: A30, B15/B40, Cw2.2) were engineered to express high levels of MICA and 4-1BBL, in addition to the endogenously expressed CD58 (Fig. 4A), because these molecules are important and play a key role in activating NK cells. These modified DCs were transduced with HLA-A2.1 to present HLA-A2–peptide complexes (Fig. 4A) and were engineered to overexpress the testis antigen MAGEA3. MAGEA3 was expressed in multiple types of human cancer, particularly in late stages of metastatic lung cancer, and the resulting DCs were named “ihv-DC1.2-MAGEA3”. HLA-A2+–matched naïve PBMCs from various donors were coincubated with ihv-DC1.2-MAGEA3 DCs for at least 2 wk. It was shown that the ihv-DC1.2-MAGEA3 cells were able to stimulate production of both CD3+ CTLs and CD3−/CD56+ NK cells from naïve PBMCs (Fig. 4B). The DC-primed T cells were sorted out using anti-CD3 beads, which demonstrated a predominance of CD8+ αβT cells, along with typically less than 10% γσ T cells (Fig. 4C).
Fig. 4.
Simultaneous induction of the MAGEA3-specific CTLs and NK cells using engineered ihv-DCs. (A) ihv-DC1 cells are engineered to express high levels of MICA, 4-1BBL, and HLA-A2.1 molecules, as shown by FACS analysis. (B). Cocultivation of ihv-DC1.2-MAGEA3 cells with HLA-A2+ naïve PBMCs produced both CD3+ T cells and CD3−/CD56+ NK cells, as shown by FACS analysis. (C) Immunophenotype of ihv-DC1.2-MAGEA3–primed, CD3-enriched T cells from at least four HLA-A2+ donors. (D) PD-L1 expression in lung cancer cell lines H1299, A549, and H441, as examined by FACS. (E) IL-10 and TGF-β expression in H1299, A549, and H441, as determined by qRT-PCR. (F) CTA expression in H1299, A549, and H441 cells, as examined by qRT-PCR. (G) MAGEA3 expression in NHF, H1299, A549, and H441 cells by anti-MAGEA3 immunoblot. (H) HLA-A2 surface expression in H1299/A2.1, A549/A2.1, and H441 cell by FACS. (I) Fluorescence imaging analysis of NHF cells and the NHF cells expressing FLAG-MAGEA3 using anti-FLAG antibody. (J) Cytotoxicity assay using NHF-Luc or NHF-Luc/MAGEA3 cells as target cells, with ihv-DC1.2-MAGEA3–primed, CD3+ T cells as killer cells. (K) Fluorescence imaging of A549/A2.1 cells and the A549/A2.1 cells expressing FLAG-MAGEA3 using anti-FLAG antibody. (L) Cytotoxicity assay using A549-Luc, A549-Luc/A2.1, or A549-Luc/A2.1/MAGEA3 cells as target cells, with ihv-DC1.2-MAGEA3–primed, CD3+ T cells as killer cells. (M) Cytotoxicity assay using H1299-Luc or H1299-Luc/A2.1 cells as target cells, with ihv-DC1.2-MAGEA3–primed, CD3+ T cells as killer cells. (N) Cytotoxicity assay using H1299-Luc/A2.1 cells as target cells, with ihv-DC1.2-MAGEA3–primed, CD3+ T cells as killer cells, in the presence of the control antibody or anti-HLA-A2 antibody.
We next selected lung cancer cells as model target cells. Three lung cancer cell lines were chosen for analyzing their expressions of immune-suppressive molecules including PD-L1 and TGF-β1/2, as well as CTAs (Fig. 4 D–F). All cancer cell lines expressed variable levels of PD-L1 and high levels of TGF-β1, with H1299 cells producing abundant levels of MAGEA3, while A549 and H441 cells produced lower levels of MAGEA3 (Fig. 4 F and G). HLA-A2.1 was transduced into HLA-A2–negative H1299 cells (HLA type: A24/A32) and A549 cells (HLA type: A30, B44, C*12/C*16) (Fig. 4H). Normal human fibroblast (NHF) cells (HLA type: A2, B51, C*7) did not express MAGEA3 (Fig. 4G), and these cells were transduced with FLAG-MAGEA3. The NHF/MAGEA3 cells showed a predominant nuclear localization of MAGEA3, as determined by fluorescence imaging (Fig. 4I). As shown in Fig. 4J, the ihv-DC1.2-MAGEA3–primed HLA-A2+, CD3+ CTLs had little cytotoxicity on NHF cells but were able to induce significant cytolysis of the MAGEA3-expressing NHF cells. Similarly, an enhanced expression of MAGEA3 in A549/A2.1 cells potentiated the killing by the ihv-DC1.2-MAGEA3–primed CD3+ CTLs (Fig. 4 K and L). Additionally, ectopic expression of HLA-A2.1 molecules rendered H1299 cells sensitive to HLA-A2–restricted killing by the ihv-DC1.2-MAGEA3–primed CD3+ CTLs, while the HLA-A2–blocking antibody effectively inhibited such killing (Fig. 4 M and N). Together, these experimental findings indicated that the engineered ihv-DCs were capable of priming naïve PBMCs to generate HLA-A2–restricted, MAGEA3-specifc CTLs, along with NK cells.
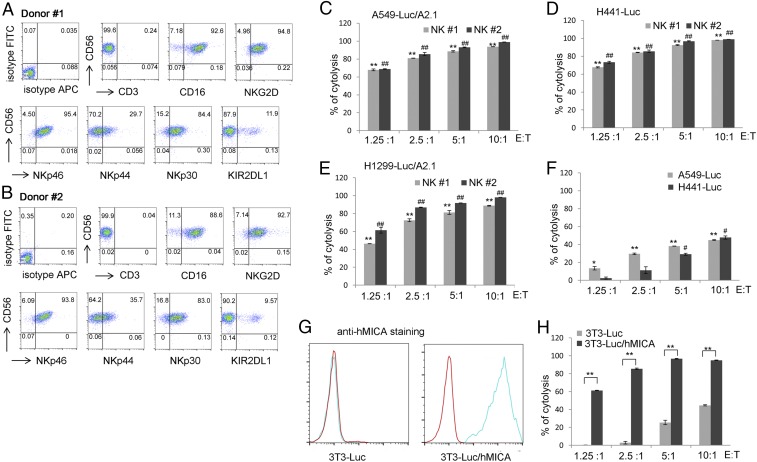
We further analyzed immunophenotypes and cytotoxicity of the ihv-DC1.2-MAGEA3–activated CD3−/CD56+ NK cells. As exemplified from NK cells generated from two donors, we found that the DC-activated NK cells expressed high levels of CD16, NKG2D, NKp46, and NKp30 and produced roughly 10% of the inhibitory receptor KIR2DL1 (Fig. 5 A and B). These DC-activated NK cells were very cytotoxic, killing A549, H441, and H1299 cells, even at low effector cell-to-target cell (E:T) ratios (Fig. 5 C–E). By comparison, the leukemic NK92 cells were much less potent in killing either A549 or H441 cells (Fig. 5F). Further, the human MICA+ NIH 3T3 cells were increasingly sensitive to the cytolysis by DC-activated NK cells at various E:T ratios (Fig. 5 G and H).
Fig. 5.
Cytotoxicity of ihv-DC1.2-MAGEA3–activated NK cells. (A and B) Immunophenotype of ihv-DC1.2-MAGEA3–activated NK cells from two HLA-A2+ donors. NK cells were negatively selected using anti-CD3 beads to deplete ihv-DC1.2-MAGEA3–primed T cells. (C–E) Cytotoxicity assay using A549-Luc/A2.1 cells (C), H441-Luc cells (D), and H1299-Luc/A2.1 cells (E) as target cells, with ihv-DC1.2-MAGEA3–activated NK cells as killer cells. (F) Cytotoxicity assay using A549-Luc and H441-Luc cells as target cells, with NK92 cells as killer cells. (G) NIH 3T3-Luc cells were stably transduced with hMICA, and the surface expression of hMICA was shown by FACS. (H) Cytotoxicity assay using 3T3-Luc and 3T3-Luc/hMICA as target cells, with ihv-DC1.2-MAGEA3–activated NK cells as killer cells.
Suppression of Lung Cancer Metastasis by ihv-DC1.2-MAGEA3–Primed Cytotoxic Lymphocytes in NSG Mice.
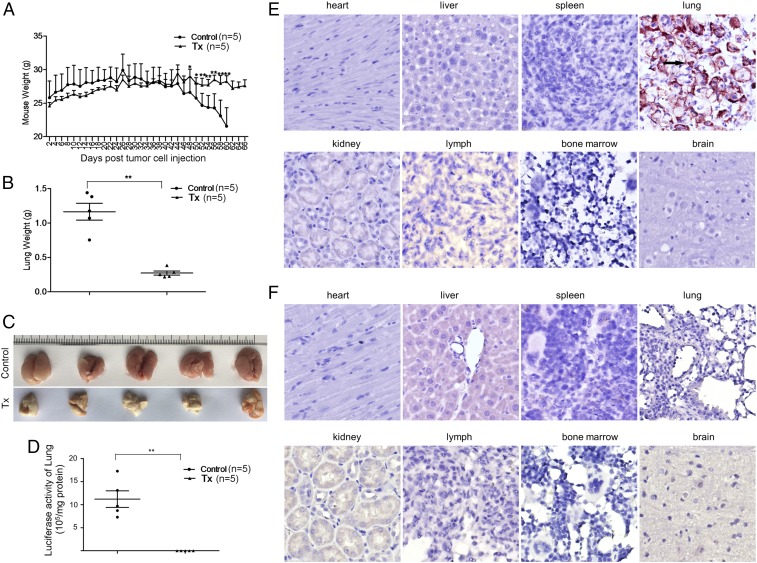
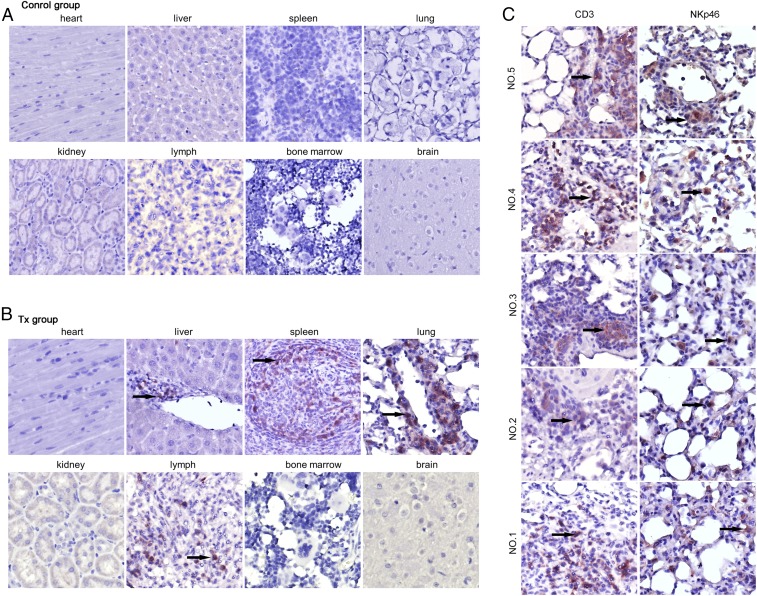
To explore the application of ihv-DC–based immunotherapy, we selected the ihv-DC1.2-MAGEA3 cells for their unique ability in priming naïve PBMCs to generate abundant amounts of HLA-A2–restricted MAGEA3-specific CTLs and NK cells, which potently induced cytolysis of human lung cancer cells ex vivo. A549-Luc/A2.1 cells were chosen because our previous study showed that these cancer cells presented a desirable lung metastasis pattern when infused through the tail vein in NSG mice. As shown in Fig. 6A, the body weights of the mice in the control group started to drop 42 d after tumor cell injection, whereas the weights of the treatment group (Tx) mice were well maintained throughout the experimental period. The lungs were significantly enlarged in the control group and exhibited high levels of luciferase activity, whereas the lungs in the Tx group appeared to be normal in appearance and weight and produced only background luciferase activity (Fig. 6 C and D). Anti-HLA-A2.1 immunohistochemistry (IHC) staining demonstrated that the A549-Luc/A2.1 cells were exclusively found in the lung tissues in the control mice (Fig. 6E), whereas the cancer cells were undetected in the Tx mice under the same condition (Fig. 6F). H&E staining verified these IHC results (Fig. S1). We next applied anti-human CD3 staining to assess the in vivo trafficking patterns of the infused CTLs. It was shown that compared with the control mice, human CD3+ T cells were detected in the spleen, lymph nodes, and lung tissues, and a few CD3+ cells were found in the liver in the Tx group mice (Fig. 7 A and B). No infiltrating T cells were found in the heart and kidney in the Tx group mice (Fig. 7B). By analyzing the lung tissues of five mice in the Tx group, we found that both CD3+ CTLs and NKp46+ NK cells were present in the lung tissues (Fig. 7C). These experimental data, therefore, indicated their desirable in vivo trafficking patterns and validated the therapeutic efficacy of the ihv-DC1.2-MAGEA3–primed cytotoxic lymphocytes in the lung cancer NSG model.
Fig. 6.
Suppression of metastatic lung cancer by ihv-DC1.2-MAGEA3–primed cytotoxic lymphocytes in NSG mice. (A) Body weights of NSG mice in the control group and the Tx group. (B) Whole lung weights of NSG mice in the control group and the Tx group at the end point. (C) Images of whole lungs of NSG mice in the control group and the Tx group at the end point. (D) The luciferase activity of the lung tissues of mice in the control group and the Tx group. (E and F) IHC staining with anti-HA (hemagglutinin) antibody to detect HLA-A2.1-HA protein in various organs of mice in the control group (E) and the Tx group (F).
Fig. 7.
ihv-DC1.2-MAGEA3–activated CTLs and NK cells infiltrate lung tissues. (A and B) Anti-CD3 IHC was analyzed for various tissue samples of mice in the control group (A) and the Tx group (B). (C) Anti-CD3 or anti-NKp46 IHC staining for the lung tissue of all five mice in the Tx group.
Discussion
We have described a method for developing human blood dendritic cell lines by selecting phytohemagglutinin (PHA)/IL-2–stimulated, Tax-transduced PBMCs from blood donors. The established ihv-DCs can be reliably grown and maintained in culture for a prolonged time without losing their maturation and activation phenotypes. We have also developed a method to amplify MoDCs by expressing Tax in GM-CSF/IL-4–induced DCs. Although we are not able to establish immortalized MoDC-Tax cells, this method can still generate adequate numbers of activated MoDCs from several million PBMCs, which is a significant advantage over the conventional MoDC method, by eliminating undesirable apheresis procedures. In addition, unlike primary DCs, the established DCs can be easily modified by expressing a given tumor antigen intracellularly, thereby presenting multiple CTA epitopes in priming CD8 T cells and enhancing the development of anticancer immunity.
We attribute our success to the effort of cell selection and culture techniques at several levels. First, PHA/IL-2 stimulates T cell proliferation, and the activated T cells secrete soluble factors that possibly enable blood immature DCs to be transduced by the Tax lentivirus, since primary immature DCs are resistant to lentiviral transduction. Second, because the majority of the Tax-transduced T cells proliferate much faster than the Tax-transduced DCs, depletion of T cells at the early selection stage is an essential step that allows low numbers of DCs to thrive. Third, most Tax-transduced DCs experience a “growth crisis” during the third month after transduction, and some of them regain their growth strength after passing this stage. Therefore, a continuous culture effort is necessary to aid the Tax-transduced DCs to become immortal. Although the DC immortalization efficiency is roughly 20%, this shortage can be improved by at least two technical modifications. One approach is to develop a DC bank from healthy blood donors, which covers frequent HLA molecules among different ethnic populations. We could select patient HLA-matched DCs from the DC bank for cancer therapy. Another approach is that we could perform a reverse-engineering technique to develop patient HLA-matched DCs. In this method, the patient’s HLA molecules are cloned and expressed in a model dendritic cell line.
A crucial phenotype of the ihv-DCs is their constitutively activated state without need for additional stimulations. Tax apparently has the capacity to promote both DC maturation and activation. We reason that Tax-induced activation of NF-κB signaling plays a key role in the process of DC maturation and activation. Upon encountering invading pathogens, some components of pathogens stimulate TLRs such as TLR3 and TLR4 in immature DCs. TLR3 or TLR4 engagement activates the receptor-associated TRAF6, which in turn induces the activation of its downstream signaling molecules, including the IκB kinase–NF-κB complex (26, 27). It is well recognized that NF-κB is the key mediator in inducing DC maturation and activation (23, 24). TRAF6 is also necessary for activating MAP kinase/AP-1 (28). Further, the Stat3 activity is crucial for IL-15–derived DCs to acquire their antigen-presenting capability and to mediate CD8+ T cell response (29). Consistent with these reports, we demonstrate that ihv-DCs exhibit the constitutive activities of NF-κB, AP-1, and Stat3 as induced by Tax, thereby contributing to their maturation and activation.
ihv-DCs have the ability to prime naïve PBMCs to generate antigen-specific CTLs that induce cytolysis of target cells in an HLA-restricted manner in an autologous setting. However, the number of tumor antigen-specific lymphocytes in healthy donors is extremely low and, therefore, it is a challenging task to generate adequate numbers of tumor antigen-specific CTLs in the cell culture condition for cancer therapy using the autologous setting. Notably, ∼7% of peripheral lymphocytes display alloreactive capability, which in turn promote generation of tumor antigen-specific CTLs (30–32). In the present study, we developed a strategy to enhance production of the antigen-specific CTLs. By introducing two allogeneic HLA-DRB1 molecules into ihv-DCs, autologous CD4 T cells can respond to these modified DCs robustly, in promoting generation of antigen-specific CD8 CTLs. This approach may have at least two levels of significance in cancer therapy. One is that sufficient amounts of CTA-specific, autologous CTLs are readily available by cocultivating with engineered DCs in a cGMP facility, which can be infused directly into patients. Another is that the engineered DCs with two allo-DRB1 molecules can serve as therapeutic cancer vaccines that induce a better inflammatory immunity in patients. These ideas need to be investigated further in clinical trials. Furthermore, we envision that simultaneous production of both CTA-specific CTLs and NK cells is a clear advantage in cancer therapy, considering the heterogeneous nature of cancer cells. The ihv-DC–activated NK cells are apparently of high quality, as exemplified by potent cytotoxicity and low levels of the inhibitory receptor KIR2DL1.
In summary, the ihv-DC models offer several notable features: (i) ihv-DCs are activated and mature DCs, thereby eliminating the need of the complex maturation and activation process to acquire functional DCs; (ii) ihv-DCs cells constitutively express abundant amounts of the costimulatory receptors, including CD80, CD86, and CD70, which stimulate antigen-specific CD8+ cytotoxic T cells to proliferate; (iii) ihv-DCs also express the chemokine receptor CCR7, the lymphoid tissue homing receptor; (iv) the ihv-DC model can provide a particularly useful tool to study DC biology; (v) these DCs can be modified genetically for investigating the role of existing or putative costimulatory receptors that mediate or enhance a protective anticancer or antiviral immunity; (vi) the engineered ihv-DCs can promote simultaneous production of CTA-specific CTLs and NK cells; and (vii) ihv-DCs are immortalized, thus allowing for their continuous availability.
Materials and Methods
All experimental procedures are described in detail in SI Materials and Methods.
Cell Lines, Antibodies, and Cytokines.
H1299, H441, A549, and U2OS cell lines were obtained from American Type Culture Collection. A375 and NHF cell lines were kindly provided by Isaiah J. Fidler, The University of Texas, MD Anderson Cancer Center, Houston, and Jieyu Zhu, Washington State University, Spokane, WA, respectively. Recombinant IL-2 was acquired from AIDS Research and Reference Reagent Program. Isotype IgG control antibody and various allophycocyanin- or FITC-labeled antibodies were purchased from BioLegend. The HLA-A2–blocking antibody was purchased from GeneTex.
Lentiviral Production.
The tax from HTLV-2 was fused with the fragment encoding enhanced green fluorescence protein, and the tax2-gfp fusion fragment was cloned into the lentiviral vector in which the human elongation factor promoter drives the expression of transgene. To generate recombinant lentiviruses, the lentiviral construct was cotransfected with the packaging plasmid mix containing the expression plasmids for VSV-G, Gag-Pol, and Rev (Invitrogen) into 293 cells using SuperFect transfection reagent (Qiagen).
Generation of ihv-DC Cell Lines.
Leukopaks were obtained from New York Blood Center. Human PBMCs were isolated from leukopaks and stimulated with PHA (5 μg/mL) for 24 h, followed by adding recombinant IL-2 (100 units/mL). The activated PBMCs were cultured for 4 to 5 d and were then transduced with the Tax2-GFP lentivirus in the presence of polybrene (10 μg/mL). The transduced cells were cultured continuously in the complete RPMI 1640 medium containing 10% FBS (Sigma) and 100 units/mL recombinant IL-2. Alternatively, the transduced cells were cultured in RPMI 1640 medium supplemented with 5% heat-inactivated human AB serum (Sigma). Two- to 3-wk after transduction, the transduced cells were negatively selected with anti-CD3 magnetic beads (Life Technologies) to deplete T cells. CD3-negative cells were maintained in culture continuously and analyzed for their immunophenotypes about 3 mo after transduction. Two dendritic cell lines (ihv-DC1 and ihv-DC2) were established from two blood donors. The ihv-DC1 cells lost CD40 during the passages in culture, and CD40 was restored by the CD40 lentiviral transduction. The ihv-DCs were grown in the RPMI 1640 medium supplemented with 5% heat-inactivated human AB serum or 10% FBS in the presence of IL-2 (50 to 100 units/mL).
Induction of Tumor-Associated Antigen-Specific CTLs by ihv-DCs.
The ihv-DCs were mixed with naïve PBMCs isolated from leukopaks at the ratio of 1:100. The mixed cells were kept in culture without adding exogenous cytokines for 2 to 3 d, followed by adding recombinant IL-2 (100 to 200 units/mL). The proliferation of the ihv-DC–reactive lymphocytes was monitored with FACS, and the presence of ihv-DCs in the mixed culture was monitored using fluorescence microscopy and qRT-PCR for detecting Tax2. Two to 3 wk after cell mixing, the ihv-DC–activated lymphocytes were analyzed with FACS and were examined for their cytotoxic activity on target cells.
Cytotoxicity Assay.
Various cell lines that were modified to express luciferase were used as targets, while the ihv-DC–activated lymphocytes generated from 14 to 21 d after mixed lymphocyte reaction served as effectors. Cancer cells were first placed in 24-well plates for 2 h for complete attachment. The ihv-DC–activated lymphocytes were then placed on cancer cells at the indicated E:T ratios. At the 4- or 16-h time point after coincubation of effector and target cells, viable cells were gently washed with PBS buffer to remove cellular debris and subjected to the luciferase activity assay using the kit from Promega. The cytotoxic activity of the ihv-DC–activated lymphocytes was determined by comparing the luciferase activities in the target cells that were treated without or with the cytotoxic lymphocytes at the indicated E:T ratios.
qRT-PCR.
Total RNA was isolated using the RNeasy kit (Qiagen), and its concentration was determined using the NanoDrop1000 spectrophotometer (Thermo Scientific). The quality and integrity of total RNA was assessed on 1% formaldehyde–agarose gels. cDNA was synthesized using the OmniScript Reverse Transcriptase Kit (Qiagen) following the manufacturer’s recommended protocol. Template samples in triplicate were subjected to qRT-PCR (Stratagene Mx3005P system) using Power SYBR Green (Applied Biosystems).
Animals and Xenograft Models.
The animal study was performed using the approved Institutional Animal Care and Use Committee protocol of the University of Maryland School of Medicine. Male NSG mice (8 wk, 18 to 22 g body weight) were obtained from the animal center at the University of Maryland School of Medicine and housed in a specific pathogen-free room. A549/A2.1 cells (2 million) were collected, resuspended in Hanks buffer, and then tail vein-injected into NSG mice. After cancer cell implantation, the mice were randomized into two groups (CTL Tx and control). The treatment began 5 d after cancer cell transplantation. The Tx group mice were injected via the tail vein with the DC-activated CTLs [q.5.d × 12 (injected every 5 d to total of 12 injections), 20 million in 200 µL in Hanks buffer] plus 1,000 units recombinant IL-2, and the control group mice were injected via the tail vein with Hanks buffer (q.5.d × 11, iv, 200 µL plus 1,000 units recombinant IL-2). The animals’ weight was measured every 2 d.
Immunohistochemistry and H&E Staining.
Tissue sections were incubated at 60 °C for 10 min and then deparaffinized in xylene and subsequently in ethanol (ETOH) and deionized water. After deparaffinization, tissue sections were immersed in a preheated antigen unmasking solution (Vector), placed into a pressure cooker for 5 to 10 min, and then cooled to room temperature under cold water. The anti-human CD3 antibody or anti-NKp46 (1:100) was used to stain the pretreated tissue sections. Next, the endogenous peroxidase activity was blocked by incubation of tissue sections with 3% hydrogen peroxide in PBS for 10 min. Biotinylated goat anti-mouse IgG antibody (H+L) (1:200) (Vector) was applied as the secondary antibody. A VECTASTAIN Elite ABC HRP Kit (Vector) was used as chromogen, and hematoxylin counterstaining was also performed.
Supplementary Material
Acknowledgments
We thank Alonso Heredia, Yiling Liu, and Suzanne Gartner for technical assistance. Research reported in this publication was supported by the University of Maryland Cancer Center and the Institute of Human Virology, University of Maryland School of Medicine (H.C.).
Footnotes
Conflict of interest statement: H.C. filed a patent application to the US Patent and Trademark Office involving the method for developing human blood dendritic cell lines. The invention is in the patent pending status.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1800550115/-/DCSupplemental.
References
- 1.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyler CJ, Doherty DG, Moser B, Eberl M. Human Vγ9/Vδ2 T cells: Innate adaptors of the immune system. Cell Immunol. 2015;296:10–21. doi: 10.1016/j.cellimm.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 3.van Beek JJ, Wimmers F, Hato SV, de Vries IJM, Sköld AE. Dendritic cell cross talk with innate and innate-like effector cells in antitumor immunity: Implications for DC vaccination. Crit Rev Immunol. 2014;34:517–536. doi: 10.1615/critrevimmunol.2014012204. [DOI] [PubMed] [Google Scholar]
- 4.Pradere J-P, Dapito DH, Schwabe RF. The yin and yang of Toll-like receptors in cancer. Oncogene. 2014;33:3485–3495. doi: 10.1038/onc.2013.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dzopalic T, Rajkovic I, Dragicevic A, Colic M. The response of human dendritic cells to co-ligation of pattern-recognition receptors. Immunol Res. 2012;52:20–33. doi: 10.1007/s12026-012-8279-5. [DOI] [PubMed] [Google Scholar]
- 6.Hammer GE, Ma A. Molecular control of steady-state dendritic cell maturation and immune homeostasis. Annu Rev Immunol. 2013;31:743–791. doi: 10.1146/annurev-immunol-020711-074929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mildner A, Jung S. Development and function of dendritic cell subsets. Immunity. 2014;40:642–656. doi: 10.1016/j.immuni.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Osugi Y, Vuckovic S, Hart DN. Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood. 2002;100:2858–2866. doi: 10.1182/blood.V100.8.2858. [DOI] [PubMed] [Google Scholar]
- 9.Kassianos AJ, Jongbloed SL, Hart DN, Radford KJ. Isolation of human blood DC subtypes. Methods Mol Biol. 2010;595:45–54. doi: 10.1007/978-1-60761-421-0_3. [DOI] [PubMed] [Google Scholar]
- 10.Jongbloed SL, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachem A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kvistborg P, Boegh M, Pedersen AW, Claesson MH, Zocca MB. Fast generation of dendritic cells. Cell Immunol. 2009;260:56–62. doi: 10.1016/j.cellimm.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Jolanda I, de Vries M, Adema GJ, Punt CJ, Figdor CG. 2005. Phenotypical and functional characterization of clinical-grade dendritic cells. Adoptive Immunotherapy: Methods and Protocols. Methods in Molecular Medicine™, eds Ludewig B., Hoffmann M.W. (Humana, New York), Vol 109, pp 113–125.
- 14.Robbins SH, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Helden SF, van Leeuwen FN, Figdor CG. Human and murine model cell lines for dendritic cell biology evaluated. Immunol Lett. 2008;117:191–197. doi: 10.1016/j.imlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Masterson AJ, et al. MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood. 2002;100:701–703. doi: 10.1182/blood.v100.2.701. [DOI] [PubMed] [Google Scholar]
- 17.Santegoets SJ, van den Eertwegh AJ, van de Loosdrecht AA, Scheper RJ, de Gruijl TD. Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J Leukoc Biol. 2008;84:1364–1373. doi: 10.1189/jlb.0208092. [DOI] [PubMed] [Google Scholar]
- 18.Santegoets SJ, et al. In vitro priming of tumor-specific cytotoxic T lymphocytes using allogeneic dendritic cells derived from the human MUTZ-3 cell line. Cancer Immunol Immunother. 2006;55:1480–1490. doi: 10.1007/s00262-006-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciminale V, Rende F, Bertazzoni U, Romanelli MG. HTLV-1 and HTLV-2: Highly similar viruses with distinct oncogenic properties. Front Microbiol. 2014;5:398. doi: 10.3389/fmicb.2014.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet Oncol. 2014;15:e517–e526. doi: 10.1016/S1470-2045(14)70202-5. [DOI] [PubMed] [Google Scholar]
- 21.Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- 22.Jain P, et al. DC-SIGN mediates cell-free infection and transmission of human T-cell lymphotropic virus type 1 by dendritic cells. J Virol. 2009;83:10908–10921. doi: 10.1128/JVI.01054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- 25.Lü M-H, et al. hTERT-based therapy: A universal anticancer approach (Review) Oncol Rep. 2012;28:1945–1952. doi: 10.3892/or.2012.2036. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi T, et al. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 2003;19:353–363. doi: 10.1016/s1074-7613(03)00230-9. [DOI] [PubMed] [Google Scholar]
- 27.Hull C, McLean G, Wong F, Duriez PJ, Karsan A. Lipopolysaccharide signals an endothelial apoptosis pathway through TNF receptor-associated factor 6-mediated activation of c-Jun NH2-terminal kinase. J Immunol. 2002;169:2611–2618. doi: 10.4049/jimmunol.169.5.2611. [DOI] [PubMed] [Google Scholar]
- 28.Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs) Oncogene. 2001;20:6482–6491. doi: 10.1038/sj.onc.1204788. [DOI] [PubMed] [Google Scholar]
- 29.Okada S, Han S, Patel ES, Yang L-J, Chang L-J. STAT3 signaling contributes to the high effector activities of interleukin-15-derived dendritic cells. Immunol Cell Biol. 2015;93:461–471. doi: 10.1038/icb.2014.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossjohn J, McCluskey J. How a home-grown T cell receptor interacts with a foreign landscape. Cell. 2007;129:19–20. doi: 10.1016/j.cell.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Gras S, Kjer-Nielsen L, Chen Z, Rossjohn J, McCluskey J. The structural bases of direct T-cell allorecognition: Implications for T-cell-mediated transplant rejection. Immunol Cell Biol. 2011;89:388–395. doi: 10.1038/icb.2010.150. [DOI] [PubMed] [Google Scholar]
- 32.Morelli AE, Thomson AW. Dendritic cells: Regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–146. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.