Significance
While stem cell therapy has become the standard of care for hematological disorders, challenges remain for the treatment of solid organ injuries. Targeting endogenous cells would overcome many hurdles associated with exogenous stem cell therapy. Alarmins are released upon tissue damage, and here we describe how upregulation of a physiological pathway by exogenous administration of a single dose of HMGB1, either locally or systemically, promotes tissue repair by targeting endogenous stem cells. We show that HMGB1 complexed with CXCL12 transitions stem cells that express CXCR4 from G0 to GAlert. These primed cells rapidly respond to appropriate activating factors released upon injury. HMGB1 promotes healing even if administered 2 wk before injury, thereby expanding its translational benefit for diverse clinical scenarios.
Keywords: HMGB1, tissue regeneration, GAlert, stem cells, immunology
Abstract
A major discovery of recent decades has been the existence of stem cells and their potential to repair many, if not most, tissues. With the aging population, many attempts have been made to use exogenous stem cells to promote tissue repair, so far with limited success. An alternative approach, which may be more effective and far less costly, is to promote tissue regeneration by targeting endogenous stem cells. However, ways of enhancing endogenous stem cell function remain poorly defined. Injury leads to the release of danger signals which are known to modulate the immune response, but their role in stem cell-mediated repair in vivo remains to be clarified. Here we show that high mobility group box 1 (HMGB1) is released following fracture in both humans and mice, forms a heterocomplex with CXCL12, and acts via CXCR4 to accelerate skeletal, hematopoietic, and muscle regeneration in vivo. Pretreatment with HMGB1 2 wk before injury also accelerated tissue regeneration, indicating an acquired proregenerative signature. HMGB1 led to sustained increase in cell cycling in vivo, and using Hmgb1−/− mice we identified the underlying mechanism as the transition of multiple quiescent stem cells from G0 to GAlert. HMGB1 also transitions human stem and progenitor cells to GAlert. Therefore, exogenous HMGB1 may benefit patients in many clinical scenarios, including trauma, chemotherapy, and elective surgery.
Adult stem cells are an essential component of tissue homeostasis with indispensable roles in both physiological tissue renewal and tissue repair following injury (1). The regenerative potential of stem cells has been very successful for hematological disorders (2). In contrast, there has been comparatively little clinical impact on enhancing the regeneration of solid organs despite continuing major scientific and public interest (3). Strategies that rely on ex vivo expansion of autologous stem cells on an individual patient basis are prohibitively expensive (4), and success in animal models has often failed to translate in late-phase clinical trials. The use of allogeneic cells would overcome the problems of limited supply but commonly entails risky lifelong immunosuppressive therapy. Some safety concerns remain about induced pluripotent stem cells (5). Furthermore, successful engraftment of exogenous stem cells to sites of tissue injury requires a supportive inductive niche, and the typical proinflammatory scarred bed in damaged recipient tissues is suboptimal (6).
An attractive alternative strategy, which overcomes many of the limitations described above, is to promote repair by harnessing the regenerative potential of endogenous stem cells (5, 7). This requires identification of key soluble mediators that enhance the activity of stem cells and can be administered systemically (8). An interesting observation was made in 1970 that a priming injury at a distant site at the time of or before the second trauma resulted in accelerated healing (9, 10). This phenomenon was explained only recently, when it was shown that a soluble mediator is released following the priming tissue injury which transitions stem cells in the contralateral limb to a state the authors termed “GAlert” (11), which is intermediate between G0 and G1. In the presence of activating factors the primed GAlert cells enter the cell cycle more rapidly than quiescent stem cells, leading to accelerated tissue repair (11). However, the identity of the soluble mediator(s) that transition stem cells to GAlert remain to be clarified.
Our long-standing interest in tissue injury (12–14) has recently centered on alarmins, a group of evolutionarily unrelated endogenous molecules with diverse homeostatic intracellular roles, which, when released from dying, injured, or activated cells, trigger an immune/inflammatory response (15). Much effort has been focused on their deleterious role in autoimmune and inflammatory conditions (15–19), and of the few studies (15, 20) that have investigated their role in tissue repair, none has used a combination of human tissues and multiple animal-injury models to characterize their effects on precise flow cytometry-defined endogenous adult stem cells in vivo. Here we show that high mobility group box 1 (HMGB1) is a key upstream mediator of tissue regeneration which acts by transitioning CXCR4+ skeletal, hematopoietic, and muscle stem cells from G0 to GAlert and that, in the presence of appropriate activating factors, exogenous administration before or at the time of injury leads to accelerated tissue repair.
Results
Alarmins Are Elevated Postinjury in Humans and Mice.
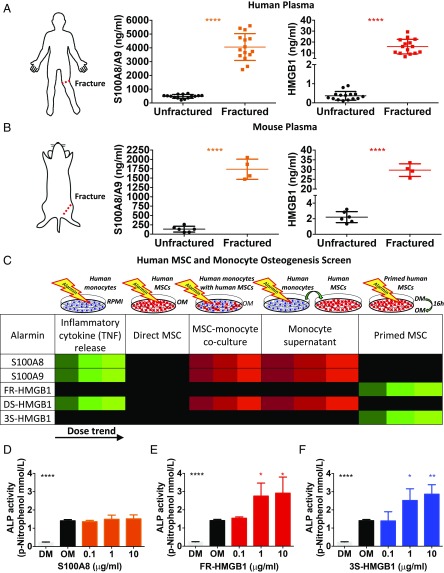
Fracture healing is a good model of tissue regeneration (21), and based on our studies of the early events in fracture healing (13), including the key role of neutrophils (14), we postulated that the alarmins HMGB1 and S100A8/A9 may play key roles in tissue regeneration. HMGB1 is a highly conserved, ubiquitous, and abundant nonhistone nuclear architectural protein that forms part of the transcription machinery (18). S100A8/A9 proteins are calcium-binding proteins that make up 40% of neutrophil cytoplasmic content (22). Both these alarmins have been associated with regulating skeletal cells (15, 23). We found elevated levels of HMGB1 and S100A8/A9 in the circulation following fracture in both human patients and mice (Fig. 1 A and B and Fig. S1 A and B).
Fig. 1.
Alarmins are elevated postinjury in humans and mice, and HMGB1 primes human MSCs for osteogenic differentiation. (A and B) Elevated plasma levels of S100A8/A9 and HMGB1 post femoral fracture in patients (A) and mice (B) collected within 4 h and at 3 h postfracture, respectively (n = 15 fractured and 15 unfractured human patients; n = 6 unfractured and 4 fractured mice). (C) Heat map showing the results of an in vitro osteogenesis screen of alarmins using hMSCs and monocytes. Green: elevated; red: reduced; black: unchanged; color brightness indicates dose trend. All data are shown and quantified in Fig. S2. (D–F) Osteogenic differentiation is unchanged in hMSCs primed with S100A8 (D) but is increased when primed with FR- (E) or 3S-HMGB1 (F), as measured by alkaline phosphatase (ALP) activity (n = 3 hMSC and 3 monocyte donors for each condition; similar results were obtained in three independent experiments; significance is versus osteogenic medium control). DM, maintenance medium; OM, osteogenic medium; RPMI, Roswell Park Memorial Institute medium. *P < 0.05, **P < 0.01, ****P < 0.0001.
HMGB1 Primes Human Mesenchymal Stem Cells for Osteogenic Differentiation.
We screened for the regenerative potential of these alarmins in humans by assessing the osteogenic differentiation of primary human mesenchymal stromal/stem cells (hMSCs) (Fig. 1C and Fig. S2). We tested different redox forms of HMGB1 because they are known to have contrasting effects (24). Fully reduced (FR) all-thiol HMGB1 promotes chemotaxis (24), whereas partially oxidized HMGB1 with a disulfide bond (DS) induces TNF production (Figs. S1C and S2A) (24). To confirm that the effect of FR-HMGB1 is due to its reduced state, we also used a recombinant nonoxidizable all-serine form (3S) of HMGB1 (24). Direct addition of alarmins to hMSCs did not promote osteogenic differentiation (Fig. S2B), while DS-HMGB1, S100A8, and S100A9 all inhibited this process in the presence of monocytes (Fig. S2C), as did the supernatants from alarmin-treated monocytes (Fig. S2D). Since alarmins are released before resident stem cells are exposed to most osteogenic signals in vivo, we modeled this temporal sequence in vitro and found that pre-exposure to only FR-HMGB1 or 3S-HMGB1, but not the proinflammatory DS-HMGB1, promoted osteogenic differentiation (Fig. 1 E and F and Fig. S2E). We confirmed that in vivo administration of FR-HMGB1 or 3S-HMGB1 did not lead to the production of TNF, IL-6, or compensatory IL-10, in contrast to DS-HMGB1 (Fig. S3 I–K). These data suggest that only FR-HMGB1 and 3S-HMGB1, which cannot be oxidized, do not induce proinflammatory cytokine production in vitro (Figs. S1C and S2A) or in vivo (Fig. S3 I–K), are viable candidates to promote fracture healing, if administered before the presence of potent osteogenic mediators.
Exogenous HMGB1 Accelerates Fracture Healing While Genetic Deletion of HMGB1 Delays Fracture Healing.
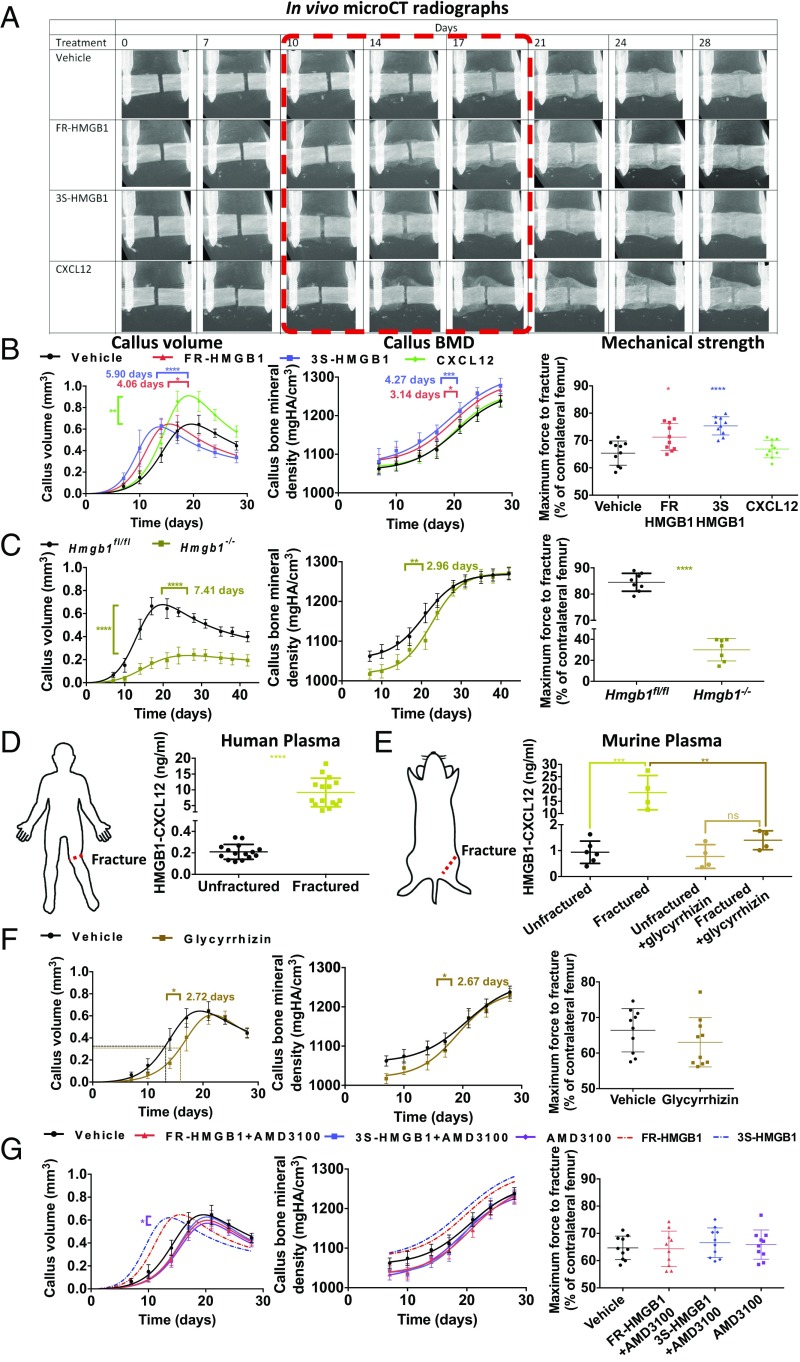
We optimized a murine fracture model (25) to permit longitudinal in vivo analysis over time (Fig. S3 A–G and Movies S1 and S2) and found that FR- or 3S-HMGB1 administered locally at the time of injury accelerated fracture repair as evidenced by in vivo micro-computed tomography (microCT) and mechanical strength testing (Fig. 2 A and B), with a clear dose response (Fig. S3H). To evaluate the contribution of endogenous HMGB1 to fracture healing, inducible whole-body Hmgb1−/− mice were generated (Fig. S4), as FR-HMGB1 in the fracture microenvironment would originate from multiple injured and activated cell types, and constitutive deletion of HMGB1 is perinatally lethal (26). Fracture healing was dramatically impaired in these animals as shown by reduced callus volume, callus bone mineral density (BMD), and mechanical strength (Fig. 2C and Fig. S5A). Thus, both exogenous and endogenous HMGB1 modulate the rate of fracture healing.
Fig. 2.
HMGB1 accelerates fracture healing via CXCL12–CXCR4. (A and B) Local addition of FR- or 3S-HMGB1 accelerates fracture healing, compared with CXCL12 or vehicle controls, as shown by in vivo microCT radiographs (A) and analysis of callus volume, callus BMD, and day 28 mechanical strength (B) (n = 10 mice for each condition). (C) Hmgb1−/− mice have markedly delayed fracture healing compared with Hmgb1fl/fl control mice as shown by reduced callus volume, callus BMD, and day 28 mechanical strength (n = 7 Hmgb1−/− mice, 8 Hmgb1fl/fl mice). (D and E) Elevated plasma levels of HMGB1–CXCL12 heterocomplex postfracture in patients (D) and mice (E) collected within 4 h and at 3 h postfracture, respectively, and inhibition of HMGB1–CXCL12 heterocomplex formation with glycyrrhizin treatment (n = 15 fractured and 15 unfractured human patients and n = 6 unfractured and 4 fractured mice). (F and G) Glycyrrhizin delays fracture healing compared with vehicle controls (F), and AMD3100 abrogates the effects of exogenous FR- or 3S-HMGB1 (G) as shown by callus volume, callus BMD, and day 28 mechanical strength (n = 10 mice for each condition). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ns, not significant.
HMGB1 Accelerates Fracture Healing via CXCL12 and CXCR4.
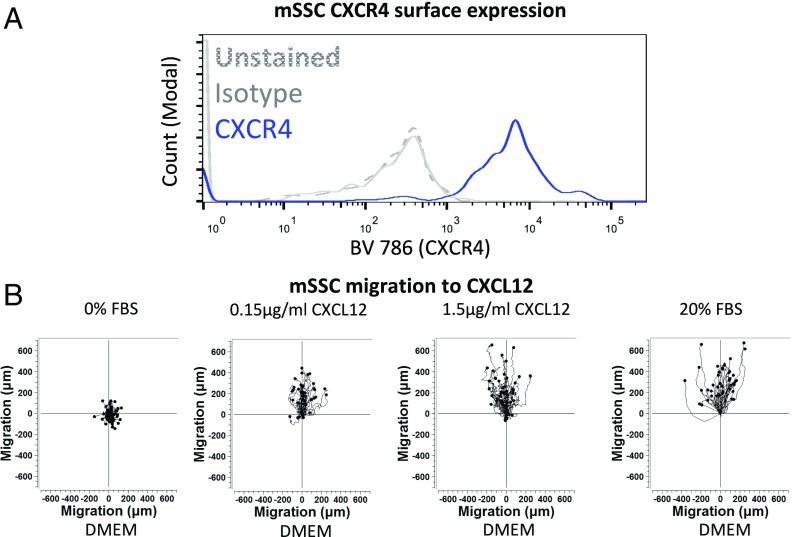
Subsequently, we sought to delineate the signaling pathways through which HMGB1 promotes regeneration. FR-HMGB1 is known to form a heterocomplex with CXCL12 (24, 27), a chemokine, which in turn binds to the receptor CXCR4 (24, 27). We found elevated plasma levels of the HMGB1–CXCL12 heterocomplex in both human patients and mice following fracture injury (Fig. 2 D and E and Fig. S5B). Glycyrrhizin is the only known inhibitor of the HMGB1–CXCL12 heterocomplex (27). It interacts with the binding sites of HMGB1 for CXCL12 but not those for RAGE on the Box regions of HMGB1 (27–29), thereby inhibiting the chemotactic activity of the heterocomplex in vitro and in vivo (27, 28). Local administration of glycyrrhizin at the fracture site inhibited the formation of the HMGB1–CXCL12 heterocomplex (Fig. 2E) and resulted in delayed fracture healing (Fig. 2F and Fig. S5C), confirming that endogenous extracellular HMGB1 modulates the rate of regeneration by forming a heterocomplex with CXCL12. We showed that murine skeletal stem cells (mSSC) (30) express functional CXCR4 (Fig. 3 and Fig. S5E) and that administration of AMD3100, a specific and clinically approved small-molecule inhibitor of CXCR4, led to impaired fracture healing in wild-type mice (Fig. 2G and Fig. S5D) and completely abolished the effects of exogenous HMGB1 (Fig. 2G and Fig. S5D). These data confirm that exogenous HMGB1 accelerates tissue regeneration through CXCR4, as was also recently noted (20). The HMGB1–CXCL12 heterocomplex causes a conformational change in CXCR4, which differs from that seen with CXCL12 alone and thereby enhances chemotaxis compared with CXCL12 (27). It was possible that the proregenerative effects of HMGB1 were simply due to enhanced CXCL12-mediated chemotaxis. To test this, we administered exogenous CXCL12 alone, and while we confirmed enhanced migration of cells to the fracture site (Fig. S3L), we found only abnormal regeneration as evidenced by a larger fracture callus without a concomitant increase in BMD or, importantly, mechanical strength (Fig. 2 A and B). Therefore, the improved regenerative effects of FR- or 3S-HMGB1 could not have been due to enhanced CXCL12-mediated cell migration alone. Taken together, these data show that, while the CXCL12–CXCR4 axis is necessary for HMGB1-mediated accelerated tissue regeneration, exogenous CXCL12 alone is insufficient to accelerate fracture healing. This suggests that the HMGB1–CXCL12 heterocomplex accelerates regeneration via an as yet unknown mechanism rather than by enhanced chemotaxis alone.
Fig. 3.
mSSCs express functional surface CXCR4. (A and B) mSSCs express surface CXCR4 as shown by the FACS histogram plot (n = 4 mice for each condition) (A) and time-lapse microscopy trajectory plots of mSSCs migrating to CXCL12, or 0% or 20% FBS control 20% FBS control (n = 50 cells for each condition) (B), with similar results observed in three independent experiments.
Exogenous HMGB1 Led to a Sustained Increase in mSSC Cell Cycling in Vivo.
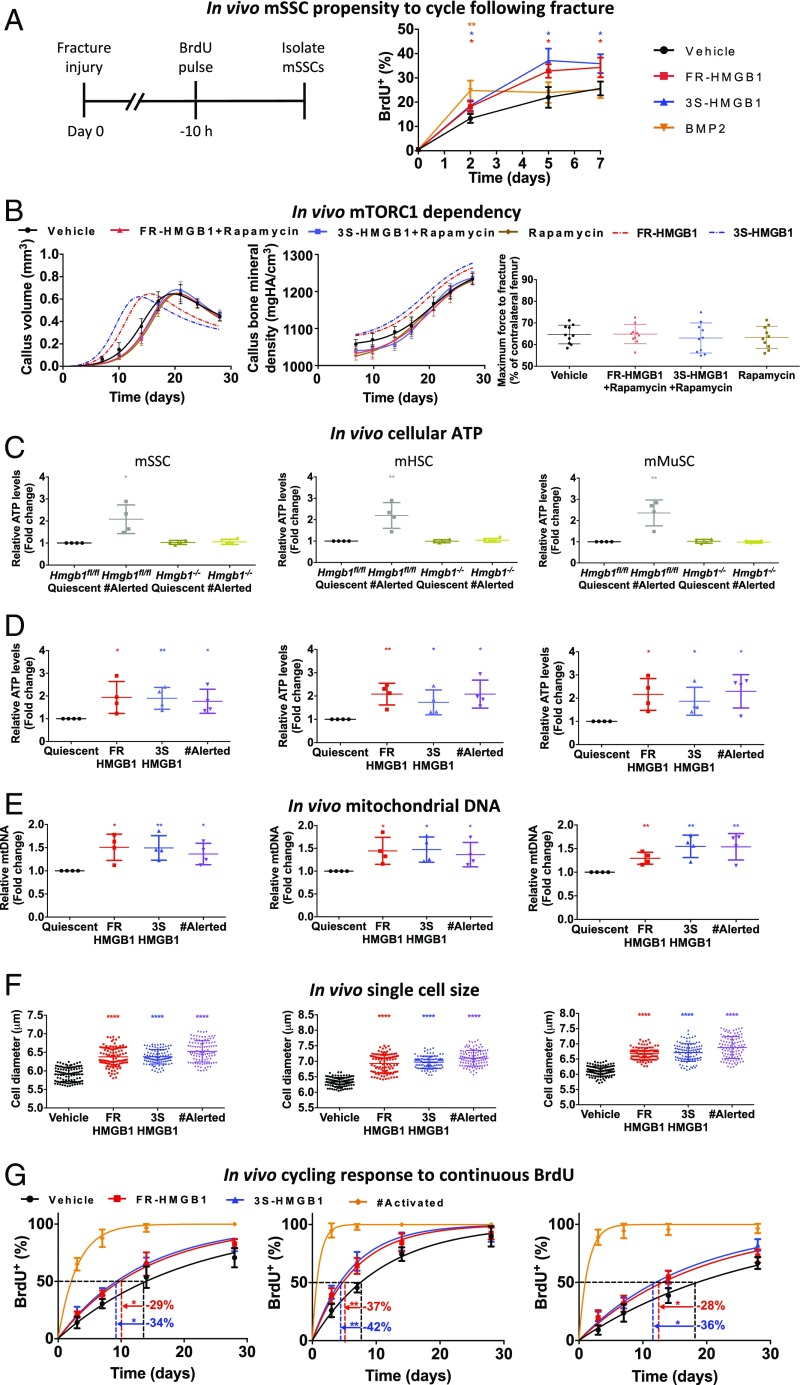
Apart from regulating chemotaxis, the CXCL12–CXCR4 axis also influences the cycling of hematopoietic stem cells (HSCs) by enforcing quiescence (31–36). Therefore, we investigated whether the HMGB1–CXCL12–CXCR4 axis additionally affects the cell cycle of stem cells to promote tissue regeneration. We analyzed the propensity to cycle of mSSCs from the fractured bones of mice that had been pulse-labeled with BrdU (Fig. 4A). mSSCs from vehicle-treated animals displayed an increasing propensity to cycle over time, which correlates with the rising levels after fracture of osteogenic mediators (21, 37) including bone morphogenetic proteins (BMPs) (30). Predictably, exogenous administration of BMP2, a known activator of mSSCs (30), resulted in an immediate increased propensity to cycle that plateaued at day 2 at levels equivalent to those of vehicle-treated controls at day 5. In comparison, mSSCs from animals treated locally with exogenous FR- or 3S-HMGB1 showed an initial increase intermediate between BMP2- and vehicle-treated controls and beyond day 2 exhibited a higher rate of cycling than cells from BMP2- or vehicle-treated animals. These data suggest that HMGB1 has an effect markedly different from that of an activator such as BMP2. Cells that have been preexposed to HMGB1 display an increased propensity to cycle when subsequently exposed to endogenous activating signals released at the fracture site, indicative of a lasting cellular effect that favors cell-cycle entry.
Fig. 4.
HMGB1 transitions stem cells to GAlert. (A) mSSCs from animals treated locally with exogenous FR- or 3S-HMGB1 dynamically adapted to the known physiologically rising levels of activating factors with a sustained higher propensity to cycle (n = 4 mice for each condition and time point). (B) Effects of exogenous FR- or 3S-HMGB1 are mTORC1 dependent in vivo because they are abrogated with rapamycin treatment, as shown by callus volume, callus BMD, and day 28 mechanical strength (n = 10 mice for each condition). (C) mSSCs, mHSCs, and mMuSCs from the limb contralateral to fracture [fracture (#) alerted] of Hmgb1−/− mice display ATP levels equivalent to those in quiescent cells from uninjured Hmgb1−/− and Hmgb1fl/fl mice (n = 4 mice for each condition). (D–F) mSSCs, mHSCs, and mMuSCs from mice treated systemically with FR- or 3S-HMGB1 display increased cellular ATP levels (D), mitochondrial DNA (E) (n = 4 mice for each condition, separate experiments for each parameter), and cell size (F) (n = 100 cells for each condition, with similar results observed in three independent experiments from n = 4 mice per condition) compared with vehicle-treated controls and equivalent to that of fracture (#) alerted cells. (G) mSSCs, mHSCs, and mMuSCs from mice treated systemically with FR- or 3S-HMGB1 display faster entry to cell cycle but more slowly than cells from the limb ipsilateral to the fracture [fracture (#) activated] cells (n = 4 mice for each condition and time point). *P < 0.05, **P < 0.01, ****P < 0.0001.
HMGB1 Transitions Multiple Human and Murine Stem and Progenitor Cells to GAlert.
An elegant series of experiments recently demonstrated that systemic mediator(s) can transition stem cells distant from the site of initial injury to a dynamic state of the cell cycle, intermediate between G0 and G1, termed “GAlert” (11). In contrast to deeply quiescent G0 stem cells, GAlert cells are more metabolically active, as evidenced by increased cellular levels of ATP, and are poised to enter the cell cycle when exposed to activating signals. As HMGB1 enhanced the in vivo cycling of mSSCs exposed to secondary activating signals, together with the elevated systemic levels of HMGB1 and HMGB1-CXCL12 postinjury in humans and mice, and our observations of accelerated fracture healing with exogenous HMGB1 treatment, we hypothesized that HMGB1 may in part accelerate fracture healing by transitioning mSSCs to the recently defined GAlert state. We also postulated that these effects may pertain to other previously well-identified and characterized stem cells known to express CXCR4, including murine hematopoietic stem cells (mHSCs) (31–36) and murine muscle stem cells (mMuSCs) (Fig. S6B) (38).
The essential criteria describing the GAlert state are increased ATP levels, mitochondrial DNA, cell size, faster entry to cell cycle, and mTORC1 dependency (11). We found that the clinically approved mTORC1 inhibitor rapamycin abolished the accelerated healing effects of exogenous HMGB1 (Fig. 4B and Fig. S6A). To investigate the other aspects of the GAlert state, we compared the cells contralateral to a fracture injury (fracture alerted) with those from mice injected i.v. with HMGB1 or vehicle control. The severity of injury is important, as only substantial injuries, such as fractures, can transition stem cells to GAlert, whereas simple venipuncture is insufficient (11). We observed that not only mSSCs but also mHSCs and mMuSCs from uninjured mice injected systemically with HMGB1 showed increased ATP levels, mitochondrial DNA, and cell size compared with vehicle-treated controls, and these levels were equivalent to those in fracture-alerted stem cells (Fig. 4 D–F). In contrast, stem cells from fractured Hmgb1−/− mice (Fig. 4C) and skeletal stem cells (SSCs) from uninjured wild-type animals treated with CXCL12 did not transition to GAlert (Fig. S6G). The essential role of exogenous HMGB1 was further confirmed by a single systemic dose of HMGB1 rescuing the elevated ATP GAlert phenotype in stem cells from Hmgb1−/− mice (Fig. S6F). The translational potential of our data is highlighted by the finding that HMGB1-treated human CD34+ hematopoietic stem and progenitor cells (hHSPCs) as well as hMSCs exhibited increased ATP levels and mitochondrial DNA upon exposure to HMGB1 but substantially less so than IFN-γ–(39) or BMP2-activated cells (Fig. S6 C and D). To assess the rate of entry into cell cycle in vivo, high-dose BrdU was continuously administered, thus utilizing the dual properties of BrdU to label cells that cycle while also acting as an injury signal that activates quiescent stem cells and recruits them into the cell cycle (40). We found that the mSSCs, mHSCs, and mMuSCs in HMGB1-treated mice entered the cell cycle faster with continuous high-dose BrdU than in vehicle-treated controls but much more slowly than activated stem cells from the injured proximal hind limb of fractured animals (fracture activated) (Fig. 4G). The previous genetic studies which demonstrated the necessity of cMet signaling for mMuSCs to transition to GAlert (11) recently led to the identification of HGF-A, an enzyme which activates HGF, a ligand for c-Met, as a stem cell alerting factor (41). Consistent with the cMet genetic studies (11), we found that in vivo cMet inhibition with PHA 665752 or anti-cMet resulted in a substantially reduced expression of surface CXCR4 on mMuSCs (Fig. S6E). Therefore, it is possible that the cMet and CXCR4 pathways are complementary. Collectively, our data show that HMGB1 transitions multiple stem cells to GAlert, priming them to cycle quickly in response to activation signals.
HMGB1 Accelerates Healing of Multiple Tissues, Even if Administered 2 Wk Before Injury.
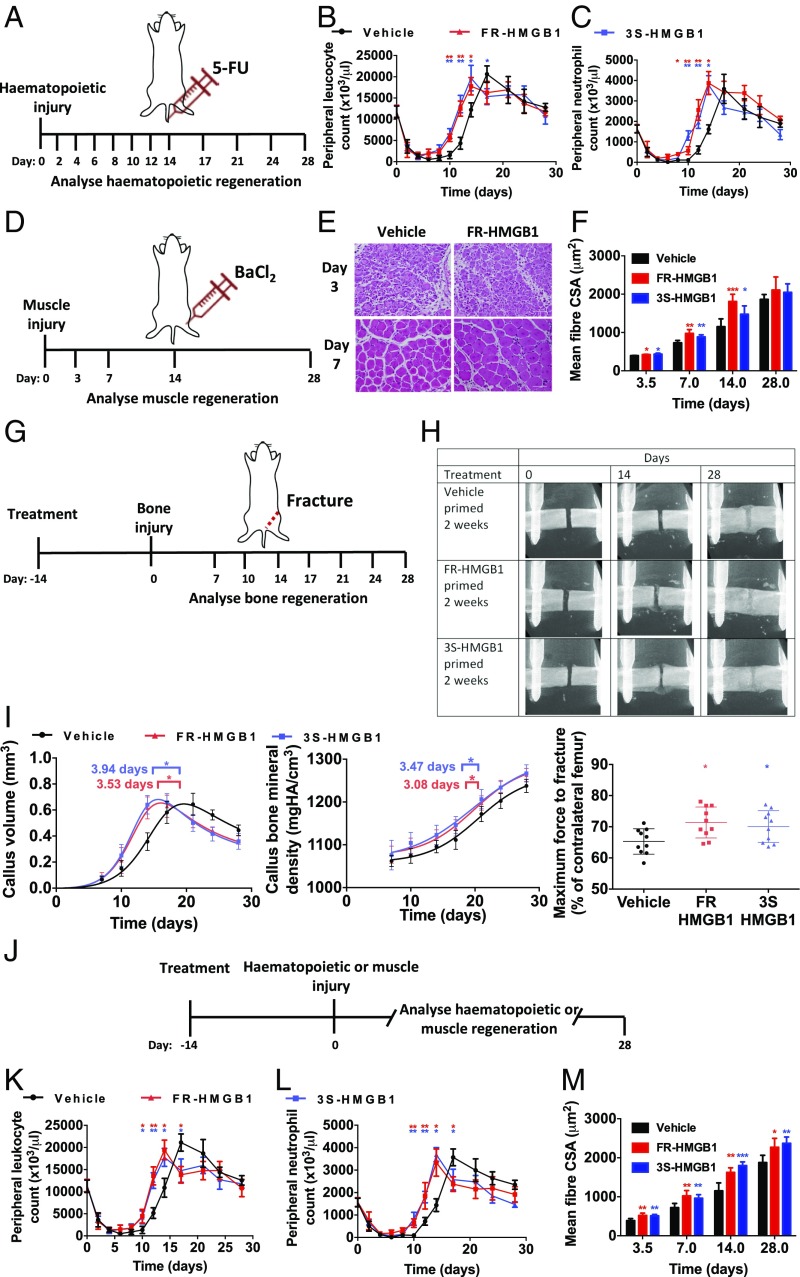
We hypothesized that HMGB1 would also lead to accelerated tissue regeneration in other tissues where stem cells could transition to GAlert, for example blood and muscle. In mice myeloablated with a common chemotherapeutic agent, 5-fluouracil (5-FU) (Fig. 5A), a single i.v. dose of HMGB1 at the time of injury led to accelerated recovery of systemic leukocyte (Fig. 5B) and neutrophil (Fig. 5C) counts. This has significant translational relevance because the duration of leucopenia and neutropenia is directly related to the risk of infection, with each day of neutropenia approximately doubling the risk of a febrile neutropenic episode (42). Febrile neutropenia is a medical emergency with a mortality rate of 6.8–9.5% (43), so accelerating hematopoietic recovery following chemotherapy would make chemotherapy safer for patients. We also found that local administration of a single dose of HMGB1 at the time of injury resulted in accelerated muscle regeneration following BaCl2 chemical injury (Fig. 5 D–F) (11). Our finding that HMGB1 resulted in mSSCs having an increased propensity to cycle that is sustained for several days (Fig. 4A) is consistent with the previous observation that, following injury, stem cells in the contralateral limb remain in GAlert for 3–4 wk (11), and we found that 2 wk post FR- or 3S-HMGB1 administration, mSSCs, mHSCs, and mMuSCs still had elevated ATP (Fig. S6H) even though circulating levels of HMGB1 had already returned to baseline (Fig. S6I). Therefore, we investigated whether pretreatment with a single i.v. dose of HMGB1 2 wk before injury would also accelerate bone, hematopoietic, and muscle tissue regeneration. We observed accelerated tissue regeneration in all these tissues (Fig. 5 G–M). However, regeneration was observed only following injury, with no ectopic tissue formation in the 2-wk period between HMGB1 treatment and injury. This indicates that HMGB1 treatment is a dynamic and adaptive form of multitissue regenerative therapy, which takes cues from the steady-state or tissue-specific activating regenerative molecular signals present at that time. The preadministration of HMGB1 would be particularly relevant in situations of planned or expected injury, including elective surgery, sports medicine, or military combat.
Fig. 5.
HMGB1 accelerates healing of multiple tissues, even if administered 2 wk before injury. (A–C) Systemic administration of FR- or 3S-HMGB1 accelerates hematopoietic recovery following 5-FU myeloablation (A), as shown by peripheral leukocyte (B) and neutrophil (C) counts (n = 8 mice each for the FR- and 3S-HMGB1 conditions; n = 9 mice for vehicle-treated controls). (D–F) Local administration of FR- or 3S-HMGB1 accelerates muscle regeneration following BaCl2 injury (D) as shown by increased muscle fiber cross-sectional area (CSA) (E and F) (n = 4 for each condition and time point). (G–M) Systemic administration of FR- or 3S-HMGB1 2 wk before injury accelerates fracture healing (G) as shown by in vivo microCT radiographs (H), callus volume, callus BMD, and mechanical strength (I) (n = 10 mice for each condition), hematopoietic recovery (J) as shown by peripheral leukocyte (K) and neutrophil (L) counts (n = 8 mice each for FR- and 3S-HMGB1 conditions; n = 9 mice for vehicle-treated controls), and muscle regeneration (J) as shown by increased muscle fiber cross-sectional area (CSA) (M) (n = 5 mice for each condition and time point). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
We have identified HMGB1 as a therapeutic target that acts on multiple endogenous adult stem cells to accelerate the physiological regenerative response to current or future injuries. These findings have broad relevance to the fields of stem cell biology and regenerative medicine and suggest a therapeutic approach to promote tissue repair. The existence of the GAlert phase, which is intermediate between G0 and G1, was described previously (11). It was noted that stem cells in GAlert enter the cell cycle faster than those in G0, and initiators of this transition would have wide-ranging implications for the field of regenerative medicine by accelerating repair.
We have demonstrated that HMGB1 accelerates the healing of multiple tissue types by forming a heterocomplex with CXCL12, which then binds to CXCR4 to transition quiescent stem cells in three different tissues to GAlert. A recent publication (20) showed that HMGB1 promotes repair in a murine model of muscle injury in part by modulating the immune response. We utilized prospective multiparameter flow cytometry isolation methodologies to study the cycling of well-defined endogenous adult stem cell populations in vivo to reduce potential in vitro artifacts and identified a mechanism of action of FR-HMGB1 during tissue repair via the initiation of the GAlert state. Furthermore, we demonstrated that this also pertains to human stem and progenitor cells.
While our work has focused on endogenous adult stem cells, it is possible that the transition to GAlert by HMGB1 may also pertain to other cell types that are usually quiescent in the steady state, can express CXCR4, and are capable of reentering the cell cycle to effect tissue repair, such as mature hepatocytes. Indeed, it was recently observed that HMGB1 treatment results in enhanced proliferation of hepatocytes following injury (20). Using clinically relevant injury models of fracture repair, the response to chemotherapy, and muscle regeneration in conjunction with human tissues and cells, we have demonstrated that FR-HMGB1 leads to accelerated regeneration of multiple tissues by transitioning the respective stem cells to GAlert.
HMGB1 has critical intracellular and extracellular functions as demonstrated by the lethality of the constitutive global knockout (44). In the nucleus HMGB1 interacts with nucleosomes, transcription factors, and histones and thus regulates gene transcription. It has recently been shown that muscle regeneration is compromised in partial Hmgb1+/− mice (20). We show here that fracture healing is dramatically impaired in conditional Hmgb1−/− with robust intracellular and extracellular protein knockdown and that stem cells fail to transition to GAlert. We confirmed at the cellular level that exogenous HMGB1 can rescue the GAlert phenotype but did not evaluate the rescue at the tissue-healing level as exogenous HMGB1 addition would not compensate for the critical intranuclear roles of HMGB1 (44).
While extracellular FR-HMGB1 enhances cell migration by forming a heterocomplex with the relatively abundant CXCL12 that is produced following injury, our data show that the enhanced regenerative effects of the heterocomplex extend beyond those explained by increased chemotaxis. Indeed, our finding that systemic pretreatment with HMGB1 2 wk before injury also accelerates tissue regeneration, with stem cells remaining in GAlert at this time point (Fig. S6H) despite no extracellular HMGB1 being detectable systemically to mediate chemotaxis or other processes (Fig. S6I), suggests that the cellular transition to GAlert is a central mechanism of the accelerated repair process. This finding also expands the use of HMGB1 into the contexts of planned or expected potential injury, such as in sports medicine, military combat, and elective surgery. The last is an area of urgent unmet medical need, as each person in the United States undergoes on average 9.2 surgical procedures in their lifetime (45).
HMGB1 is a pleotropic factor, with contrasting effects depending on the redox status. Our in vitro screen confirmed that only priming of human bone marrow-derived MSCs by FR- or 3S-HMGB1 promoted osteogenesis on subsequent exposure to osteogenic factors. We did not find that exogenous administration of FR-HMGB1 either locally or systemically resulted in any untoward inflammation, suggesting that potential conversion to the proinflammatory disulfide form may not be a limitation when considering the development of a therapeutic. Furthermore, we did not observe any significant difference in the regenerative effects of 3S- compared with FR-HMGB1.
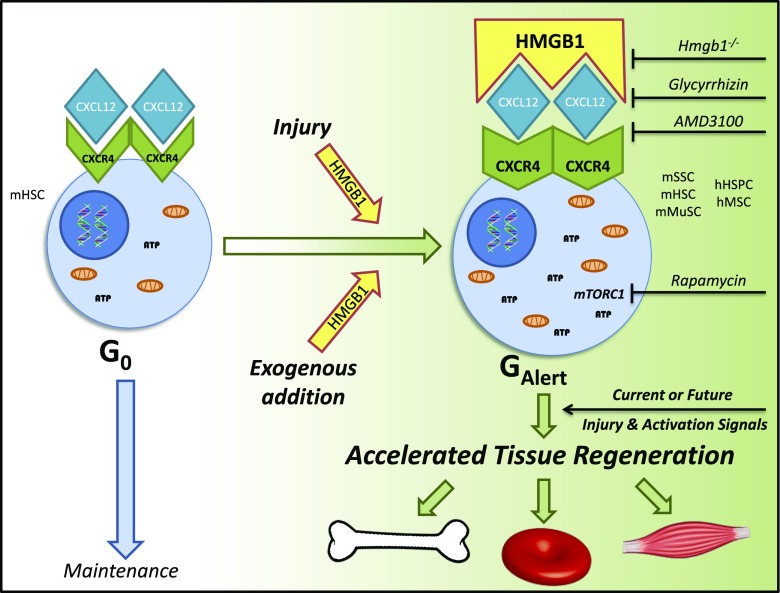
We propose a model (Fig. 6) in which a highly conserved injury signal, HMGB1, acts via a well-established maintenance-signaling pathway, CXCL12–CXCR4, to ultimately promote tissue regeneration. Potentially this pathway may be targeted to accelerate healing in any tissue that relies on repair by cells that express CXCR4 and can transition to GAlert. In the clinic, we envisage that FR-HMGB1 would be administered as a single dose either locally or systemically soon after injury or even up to 2 wk beforehand to accelerate healing.
Fig. 6.
Schematic of accelerated tissue regeneration pathway via the dynamic and adaptive HMGB1–CXCL12–CXCR4–GAlert axis. The CXCL12–CXCR4 axis is known to maintain stem cell quiescence. The highly conserved alarmin, HMGB1, when released following injury or administered in its fully reduced state, forms a heterocomplex with CXCL12, which then binds to CXCR4, to transition resident stem cells to GAlert. Stem cells remain in this primed state for at least 2 wk, and when they are exposed to activating factors, such as those released following current or future injury, the cells in GAlert enter the cell cycle faster than cells in G0. This results in accelerated regeneration of multiple tissues.
Materials and Methods
The objective of this study was to understand the role of alarmins in tissue regeneration in vivo through their effects on adult stem cells and the translational relevance of these findings. We used human samples and primary human cells and multiple murine models of injury and regeneration. For prospective multiparameter flow cytometry assays, we used well-established skeletal, hematopoietic, and muscle stem cell-surface markers and published isolation protocols (30, 40, 46). Sample sizes (n values) are reported as biological replicates of human donors and mice. The data presented were compiled over the course of 3 y as human samples and mice with the appropriate genotype became available. The magnitude of the effect and variability in the measurements were used to determine sample size and replication of data. Although samples were not specifically randomized or blinded, mouse identification numbers were used when possible as sample identifiers. Therefore, the genotypes and experimental conditions of each mouse/sample were not readily known to the experimenters during sample processing and data collection. Animals were excluded from the study only if their health status was compromised. In these cases, the animals were handled in accordance with Home Office guidelines.
All animal procedures were approved by the institutional ethics committee and the United Kingdom Home Office (PLL 71/7161 and PLL 30/3330). Plasma samples from human patients who had sustained femoral fractures and from healthy unfractured controls were obtained from the John Radcliffe Hospital (Research Ethics Committee Reference: 16/SW/0263, Oxford University Hospital Research and Development Project Identification Reference: 12229, Integrated Research Application System Project Identification: 213014).
See SI Materials and Methods for a more detailed description of the materials and methods used.
Supplementary Material
Acknowledgments
We thank T. Taniguchi, H. Yanai, and J. Nishio for supplying Hmgb1fl/fl mice; E. Venereau and M. Bianchi for facilitating the purchase of HMGB1 reagents from HMGBiotech; C. Chan for SSC expertise; A. Grover and C. Nerlov for HSC expertise; L. Chantranupong for mTOR expertise; C. Peele and O. Dushek for assistance with mathematical modeling; M. Uguccioni for sharing the HMGB1–CXCL12 heterocomplex hybrid ELISA protocol; E. Abu Shah for technical assistance with time-lapse microscopy; V. Kumar and A. Vinals Guitart for computational programming for migration analysis; N. Mullee, T. James, B. Shine, P. Sangeetha, K. Lewis, E. Tutton, M. Costa, and K. Willett for acquisition of human fractured plasma samples; E. Abu Shah, H. Novak, and P. Demetriou for sharing human unfractured plasma samples; E. Clutterbuck for assistance with magnetically activated cell sorting (MACS); J. Webber for flow cytometry and FACS services; and B. Stott, I. Parisi, and M. Curtinha for histology services. This work was supported by Medical Research Council Grant MR/K007939/1 (to M.F., N.J.H. and J.N.), Arthritis Research UK Grant 21190 (to A.I.E.S., M.F., N.J.H., and J.N.), Academy of Medical Sciences Grant SGL014/1003 (to J.K.C.), and Kennedy Trust for Rheumatology Grants MSP131411 and KENN151611 (to G.L., A.I.E.S., M.F., N.J.H., and J.N.).
Footnotes
Conflict of interest statement: The University of Oxford has filed patent applications on behalf of the investigators (G.L., A.I.E.S., M.F., N.J.H., J.K.C., and J.N.) claiming some of the concepts described in this publication.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802893115/-/DCSupplemental.
References
- 1.Weissman IL. Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.Gratwohl A, et al. Worldwide Network for Blood and Marrow Transplantation (WBMT) One million haemopoietic stem-cell transplants: A retrospective observational study. Lancet Haematol. 2015;2:e91–e100. doi: 10.1016/S2352-3026(15)00028-9. [DOI] [PubMed] [Google Scholar]
- 3.Brooks M. Stem cell research: Time for a dose of realism. BMJ. 2017;356:j443. doi: 10.1136/bmj.j443. [DOI] [PubMed] [Google Scholar]
- 4.Trainor N, Pietak A, Smith T. Rethinking clinical delivery of adult stem cell therapies. Nat Biotechnol. 2014;32:729–735. doi: 10.1038/nbt.2970. [DOI] [PubMed] [Google Scholar]
- 5.Dimmeler S, Ding S, Rando TA, Trounson A. Translational strategies and challenges in regenerative medicine. Nat Med. 2014;20:814–821. doi: 10.1038/nm.3627. [DOI] [PubMed] [Google Scholar]
- 6.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat Med. 2014;20:857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 7.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32:795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, et al. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science. 2015;348:aaa2340. doi: 10.1126/science.aaa2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph J, Dyson M. The effect of abdominal wounding on the rate of tissue regeneration. Experientia. 1970;26:66–67. doi: 10.1007/BF01900396. [DOI] [PubMed] [Google Scholar]
- 10.Davis TA, Longcor JD, Hicok KC, Lennon GG. Prior injury accelerates subsequent wound closure in a mouse model of regeneration. Cell Tissue Res. 2005;320:417–426. doi: 10.1007/s00441-005-1107-7. [DOI] [PubMed] [Google Scholar]
- 11.Rodgers JT, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harry LE, et al. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res. 2008;26:1238–1244. doi: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 13.Glass GE, et al. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci USA. 2011;108:1585–1590. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan JK, et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol Med. 2015;7:547–561. doi: 10.15252/emmm.201404487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan JK, et al. Alarmins: Awaiting a clinical response. J Clin Invest. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 17.Terrando N, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris HE, Andersson U, Pisetsky DS. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 19.Horiuchi T, et al. Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J Biol Chem. 2017;292:8436–8446. doi: 10.1074/jbc.M116.769380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirone M, et al. High mobility group box 1 orchestrates tissue regeneration via CXCR4. J Exp Med. 2018;215:303–318. doi: 10.1084/jem.20160217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Einhorn TA, Gerstenfeld LC. Fracture healing: Mechanisms and interventions. Nat Rev Rheumatol. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–7713. [PubMed] [Google Scholar]
- 23.Zreiqat H, Howlett CR, Gronthos S, Hume D, Geczy CL. S100A8/S100A9 and their association with cartilage and bone. J Mol Histol. 2007;38:381–391. doi: 10.1007/s10735-007-9117-2. [DOI] [PubMed] [Google Scholar]
- 24.Venereau E, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zwingenberger S, et al. Establishment of a femoral critical-size bone defect model in immunodeficient mice. J Surg Res. 2013;181:e7–e14. doi: 10.1016/j.jss.2012.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanai H, et al. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc Natl Acad Sci USA. 2013;110:20699–20704. doi: 10.1073/pnas.1320808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiraldi M, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mollica L, et al. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Huttunen HJ, Fages C, Kuja-Panula J, Ridley AJ, Rauvala H. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res. 2002;62:4805–4811. [PubMed] [Google Scholar]
- 30.Chan CKF, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Nie Y, Han Y-C, Zou Y-R. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzeng Y-S, et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–439. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 35.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho T-J, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor β superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 38.Maesner CC, Almada AE, Wagers AJ. Established cell surface markers efficiently isolate highly overlapping populations of skeletal muscle satellite cells by fluorescence-activated cell sorting. Skelet Muscle. 2016;6:35. doi: 10.1186/s13395-016-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers JT, Schroeder MD, Ma C, Rando TA. HGFA is an injury-regulated systemic factor that induces the transition of stem cells into GAlert. Cell Rep. 2017;19:479–486. doi: 10.1016/j.celrep.2017.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 43.Lyman GH, et al. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010;116:5555–5563. doi: 10.1002/cncr.25332. [DOI] [PubMed] [Google Scholar]
- 44.Kang R, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee P, Regenbogen S, Gawande AA. 2008 How many surgical procedures will Americans experience in an average lifetime?: Evidence from three states. 55th Annual Meeting (Massachusetts Chapter of the American College of Surgeons, Boston). Available at www.mcacs.org/abstracts/2008/p15.cgi. Accessed June 6, 2017.
- 46.Liu L, Cheung TH, Charville GW, Rando TA. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat Protoc. 2015;10:1612–1624. doi: 10.1038/nprot.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gregor T, et al. Correlating micro-CT imaging with quantitative histology. In: Goswami T, editor. Injury and Skeletal Biomechanics. InTech, Rijeka; Croatia: 2012. [Google Scholar]
- 48.Particelli F, et al. A comparison between micro-CT and histology for the evaluation of cortical bone: Effect of polymethylmethacrylate embedding on structural parameters. J Microsc. 2012;245:302–310. doi: 10.1111/j.1365-2818.2011.03573.x. [DOI] [PubMed] [Google Scholar]
- 49.O’Neill KR, et al. Micro-computed tomography assessment of the progression of fracture healing in mice. Bone. 2012;50:1357–1367. doi: 10.1016/j.bone.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Steiner M, et al. Comparison between different methods for biomechanical assessment of ex vivo fracture callus stiffness in small animal bone healing studies. PLoS One. 2015;10:e0119603. doi: 10.1371/journal.pone.0119603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peytour Y, et al. Obtaining of CD34+ cells from healthy blood donors: Development of a rapid and efficient procedure using leukoreduction filters. Transfusion. 2010;50:2152–2157. doi: 10.1111/j.1537-2995.2010.02683.x. [DOI] [PubMed] [Google Scholar]
- 52.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.