“Be afraid, be very afraid!” Kate Jones warned me over coffee in London. She is an expert on global emergent diseases. Her work showed that new diseases are on the increase and that they usually originate from contact with wild animals as our species occupies and further exploits natural areas on the planet (1). I remembered her warning a few years later in 2014–2015 when an unprecedented Ebola epidemic broke out in West Africa and threatened the rest of the planet. This lethal epidemic likely started because someone, somewhere, cooked and ate a virus-laden bat. In PNAS, Anderson et al. (2) provide another reason to be very afraid: a “mega-pest” of food and fiber has recently invaded the New World from the Old and has begun to mix its genome with a native mega-pest.
The genus Helicoverpa belongs to a group of heliothine moths in the family Noctuidae that are considered mega-pests because of the immense crop damage they cause (3). The Helicoverpa corn earworm and cotton bollworm complex of moth pests (Lepidoptera) are among the world’s most devastating pests of food and fiber crops. Helicoverpa zea is the New World representative, mainly known for its depredations on cotton and corn, and also found on over 100 other plant species belonging to 29 plant families (4). It causes hundreds of millions of dollars of annual damage. Its close relative, the Old World Helicoverpa armigera, is even more devastating, particularly in tropical Africa and Asia. This second pest feeds on over 300 species belonging to 68 plant families and is estimated to inflict over $5 billion of damage annually to crops worldwide (4). Helicoverpa caterpillars are particularly challenging because they prefer to bore into plant reproductive parts, the fruits and/or flowers of crops, the very same parts that are valuable to humans (Fig. 1). Helicoverpa are among the major pests controlled using insecticides and via transgenic crops with insecticidal properties. These control measures are only partially successful, as H. armigera has shown a remarkable penchant for evolving resistance to virtually all insecticidal groups, and also because insect sprays tend to reduce populations of the caterpillars’ parasites and predators, leading to frequent outbreaks of Helicoverpa as “secondary” pests. Helicoverpa zea, in contrast, remains susceptible to many major insecticides and tends to cause less damage than its Old World relative (4).
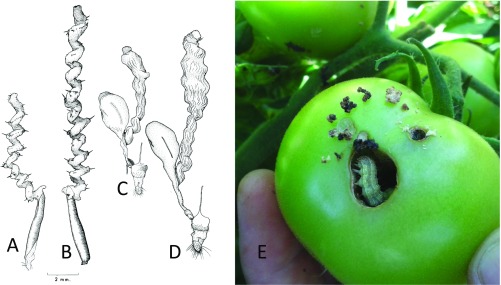
Fig. 1.
Genitalia and larva of Helicoverpa. (A) Male genitalia of H. armigera, showing the aedeagus below and spiral extension above. (B) Male genitalia of H. zea. (C) Female genitalia of H. armigera, showing the spiral diverticulum off the bursa copulatrix (sac on Left of drawing). (D) Female genitalia of H. zea. (E) Larva of H. armigera or a hybrid with H. zea feeding on developing tomato fruit, in Goiás, Brazil. [(A–D) Reproduced from ref. 7, with permission from the Minister of Public Works and Government Services of Canada, 2018; (E) Image courtesy of Cecília Czepak (Universidade Federal de Goiás, Goiânia, Brazil).]
In 2013, H. armigera was found in the New World for the first time (5). The moth was probably introduced to Brazil as caterpillars via trade in fresh fruit or vegetables. Some genetic evidence suggested hybrids between H. armigera and H. zea (6). However, could they hybridize? The two species are closely related (3), but H. zea populations contain only about one-half the genetic diversity of H. armigera, suggesting that H. zea speciated as a result of transoceanic colonization around 1.5 My ago and was thereafter subject to population bottlenecks (4). Anderson et al. (2) use genome resequencing data to show convincingly that H. armigera and hybrids between the two species are indeed present in Brazil and that the source of the recent invasion was from an African/European population.
The Latin name Helicoverpa (“spiral penis”) refers to an unusual feature of this moth genus (7): Helicoverpa have among the most elaborate genitalia of any lepidopteran. The males are equipped with an enormous, inflatable, spiny, spiral extension to the aedeagus (the name applied to the penis of an insect) (Fig. 1 A and C). The females have a matching spiral diverticulum, the appendix bursa, off their bursa copulatrix (Fig. 1 B and D). The bursa copulatrix is the name given to the internal sac that stores the lepidopteran spermatophore—a proteinaceous package containing the sperm that the male transfers during mating. Hardwick (7) fixed mating pairs in the act, so to speak, by dropping 48 pairs of Helicoverpa armigera at various stages of copulation and mating into boiling water. He then preserved these pairs in alcohol and dissected them, and so was able to deduce the mechanism and time course of mating. After successful courtship, the male first inserts his chitinous aedeagus into the female’s mating canal. From within the aedeagus, the spiny extension then begins to be inflated along the female appendix bursae. After full inflation of the penis extension, the soft spermatophore is extruded as a flexible mass from the tip of the penis extension and travels back down the central canal formed by the spiral appendix bursae, now fully stretched by the turgid extension of the aedeagus, into the main chamber of the bursa, where it expands rapidly to a balloon-like shape. The spiral penis extension is then slowly retracted from the tip, coil by coil, into the aedeagus. Finally, if all is well, the aedeagus is then withdrawn, leaving a slowly hardening spermatophore with its precious packet of sperm in place in the bursa (7). Successful mating took around 90 min on average. Hardwick also found that successfully mated females often displayed scar tissue in the appendix bursae due to the stabbing effect of the spines on the penis extension (Fig. 1 A and B). Hardwick’s and subsequent work led to an invention by Australia’s Commonwealth Scientific and Industrial Research Organisation of the “Phalloblaster,” a specialized kit for inflating and hardening lepidopteran aedeagus extensions for identification and specimen preservation (8).
The reader at this point might infer that the author is obsessed with details about genitalia. Lepidopterists are indeed renowned for this, but there is an important biological issue here. Why do Helicoverpa have such complex copulation mechanics, and how does it affect mating between species? We do not know, but penis elaboration is an expected vagary of sexual selection and occurs across all animals (9). Regardless of its origin, penis complexity seems to cause problems. A frequent outcome of mating between Helicoverpa species, for example, H. zea × H. armigera matings, is to “lock up,” leading to death without reproduction (7, 10). The male is for some reason unable successfully to withdraw his elaborate penis extension from the female; perhaps the hardening spermatophore seals the mating partners together. The penis extension of H. zea is much larger than that of H. armigera (Fig. 1 A and B), as is the corresponding diverticulum of the female bursa (Fig. 1 C and D), and so it seems likely that the “lock-and-key” mechanism will often fail in interspecific matings.
My superficial reading of Hardwick’s paper led to my citing this in lectures on speciation as a good example of mechanical reproductive isolation between species. On rereading Hardwick (7), I now realize that I oversimplified my teaching. Lock-ups do not always occur between H. zea and H. armigera, and fertile hybrids are produced in a fraction of interspecific matings in the laboratory. Although lock-up can occur in matings between species, some matings are successful, and lock-up also occurs within species (7, 10)—a cost, perhaps, of the runaway sexual selection that led to penis elaboration. Therefore, it is possible that some hybridization and backcrossing between the two species could occur, albeit with reduced rates of success, as has now been demonstrated by the new genomic data from Brazil.
Peculiarly, most of the resequenced hybrids appear closer to H. armigera than to H. zea, suggesting that for the moment few nuclear loci from H. armigera are flowing into H. zea (2). The apparent rarity of backcrossing to H. zea in the Brazilian hybrid individuals suggests that hybridization will perhaps not introduce novel insecticide resistance and other genes to H. zea as feared. However, the reverse introduction is just as worrying because it suggests that locally adapted H. zea genes may help H. armigera spread in the New World.
Could it be that we are witnessing the birth of a new hybrid species? This is hard to predict. However, hybrid origin has led repeatedly to other invasive species. For instance, Spartina anglica, a maritime grass that aggressively colonizes salt marshes, arose in 19th-century England as a result of an allopolyploid hybridization between a native species and an introduced North American species (11). An extraordinary recent case is the marbled crayfish Procambarus virginalis, which seems to have originated via a hybrid between two North American Procambarus species and was likely spread via the pet trade. The marbled crayfish is a triploid hybrid, very likely created in captivity, and is entirely parthenogenetic. After escaping from captivity, it has since spread to become invasive in many European countries as well as in Madagascar (12). Finally, “coywolves,” hybrids between coyote and wolf populations, appear to be spreading in eastern North America. The hybrids have genes from both parents that may form combinations that are adaptive in an increasingly anthropogenic landscape (13).
Evolutionary biologists expect species to evolve via millions of years of adaptive evolution, so a priori it seems highly unlikely that a successful invasive species would ever originate in a pet store. However, this does appear to have happened with the marbled crayfish. The trouble is, natural selection is very good at amplifying rare but advantageous combinations of genes. As unlikely as some of the origins of these invasive hybrid species might sound, these cases prove that human transport of organisms to new environments can lead to invasive species that gain variation from more than one parent. With the H. armigera/H. zea hybrids, we may be witnessing the start of another case of hybridization leading to an even worse mega-pest than we had before.
Invasive hybrid species are part of a larger problem of invasive species in general. Recently, another major polyphagous noctuid pest, the fall armyworm Spodoptera frugiperda (14, 15), has been confirmed to have caused outbreaks on corn and other crops in tropical Africa. This species has travelled in the opposite direction, invading Africa from the Americas. Although no hybridization with native Spodoptera has yet been confirmed, the invasive forms appear to represent divergent genotypes not hitherto recorded in typical corn or rice strain S. frugiperda (15). Even if not hybridizing, this new armyworm for Africa is inflicting major damage on subsistence crops in a region of the world where famines still regularly occur. Invasive species, whether hybridizing or not, are a kind of venereal disease of continents increasingly interconnected by trade. We should be afraid, and we need better research effort to design strategies and guard against such episodes in future.
Acknowledgments
I thank David G. Heckel for catching a number of errors, for personal communications on his unpublished study of H. armigera × H. zea hybridization, and for his suggestions for including the fall armyworm invasion information.
Footnotes
The author declares no conflict of interest.
See companion article on page 5034.
References
- 1.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson CJ, et al. Hybridization and gene flow in the mega-pest lineage of moth, Helicoverpa. Proc Natl Acad Sci USA. 2018;115:5034–5039. doi: 10.1073/pnas.1718831115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho S, et al. Molecular phylogenetics of heliothine moths (Lepidoptera: Noctuidae: Heliothinae), with comments on the evolution of host range and pest status. Syst Entomol. 2008;33:581–594. [Google Scholar]
- 4.Pearce SL, et al. Genomic innovations, transcriptional plasticity and gene loss underlying the evolution and divergence of two highly polyphagous and invasive Helicoverpa pest species. BMC Biol. 2017;15:63. doi: 10.1186/s12915-017-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czepak C, Albernaz KC, Vivan LM, Guimarães HO, Carvalhais T. Primeiro registro de ocorrência de Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) no Brasil. Pesqui Agropecu Trop. 2013;43:110–113. [Google Scholar]
- 6.Anderson CJ, Tay WT, McGaughran A, Gordon K, Walsh TK. Population structure and gene flow in the global pest, Helicoverpa armigera. Mol Ecol. 2016;25:5296–5311. doi: 10.1111/mec.13841. [DOI] [PubMed] [Google Scholar]
- 7.Hardwick DF. The corn earworm complex. Mem Entomol Soc Can. 1965;97:5–247. [Google Scholar]
- 8.Matthews M. The CSIRO vesica everter: A new apparatus to inflate and harden eversible and other weakly sclerotised structures in insect genitalia. J Nat Hist. 1998;32:317–327. [Google Scholar]
- 9.Eberhard WG. Sexual Selection and Animal Genitalia. Harvard Univ Press; Cambridge, MA: 1985. [Google Scholar]
- 10.Laster ML, Sheng CF. Search for hybrid sterility for Helicoverpa zea in crosses between the North American H. zea and H. armigera (Lepidoptera: Noctuidae) from China. J Econ Entomol. 1995;88:1288–1291. [Google Scholar]
- 11.Ainouche ML, Baumel A, Salmon A. Spartina anglica C. E. Hubbard: A natural model system for analysing early evolutionary changes that affect allopolyploid genomes. Biol J Linn Soc Lond. 2004;82:475–484. [Google Scholar]
- 12.Gutekunst J, et al. Clonal genome evolution and rapid invasive spread of the marbled crayfish. Nat Ecol Evol. 2018;2:567–573. doi: 10.1038/s41559-018-0467-9. [DOI] [PubMed] [Google Scholar]
- 13.vonHoldt BM, Kays R, Pollinger JP, Wayne RK. Admixture mapping identifies introgressed genomic regions in North American canids. Mol Ecol. 2016;25:2443–2453. doi: 10.1111/mec.13667. [DOI] [PubMed] [Google Scholar]
- 14.Goergen G, Kumar PL, Sankung SB, Togola A, Tamò M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One. 2016;11:e0165632. doi: 10.1371/journal.pone.0165632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagoshi RN, et al. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci Rep. 2018;8:3710. doi: 10.1038/s41598-018-21954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]