Abstract
Background
The aim of this study was to investigate the effect of curcumin treatment on the expression of the N-methyl-D-aspartate receptor (NMDAR) subunit, NR2A, in a rat PC12 cell line treated with the acetyl amyloid-β peptide, Aβ(25–35), in an in vitro model of Alzheimer’s disease.
Material/Methods
PC12 cells, derived from rat phaeochromocytoma, were treated for 24 hours with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of the acetyl amyloid-β peptide, Aβ(25–35). A Cell Counting Kit-8 (CCK-8) assay was used to determine cell viability, and flow cytometry was used to measure cell apoptosis. In the supernatant of the treated PC12 cells, Western blotting was used to measure the cell injury biomarker, lactate dehydrogenase (LDH), and the biomarker for oxidative stress, malondialdehyde (MDA). Expression of the N-methyl-D-aspartate receptor (NMDAR) subunit, NR2A, was analyzed by Western blotting and quantitative reverse transcription-polymerase chain reaction (qRT-PCR).
Results
Curcumin treatment protected the rat PC12 cells from Aβ(25–35)-induced reduction in cell viability, apoptosis, release of LDH, and MDA production. Curcumin treatment of PC12 cells was associated with increased expression of the NMDAR subunit, NR2A.
Conclusions
The findings of this study showed a neuroprotective effect of curcumin treatment in an in vitro model of Alzheimer’s disease that was associated with the increased expression of the NMDAR subunit, NR2A.
MeSH Keywords: Alzheimer Disease; Apoptosis; Cell Survival; Curcumin; Receptors, N-Methyl-D-Aspartate
Background
Alzheimer’s disease is the most common type of dementia in elderly people and is a progressive neurodegenerative disease [1,2]. Progressive cognitive impairment and memory loss are the major features of Alzheimer’s disease [3]. The accumulation of beta-amyloid (Aβ) has been shown to induce neurotoxicity and oxidative stress, which are involved in the pathogenesis of Alzheimer’s disease [4].
Knowledge of Aβ-induced neuronal cell injury in Alzheimer’s disease has resulted in the widespread use of in vitro cell models to study this disease [5]. Previously, in vitro studies have been an important component of preclinical studies on the effects of some approved drugs for Alzheimer’s disease, such as rivastigmine, galantamine, and donepezil [4,5]. Although there have been an increasing number of preclinical and clinical studies on the pathogenesis and treatment of Alzheimer’s disease, there are still no effective treatments. Therefore, there remains an urgent need to find novel approaches for the treatment of Alzheimer’s disease.
Curcumin, or 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, is a natural active ingredient extracted from the rhizomes of turmeric plants. Several studies have now shown that curcumin has a variety of pharmacological properties that can affect cancer, inflammation, and result in neuroprotection [6–9]. Previously published studies have been undertaken to determine the potential therapeutic effects of curcumin in Alzheimer’s disease [10]. Curcumin has been shown to reduce Aβ oligomer and fibril formation [11]. Previous studies also have shown that curcumin inhibited Aβ-induced neurotoxicity in the brain [12,13]. Studies have also found that curcumin suppressed Aβ-induced inflammation and reduced the levels of inducible nitric oxide synthase (iNOS) and interleukin (IL)-1β in the brain of transgenic mouse models [14–16]. Also, in 2008, the findings of a pilot randomized, controlled, clinical trial showed beneficial effects of curcumin in patients with Alzheimer’s disease [17]. However, the mechanism of neuroprotection exerted by curcumin remains unknown.
The N-methyl-D-aspartate receptor (NMDAR) is a glutamate receptor and ion channel protein found in nerve cells. NMDAR contains the NR1 and NR2 receptor sub-units; the NR2 subunit of NMDAR is a key factor the organization and function of neuronal synapses and in NMDAR function. Previously published studies have shown that NR2A and NR2B were abundant in brain tissues, with the highest levels in the hippocampus and cerebral cortex, with NR2A and NR2B regulating the NMDA receptor-mediated excitatory postsynaptic potential (EPSP), and inducing long-term potentiation (LTP) formation, all of which are factors that affect the synaptic plasticity of the central nervous system [18]. NR2A has been shown to promote neuronal survival and protect against neuronal damage [19].
The aim of this study was to investigate the effect of curcumin treatment on the expression of the NMDAR subunit, NR2A, in a rat PC12 cell line treated with the acetyl amyloid-β peptide, Aβ(25–35), in an in vitro model of Alzheimer’s disease.
Material and Methods
Preparation of the acetyl amyloid-β peptide, Aβ(25–35)
Acetyl amyloid-β peptide, Aβ(25–35), aggregation was prepared as previously described [20]. Briefly, lyophilized Aβ(25–35) was dissolved in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and incubated at 37°C with constant mixing for three days to induce aggregation. The aggregated Aβ(25–35) was then diluted to 100 μg/mL (100 μM) and stored at −20°C until required.
PC12 cell culture and the development of the in vitro Alzheimer’s disease model
The PC12 rat pheochromocytoma cell line was obtained from American Type Culture Collection (ATCC). PC12 cells were grown in DMEM medium supplemented with 10% fetal bovine serum (FBS) (Sangon Biotech Co. Ltd., Shanghai, China), including 1% penicillin and streptomycin solution, at 37°C with 5% CO2. Cells were passaged when 80% confluence was reached.
For the in vitro cell model, PC12 cells were seeded in 96-well plates, at 3×104 cells in 100 μl per well. After incubation for 24 h, 20 μM of aggregated Aβ(25–35) dissolved in serum-free DMEM was added, followed by incubation for 24 h at 37°C. PC12 cells (3×104 cells per well) were treated with for 24 h with increasing concentrations of curcumin (5, 10, 20, 30 μM/l) in the presence of 20 μM of Aβ(25–35).
Cell viability assay
PC12 cells were plated into 96-well plates, at 3×104 cells per well, and cultured in DMEM medium at 37°C for 24 h. The cells were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of 20 μM of Aβ(25–35) for 24 h. Cells in the control group were treated with the same volume of DMEM medium. At the end of the experiment, cell viability was assessed using a Cell Counting Kit-8 (CCK-8) assay kit, according to the manufacturer’s instructions. The assays were performed in triplicate.
Detection of cell apoptosis
PC12 cells, at 3×104 cells per well, were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of 20 μM of Aβ(25–35) for 24 h. PC12 cells were then harvested and washed with cold PBS. PC12 cell apoptosis was determined using a cell apoptosis assay. Briefly, PC12 cells (1×106) from each group were re-suspended in binding buffer and then labeled with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) using an Annexin-V FITC/PI kit (BD Pharmingen, San Diego, CA, USA), according to the manufacturer’s instructions. Flow cytometry analysis was performed using the BD FACS Aria (BD Biosciences, Franklin Lakes, NJ, USA) to analyze and quantify the cells. The assays were performed in triplicate.
Lactate dehydrogenase (LDH) and malondialdehyde (MDA) measurements
PC12 cells (3×104 cells per well) were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of 20 μM of Aβ(25–35) for 24 h. Cell injury was assessed by measuring the LDH activity in the supernatant of PC12 cells, using an LDH kit, according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Measurement of the oxidative stress biomarker, malondialdehyde (MDA) in the supernatant of the PC12 cells was detected using a commercial kit (Nanjing Jiancheng Bioengineering Institute) following the manufacturer’s instructions. The results were compared with the non-treated control. The assays were performed in triplicate.
Western blot analysis
PC12 cells, at 3×104 cells per well, were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of 20 μM Aβ(25–35) for 24 h. Then log-phase PC12 cells were collected, and RIPA buffer (Auragene, Changsha, China) was used to extract the total cellular proteins. A BCA protein quantitation kit (Thermo, USA) was used to detect the concentration of protein samples. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis was performed to separate the protein samples. The separated proteins were then transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% skimmed milk powder at room temperature for 1 h, and incubated at 4°C overnight with primary polyclonal rabbit antibodies that included anti-β-actin (1: 1000), anti-NR2A (1: 1000), anti-caspase-3 (1: 1000), anti-pAKT (1: 1000) (Cell Signaling Technology, Inc., Danvers, MA, USA). The membranes were washed and then incubated with a horseradish peroxidase (HRP)-conjugated secondary anti-rabbit IgG (1: 5,000) at room temperature for 1 h. To visualize the protein bands, an enhanced chemiluminescence (ECL) kit (Applygen Technologies Inc., Beijing, China) was used, according to the manufacturer’s instructions.
Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
To extract the total RNA from the PC12 cells, TRIZOL reagent (Takara, Japan) was used, according to the manufacturer’s instructions. The A260/A280 ratio was used to evaluate the quality and integrity of the RNA. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control. The PrimeScript™ RT reagent kit (Takara, Japan) was used to generate cDNAs. SYBR Premix Ex Taq (Takara, Japan) was used for RT-PCR analysis, according to the manufacturer’s instructions. Primer sequences used for real-time PCR were obtained from Gen Script Co., Ltd., Nanjing, China, as required.
Statistical analysis
All data were presented as the mean ± standard deviation (SD). The SPSS 17.0 statistical software (SPSS, Chicago, IL, USA) was used for statistical analysis. The Student’s t-test or analysis of variance (ANOVA) were used for statistical comparison between groups. Statistical significance was assumed to be p<0.05.
Results
Effects of curcumin on the cell viability of Aβ(25–35)-treated PC12 cells
Acetyl amyloid-β peptide, Aβ(25–35)-treated PC12 cells were used as an in vitro cell model for Alzheimer’s disease. The effect of curcumin on PC12 cell viability was determined by performing a Cell Counting Kit-8 (CCK-8) assay. As shown in Figure 1, Aβ(25–35) suppressed PC12 cell viability when compared with the control group. Compared with the Aβ(25–35) treatment alone of PC12 cells, curcumin treatment significantly improved PC12 cell viability, in a dose-dependent manner (p<0.01).
Figure 1.
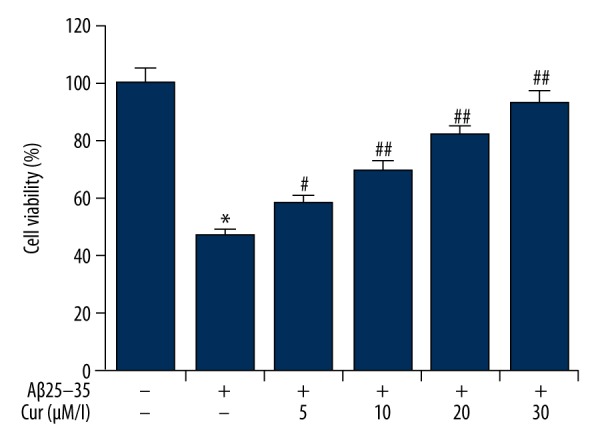
Effects of curcumin on the cell viability in Aβ(25–35)-treated PC12 cells. PC12 cells were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of 20 μM of Aβ(25–35) for 24 h. Cell viability was assessed using the Cell Counting Kit-8 (CCK-8) assay. Data are shown as mean ±SD. * p<0.05 vs. non-treated group; #, ## p<0.05, 0.01 vs. the Aβ(25–35) treatment alone group. Cur – curcumin.
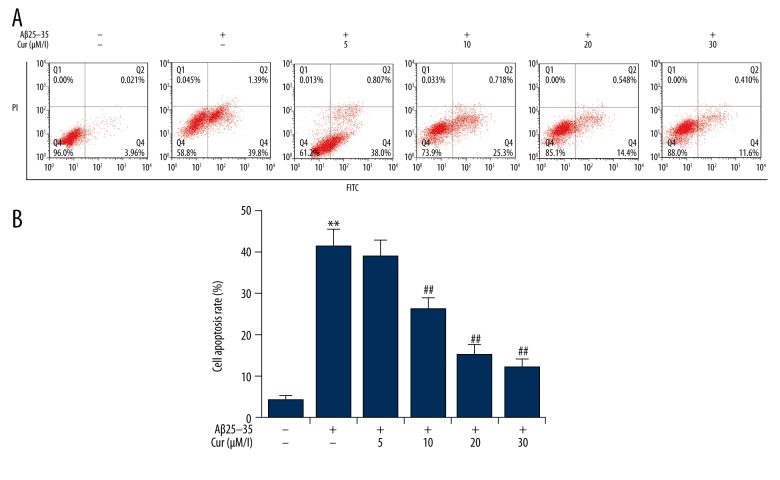
Effects of curcumin on Aβ(25–35)-induced apoptosis in PC12 cells
The effect of curcumin on cell apoptosis in Aβ(25–35)-treated PC12 cells was analyzed by flow cytometry. The findings were that PC12 cell apoptosis was significantly induced by Aβ(25–35) treatment. Curcumin treatment significantly reduced Aβ(25–35)-induced PC12 cell apoptosis, in a dose-dependent manner (Figure 2).
Figure 2.
Effects of curcumin on the cell apoptosis in Aβ(25–35)-treated PC12 cells. PC12 cells were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/l) in the presence of 20 μM Aβ(25–35) for 24 h. Cell apoptosis was determined by flow cytometry. Data are shown as mean ±SD. ** p<0.01 vs. non-treated group; ## p<0.01 vs. Aβ(25–35) treatment alone group. Cur – curcumin.
Effects of curcumin on the release of lactate dehydrogenase (LDH) in Ab(25–35)-treated PC12 cells
The release of cellular lactate dehydrogenase (LDH) was considered to be an indicator for Ab(25–35)-induced cytotoxicity. As shown in Figure 3, Ab(25–35) treatment increased the release of LDH from PC12 cells when compared with the control group. Curcumin treatment significantly reduced Ab(25–35)-induced LDH release by PC12 cells, in a dose-dependent manner.
Figure 3.
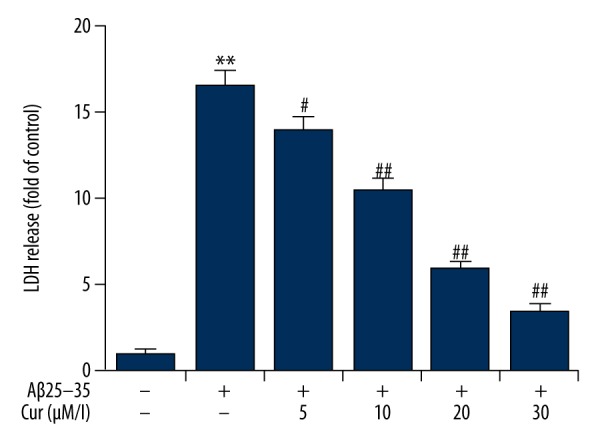
Effects of curcumin on the release of lactate dehydrogenase (LDH) in Aβ(25–35)-treated PC12 cells. PC12 cells were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of 20 μM Aβ(25–35) for 24 h. The release of LDH was measured by using a LDH Cytotoxicity Assay kit, and the result is expressed as the fold increase of the control (non-treated) group. Data are shown as mean ±SD. ** p<0.01 vs. non-treated group; #, ## p<0.05, 0.01 vs. the Aβ(25–35) treatment alone group. Cur – curcumin.
Effects of curcumin on malondialdehyde (MDA) production in Aβ(25–35)-treated PC12 cells
Levels of the oxidative stress biomarker, malondialdehyde (MDA), were determined in this study. The findings were that Aβ(25–35) significantly increased the production of MDA in the supernatant of PC12 cells, and this increase was significantly inhibited by curcumin treatment, in a dose-dependent manner (Figure 4).
Figure 4.

Effects of curcumin on the malondialdehyde (MDA) content of Aβ(25–35)-treated PC12 cells. PC12 cells were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of 20 μM Aβ(25–35) for 24 h. The levels of MDA level was measured by using an MDA detection kit, and the result is expressed as the fold increase of the control (non-treated) group. Data are shown as mean ±SD. ** p<0.01 vs. non-treated group; ## p<0.01 vs. Aβ(25–35) treatment alone group. Cur – curcumin.
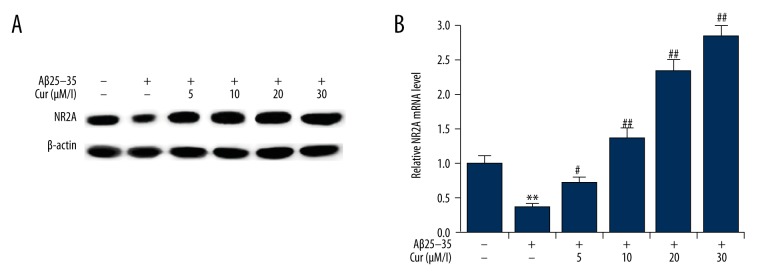
Effects of curcumin on the expression of NR2A in Aβ(25–35)-treated PC12 cells
Because the expression of the N-methyl-D-aspartate receptor (NMDAR) subunit, NR2A, has previously been demonstrated to be neuroprotective, the effects of curcumin on NR2A expression were in this in vitro cell model. When compared with the control group, the expression of NR2A was significantly decreased by treatment with Aβ(25–35). Compared with the group of cells treated with Aβ(25–35) alone, curcumin treatment promoted NR2A expression in Aβ(25–35)-treated PC12 cells, in a dose-dependent manner (Figure 5).
Figure 5.
Effects of curcumin on the expression of NR2A expression in Aβ(25–35)-treated PC12 cells. PC12 cells were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of 20 μM Aβ(25–35) for 24 h. The protein (A) and mRNA (B) expression levels of NR2A were detected by Western blotting and quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Data are shown as mean ±SD. ** p<0.01 vs. non-treated group; #, ## p<0.05, 0.01 vs. Aβ(25–35) treatment alone group. Cur – curcumin.
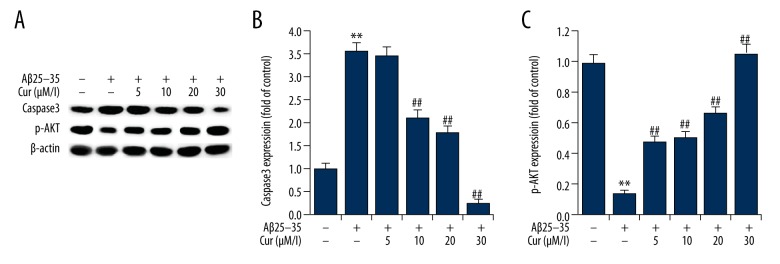
Effects of curcumin on the expression of caspase-3 and phospho-AKT (p-AKT) in Aβ(25–35)-treated PC12 cells
The activation of caspase-3 is a marker of cell apoptosis. The findings of this study showed that Aβ(25–35) treatment significantly increased caspase-3 expression in PC12 cells when compared with the control group. Curcumin treatment significantly reduced the expression of caspase-3 expression in PC12 cells, in a dose-dependent manner (Figure 6). The phosphorylation of AKT (p-AKT) was analyzed in the present study, and the results showed that Aβ(25–35) treatment significantly decreased the expression levels of p-AKT in PC12 cells, and this reduction was markedly inhibited by curcumin treatment, in a dose-dependent manner (Figure 6).
Figure 6.
Effects of curcumin on the expression of caspase-3 and p-AKT in Aβ(25–35)-treated PC12 cells. PC12 cells were treated with increasing concentrations of curcumin (5, 10, 20, 30 μM/L) in the presence of 20 μM Aβ(25–35) for 24 h. (A) The protein expression levels of caspase-3 and p-AKT were detected by Western blotting. (B, C) Protein expression of caspase-3 and p-AKT are presented as the fold increase of the control (non-treated) group. Data are shown as mean ±SD. ** p<0.01 vs. non-treated group; ## p<0.01 vs. Aβ(25–35) treatment alone group. Cur – curcumin.
Discussion
As the most common cause of dementia in the elderly, Alzheimer’s disease, seriously affects the quality of life for patients. However, at this time, there is no effective treatment, and the underlying pathogenesis of Alzheimer’s disease has not been fully elucidated. Recently published research findings showed that immunity and inflammation were involved in the occurrence of Alzheimer’s disease [21–23]. Furthermore, studies have shown that oxidative stress was the main mechanism underlying β-amyloid (Aβ)-mediated neurotoxicity in Alzheimer’s disease [24–26].
Curcumin, the yellow pigment and active constituent of turmeric, is commonly used in Indian foods, and parts of India have been reported to have a low prevalence of Alzheimer’s disease [27]. Several studies have determined the effects of curcumin on the progression of Alzheimer’s disease, and several mechanisms have been proposed for the effects of curcumin on the pathogenesis of Alzheimer’s disease, including anti-oligomerization, anti-phosphorylation, and anti-inflammatory effects. This study investigated the effect of curcumin on Alzheimer’s disease in an in vitro model, to explore whether the N-methyl-D-aspartate receptor (NMDAR) subunit, NR2A, had a role in the effects of curcumin in Alzheimer’s disease prevention or treatment.
The acetyl amyloid-β peptide, Aβ(25–35) has been commonly used in models of Alzheimer’s disease [28]. In this study, an in vitro model of Alzheimer’s disease was constructed that included Aβ(25–35)-induced PC12 cell injury. In this study, pretreatment with curcumin protected PC12 cells from Aβ(25–35)-induced loss of cell viability and apoptosis. Because the release of lactate dehydrogenase (LDH from cells is a sign of cells damage, and the presence of reactive oxygen species (ROS) due to oxidative stress results in the production of malondialdehyde (MDA), LDH and MDA levels were measured in this study to determine the extent of the oxidative damage. The findings were that Aβ(25–35) treatment enhanced the production of LDH and MDA in the supernatant of PC12 cells and that curcumin treatment reduced LDH and MDA production, in a dose-dependent manner.
In previously published studies, NR2A expression has been shown to have neuroprotective effects [19,29]. In the present study, the effect of curcumin on NR2A expression in PC12 cells, when compared with the non-treated control, showed that NR2A expression was inhibited by Aβ(25–35) treatment, and these effects were inhibited by curcumin treatment, in a dose-dependent manner.
In this study, the effects of curcumin on the expression of caspase-3 and p-AKT in Aβ(25–35)-treated PC12 cells were also studied. The results showed that Aβ(25–35) treatment significantly increased caspase-3 expression in PC12 cells when compared with the control group, and this increase was prevented by curcumin treatment. The findings of this study also showed that the phosphorylation of AKT in PC12 cells was decreased by Aβ(25–35) treatment, and this reduction was inhibited by curcumin treatment.
The findings of the present study showed that curcumin could protect PC12 cells from Aβ(25–35)-induced inhibition of cell viability, apoptosis, and release of cellular LDH, as well as the production of MDA. The expression of NR2A was involved in this cellular protection, supporting the potential neuroprotective role of curcumin in Alzheimer’s disease.
Conclusions
The findings of this study showed a neuroprotective effect of curcumin treatment in an in vitro model of Alzheimer’s disease that was associated with the increased expression of the NMDAR subunit, NR2A. For the first time, these findings have shown that the neuroprotective role that curcumin may have in Alzheimer’s disease could occur via the regulation of oxidative stress and NR2A expression. Further studies, including future controlled clinical studies, are recommended to determine whether curcumin is a potential treatment for Alzheimer’s disease.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Jiangsu Provincial Traditional Chinese Medicine Administration Project (No. LB13044)
References
- 1.De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. Alzheimer’s disease. Subcell Biochem. 2012;65:329–52. doi: 10.1007/978-94-007-5416-4_14. [DOI] [PubMed] [Google Scholar]
- 2.Uzun S, Kozumplik O, Folnegović Smalc V. Alzheimer’s dementia: Current data review. Coll Antropol. 2011;35:1333–37. [PubMed] [Google Scholar]
- 3.Solfrizzi V, Custodero C, Lozupone M, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer’s disease and late-life cognitive disorders: A systematic review. J Alzheimers Dis. 2017;59:815–49. doi: 10.3233/JAD-170248. [DOI] [PubMed] [Google Scholar]
- 4.Amorim JA, Canas PM, Tomé AR, et al. Mitochondria in excitatory and inhibitory synapses have similar susceptibility to amyloid-β peptides modeling Alzheimer’s disease. J Alzheimers Dis. 2017;60(2):525–36. doi: 10.3233/JAD-170356. [DOI] [PubMed] [Google Scholar]
- 5.Tayeb HO, Yang HD, Price BH, Tarazi FI. Pharmacotherapies for Alzheimer’s disease: Beyond cholinesterase inhibitors. Pharmacol Ther. 2012;134:8–25. doi: 10.1016/j.pharmthera.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Dutta S, Padhye S, Priyadarsini KI, Newton C. Antioxidant and antiproliferative activity of curcumin semicarbazone. Bioorg Med Chem Lett. 2005;15:2738–44. doi: 10.1016/j.bmcl.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Biswas SK, McClure D, Jimenez LA, et al. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: Mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6:259–70. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Li Q, Wang X, et al. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 Pathway. PLoS One. 2013;8:e59843. doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong Z, Hongmei Z, Lu S, Yu L. Curcumin mediates presenilin-1 activity to reduce beta-amyloid production in a model of Alzheimer’s Disease. Pharmacol Rep. 2011;63:1101–8. doi: 10.1016/s1734-1140(11)70629-6. [DOI] [PubMed] [Google Scholar]
- 12.Sun Q, Jia N, Wang W, et al. Activation of SIRT1 by curcumin blocks the neurotoxicity of amyloid-beta25-35 in rat cortical neurons. Biochem Biophys Res Commun. 2014;448:89–94. doi: 10.1016/j.bbrc.2014.04.066. [DOI] [PubMed] [Google Scholar]
- 13.Jiang T, Zhi XL, Zhang YH, et al. Inhibitory effect of curcumin on the Al(III)-induced Abeta(4)(2) aggregation and neurotoxicity in vitro. Biochim Biophys Acta. 2012;1822:1207–15. doi: 10.1016/j.bbadis.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Lu Z, Shen Y, Wang T, et al. Curcumin promotes neurite outgrowth via Reggie-1/flotillin-2 in cortical neurons. Neurosci Lett. 2014;559:7–12. doi: 10.1016/j.neulet.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Giri RK, Rajagopal V, Kalra VK. Curcumin, the active constituent of turmeric, inhibits amyloid peptide-induced cytochemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating early growth response-1 transcription factor. J Neurochem. 2004;91:1199–210. doi: 10.1111/j.1471-4159.2004.02800.x. [DOI] [PubMed] [Google Scholar]
- 16.Begum AN, Jones MR, Lim GP, et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther. 2003;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum L, Lam CW, Cheung SK, et al. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer’s disease. J Clin Psychopharmacol. 2008;28:110–13. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan PS, Misra UK, Kalita J. A study of glutamate levels, NR1, NR2A, NR2B receptors and oxidative stress in a rat model of Japanese encephalitis. Physiol Behav. 2017;171:256–67. doi: 10.1016/j.physbeh.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Zhao Y, Zheng W, et al. Neuroprotective effect of curcumin on transient focal cerebral ischemia in rats. Brain Res. 2008;1229:224–32. doi: 10.1016/j.brainres.2008.06.117. [DOI] [PubMed] [Google Scholar]
- 20.Park SY, Kim HS, Cho EK, et al. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem Toxicol. 2008;46:2881–87. doi: 10.1016/j.fct.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 21.Heppner FL, Ransoho RM, Becher B. Immune attack: The role of inflammation in Alzheimer disease. Nat Rev. 2015;16:358–72. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 22.Kumar DK, Choi SH, Washicosky KJ, et al. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer’s disease. Sci Transl Med. 2016;8:340ra72. doi: 10.1126/scitranslmed.aaf1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minter MR, Zhang C, Leone V, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep. 2016;6:30028. doi: 10.1038/srep30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonnen JA, Breitner JC, Lovell MA, et al. Free radical-mediated damage to the brain in Alzheimer’s disease and its transgenic mouse models. Free Radical Biol Med. 2008;45:219–30. doi: 10.1016/j.freeradbiomed.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–95. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 26.Trushina E, McMurray C. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–48. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- 27.Chandra V, Pandav R, Dodge HH, et al. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–89. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- 28.Yoon JH, Youn K, Ho CT, et al. p-Coumaric acid and ursolic acid from Corni fructus attenuated β-Amyloid 25-35-induced toxicity through regulation of the NF-κB signaling pathway in PC12 cells. J Agricult Food Chem. 2014;62:4911–16. doi: 10.1021/jf501314g. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Zhang Q, Tu J, et al. Prosurvival NMDA 2A receptor signaling mediates postconditioning neuroprotection in the hippocampus. Hippocampus. 2015;25:286–96. doi: 10.1002/hipo.22372. [DOI] [PubMed] [Google Scholar]