Summary
Background
Gene fusions involving NTRK1, NTRK2, or NTRK3 (TRK fusions) are found in a broad range of paediatric and adult malignancies. Larotrectinib, a highly selective small-molecule inhibitor of the TRK kinases, had demonstrated activity in preclinical models and in adults with tumours harbouring TRK fusions. The primary aim of this study was to assess the safety of larotrectinib in paediatric patients.
Methods
This multicentre, phase 1 study enrolled infants, children and adolescents aged 1 month to 21 years with locally advanced or metastatic solid or central nervous system tumours regardless of TRK fusion status. Other key inclusion criteria included evaluable and/or measurable disease according to disease specific criteria, Karnofsky (≥16 years of age) or Lansky (<16 years of age) performance score of ≥50, adequate organ function, and full recovery from the acute toxic effects of all prior anticancer therapy. Larotrectinib was administered orally, twice daily (BID) on a continuous 28-day schedule, in increasing dose levels, according to a rolling six design. The primary endpoint of the phase 1 dose escalation component was the safety of larotrectinib, including dose limiting toxicity, in paediatric patients with advanced solid or primary central nervous system tumours treated with at least one dose of larotrectinib. Secondary endpoints included the maximum tolerated dose or appropriate dose of larotrectinib for further clinical investigation, pharmacokinetics, and an assessment of antitumour activity including the objective response rate (ORR). Reported here are the patients enrolled to the phase 1 dose escalation cohort which has completed enrolment. Follow-up of these patients and enrolment to phase 2 cohorts are ongoing on this protocol. This trial is registered with ClinicalTrials.gov, number NCT02637687.
Results
Twenty-four patients (17 with tumours with TRK fusions, seven without) with a median age of 4·5 years (range 0·1–18) were enrolled to three dose cohorts. Patients with TRK fusion cancers had diagnoses of infantile fibrosarcoma (n=8), other soft tissue sarcomas (n=7) and papillary thyroid cancer (n=2). Adverse events were predominantly grade 1; the most common were increased alanine and aspartate aminotransferase [10 (42%) of 24 each] and leukopenia and decreased neutrophil count [5 (21%) of 24 each]. Grade 3 alanine aminotransferase elevation in a patient without a TRK fusion with progressive disease was the only dose limiting toxicity and resulted in larotrectinib discontinuation. No other patients discontinued larotrectinib for adverse events. No grade 3 treatment-related adverse events occurred in more than one patient and no grade 4 or 5 treatment-related adverse events were observed. Two larotrectinib related serious adverse events were observed: grade 3 nausea and grade 3 ejection fraction decrease during the 28-day follow-up after discontinuing larotrectinib and while on anthracyclines [1 (4%) of 24 each]. The maximum tolerated dose was not defined. A dose of 100 mg/m2 (maximum of 100 mg/dose) BID was determined to be the recommended phase 2 dose based on pharmacokinetics and antitumor activity. In patients with TRK fusion cancers and measurable disease by Response Evaluation Criteria In Solid Tumors (RECIST) v1.1, the objective response rate (ORR) was 93% (14 of 15 patients), with the remaining patient that did not meet RECIST partial response criteria showing tumour regression. In patients without documented TRK fusion cancers, the ORR was 0% (0 of 7 patients).
Interpretation
The TRK inhibitor larotrectinib was well tolerated in paediatric patients. A recommended phase 2 dose of 100mg/m2 (cap of 100 mg) was defined for infants, children, and adolescents, regardless of age. Larotrectinib demonstrated antitumour activity in all patients with TRK fusion-positive tumours.
Funding
Loxo Oncology
Introduction
The TRK family of neurotrophin tyrosine kinase receptors, TRKA, TRKB, and TRKC, encoded respectively by the NTRK1, NTRK2, and NTRK3 genes, are involved in the growth, differentiation, and survival of neurons.1,2 Gene fusions involving NTRK1, NTRK2, and NTRK3 (TRK fusions) have been identified in a broad range of paediatric and adult malignancies.3 Typically, the 3′ region of the NTRK gene is joined with the 5′ region of an unrelated gene, with the encoded fusion protein comprising the kinase domain of the TRK protein joined in-frame with the fusion partner. The resultant novel fusion oncoprotein is both aberrantly expressed and constitutively active, leading to the activation of downstream pro-oncogenic pathways.
TRK fusions occur relatively infrequently in many common adult malignancies and paediatric cancers.4–8 In contrast, in certain rare paediatric tumours, including infantile fibrosarcoma (IFS),9,10 cellular congenital mesoblastic nephroma,11,12 and papillary thyroid cancer,13 TRK fusions are found at higher frequencies. TRK fusions may therefore represent a clinically targetable driver alteration in many tumour types. IFS is particularly noteworthy, as these tumours are often locally advanced and infiltrative, necessitating chemotherapy and/or potentially morbid surgery in order to achieve a cure.14,15
Larotrectinib (LOXO-101) is an orally administered ATP-competitive inhibitor of TRKA, TRKB and TRKC, with a 50% inhibitory concentration (IC50) of 5–11 nM in vitro, and >100-fold selectivity for TRK over other kinases.16 In tumour cell lines harbouring TRK fusions, cells were sensitive to larotrectinib with IC50 values in the low nanomolar range. Larotrectinib has recently been shown to have clinical activity in adult patients with TRK fusion cancers, with an adult recommended phase 2 dose of 100 mg twice daily (BID).16,17
Given the early evidence of antitumour activity in the adult setting, and the burden of TRK fusions in specific paediatric cancers, we designed a phase 1 trial to determine the safety of larotrectinib, including dose limiting toxicities, and preliminary efficacy in paediatric patients with advanced solid tumours. The high intrinsic solubility of larotrectinib allowed use of a liquid formulation for very young patients unable to swallow capsules.
Methods
Study design and participants
We conducted this multicentre, open-label phase 1/2 study at eight sites in the United States (appendix p2). Only the phase 1 dose-escalation component is reported here; the phase 2 component is ongoing. Eligible patients included those 1 month to 21 years of age with any locally advanced or metastatic solid tumour or primary central nervous system (CNS) tumour that had relapsed, progressed or had an inadequate response to available therapies and for which no standard or available systemic curative therapy existed, regardless of histology. Presence of a TRK fusion was not required, except for infants 1 month to less than 1 year of age. However, TRK fusion testing was performed locally prior to enrolment, resulting in investigators enriching the study population with patients with TRK fusion cancers. Following protocol amendment on 12 September 2016 due to initial activity seen in patients with IFS, and at the request of the US Food and Drug Administration (FDA), those with locally advanced IFS who would require disfiguring surgery or limb amputation to achieve a complete surgical resection could also be included. Other key inclusion criteria included evaluable and/or measurable disease according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1,18 Response Assessment in Neuro-Oncology criteria,19 or International Neuroblastoma Response Criteria;20 Karnofsky (≥16 years of age) or Lansky (<16 years of age) performance score of ≥50; absolute neutrophil count ≥1·0 × 109/L and platelet count ≥100·0 × 109/L; haemoglobin ≥8·0 g/dL; bilirubin ≤1·5 × upper limit of normal for age; alanine aminotransferase ≤135 U/L; an estimated glomerular filtration rate ≥30 mL/minute or serum creatinine below a predefined level based on age and gender; and full recovery from the acute toxic effects of all prior anticancer therapy, with a minimum of 21 days from myelosuppressive chemotherapy (42 days for nitrosourea), 14 days from haematopoietic growth factors and local palliative radiation therapy, 42 days from substantial bone marrow radiation, 56 days from stem cell infusion, and the shorter of 2 weeks or five half-lives from prior investigational agents. Exclusion criteria included major surgery within 14 days prior to the start of study treatment; clinically significant cardiovascular disease or corrected QT interval >480 milliseconds; active uncontrolled systemic infection; and conditions affecting oral absorption.
The protocol was approved by the institutional review boards of all participating centres. The study was conducted in accordance with the protocol and the principles expressed in the Declaration of Helsinki. All patients and/or their parents provided written informed consent before any study specific procedures were conducted.
Procedures
Patients received larotrectinib orally (capsule or liquid formulation), BID, in 28-day cycles of continuous dosing. Treatment was continued until disease progression or unacceptable toxicity with no prespecified maximum number of cycles. Patients with progression could continue treatment if the investigator determined they were experiencing clinical benefit. Dose escalation proceeded through planned cohorts until the maximum tolerated dose (MTD) was reached, according to the occurrence of dose-limiting toxicity (DLT) in cycle 1, or until it was determined that a suitable recommended phase 2 dose had been achieved based on pharmacokinetic exposure.
Patients were enrolled to three cohorts using a modified rolling six design;21 however, enrolment to cleared cohorts remained open to patients with TRK fusion cancers during toxicity assessments and protocol amendments, without an enrolment cap. Cohorts 1 and 2 used dosing nomograms that assigned doses based on both age and weight predicted on SimCyp (SimCyp Ltd., Sheffield, UK) simulation modelling to achieve an area under the curve (AUC) equivalent to adult doses of 100 mg (the adult recommended phase 2 dose) and 150 mg BID, respectively. Modelling predicted that infants and children less than 6 years of age should be treated with lower larotrectinib doses on a body surface area (BSA) basis than older children, adolescents, and adults to achieve the same AUC, with the lowest doses on a BSA basis assigned to the youngest infants. Thus, based on age, patients in cohorts 1 and 2 were assigned doses ranging from 17–96% and 30–208% of the BSA-adjusted adult recommended phase 2 dose of 100 mg BID, respectively. After review of data from cohorts 1 and 2, the protocol was subsequently amended on 12 September 2016 such that patients enrolled to cohort 3 were assigned to receive 100 mg/m2 BID (maximum of 100 mg/dose), regardless of age, equating to a maximum of 173% of the adult recommended phase 2 dose.
At the treating physician’s discretion, the protocol allowed intrapatient dose escalation as soon as cycle 1 day 8 and once every cycle thereafter, with a 50% increase in dose for patients who did not achieve an AUC0–24 of 3500 ng*h/mL (approximately 70% of that in adults treated with 100 mg BID). Patients who were dose escalated continued to contribute data to their assigned cohort for the purpose of DLT evaluation. Dose interruptions of up to 21 days were specified for clinically significant grade 3 or 4 adverse events. Patients in whom this toxicity was judged related to larotrectinib restarted at a lower protocol-defined dose upon recovery.
Patients with locally advanced sarcomas were able to undergo local control surgery after adequate tumour response. Patients with UICC-R022 (negative margin) resection discontinued larotrectinib and were followed with radiographic imaging every 3 months. Patients with R1 (marginal) or R2 (gross residual) surgery were eligible to restart larotrectinib immediately following surgery. Responding patients were able to enter a “wait and see” drug discontinuation period after a minimum of 6 cycles of treatment and could be retreated if they had disease progression after drug discontinuation.
Disease status was assessed by investigators according to the aforementioned disease-specific criteria at baseline and then on day 1 of every other cycle, with an optional assessment on day 1 of cycle 2. Disease assessments included computerised tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) of chest, abdomen, pelvis, and any other areas with suspected disease involvement. Patients with locally advanced infantile fibrosarcoma could be evaluated with an x-ray of the chest instead of CT scan. Patients with neuroblastoma also had bilateral bone marrow aspirates and biopsies. Response in one patient has previously been described.23 Blinded independent central review of imaging was subsequently performed and is reported here as best objective response data. Adverse events were monitored throughout the study and for 28 days after treatment and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Laboratory monitoring for toxicity and symptom directed neurological examinations for close monitoring for neurological toxicities were performed weekly during cycle 1 and every 4 weeks thereafter.
Serial blood samples were collected for pharmacokinetic analyses. Plasma concentrations of larotrectinib were determined by liquid chromatography/mass spectrometry. Patients undergoing a standard of care cerebrospinal fluid (CSF) sampling while on study (n=2) had larotrectinib concentrations analysed in CSF, along with a concurrent plasma sample. Interim pharmacokinetic parameters were calculated throughout the study for all enrolled patients using Excel (Microsoft).
In patients 3 years of age or older, pain was assessed using the Wong-Baker Faces Scale. Health-related quality of life (HRQoL) assessments were based on the PedsQL 4.0™ for patients aged 2 years or older and Peds QL Infant™ for infants aged less than 2 years. Impaired HRQoL was defined as a total score >2 standard deviations below the population mean.24,25 Scores consistent with impaired HRQoL were total scores ≤49·5 for children and adolescents aged 2–18 years, ≤62·57 for infants aged 1–12 months and ≤68·07 for infants aged 13–24 months.26,27
TRK fusion status was assessed locally, prior to enrolment, in a CLIA-certified laboratory by either fluorescence in situ hybridization (n=3), reverse transcription polymerase chain reaction (n=2) or next-generation sequencing (n=15) and was not centrally tested on this protocol. If patients did not have tumour available for such analyses (n=4), they were considered not to have TRK fusions for the purpose of this report.
Outcomes
The primary objective was to determine the safety of oral larotrectinib, including DLT, in paediatric patients with advanced solid or primary CNS tumors. All patients who received at least one dose of larotrectinib were included in the safety analyses. Secondary objectives included the determination of the MTD or the appropriate dose of larotrectinib for further clinical investigation, the pharmacokinetics (AUC, Cmax, Tmax, and T1/2), an assessment of antitumour activity, including the objective response rate (ORR), progression-free survival (PFS), and overall survival, and an evaluation of pain and HRQoL. PFS was defined as interval from the date of the first dose of study drug to the earliest of documented PD or death. OS was defined as the interval from the date of the first dose of study drug to the date of death due to any cause. Evaluation of potential biomarkers of response and resistance to larotrectinib was an exploratory objective. Evaluation of the objective response rate in patients with TRK fusion tumours was performed as a post hoc analysis.
DLT was defined as any of the following treatment-emergent adverse events, if they occurred during the first cycle and were attributed as related to larotrectinib: grade 3 or higher nonhaematological toxicity, with the exception of grade 3 fatigue or nausea or grade 3 or 4 vomiting or diarrhoea persisting for less than 48 hours; any toxicity, regardless of grade, resulting in discontinuation or dose reduction of larotrectinib; grade 4 thrombocytopenia or grade 3 thrombocytopenia with grade 1 or higher bleeding; grade 4 anaemia lasting more than 7 days; or grade 4 neutropenia lasting more than 7 days.
Statistical analysis
Safety and antitumour activity data were summarised descriptively. Continuously distributed data were summarised based on the median and range of values. Categorical data were summarised based on the number and percentage of patients in each category. Adverse events were summarised using the standardised preferred term assigned by the Medical Dictionary for Regulatory Activities (MedDRA) version 18.1. The safety population comprised all patients who received one or more doses of larotrectinib. It was anticipated that enrolment of up to 36 patients might be required in order to define the MTD of larotrectinib, with the actual number dependent on the safety profile. A safety review committee was convened to review safety and pharmacokinetic data and render dose-escalation decisions prior to each dose escalation. Antitumour activity was assessed in all enrolled patients; the ORR was calculated as the proportion of patients with measurable disease at baseline by RECIST v1.1 with a complete response (CR) or partial response (PR), as specified in the protocol and recommended by the US FDA. Estimates of the ORR are accompanied by two-sided exact binomial 95% confidence intervals (CI) using the Clopper-Pearson method. Duration of response was summarized descriptively using the Kaplan-Meier method with the 95% CI about the median calculated using Greenwood’s formula (whenever estimable). Statistical analyses were performed using SAS (version 9.4).
This trial is registered with ClinicalTrials.gov, number NCT02637687.
Role of the funding source
The study was designed by representatives of the funder, Loxo Oncology, in conjunction with the lead investigators (TWL, SGD, LM, ASP, DSH). The funder collected the study data and analysed and interpreted these data in collaboration with the authors. Loxo Oncology commissioned medical writing services to support the drafting of our report. All authors had full access to all study data and TWL, SGD, ASP and DSH had the final responsibility for the decision to submit for publication.
Results
Between 21 December 2015 and 13 April 2017, 24 patients (12 boys/12 girls) with a median age of 4·5 years (IQR 1·3–13·3 years, minimum 1 month, maximum 18 years) were enrolled to three cohorts; baseline characteristics are summarised in table 1. A data cutoff date of 17 July 2017 was used for this analysis. While the presence of a TRK fusion was not required for enrolment, the study was enriched for patients with such lesions, with 17 having tumours that harboured TRK fusions, involving NTRK1 (n=9), NTRK2 (n=1) or NTRK3 (n=7); seven patients had cancers without a documented TRK fusion. Patients with TRK fusions had primary diagnoses of IFS (n=8, 2 NTRK1, 6 NTRK3), other soft tissue sarcoma (n=7, 6 NTRK1, 1 NTRK2), and papillary thyroid cancer (n=2, 1 NTRK1, 1 NTRK3). Eleven (65%) of 17 patients with TRK fusion cancers had locally advanced disease, including two patients with IFS who enrolled without prior systemic therapy.
Table 1.
Demographic and disease characteristics at baseline
| Patients (n=24) |
|
|---|---|
| Age | |
| 1 month to <2 years | 7 (29%) |
| 2 years to <12 years | 10 (42%) |
| 12 years to 18 years | 7 (29%) |
| Median (IQR) | 4·5 years (IQR 1·3 – 13·3) |
| Range | 0·1 – 18 years |
| Sex | |
| Male | 12 (50%) |
| Female | 12 (50%) |
| Performance status (Karnofsky/Lansky) | |
| 50 – 60 | 1 (4%) |
| 70 – 80 | 8 (33%) |
| 90 – 100 | 15 (63%) |
| Tumour | |
| Infantile fibrosarcoma | 8 (33%) |
| Other soft tissue sarcomas* | 7 (29%) |
| Papillary thyroid cancer | 2 (8%) |
| Other** | 7 (29%) |
| NTRK gene fusion | |
| NTRK1 | 9 (38%) |
| NTRK2 | 1 (4%) |
| NTRK3 | 7 (29%) |
| None documented | 7 (29%) |
| Not detected | 3 (13%) |
| Tumour unavailable for analysis | 4 (17%) |
| Stage at enrolment | |
| Localised/unresectable | 11 (46%) |
| Metastatic | 8 (33%) |
| Other*** | 5 (21) |
| Prior cancer treatments | |
| Systemic therapy | 17 (71%) |
| Surgery | 17 (71%) |
| Radiotherapy | 8 (33%) |
| Number of prior systemic regimens | |
| 0 | 7 (29%) |
| 1 | 7 (29%) |
| ≥2 | 10 (42%) |
Data are n (%), unless otherwise stated.
Other soft tissue sarcomas include spindle cell sarcomas (n=4), undifferentiated sarcoma (n=1), and tumors with myopericytic/myofibromatous differentiation (n=2).
Other includes diffuse intrinsic pontine glioma (n=2) and 1 each of osteosarcoma, neuroblastoma, intracranial Ewing-like sarcoma, medulloblastoma, and pineoblastoma (none having a documented TRK fusion).
Other includes 4 patients with primary central nervous system tumours and 1 with intracranial Ewing-like sarcoma.
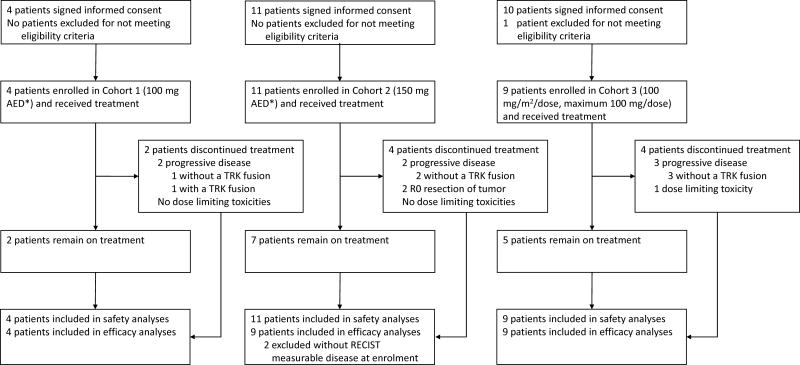
Four patients were enrolled to cohort 1, 11 to cohort 2, and 9 to cohort 3; all were evaluable for safety (figure 1). Three (75%) of four patients in cohort 1 and two (18%) of 11 patients in cohort 2 were dose escalated one to four times each. In cohort 3, one patient with a TRK fusion-negative neuroblastoma experienced a grade 3 dose limiting alanine aminotransferase elevation. This patient had not undergone intrapatient dose escalation and discontinued therapy due to this event. No other patient had a DLT or discontinued larotrectinib for adverse events. The MTD was not reached. Following analysis of the safety, pharmacokinetics, and responses observed at the completion of enrolment to cohort 3, this dose level (100 mg/m2 BID, maximum 100 mg/dose) was subsequently declared the recommended phase 2 dose in paediatric patients, based on pharmacokinetic parameters similar to those reported in adults treated with 100 mg/dose, which were associated with clinical response.
Figure 1. CONSORT Diagram.
* Adult Equivalent Dose (AED) by SimCyp modelling
Larotrectinib related adverse events for all patients, and for those treated at the recommended phase 2 dose, are shown in table 2 and the appendix (p3, 4). The majority of adverse events were grade 1 or 2, with only four (17%) of 24 patients experiencing grade 3 treatment-related adverse events. No single grade 3 treatment-related adverse event occurred in more than one patient. No grade 4 or 5 adverse events were attributed to larotrectinib. There were no larotrectinib related deaths or deaths on treatment. One patient died of progressive disease within the 28-day follow-up period after discontinuing larotrectinib. The most common treatment-related adverse events were low grade increases in liver enzyme levels, haematological toxicity and vomiting. Grade 1 fatigue was observed in three (13%) of 24 patients; other neurological toxicities were rarely seen. Two larotrectinib related serious adverse events were observed: grade 3 nausea and grade 3 ejection fraction decrease in one (4%) of 24 patients each. The grade 3 ejection fraction decrease occurred during a patient’s 28-day follow-up period off-larotrectinib after discontinuation for progressive disease; this patient received anthracyclines before and after larotrectinib treatment (320 mg/m2 lifetime dose of doxorubicin). No other patients had decreased ejection fraction. One patient in cohort 3 had a dose reduction during cycle 2 of therapy due to neutropenia; no other patient required dose reduction for toxicity.
Table 2.
Larotrectinib related adverse events occurring in >10% of patients at grade 1 or 2 in severity and all grade 3 or worse in severity.
| Overall (n=24) | 100 mg/m2 BID (n=9) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Preferred Term | Any grade |
Grades 1–2 |
Grade 3 |
Grade 4 |
Grade 5 |
Any grade |
Grades 1–2 |
Grade 3 |
Grade 4 |
Grade 5 |
| At least one related TEAE | 22 (92%) | 21 (88%) | 4 (17%) | 0 | 0 | 8 (89%) | 8 (89%) | 2 (22%) | 0 | 0 |
| Alanine aminotransferase increased | 10 (42%) | 9 (38%) | 1 (4%) | 0 | 0 | 3 (33%) | 2 (22%) | 1 (11%) | 0 | 0 |
| Aspartate aminotransferase increased | 10 (42%) | 10 (42%) | 0 | 0 | 0 | 4 (44%) | 4 (44%) | 0 | 0 | 0 |
| Leukocyte count decreased | 5 (21%) | 5 (21%) | 0 | 0 | 0 | 2 (22%) | 2 (22%) | 0 | 0 | 0 |
| Neutrophil count decreased | 5 (21%) | 4 (17%) | 1 (4%) | 0 | 0 | 3 (33%) | 2 (22%) | 1 (11%) | 0 | 0 |
| Vomiting | 5 (21%) | 5 (21%) | 0 | 0 | 0 | 2 (22%) | 2 (22%) | 0 | 0 | 0 |
| Anaemia | 4 (17%) | 4 (17%) | 0 | 0 | 0 | 2 (22%) | 2 (22%) | 0 | 0 | 0 |
| Constipation | 4 (17%) | 4 (17%) | 0 | 0 | 0 | 2 (22%) | 2 (22%) | 0 | 0 | 0 |
| Hypoalbuminaemia | 4 (17%) | 4 (17%) | 0 | 0 | 0 | 1 (11%) | 1 (11%) | 0 | 0 | 0 |
| Nausea | 4 (17%) | 3 (13%) | 1 (4%) | 0 | 0 | 3 (33%) | 2 (22%) | 1 (11%) | 0 | 0 |
| Blood creatinine increased | 3 (13%) | 3 (13%) | 0 | 0 | 0 | 1 (11%) | 1 (11%) | 0 | 0 | 0 |
| Fatigue | 3 (13%) | 3 (13%) | 0 | 0 | 0 | 1 (11%) | 1 (11%) | 0 | 0 | 0 |
| Ejection fraction decreased | 1 (4%) | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Weight increased | 1 (4%) | 0 | 1 (4%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Data are number (%). BID=twice daily; TEAE=treatment-emergent adverse event
The larotrectinib AUC and Cmax increased proportionately with dose per BSA. There was sustained IC90 target coverage and similar pharmacokinetics among infants, children and adolescents treated in cohort 3 and adults treated at the recommended phase 2 dose (appendix p5). There was no apparent difference in Cmax or AUC in patients treated with capsule versus liquid formulation or among different paediatric age groups (appendix p5). Larotrectinib was detectable in CSF in both patients who had sampling, with CSF to plasma concentrations of 28% via Ommaya (corrected for protein binding) and 123% via lumbar puncture. Pharmacokinetic-based dose adjustment for patients enrolled to cohorts 1 and 2 is summarised in the appendix (p1).
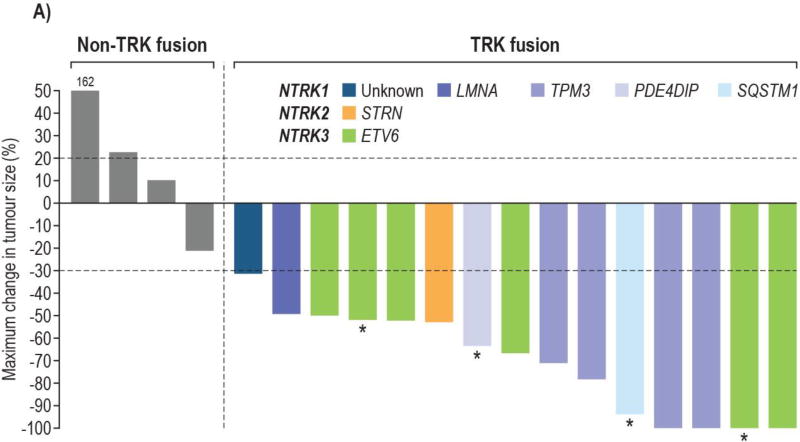
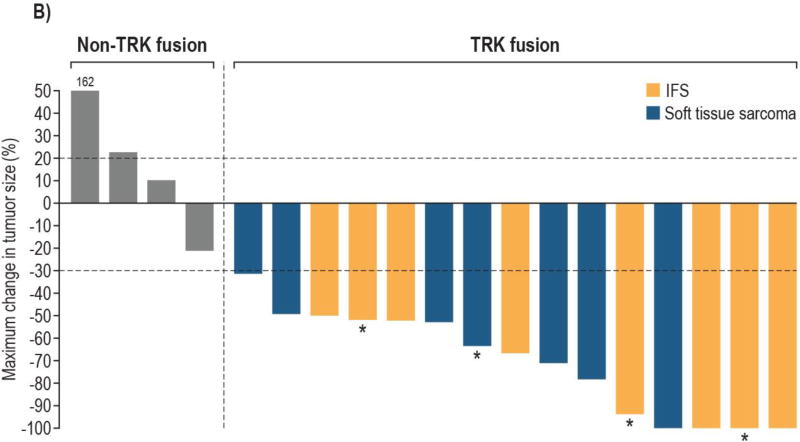
Of the 24 patients enrolled on study, 22 were evaluable for objective response with measurable disease by RECIST 1.1 at enrolment; 2 patients without measurable disease were excluded. The ORR among these patients was 64% (14 of 22; 95% CI: 41, 83), with responses seen only in patients with TRK fusions. Among the 15 evaluable patients with TRK fusions, the ORR by investigator review was 93% (95% CI: 68, 100; CR, n=4; PR, n=10 with two pending confirmation at the cutoff subsequently confirmed); one patient had an initial PR which became stable disease at a subsequent assessment (figure 2). Independent radiology review was concordant, with a confirmed ORR of 93% (95% CI: 68, 100; CR, n=2; PR, n=12; appendix p6). All seven patients without documented TRK fusions had PD as best response.
Figure 2. Waterfall plot of maximal change in tumour size for patients with RECIST measurable tumours b investigator assessment.
A. Bars are colour coded by NTRK gene and fusion partner
5 enrolled patients are not shown: 3 non-TRK fusion patients due to clinical disease progression without post-baseline tumour measurements and 2 TRK fusion patients due to having non-measurable disease at baseline
*Locally advanced patients who underwent surgery
B. Bars are colour coded by histological diagnosis
5 enrolled patients are not shown: 3 non-TRK fusion patients due to clinical disease progression without post-baseline tumour measurements and 2 TRK fusion patients due to having non-measurable disease at baseline
*Locally advanced patients who underwent surgery
All 15 patients with TRK fusion cancers and measurable disease by RECIST v1.1 experienced reductions in tumour burden. Responses occurred in patients with fusions in each of the NTRK genes: NTRK1 (n=7); NTRK2 (n=1); NTRK3 (n=6) and in both IFS (n=8) and other soft tissue sarcomas (n=6). Responses were seen in all cohorts, but following intrapatient dose escalation and a protocol modification to BSA based dosing for cohort 3, only three (18%) of 17 patients with TRK fusions were treated at doses of less than 80% of the recommended phase 2 dose.
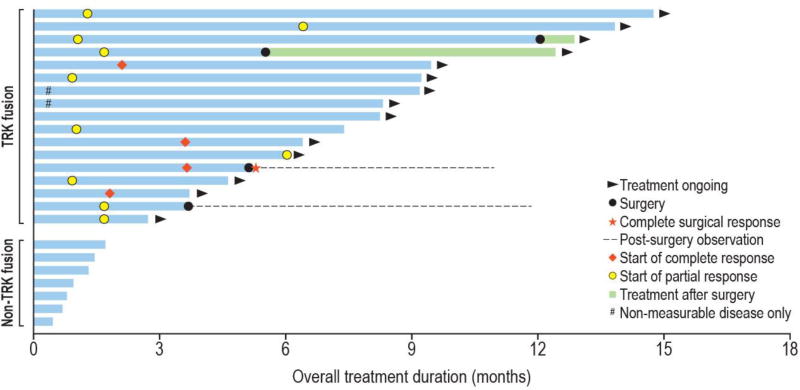
Responses occurred at a median of 1·7 months (IQR 1·0–2·9 months), consistent with the first protocol mandated response assessment, with some patients having response apparent on exam within days (figure 3). Of the 17 patients with TRK fusions, all but one (6%) remained on treatment (n=14) or had undergone surgery with curative intent (n=2) after a median of 8·2 months (IQR 5·2–9·5 months). The median duration of response had not been reached (95% CI: 5.6 months, upper limit not calculable; appendix, p7). Two patients with TRK fusion-positive papillary thyroid cancer did not have measurable disease at enrolment, but both remained on treatment without progression as of the data cutoff (>7 months).
Figure 3. Swimmer plot showing all patients enrolled on study.
Includes all 24 patients who enrolled in the study
Two patients with TRK fusions had progressive disease while on study. One patient with locally advanced IFS had a partial response and entered a “wait and see” drug discontinuation period after 12 cycles of therapy without surgical resection. This patient had tumour progression 1 month after stopping larotrectinib but demonstrated significant tumour shrinkage again after restarting therapy and remains on treatment, now at month 14 since initial enrolment. A 1-year-old girl with IFS was the only patient with a TRK fusion cancer to progress while on therapy, following a response to treatment. A G623R TRKC solvent front resistance mutation was identified in the recurrent tumour, as previously reported.28 Circulating DNA obtained from plasma at the time of progression also confirmed both the presence of the ETV6-NTRK3 fusion and the G623R-encoding resistance mutation (data not shown).
Four patients with locally advanced disease (IFS, n=2; other soft tissue sarcoma, n=2) achieved partial responses to therapy with larotrectinib by imaging and underwent on-study resection. Two of four achieved a UICC-R0 resection, defined by pathological clear margins. One of these patients had no viable tumour on microscopic examination and was reclassified as a pathological complete response. Both patients with R0 resections continue to be followed on-study without recurrence; now >6 months off larotrectinib. The other two patients had R1 resections and restarted therapy with larotrectinib.
Only one of seven patients with available baseline Wong-Baker scores had pain at baseline; his score decreased from 8 to 2 after 2 cycles of larotrectinib. Among 16 of 17 patients with TRK fusions for whom baseline HRQoL data were available, none had baseline total scores indicating impaired HRQoL. Fourteen of these patients had HRQoL data available at the start of cycle 6 and none had evidence of impaired HRQoL based on total scores.
Discussion
In this paediatric phase 1 trial, larotrectinib was well tolerated and induced sustained tumour regressions in over 90% of infants, children and adolescents with TRK fusions. The recommended phase 2 dose of 100 mg/m2 BID (maximum of 100 mg/dose) was tolerable, achieved an AUC comparable with adults treated with 100 mg BID, and was highly active. The most common adverse events were mild elevations of liver enzyme levels, cytopenias, and vomiting. HRQoL among infants, children and adolescents with TRK fusions appeared to be acceptable while receiving larotrectinib.
The nearly universal response to larotrectinib in infants, children and adolescents with TRK fusion-positive solid tumours occurred in patients with fusions of each of the three NTRK genes. While the objective responses occurred in patients with sarcoma, two patients with papillary thyroid cancer and RECIST-nonmeasurable disease nevertheless remained on treatment as of the data cutoff for more than 7 months.
Two patients with locally advanced IFS were able to undergo R0 surgical resection for local control of their disease, discontinue all therapy, and enter follow-up observation following response to larotrectinib. Two additional patients, who continued larotrectinib after R1 surgical resection have avoided morbid surgery to date. Considering these patients may have otherwise undergone disfiguring surgery, therapy with larotrectinib may ultimately represent a new standard of care in these patients by allowing less morbid surgical options. The response rate and side effect profile observed here compares favourably to that reported for infants with IFS treated with vincristine and actinomycin as upfront therapy.14
With a median follow-up of 8·2 months (IQR 5·2–9·5 months), all patients with TRK fusions except one remain on treatment or have undergone potentially curative surgery. The single patient with acquired resistance was associated with the occurrence of an NTRK3 mutation resulting in a G623R substitution that has been shown to confer resistance to larotrectinib in preclinical models and to be associated clinically with acquired resistance to the multikinase inhibitor entrectinib.29 This patient subsequently responded to a next generation selective TRK inhibitor, LOXO-195, which inhibits both wild-type TRK and TRK harbouring resistance mutations.28 Clinical trials of LOXO-195 in adults, infants, children and adolescents with acquired resistance to TRK inhibition are ongoing (NCT03215511).
We used intrapatient dose escalation to more quickly define the recommended phase 2 dose. Cohorts 1 and 2 used dosing nomograms based on SimCyp modelling to assign doses accounting for both age and BSA. Modelling assigned lower BSA based doses to the youngest infants due to the predicted developmental immaturity of drug elimination pathways. However, using data from cohorts 1 and 2, we observed that this approach reduced rather than enhanced our ability to deliver the desired AUC in patients, and we consequently modified the dosing strategy for cohort 3 to use only BSA based dosing. The use of modelling-based doses for the first two cohorts may have increased the number of patients required to define the recommended phase 2 dose. Responses were seen at all dose levels; thus it was not possible to define a dose or exposure response relationship for larotrectinib in TRK fusion cancers.
This trial is noteworthy for the simultaneous development of larotrectinib in pediatric and adult patients. We designed this study to permit enrolment of infants as young as 1 month of age because TRK fusion malignancies are enriched in very young patients. Pharmacokinetics were similar between capsule and liquid formulations, allowing dosing in infants and children unable to swallow capsules. Real-time pharmacokinetic assessment was critical to our ability to enrol infants on study, as drug exposure in very young infants can be difficult to predict due to incompletely developed drug absorption, distribution, metabolism, and elimination.30
We observed little CNS toxicity despite measuring meaningful larotrectinib levels in the CSF in both patients evaluated. As only two patients had CSF sampling, the differences observed in CNS penetration could be due to inter-individual variability rather than due to true differences in ventricular and lumbar concentrations. Published literature and experimental data predicted that continuous TRK inhibition in the CNS might cause on-target DLTs that could compromise TRK fusion target coverage systemically. The relatively low frequency and grade of larotrectinib-associated neurological toxicities seen suggests that the therapeutic index of larotrectinib is wide, while preserving the potential to address disease in the CNS. Additional follow-up of patients enrolled on this study to evaluate longer term safety and efficacy outcomes is ongoing and the PFS and overall survival of these patients will be reported elsewhere.
Limitations of this study include the inability to estimate the relative frequency of TRK aberrations in paediatric cancers since testing for TRK fusions was not performed as part of the trial. Due to the mechanism of action of larotrectinib, this study was enriched for patients with TRK fusions, which resulted in a high response rate for all patients enrolled on this trial. The response rate seen here is particularly notable given that only 1-dimensional response assessment by RECIST v1.1 was used, which has been shown to potentially underestimate response in paediatric soft tissue sarcomas compared with 3-dimensional response assessment.31 While this trial was designed as a phase 1/2 study, the phase 2 component of this trial evaluating larotrectinib in paediatric patients with TRK fusion cancers is ongoing and will be reported separately.
In conclusion, larotrectinib is tolerable and demonstrated a high response rate in infants, children and adolescents with advanced TRK fusion tumours. In addition to delivering durable responses in patients with advanced metastatic disease, larotrectinib may also offer a new treatment paradigm for patients with locally advanced TRK fusion disease otherwise facing morbid surgery. Screening for TRK fusions should be strongly considered in patients with advanced paediatric tumours and efforts are ongoing to identify optimal testing methods. Currently, primary therapy for many patients with these diagnoses includes cytotoxic chemotherapy, radiotherapy, and in some cases morbid surgery. In survivors, these modalities are associated with a significant burden of late effects. Further study of larotrectinib in infants, children, and adolescents with TRK fusions, including the ongoing phase 2 component of this protocol, and future trials evaluating larotrectinib in the initial management of such patients are warranted.
Supplementary Material
Research in context.
Evidence before this study
Prior to our study, there was a paucity of information about the role of selective TRK inhibition in infants, children and adolescents with cancer. We conducted a PubMed search on November 6, 2017 and included the following search terms: paediatric; NTRK inhibitor or TRK inhibitor; and cancer. The search yielded one prior clinical trial of the non-selective inhibitor lestaurtinib in children with neuroblastoma and one prior clinical trial of the non-selective inhibitor crizotinib in refractory solid tumours and anaplastic large cell lymphoma. Neither trial enrolled any patients known to have TRK fusions. One case report was found of a child with IFS treated with larotrectinib on the clinical trial that is the subject of the current report. One case report of a child with IFS treated with the non-selective inhibitor crizotinib was found.
Added value of this study
To our knowledge, our results provide the first proof-of-concept of a very high response rate and durable responses to selective TRK inhibition in infants, children and adolescents with TRK fusion cancers. These results establish TRK fusions as a tractable target in these patients. Our results will allow infants and children to be dosed safely with larotrectinib using either a liquid or capsule formulation.
Implications of all the available evidence
The combination of a favourable toxicity profile and nearly universal response rate argues for careful strategies to identify TRK fusions in infants, children and adolescents with advanced cancers. Moreover, systematic evaluation of larotrectinib earlier in the course of these diseases should be strongly considered.
Acknowledgments
We thank the participating patients and their families and contributing clinical staff across all sites and Alturas Analytics, Inc. for providing real-time bioanalytical assessments. This trial was funded by Loxo Oncology, Inc., Stamford, CT. Medical writing services were provided by Jim Heighway PhD of Cancer Communications and Consultancy Ltd, Knutsford, UK and were funded by Loxo Oncology, Inc. Additional support was provided by the Alex’s Lemonade Stand Foundation Center of Excellence Award (SGD) and the NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 (NF).
TWL has received fees for consulting and advisory board roles from Loxo Oncology, Eli Lilly, and Novartis and TWL’s institution has received research funding from Pfizer. SGD and NF have received fees for consulting and advisory board roles from Loxo Oncology. RN has received non-financial support from Loxo Oncology. MR is a consultant for and holds a patent 62/318,041 issued to Loxo Oncology. MCC is an employee of and owns stock in Loxo Oncology and holds a patent 62/318,041 issued to Loxo Oncology. BBT and KTE are employees of and own stock in Loxo Oncology. SS and SC are consultants for Loxo Oncology. DSH has received travel expenses from Loxo Oncology.
Footnotes
Contributors
TWL, SGD, LM, SS, SC, MCC, ASP, and DSH contributed to study conception and design. TWL, SGD, BT, NF, LM, CMA, RN, ASP, and DSH contributed to patient recruitment. SS performed the pharmacokinetic analysis. SC performed the statistical analysis. All authors contributed to data analysis and interpretation. All authors contributed to the drafting of the report and approved the final version for submission.
Declaration of interests
All other authors declare no competing interests.
References
- 1.Nakagawara A. Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett. 2001;169:107–14. doi: 10.1016/s0304-3835(01)00530-4. [DOI] [PubMed] [Google Scholar]
- 2.Rubin JB, Segal RA. Growth, survival and migration: the Trk to cancer. Cancer Treat Res. 2003;115:1–18. doi: 10.1007/0-306-48158-8_1. [DOI] [PubMed] [Google Scholar]
- 3.Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1:e000023. doi: 10.1136/esmoopen-2015-000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creancier L, Vandenberghe I, Gomes B, et al. Chromosomal rearrangements involving the NTRK1 gene in colorectal carcinoma. Cancer Lett. 2015;365:107–11. doi: 10.1016/j.canlet.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319:743–8. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- 6.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlick D, Schrock AB, Malicki D, et al. Identification of NTRK fusions in pediatric mesenchymal tumors. Pediatr Blood Cancer. 2017;64:e26433. doi: 10.1002/pbc.26433. [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Diaz AK, Paugh BS, et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46:444–50. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheng WQ, Hisaoka M, Okamoto S, et al. Congenital-infantile fibrosarcoma. A clinicopathologic study of 10 cases and molecular detection of the ETV6-NTRK3 fusion transcripts using paraffin-embedded tissues. Am J Clin Pathol. 2001;115:348–55. doi: 10.1309/3H24-E7T7-V37G-AKKQ. [DOI] [PubMed] [Google Scholar]
- 10.Bourgeois JM, Knezevich SR, Mathers JA, Sorensen PH. Molecular detection of the ETV6-NTRK3 gene fusion differentiates congenital fibrosarcoma from other childhood spindle cell tumors. Am J Surg Pathol. 2000;24:937–46. doi: 10.1097/00000478-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Knezevich SR, Garnett MJ, Pysher TJ, Beckwith JB, Grundy PE, Sorensen PH. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998;58:5046–8. [PubMed] [Google Scholar]
- 12.El Demellawy D, Cundiff CA, Nasr A, et al. Congenital mesoblastic nephroma: a study of 19 cases using immunohistochemistry and ETV6-NTRK3 fusion gene rearrangement. Pathology. 2016;48:47–50. doi: 10.1016/j.pathol.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Prasad ML, Vyas M, Horne MJ, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–107. doi: 10.1002/cncr.29887. [DOI] [PubMed] [Google Scholar]
- 14.Orbach D, Brennan B, De Paoli A, et al. Conservative strategy in infantile fibrosarcoma is possible: The European paediatric Soft tissue sarcoma Study Group experience. Eur J Cancer. 2016;57:1–9. doi: 10.1016/j.ejca.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Orbach D, Rey A, Cecchetto G, et al. Infantile fibrosarcoma: management based on the European experience. J Clin Oncol. 2010;28:318–23. doi: 10.1200/JCO.2009.21.9972. [DOI] [PubMed] [Google Scholar]
- 16.Doebele RC, Davis LE, Vaishnavi A, et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov. 2015;5:1049–57. doi: 10.1158/2159-8290.CD-15-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyman D, Laetsch T, Kummar S, et al. The efficacy of larotrectinib (LOXO-101), a selective tropomyosin receptor kinase (TRK) inhibitor, in adult and pediatric TRK fusion cancers. J Clin Oncol. 2017;35(suppl) abstr LBA2501. [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 20.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–77. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 21.Skolnik JM, Barrett JS, Jayaraman B, Patel D, Adamson PC. Shortening the timeline of pediatric phase I trials: the rolling six design. J Clin Oncol. 2008;26:190–5. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 22.Hermanek P, Sobin LH, et al. TNM Classification of Malignant Tumors. 4. Berlin, Germany: Springer-Verlag; 1987. [Google Scholar]
- 23.Nagasubramanian R, Wei J, Gordon P, Rastatter JC, Cox MC, Pappo A. Infantile fibrosarcoma with NTRK3-ETV6 fusion successfully treated with the tropomyosin-related kinase inhibitor LOXO-101. Pediatr Blood Cancer. 2016;63:1468–70. doi: 10.1002/pbc.26026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Varni JW, Limbers CA, Neighbors K, et al. The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011;20:45–55. doi: 10.1007/s11136-010-9730-5. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell HR, Lu X, Myers RM, et al. Prospective, longitudinal assessment of quality of life in children from diagnosis to 3 months off treatment for standard risk acute lymphoblastic leukemia: Results of Children's Oncology Group study AALL0331. Int J Cancer. 2016;138:332–9. doi: 10.1002/ijc.29708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung L, Klaassen RJ, Dix D, et al. Identification of paediatric cancer patients with poor quality of life. Br J Cancer. 2009;100:82–8. doi: 10.1038/sj.bjc.6604826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drilon A, Nagasubramanian R, Blake JF, et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov. 2017;7:963–972. doi: 10.1158/2159-8290.CD-17-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drilon A, Li G, Dogan S, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC) Ann Oncol. 2016;27:920–6. doi: 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funk RS, Brown JT, Abdel-Rahman SM. Pediatric pharmacokinetics: human development and drug disposition. Pediatr Clin North Am. 2012;59:1001–16. doi: 10.1016/j.pcl.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Orsatti G, Beltrame V, Crimi F, Frigo AC, Bisogno G, Stramare R. Radiologic Response Assessment in Pediatric Soft Tissue Sarcoma: Computed-Assisted Volume Evaluation. The Journal of pediatrics. 2017;182:327–34. e2. doi: 10.1016/j.jpeds.2016.11.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.