Abstract
Phosgene Oxime (CX, Cl2CNOH), a halogenated oxime, is a potent chemical weapon that causes immediate acute injury and systemic effects. CX, grouped together with vesicating agents, is an urticant or nettle agent with highly volatile, reactive, corrosive, and irritating vapor, and has considerably different chemical properties and toxicity compared to other vesicants. CX is absorbed quickly through clothing with faster cutaneous penetration compared to other vesicating agents causing instantaneous and severe damage. For this reason, it could be produced as a weaponized mixture with other chemical warfare agents to enhance their deleterious effects. The immediate devastating effects of CX and easy synthesis makes it a dangerous chemical with both military and terrorist potentials. Although CX is the most potent vesicating agent, it is one of the least studied chemical warfare agents and the pathophysiology as well as long term effects are largely unknown. CX exposure results in immediate pain and inflammation, and it mainly affects skin, eye and respiratory system. There are no antidotes available against CX-induced injury and the treatment is only supportive. This review summarizes existing knowledge regarding exposure, toxicity and the probable underlying mechanisms of CX compared to other important vesicants’ exposure.
Keywords: Phosgene oxime, vesicating agent, nettle agent, skin damage, urticaria, systemic toxicity
1. INTRODUCTION
Chemical warfare agents (CWAs), because of their low cost of manufacturing, easy synthesis and devastating multi-organ toxic effects have been used extensively in warfare (Dacre and Goldman, 1996; Ganesan et al., 2010). The first reported use of CWAs dates back to 1915, when chlorine was used by German army against allied forces at Ypres (Ganesan et al., 2010). Subsequently, large quantities of various CWAs (Choking agents, lachrymators, vesicants, nerve agents, and central nervous system-disabling agents) were produced and stockpiled by several nations, which poses an additional accidental exposure risk, apart from their use in conflicts and feared use by terrorists (Geraci, 2008; Saladi et al., 2006; Watson and Griffin, 1992).
Among the various CWAs developed, vesicants/vesicating agents consist of chemicals that lead to the formation of vesicles/blisters apart from their ability to cause acute and debilitating injuries to multiple organs. These include: 1) mustard agents such as sulfur mustard [bis(2-chloroethyl)sulfide; HD; SM; Lost; Yperite; CAS # 505-60-2 (Fig. 1B)], and nitrogen mustards [HN1 (bis(2-chloroethyl) ethylamine; CAS # 538-07-8), HN2 (2,2′-dichloro-N-methyldiethylamine; CAS # 51-75-2), and HN3 (tris(2-chloroethyl)amine hydrochloride); CAS # 555-77-1]; 2) arsenical vesicants such as lewisite [L or L-1; LEW; dichloro(2-chlorovinyl) arsine; CAS # 541-25-3 (Fig. 1C)]; and 3) nettle agent phosgene oxime (CX; dichloroformoxime; CAS # 1794-86-1 (Fig. 1A) (TOXNET). Of these, SM has been the most extensively used vesicating agent in various conflicts (Dacre and Goldman, 1996; Saladi et al., 2006), with first reported use in 1917, during the World War I, by the German army against the allied forces near the town of Ypres, Belgium (Prevention, 2011). The year 2017 marks the one hundred years of use of vesicating agent SM in warfare. Extensive use of SM in numerous combats has resulted in large number of causalities which led it to earn the nick name the “King of The Battle Gasses” (Geraci, 2008; McManus and Huebner, 2005).
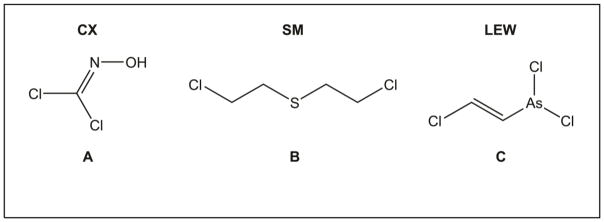
Figure 1.
Chemical structures of nettle vesicant CX (A), mustard vesicant SM (B), and arsenical vesicant LEW (C). The structures were drawn using Chem Draw software (Chem Draw Professional 17.0).
Despite international efforts to de-arm nations of chemical weapons, multiple countries including Iran, Libya, North Korea, and Syria have huge stockpiles of these agents. Most recently, use of nerve agent, Sarin (GB) against civilians in Syria and the use of SM by Islamic State (ISIL) against Kurdish fighters in Syria, and against civilians in Iraq was reported (Chulov, 2017; Kohnavard, 2016; Nebehay, 2017; SAMSFoundation, 2015). These latest deployments serve as reminder that SM and other CWAs still pose a potential threat and could be used by individuals/groups motivated to cause mass casualties, highlighting the need to step up research efforts to understand the injury mechanisms and to develop targeted therapies.
SM has been the most extensively studied vesicating agent (Dacre and Goldman, 1996; Ghabili et al., 2011; Ghabili et al., 2010). A variety of animal models including mice, rabbits, rats, weanling pigs, and hairless guinea pigs, have been used to study the toxicity and pathology of SM, and to understand the underlying mechanisms (Allon et al., 2009; Banin et al., 2003; Dachir et al., 2010; Greenberg et al., 2006; Joseph et al., 2011; Kadar et al., 2009; Kadar et al., 2001; Kan et al., 2003; Morad et al., 2005; Paromov et al., 2007; Petrali et al., 2000; Shakarjian et al., 2010; Smith et al., 1997; Smith et al., 1995). Monofunctional analogs such as 2-chloroethyl ethyl sulfide (CEES) and bifunctional analogues, NMs, have been used to study SM induced toxicity and associated mechanisms as synthesis and use of SM is highly restricted (Banin et al., 2003; Black et al., 2010; Gordon et al., 2010; Goswami et al., 2015; Goswami et al., 2016b; Han et al., 2004; Hardej and Billack, 2007; Inturi et al., 2011; Jain et al., 2011a; Jain et al., 2014a; Jain et al., 2011b; Jowsey et al., 2009; Mangerich et al., 2016; O’Neill et al., 2011; Rancourt et al., 2012; Tewari-Singh et al., 2011; Tewari-Singh et al., 2010; Tewari-Singh et al., 2014; Tewari-Singh et al., 2012).
Arsenical vesicating agent LEW was developed as a chemical warfare agent during World War I. Though not used in warfare, its stockpiles are known to exist and it has been reported to be mixed with SM or other chemical warfare agents to achieve greater effectiveness in combat (Goldman and Dacre, 1989; Kohnavard, 2016; Tewari-Singh et al., 2016). Compared to SM, there are fewer studies on the toxicity mechanisms of LEW (Li et al., 2016b; Mann et al., 1946, 1947; Mouret et al., 2013; Nguon et al., 2014; Tewari-Singh et al., 2016; TOXNET).
The nettle agent and vesicant CX is reported to be stockpiled during World War II as a potent chemical weapon which could be used alone or with other chemical warfare agents to cause startlingly rapid incapacitation and death. Though, this is the most dangerous chemical agent among the vesicants, it is the least studied agent (Augerson, 2000; Patocka, 2011; Tewari-Singh et al., 2017) (Table 1).
Table 1.
Physical and chemical properties of nettle vesicant CX (A), mustard vesicant SM (B), and arsenical vesicant LEW (C).
| Agent | Phosgene oxime CX Dichloroformoxime | Sulfur mustard Bis(2-chloroethyl) sulfide, SM, HD, Yperite, LOST | Lewisite Agent L Dichloro (2-chlorovinyl) arsine |
|---|---|---|---|
| Molecular weight | 113.93 | 159.07 | 207.31 |
| Physical state (at 20 °C) | colorless, crystalline solid or yellowish-brown liquid (munitions-grade) | Oily, colorless (pure) to yellowish dark-brown (munitions-grade) liquid | Oily colorless liquid |
| Aqueous solubility | Soluble | Slightly soluble | Slightly soluble |
| Vapor density (compared to air) | 3.9 | 5.4 | 7.1 |
| Vapor pressure (mm Hg at 20 °C) | 11.2 | 0.06–0.11 | 0.395 |
| Volatility (mg/m3 at 20 °C) | 1800 | 610 | 4480 |
| Boiling point (°C) | 128 | 215–217 | 190 |
| Melting point (°C) | 35–40 | 13–14.4 | 0.1 |
| Decomposition temperature (°C) | <128 | 149–177 | >100 |
| Odor | Disagreeable, prickling odor | Almost odorless (in pure state at typical field concentrations); horseradish, garlic or mustard odor at higher concentrations | Faint geranium like odor |
Sources: (Augerson, 2000; Dacre and Goldman, 1996; TOXNET)
Exposure to vesicating agents causes damage to the ocular, skin and pulmonary systems at even at low doses while higher dose exposures lead to multi-organ toxicity including systemic effects (Augerson, 2000) (Table 2). Mustard agents cause acute and chronic debilitating injuries from ocular and dermal absorption, as well as lung inhalation, resulting in severe injury to these tissues as well as systemic toxic effects including the gastrointestinal, hematological, immunological, mucoskeletal, reproductive, nervous, and cardiac systems at higher exposure doses (Ghabili et al., 2011; Ghasemi et al., 2013; Graham and Schoneboom, 2013; Panahi et al., 2013). Injury from SM is biphasic with symptoms of delayed injury appearing as long as 40 years after the initial exposure (Balali-Mood and Hefazi, 2006; Balali-Mood et al., 2008; Etezad-Razavi et al., 2006; Ghabili et al., 2010; Ghanei et al., 2010; Hefazi et al., 2006; Kehe and Szinicz, 2005; Keramati et al., 2013; Korkmaz et al., 2008; Shohrati et al., 2007) (Table 2). At the molecular level, these effects could be attributed to SMs alkylating properties and/or thiol-depleting properties, resulting in the activation of signaling pathways related to DNA damage, oxidative stress, and inflammation (Kehe et al., 2009; Paromov et al., 2007; Sabourin et al., 2002; Shakarjian et al., 2010) (Table 3).
Table 2.
Injury symptoms upon exposure to nettle vesicant CX (A), mustard vesicant SM (B), and arsenical vesicant LEW (C).
| Ocular injury | Immediate pain, conjunctivitis, edema, keratitis, iritis, lacrimation, vision loss, temporary blindness |
Acute/early: Irritation, foreign body sensation, pain, conjunctivitis, photophobia, edema, corneal ulceration, opacity, lacrimation, blepharospasm, vesication, temporary blindness Chronic/delayed: Vasculitis, corneal scarring, opacification, ulceration, perforation and erosions, limbal stem cell deficiency, neovascularization, endothelial cell damage |
Immediate pain, blepharospasm, conjunctivitis, photophobia, lacrimation, corneal ulceration and opacity, iritis, temporary blindness |
| Pulmonary injury | Irritation, pulmonary edema, necrotizing bronchitis, pulmonary venule thrombosis |
Acute/early: Coughing, choking, dyspnea, hypoxia, pseudomembrane formation, bronchospasm, pulmonary edema, rhinorrhea, tachypnea Chronic/delayed: Decreased lung capacity, pulmonary fibrosis, increased incidences of lung cancer, emphysema, chronic bronchitis, bronchiolitis |
Sneezing, coughing, rhinitis, pulmonary edema |
| Cutaneous injury | Immediate itching, erythema, edema, hives, blanching (skin whitening), urticaria, tissue necrosis/eschar |
Acute/early: Itching, erythema, blisters/vesication, desquamation, hyperesthesia, necrosis/eschar, pruritis, purpura, hyper/hypo-pigmentation Chronic/delayed: Atrophy, scarring, eczema, popular rash, keloids, cherry angiomas, scaling, seborrheic dermatitis |
Immediate itching/stinging followed by erythema, blisters/vesication |
| Other organ systems affected | GI tract, liver, cardiovascular, kidneys, spleen, and Immune system | GI tract, liver, cardiovascular, CNS, kidneys, spleen, immune system, bone marrow, lymphatic, reproductive, and mucoskeletal system | GI tract, liver, cardiovascular, and CNS |
Table 3.
Molecular mechanisms involved in nettle vesicant CX (A), mustard vesicant SM (B), and arsenical vesicant LEW (C) induced toxicity.
| CX | SM | LEW |
|---|---|---|
|
|
|
Arsenical vesicant LEW exposure also causes debilitating effects on its primary target organs eyes, skin and the respiratory systems with more severe lesions. However, as compared to mustard vesicants, its toxicity is associated with severe pain within minutes of exposure and its faster cutaneous absorption causes more sever systemic effects (Li et al., 2016a; Mouret et al., 2013; Nguon et al., 2014; Tewari-Singh et al., 2016; TOXNET)(Table 2). However, there is limited information available on the toxic effects of LEW (Augerson, 2000; Goldman and Dacre, 1989; McManus and Huebner, 2005; Prevention, 2011). The toxic outcomes of LEW exposure could be attributed to its ability to combine with thiol groups, react with biological sulfhydryl groups and glutathione, and to release hydrochloric acid. At molecular level, oxidative stress, unfolded protein response, inflammation and apoptosis in addition to heavy metal toxicity are plausible mechanisms responsible for LEW toxicity (CDC, 2011; Li et al., 2016b; Mouret et al., 2013; Nguon et al., 2014; Tewari-Singh et al., 2016) (Table 3).
CX was first synthesized in 1929 and its potential as a chemical warfare agent was recognized due to its fast penetration and immediate injuries (Patocka, 2011). Although it was stockpiled during World War II, there are no records of its use in battlefield. It was produced alone or as a mixture with LEW and SM to enhance their penetration. CX is a halogenated oxime and is a colorless, crystalline solid with a strong, disagreeable odor and violently irritating vapor (Patocka, 2011; Schraga, 2016). CX is generally produced by reduction of chloropicrin by tin in presence of hydrochloric acid (Patocka, 2011). CX exists in vapor form at ambient atmosphere as it has a vapor pressure of 11.2 mmHg at 25 °C. It is heavier than air thus settles in low-lying areas. CX is relatively non-persistent in soil, as it is highly unstable and decomposes rapidly before it could volatilize. CX is expected to have high mobility in soil due to a soil adsorption coefficient (Koc, based on structure estimation method) of 68, and it exists partially as an anion as it’s a weak acid (estimated pKa 6.5)(Meylan et al., 1992; Swann et al., 1983). It is soluble in water and organic solvents and hydrolyses very rapidly, particularly in the presence of an alkali to form hydrogen chloride and hydrolamines (Bartelt-Hunt et al., 2006b; Ellison, 2007; Wismer, 2009). CX has a reported half-life of 83 days at unspecified pH and temperature (Bartelt-Hunt et al., 2006a). Volatilization of CX from aqueous solutions is not expected to be a major fate because of its presence as an anion. Bioconcentration is not expected as CX has an estimated bioconcentration factor (BCF) of 3. (ATSDR, 2014; Augerson, 2000; CDC, 2011). Physical and chemical properties of CX, SM and LEW are summarized in Table 1.
Although CX is grouped together with vesicants, this is an urticant or nettle agent and not a pure vesicant as it does not lead to blister/vesicles formation. It produces intense itching and rash resembling hives upon cutaneous exposure (Augerson, 2000; Patocka, 2011). Its exposure in both liquid and vapor forms can cause more severe damage to the skin, eye, and lung tissues than other vesicants due to its fast penetration, immediate pain and tissue destruction. In addition, its rapid absorption through the skin can lead to immediate skin damage and severe systemic toxicity that can lead to rapid mortality. The nature of injuries caused by CX resembles those caused by acids, therefore it is often referred to as a corrosive agent (Patocka, 2011). The mechanism of action of CX is unknown; however, it likely possesses alkylating and nucleophilic properties resembling mustard vesicants, and its effect could be direct involving corrosive injury, cell death and tissue destruction, or indirect involving inflammatory response causing delayed tissue injury. Unlike SM and LEW, reports on the effects of CX exposure and the mechanism of injury, and long term effects are unknown (Augerson, 2000). We have employed SKH-1 hairless mouse model in our recently published report to understand CX-induced acute injury and the underlying molecular mechanisms (Tewari-Singh et al., 2017). Further studies are needed to understand the injury mechanism of the rapid onset of severe and prolonged effect of CX exposure to develop effective therapies against this most potent vesicant.
2. INJURY SYMPTOMS & TARGET ORGANS
The injury symptoms upon vesicants’ exposure vary depending on the dose, route and form of exposure. CX is absorbed rapidly and has faster penetration (it can even penetrate rubber gloves) than other vesicating agents. The symptoms upon CX exposure appear instantly in comparison to SM or LEW; it takes few seconds to minutes for the symptoms to appear upon LEW exposure, and for SM, the latency period is in hours (Augerson, 2000). A comparison of injury symptoms upon CX, SM, and LEW exposure is summarized in Table 2. Skin and mucous membrane irritation can begin within seconds of exposure to low doses of CX (0.2mg.min/m3), while unbearable pain and irritation could occur minutes after exposure to a dose of 3mg.min/m3. Lethal systemic dose estimation [LCt50 (concentration-time product capable of killing 50% of exposures)] is 1500–2000mg.min/m3 (ATSDR, 2014; Schraga, 2016).
The rapid skin damage caused by CX makes the skin susceptible to injury from other chemical agents. CX exposure at higher concentrations is more damaging and causes instant pain followed by tissue necrosis, systemic effects and mortality (Augerson, 2000). People could be exposed to CX by air, by breathing the gas and skin and eye contact. If liquid CX is released into the water or food, people can be exposed by drinking water or eating the contaminated food (Patocka, 2011). Immediate eye and respiratory irritation occurs upon CX vapor exposure. The symptoms include cough, throat pain, increased lachrymation and impaired vision (Augerson, 2000).
3.1. Eye
Eyes are the most sensitive organ to vesicant exposure (Ghasemi et al., 2013; Gordon et al., 2009; Goswami et al., 2016a; Kadar et al., 2013a; Kadar et al., 2009; Kadar et al., 2013b; McNutt et al., 2012; Tewari-Singh et al., 2016). CX exposure of the eye results in immediate and severe pain, irritation, edema, lacrimation, conjunctivitis, and blepharospasm with more severe exposure resulting in keratitis, iritis, corneal perforation and blindness (Table 2) (Patocka, 2011). Unlike SM, there are no reports available on the long-term ocular effects of LEW and CX. SM exposure is known to cause a biphasic injury with the symptoms appearing few hours after the exposure and comprising of an acute phase with inflammation, conjunctivitis, lachrymation, photophobia, keratitis, corneal ulceration and erosion and a delayed phase consisting of persistent epithelial defects, keratitis, corneal scarring, neovascularization, endothelial cell damage and limbal stem cell deficiency (Gordon et al., 2009; McNutt et al., 2012). LEW exposure of the eyes is reported to cause instant pain, inflammation, irritation, swelling and tearing, edema of eyelids, massive corneal necrosis and blindness (Goldman and Dacre, 1989; Olajos et al., 1998; Tewari-Singh et al., 2016).
3.2. Respiratory system
CX is quickly absorbed upon inhalation exposure causing immediate and incapacitating irritation, pain, runny nose, hoarseness, as well as local tissue destruction of the upper airways at low exposure doses, while serious complications such as pulmonary edema followed by tachypnea, dyspnea, and cyanosis occur at higher exposure doses (Augerson, 2000; Patocka, 2011; Schraga, 2016). Exposure to aerosol could result in necrotizing bronchiolitis, pulmonary edema with pulmonary vein thrombosis (Augerson, 2000). Unlike SM, the long term respiratory effects of CX are unknown, although it is believed to result in the development of pulmonary fibrosis (Augerson, 2000). In comparison, the respiratory injury symptoms from SM exposure include sneezing, nasal and throat irritation, loss of taste and smell at lower exposures doses, while higher dose exposure results in laryngitis, aphonia, bronchitis, coughing, pseudomembrane formation, dyspnea, and hypoxia (Weinberger et al., 2016; White et al., 2016). Long term effects of SM exposure include chronic bronchitis, decreased lung capacity, pulmonary fibrosis, and increased incidences of lung cancer (Balali-Mood et al., 2008; Beheshti et al., 2006; Ghanei et al., 2008; Ghanei and Harandi, 2007; Ghasemi et al., 2013; Hefazi et al., 2005). Respiratory symptoms upon LEW exposure resemble those of upper respiratory infections, with sneezing, nausea, coughing, rhinitis, mucous membrane erythema. Severe LEW exposure results in coughing, laryngitis and aphonia (Augerson, 2000; McManus and Huebner, 2005; TOXNET).
3.3 Skin
CX is absorbed quickly upon dermal exposure and results in immediate itching, pain, skin blanching, erythema, edema, hives formation, pruritic, pigmentation and severe necrosis. Desquamation with necrosis of the skin could be followed by eschar formation and polymorphonuclear infiltrates and the complete healing could take months (Augerson, 2000; Tewari-Singh et al., 2017). Severe exposure of the skin could result in systemic effects, but long-term effects are unknown (Augerson, 2000; Patocka, 2011; Tewari-Singh et al., 2017). Exposure of the skin to vesicant SM results in acute and chronic lesions, with varying severity and symptoms depending on the dose and duration of the exposure. SM is a very lipophilic molecule and readily penetrates the skin. Appearance of symptoms could take few hours to days and include edema, erythema, inflammation, epidermal-dermal separation, blistering, ulceration, desquamation, and necrosis. Delayed effects include altered pigmentation, presence of cherry angiomas, eczema, hypertrophy, and dry and sensitive skin (Balali-Mood and Hefazi, 2005, 2006; Dacre and Goldman, 1996; Ghabili et al., 2011; Ghabili et al., 2010). LEW exposure of the skin results in immediate itching, erythema, edema, desquamation, inflammation, vesication, and degenerative necrotic changes (Augerson, 2000; Li et al., 2016b; Mouret et al., 2013; Nguon et al., 2014).
3.4. Systemic toxicity
Apart from the toxic effects of CX exposure on eyes, respiratory, and skin system, CX exposure has also been shown to induce severe systemic toxic effects in our recent study (Tewari-Singh et al., 2017). Histopathological analyses of the lung, liver, spleen, kidney, and heart tissue showed dilatation of the peripheral vessels (including capillaries and sinusoids) and the pooling of red blood cells (RBCs) in the vessels (Tewari-Singh et al., 2017). This severe vascular dilation could result in a marked loss of blood from the vessels into the surrounding tissue and could lead to low blood pressure, relative hypoxia, and shock leading to possible mortality. Similar effect has been observed for high dose exposure of LEW, where death may result from fluid loss, hypovolemia secondary to capillary leakage - known as the “Lewisite shock” (Smith et al., 1997; Watson and Griffin, 1992). Higher dose exposures of SM affect the rapidly-dividing cells in the GI tract and bone marrow. In the GI tract, destruction of the mucosa and perforations of the GI walls lead to GI bleeding (Ghasemi et al., 2013), while in the bone marrow, it results in bone-marrow suppression. Pancytopenia, increase in the number of RBCs and hematocrit in long-term, loss of spleen cells, depression of cell-mediated immunity are also observed. SM is also classified as a carcinogen and few reports have shown that it affects the reproductive, cardiovascular, renal, hepatic, and central nervous system and causes psychological complications (Balali-Mood and Hefazi, 2005; Geraci, 2008; Ghabili et al., 2010; Ghasemi et al., 2013).
3. PATHOPHYSIOLOGY
Among vesicants, molecular mechanism of SM toxicity has been extensively studied. SM is a highly reactive chemical and in aqueous solutions forms the sulfonium ion that reacts readily with all major macromolecules in the cell (Dacre and Goldman, 1996; Kehe et al., 2009). DNA damage is one of the key events after SM exposure leading to H2A.X and p53 phosphorylation (Joseph et al., 2011; Paromov et al., 2007). PARP activation leads to cellular NAD+ depletion, cell cycle arrest, and activation of DNA damage repair. The cell could undergo apoptosis/necrosis depending upon the extent of damage. SM is also known to cause ER stress resulting in changes in Ca++ homeostasis, reduction in cellular glutathione levels, NO signaling and oxidative stress. Release of inflammatory mediators like cyclooxygenase-2 (COX-2) and cytokines [tumor necrosis factor- alpha (TNF-α), interleukins (IL)-1α/β, IL-6 and IL-8) as well as activation of matrix metalloprotease-9 (MMP-9), Nuclear factor-kappa B (NF-κB) and mitogen activated protein kinase (MAPK) pathways are also reported to play a major role in SM-induced toxicity (Dacre and Goldman, 1996; Kehe et al., 2009; Mouret et al., 2015; Shakarjian et al., 2006; Shakarjian et al., 2010) (Table 3). Similarly, in LEW injury, DNA damage, apoptotic cell death, unfolded protein response (UPR) pathway, oxidative stress, decrease in cellular glutathione levels, release of inflammatory mediators (COX-2) and cytokines (TNF- α, IL-1β, IL-6, and IL-8), and activation of MMP-9 and NF-κB pathways have been reported. Arsenic poisoning and inhibition of carbohydrate metabolism are also reported to be involved in LEW- induced toxicity (Augerson, 2000; Goldman and Dacre, 1989; Kehe et al., 2001; Li et al., 2016b; Mouret et al., 2013; Nelson et al., 2006; Nguon et al., 2014; Srivastava et al., 2016) (Table 3). There are very few reports on the toxic effects of CX, and the mechanism of injury is unknown. Possible molecular mechanisms involved in SM-, LEW- and CX-induced injury have been summarized in Table 3. The toxic effects upon CX exposure could be attributed to its alkylating properties, or to the effect of chlorine, oxime or carbonyl groups, resulting in direct (enzyme inactivation, corrosive injury and cell death with rapid tissue destruction) or indirect toxicity (involving activation of alveolar macrophages, recruitment of neutrophils, and release of hydrogen peroxide), resulting in delayed tissue injury, such as pulmonary edema (Augerson, 2000; Tewari-Singh et al., 2017).
In our recent report, we analyzed the effect of acute cutaneous phosgene-exposure in SKH-1 hairless mice. Cutaneous CX exposure (4 min exposure on two 12-mm sites on the dorsal surface) resulted in 20% mortality within 8 h. In the exposed skin area, blanching was observed within minutes of exposure with the center surrounded by an erythematosus ring. Urticaria (red hives-like area), necrosis and wheal formation was also observed within the blanched skin area (Tewari-Singh et al., 2017). Immediate increase in skin injury parameters, including doubling of skin bi-fold thickness, moderate erythema and edema, and necrosis was observed that maxed at 2h post-exposure. Histopathologic analyses were consistent with the observed injury parameters and were similar to skin urticaria due to allergic and non-allergic reactions to various environmental substances (Jain, 2014). In addition, an increase in the inflammatory cells mostly neutrophils and degranulated mast cells was observed. The neutrophil infiltration was further confirmed by increased myeloperoxidase (MPO) expression in the skin lysates. Neutrophil infiltration has also been shown to play a key role in skin inflammation related to mustard vesicating agents (Jain et al., 2014b; Shakarjian et al., 2010; Wormser et al., 2005).
DNA damage, p53 phosphorylation and accumulation have also been shown to play an important role in vesicating agents-induced apoptotic cell death, and have also been associated with the injury (Goswami et al., 2016b; Kehe and Szinicz, 2005; Paromov et al., 2007). Like mustards, an increase in phosphorylation of p53 at ser15 and its accumulation in the skin tissue samples upon CX exposure were observed. Cutaneous exposure to CX also resulted in an increase in TNFα and COX-2 levels in the skin tissue, which has been also observed with vesicating agents exposure of the skin tissue (Shakarjian et al., 2010; Tewari-Singh et al., 2017; Tewari-Singh et al., 2009).
The systemic effects seen in our reported study with CX further support the fact that CX is absorbed instantaneously, leading to more severe systemic toxicity and death compared to other vesicating agents. Although, cutaneous exposure to other vesicants at higher doses, results in damage to multiple organ systems but mortality is rare (Dacre and Goldman, 1996; Goswami et al., 2015; Kehe et al., 2008; Patocka, 2011).
4. TREATMENT
Among vesicating agents, effective anti-dotes are available only for LEW-induced toxicity, in the form of British Anti-Lewisite (BAL; dimercaprol) and derivatives, meso-2,3-dimercaptosuccinic acid (DMSA) and 2,3-dimercapto-1-propane- sulphonic acid (DMPS) (Goswami et al., 2016a; Hughes, 1946, 1947; Mann et al., 1947; Mouret et al., 2013). However, there are still limitations with the use of these therapies including narrow therapeutic window, toxicity and difficulty in administration, thus, requiring the need for the development of better and safe antidotes. There are no effective approved antidotes available for SM. Although a number of compounds including anti-oxidants, protease inhibitors, PARP inhibitors, angiogenesis inhibitors, calcium modulators, anti-inflammatory agents, and flavanones have been shown to be effective to various extents in laboratory studies (Balszuweit et al., 2013; Goswami et al., 2016a; Kadar et al., 2014; Kadar et al., 2009; Laskin et al., 2010; McElroy and Day, 2016; Paromov et al., 2007; Smith, 2009; Tewari-Singh and Agarwal, 2016; Weinberger et al., 2016). There is no specific antidote available against CX-induced injuries and the treatment is mostly supportive to reduce symptoms, prevent infections and help healing. For oral exposures, dilution with water or milk could be helpful. For ocular injury, irrigation with copious amount of water could be helpful while for necrotic skin lesions, surgical intervention may be required. Recovery depends on the extent of injury and could take anywhere several months (Patocka, 2011). Since CX is absorbed within seconds, the timing of decontamination is very crucial. Systemic analgesics are preferred over topical anesthetics, as use of later may increase the severity of corneal damage (Schraga, 2016). Our recently published study shows that some of the molecular events upon CX-induced injury, could be similar to those induced by mustard-induced toxicity, including p53 phosphorylation and accumulation, increased COX-2 and TNFα levels, and increased MPO activity (Joseph et al., 2011; Mouret et al., 2015; Smith et al., 1997; Tewari-Singh et al., 2017; Tewari-Singh et al., 2013). Hence, agents identified for treating mustard induced injuries could also be tested as potential therapies for CX-induced injury. Since mast cell activation and histamine release could be involved in CX-induced instantaneous inflammation and urticarial; anti-histamine, anti-inflammatory and immunosuppressant drugs could useful to reduce the inflammatory response and mortality associated with CX-induced toxicity (Hennino et al., 2006; Jain, 2014; Tewari-Singh et al., 2017). Analgesics and antibiotics could be given to reduce the pain and prevent infections and promote healing.
5. CONCLUSIONS
CX is a dangerous, corrosive, and fast penetrating urticant, which can cause serious immediate toxic effects and incapacitation with fast mortality due to systemic effects. It is known to cause more severe tissue damage than other vesicating agents; however, its toxicity has not been well studies and its mechanism of action is unknown. Although it has never been used in warfare, its potent nature and toxic consequences make it a potential military and terrorist weapon. It could be produced as a weaponized mixture with other chemical warfare agents to enhance their deleterious effects. There are no antidotes available for CX, only removal of causalities from the source of exposure and rapid decontamination are the key factors in reducing causalities. Further comprehensive studies to investigate pathophysiology of the toxic effects of CX are needed to develop effective therapies.
Highlights.
Phosgene oxime (CX) is a nettle agent grouped together with vesicants.
CX is the most potent but least studied vesicating agent.
CX is absorbed quickly and causes immediate pain and inflammation.
CX with faster penetration causes severe tissue damage and systemic toxicity.
CX could be produced as a weaponized mixture with other chemical warfare agents.
Acknowledgments
We thank the support from Countermeasures Against Chemical Threats (CounterACT) Program, Office of the Director National Institutes of Health (NIH OD) and the National Eye Institute (NEI) [Grant Number U01EY023143] to Rajesh Agarwal and CounterACT Program, (NIH OD) and the National Institute of Neurological Disorders and Stroke (NINDS) [Grant Number R21 AR073544] to Neera Tewari-Singh.
ABBREVIATIONS
- BAL
British Anti-Lewisite (dimercaprol)
- COX-2
cyclooxygenase-2
- CWAs
Chemical warfare agents
- CX
phosgene oxime (dichloroformoxime)
- DMPS
2,3-dimercapto-1-propane- sulphonic acid
- IL
Interleukins
- LEW
Lewisite; dichloro(2-chlorovinyl) arsine
- MAPK
mitogen activated protein kinase
- MMP-9
matrix metalloprotease-9
- MPO
myeloperoxidase
- NM
nitrogen mustard
- RBCs
red blood cells
- SM
sulfur mustard
- TNF-α
tumor necrosis factor- alpha
- UPR
unfolded protein response
Footnotes
CONFLICT OF INTEREST
The Authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allon N, Amir A, Manisterski E, Rabinovitz I, Dachir S, Kadar T. Inhalation exposure to sulfur mustard in the guinea pig model: clinical, biochemical and histopathological characterization of respiratory injuries. Toxicology and applied pharmacology. 2009;241:154–162. doi: 10.1016/j.taap.2009.08.006. [DOI] [PubMed] [Google Scholar]
- ATSDR. Medical Management Guidelines (MMGs): Phosgene Oxime. 2014. [Google Scholar]
- Augerson W. Skin Damaging Agents. RAND Corporation; Santa Monica, CA: 2000. A Review of the Scientific Literature as it Pertains to Gulf War Illnesses: Volume 5: Chemical and Biological Warfare Agents; pp. 15–52. [Google Scholar]
- Balali-Mood M, Hefazi M. The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundamental & clinical pharmacology. 2005;19:297–315. doi: 10.1111/j.1472-8206.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- Balali-Mood M, Hefazi M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic & clinical pharmacology & toxicology. 2006;99:273–282. doi: 10.1111/j.1742-7843.2006.pto_429.x. [DOI] [PubMed] [Google Scholar]
- Balali-Mood M, Mousavi S, Balali-Mood B. Chronic health effects of sulphur mustard exposure with special reference to Iranian veterans. Emerging health threats journal. 2008;1:e7. doi: 10.3134/ehtj.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balszuweit F, John H, Schmidt A, Kehe K, Thiermann H, Steinritz D. Silibinin as a potential therapeutic for sulfur mustard injuries. Chemico-biological interactions. 2013;206:496–504. doi: 10.1016/j.cbi.2013.06.010. [DOI] [PubMed] [Google Scholar]
- Banin E, Morad Y, Berenshtein E, Obolensky A, Yahalom C, Goldich J, Adibelli FM, Zuniga G, DeAnda M, Pe’er J, Chevion M. Injury induced by chemical warfare agents: characterization and treatment of ocular tissues exposed to nitrogen mustard. Investigative ophthalmology & visual science. 2003;44:2966–2972. doi: 10.1167/iovs.02-1164. [DOI] [PubMed] [Google Scholar]
- Bartelt-Hunt SL, Barlaz MA, Knappe DR, Kjeldsen P. Fate of chemical warfare agents and toxic industrial chemicals in landfills. Environmental science & technology. 2006a;40:4219–4225. doi: 10.1021/es052400y. [DOI] [PubMed] [Google Scholar]
- Bartelt-Hunt SL, Barlaz MA, Knappe DRU, Kjeldsen P. Fate of Chemical Warfare Agents and Toxic Industrial Chemicals in Landfills. Environmental science & technology. 2006b;40:4219–4225. doi: 10.1021/es052400y. [DOI] [PubMed] [Google Scholar]
- Beheshti J, Mark EJ, Akbaei HM, Aslani J, Ghanei M. Mustard lung secrets: long term clinicopathological study following mustard gas exposure. Pathol Res Pract. 2006;202:739–744. doi: 10.1016/j.prp.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Black AT, Joseph LB, Casillas RP, Heck DE, Gerecke DR, Sinko PJ, Laskin DL, Laskin JD. Role of MAP kinases in regulating expression of antioxidants and inflammatory mediators in mouse keratinocytes following exposure to the half mustard, 2-chloroethyl ethyl sulfide. Toxicology and applied pharmacology. 2010;245:352–360. doi: 10.1016/j.taap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. PHOSGENE OXIME (CX): Blister Agent. 2011. [Google Scholar]
- Chulov M. Sarin used in April Syria attack, chemical weapons watchdog confirms. The Guardian 2017 [Google Scholar]
- Dachir S, Cohen M, Fishbeine E, Sahar R, Brandies R, Horwitz V, Kadar T. Characterization of acute and long-term sulfur mustard-induced skin injuries in hairless guinea-pigs using non-invasive methods. Skin Res Technol. 2010;16:114–124. doi: 10.1111/j.1600-0846.2009.00409.x. [DOI] [PubMed] [Google Scholar]
- Dacre JC, Goldman M. Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacological reviews. 1996;48:289–326. [PubMed] [Google Scholar]
- Ellison DH. Handbook of Chemical and Biological Warfare Agents: Chapter 5 Urticants. CRC Press; Boca Raton: 2007. p. 800. [Google Scholar]
- Etezad-Razavi M, Mahmoudi M, Hefazi M, Balali-Mood M. Delayed ocular complications of mustard gas poisoning and the relationship with respiratory and cutaneous complications. Clin Experiment Ophthalmol. 2006;34:342–346. doi: 10.1111/j.1442-9071.2006.01220.x. [DOI] [PubMed] [Google Scholar]
- Ganesan K, Raza SK, Vijayaraghavan R. Chemical warfare agents. Journal of pharmacy & bioallied sciences. 2010;2:166–178. doi: 10.4103/0975-7406.68498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci MJ. Mustard gas: imminent danger or eminent threat? The Annals of pharmacotherapy. 2008;42:237–246. doi: 10.1345/aph.1K445. [DOI] [PubMed] [Google Scholar]
- Ghabili K, Agutter PS, Ghanei M, Ansarin K, Panahi Y, Shoja MM. Sulfur mustard toxicity: history, chemistry, pharmacokinetics, and pharmacodynamics. Critical reviews in toxicology. 2011;41:384–403. doi: 10.3109/10408444.2010.541224. [DOI] [PubMed] [Google Scholar]
- Ghabili K, Agutter PS, Ghanei M, Ansarin K, Shoja MM. Mustard gas toxicity: the acute and chronic pathological effects. Journal of applied toxicology: JAT. 2010;30:627–643. doi: 10.1002/jat.1581. [DOI] [PubMed] [Google Scholar]
- Ghanei M, Adibi I, Farhat F, Aslani J. Late respiratory effects of sulfur mustard: how is the early symptoms severity involved? Chron Respir Dis. 2008;5:95–100. doi: 10.1177/1479972307087191. [DOI] [PubMed] [Google Scholar]
- Ghanei M, Harandi AA. Long term consequences from exposure to sulfur mustard: a review. Inhalation toxicology. 2007;19:451–456. doi: 10.1080/08958370601174990. [DOI] [PubMed] [Google Scholar]
- Ghanei M, Poursaleh Z, Harandi AA, Emadi SE, Emadi SN. Acute and chronic effects of sulfur mustard on the skin: a comprehensive review. Cutaneous and ocular toxicology. 2010;29:269–277. doi: 10.3109/15569527.2010.511367. [DOI] [PubMed] [Google Scholar]
- Ghasemi H, Owlia P, Jalali-Nadoushan MR, Pourfarzam S, Azimi G, Yarmohammadi ME, Shams J, Fallahi F, Moaiedmohseni S, Moin A, Yaraee R, Vaez-Mahdavi MR, Faghihzadeh S, Mohammad Hassan Z, Soroush MR, Naghizadeh MM, Ardestani SK, Ghazanfari T. A clinicopathological approach to sulfur mustard-induced organ complications: a major review. Cutaneous and ocular toxicology. 2013;32:304–324. doi: 10.3109/15569527.2013.781615. [DOI] [PubMed] [Google Scholar]
- Goldman M, Dacre JC. Lewisite: its chemistry, toxicology, and biological effects. Reviews of environmental contamination and toxicology. 1989;110:75–115. doi: 10.1007/978-1-4684-7092-5_2. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Desantis A, Deshmukh M, Lacey CJ, Hahn RA, Beloni J, Anumolu SS, Schlager JJ, Gallo MA, Gerecke DR, Heindel ND, Svoboda KK, Babin MC, Sinko PJ. Doxycycline hydrogels as a potential therapy for ocular vesicant injury. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2010;26:407–419. doi: 10.1089/jop.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MK, Enzenauer RW, Babin MC. Ocular toxicity of sulfur mustard. In: Gupta RC, editor. Handbook of Toxicology of Chemical Warfare Agents. Academic Press; 2009. [Google Scholar]
- Goswami DG, Kumar D, Tewari-Singh N, Orlicky DJ, Jain AK, Kant R, Rancourt RC, Dhar D, Inturi S, Agarwal C, White CW, Agarwal R. Topical nitrogen mustard exposure causes systemic toxic effects in mice. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie. 2015;67:161–170. doi: 10.1016/j.etp.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami DG, Tewari-Singh N, Agarwal R. Corneal toxicity induced by vesicating agents and effective treatment options. Annals of the New York Academy of Sciences. 2016a;1374:193–201. doi: 10.1111/nyas.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami DG, Tewari-Singh N, Dhar D, Kumar D, Agarwal C, Ammar DA, Kant R, Enzenauer RW, Petrash JM, Agarwal R. Nitrogen Mustard-Induced Corneal Injury Involves DNA Damage and Pathways Related to Inflammation, Epithelial-Stromal Separation, and Neovascularization. Cornea. 2016b;35:257–266. doi: 10.1097/ICO.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JS, Schoneboom BA. Historical perspective on effects and treatment of sulfur mustard injuries. Chemico-biological interactions. 2013 doi: 10.1016/j.cbi.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Greenberg S, Kamath P, Petrali J, Hamilton T, Garfield J, Garlick JA. Characterization of the initial response of engineered human skin to sulfur mustard. Toxicological sciences: an official journal of the Society of Toxicology. 2006;90:549–557. doi: 10.1093/toxsci/kfi306. [DOI] [PubMed] [Google Scholar]
- Han S, Espinoza LA, Liao H, Boulares AH, Smulson ME. Protection by antioxidants against toxicity and apoptosis induced by the sulphur mustard analog 2-chloroethylethyl sulphide (CEES) in Jurkat T cells and normal human lymphocytes. British journal of pharmacology. 2004;141:795–802. doi: 10.1038/sj.bjp.0705591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardej D, Billack B. Ebselen protects brain, skin, lung and blood cells from mechlorethamine toxicity. Toxicology and industrial health. 2007;23:209–221. doi: 10.1177/0748233707083541. [DOI] [PubMed] [Google Scholar]
- Hefazi M, Attaran D, Mahmoudi M, Balali-Mood M. Late respiratory complications of mustard gas poisoning in Iranian veterans. Inhalation toxicology. 2005;17:587–592. doi: 10.1080/08958370591000591. [DOI] [PubMed] [Google Scholar]
- Hefazi M, Maleki M, Mahmoudi M, Tabatabaee A, Balali-Mood M. Delayed complications of sulfur mustard poisoning in the skin and the immune system of Iranian veterans 16–20 years after exposure. International journal of dermatology. 2006;45:1025–1031. doi: 10.1111/j.1365-4632.2006.03020.x. [DOI] [PubMed] [Google Scholar]
- Hennino A, Berard F, Guillot I, Saad N, Rozieres A, Nicolas JF. Pathophysiology of urticaria. Clinical reviews in allergy & immunology. 2006;30:3–11. doi: 10.1385/CRIAI:30:1:003. [DOI] [PubMed] [Google Scholar]
- Hughes WF., Jr Clinical uses of 2,3-dimercaptopropanol (BAL); the treatment of lewisite burns of the eye with BAL. The Journal of clinical investigation. 1946;25:541–548. doi: 10.1172/JCI101736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes WF., Jr Treatment of lewisite burns of the eye with dimercaprol (BAL) Arch Ophthal. 1947;37:25–41. doi: 10.1001/archopht.1947.00890220030004. [DOI] [PubMed] [Google Scholar]
- Inturi S, Tewari-Singh N, Gu M, Shrotriya S, Gomez J, Agarwal C, White CW, Agarwal R. Mechanisms of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced DNA damage in skin epidermal cells and fibroblasts. Free radical biology & medicine. 2011;51:2272–2280. doi: 10.1016/j.freeradbiomed.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Gu M, Inturi S, White CW, Agarwal R. Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin. Toxicology letters. 2011a;205:293–301. doi: 10.1016/j.toxlet.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Inturi S, Orlicky DJ, White CW, Agarwal R. Histopathological and immunohistochemical evaluation of nitrogen mustard-induced cutaneous effects in SKH-1 hairless and C57BL/6 mice. Experimental and toxicologic pathology: official journal of the Gesellschaft fur Toxikologische Pathologie. 2014a;66:129–138. doi: 10.1016/j.etp.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Inturi S, Orlicky DJ, White CW, Agarwal R. Myeloperoxidase deficiency attenuates nitrogen mustard-induced skin injuries. Toxicology. 2014b;320:25–33. doi: 10.1016/j.tox.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tewari-Singh N, Orlicky DJ, White CW, Agarwal R. 2-Chloroethyl ethyl sulfide causes microvesication and inflammation-related histopathological changes in male hairless mouse skin. Toxicology. 2011b;282:129–138. doi: 10.1016/j.tox.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. Pathogenesis of chronic urticaria: an overview. Dermatology research and practice. 2014;2014:674709. doi: 10.1155/2014/674709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph LB, Gerecke DR, Heck DE, Black AT, Sinko PJ, Cervelli JA, Casillas RP, Babin MC, Laskin DL, Laskin JD. Structural changes in the skin of hairless mice following exposure to sulfur mustard correlate with inflammation and DNA damage. Experimental and molecular pathology. 2011;91:515–527. doi: 10.1016/j.yexmp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowsey PA, Williams FM, Blain PG. DNA damage, signalling and repair after exposure of cells to the sulphur mustard analogue 2-chloroethyl ethyl sulphide. Toxicology. 2009;257:105–112. doi: 10.1016/j.tox.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Kadar T, Amir A, Cohen L, Cohen M, Sahar R, Gutman H, Horwitz V, Dachir S. Anti-VEGF therapy (bevacizumab) for sulfur mustard-induced corneal neovascularization associated with delayed limbal stem cell deficiency in rabbits. Current eye research. 2014;39:439–450. doi: 10.3109/02713683.2013.850098. [DOI] [PubMed] [Google Scholar]
- Kadar T, Cohen M, Cohen L, Fishbine E, Sahar R, Brandeis R, Dachir S, Amir A. Endothelial cell damage following sulfur mustard exposure in rabbits and its association with the delayed-onset ocular lesions. Cutaneous and ocular toxicology. 2013a;32:115–123. doi: 10.3109/15569527.2012.717571. [DOI] [PubMed] [Google Scholar]
- Kadar T, Dachir S, Cohen L, Sahar R, Fishbine E, Cohen M, Turetz J, Gutman H, Buch H, Brandeis R, Horwitz V, Solomon A, Amir A. Ocular injuries following sulfur mustard exposure--pathological mechanism and potential therapy. Toxicology. 2009;263:59–69. doi: 10.1016/j.tox.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Kadar T, Dachir S, Cohen M, Gutman H, Cohen L, Brandeis R, Horwitz V, Amir A. Prolonged impairment of corneal innervation after exposure to sulfur mustard and its relation to the development of delayed limbal stem cell deficiency. Cornea. 2013b;32:e44–50. doi: 10.1097/ICO.0b013e318262e885. [DOI] [PubMed] [Google Scholar]
- Kadar T, Turetz J, Fishbine E, Sahar R, Chapman S, Amir A. Characterization of acute and delayed ocular lesions induced by sulfur mustard in rabbits. Current eye research. 2001;22:42–53. doi: 10.1076/ceyr.22.1.42.6975. [DOI] [PubMed] [Google Scholar]
- Kan RK, Pleva CM, Hamilton TA, Anderson DR, Petrali JP. Sulfur mustard-induced apoptosis in hairless guinea pig skin. Toxicologic pathology. 2003;31:185–190. doi: 10.1080/01926230390183661. [DOI] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Emmler J, Kreppel H, Jochum M, Thiermann H. Sulfur mustard research-strategies for the development of improved medical therapy. Eplasty. 2008;8:e32. [PMC free article] [PubMed] [Google Scholar]
- Kehe K, Balszuweit F, Steinritz D, Thiermann H. Molecular toxicology of sulfur mustard-induced cutaneous inflammation and blistering. Toxicology. 2009;263:12–19. doi: 10.1016/j.tox.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Kehe K, Flohe S, Krebs G, Kreppel H, Reichl FX, Liebl B, Szinicz L. Effects of Lewisite on cell membrane integrity and energy metabolism in human keratinocytes and SCL II cells. Toxicology. 2001;163:137–144. doi: 10.1016/s0300-483x(01)00389-4. [DOI] [PubMed] [Google Scholar]
- Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Keramati MR, Balali-Mood M, Mousavi SR, Sadeghi M, Riahi-Zanjani B. Biochemical and hematological findings of Khorasan veterans 23 years after sulfur mustard exposure. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2013;18:855–859. [PMC free article] [PubMed] [Google Scholar]
- Kohnavard N. BBC Persian. BBC World News; Taza, Northern Iraq: 2016. Iraqi town Taza ‘hit in IS chemical attack’ appeals for help. [Google Scholar]
- Korkmaz A, Tan DX, Reiter RJ. Acute and delayed sulfur mustard toxicity; novel mechanisms and future studies. Interdisciplinary toxicology. 2008;1:22–26. doi: 10.2478/v10102-010-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin JD, Black AT, Jan YH, Sinko PJ, Heindel ND, Sunil V, Heck DE, Laskin DL. Oxidants and antioxidants in sulfur mustard-induced injury. Annals of the New York Academy of Sciences. 2010;1203:92–100. doi: 10.1111/j.1749-6632.2010.05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Srivastava RK, Athar M. Biological and environmental hazards associated with exposure to chemical warfare agents: arsenicals. Annals of the New York Academy of Sciences. 2016a;1378:143–157. doi: 10.1111/nyas.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Srivastava RK, Weng Z, Croutch CR, Agarwal A, Elmets CA, Afaq F, Athar M. Molecular Mechanism Underlying Pathogenesis of Lewisite-Induced Cutaneous Blistering and Inflammation: Chemical Chaperones as Potential Novel Antidotes. The American journal of pathology. 2016b;186:2637–2649. doi: 10.1016/j.ajpath.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangerich A, Debiak M, Birtel M, Ponath V, Balszuweit F, Lex K, Martello R, Burckhardt-Boer W, Strobelt R, Siegert M, Thiermann H, Steinritz D, Schmidt A, Bürkle A. Sulfur and nitrogen mustards induce characteristic poly(ADP-ribosyl)ation responses in HaCaT keratinocytes with distinctive cellular consequences. Toxicology letters. 2016;244:56–71. doi: 10.1016/j.toxlet.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Mann I, Pirie A, Pullinger BD. A study of lewisite lesions of the eyes of rabbits. American journal of ophthalmology. 1946;29:1215–1227. doi: 10.1016/0002-9394(46)91629-7. [DOI] [PubMed] [Google Scholar]
- Mann I, Pirie A, Pullinger BD. The treatment of lewisite and other arsenical vesicant lesions of the eyes of rabbits with British anti-lewisite (BAL) American journal of ophthalmology. 1947;30:421–435. doi: 10.1016/0002-9394(47)91183-5. [DOI] [PubMed] [Google Scholar]
- McElroy CS, Day BJ. Antioxidants as potential medical countermeasures for chemical warfare agents and toxic industrial chemicals. Biochemical pharmacology. 2016;100:1–11. doi: 10.1016/j.bcp.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus J, Huebner K. Vesicants. Critical care clinics. 2005;21:707–718. vi. doi: 10.1016/j.ccc.2005.06.005. [DOI] [PubMed] [Google Scholar]
- McNutt P, Hamilton T, Nelson M, Adkins A, Swartz A, Lawrence R, Milhorn D. Pathogenesis of acute and delayed corneal lesions after ocular exposure to sulfur mustard vapor. Cornea. 2012;31:280–290. doi: 10.1097/ICO.0B013E31823D02CD. [DOI] [PubMed] [Google Scholar]
- Meylan W, Howard PH, Boethling RS. Molecular topology/fragment contribution method for predicting soil sorption coefficients. Environmental science & technology. 1992;26:1560–1567. [Google Scholar]
- Morad Y, Banin E, Averbukh E, Berenshtein E, Obolensky A, Chevion M. Treatment of ocular tissues exposed to nitrogen mustard: beneficial effect of zinc desferrioxamine combined with steroids. Investigative ophthalmology & visual science. 2005;46:1640–1646. doi: 10.1167/iovs.04-1165. [DOI] [PubMed] [Google Scholar]
- Mouret S, Wartelle J, Batal M, Emorine S, Bertoni M, Poyot T, Clery-Barraud C, Bakdouri NE, Peinnequin A, Douki T, Boudry I. Time course of skin features and inflammatory biomarkers after liquid sulfur mustard exposure in SKH-1 hairless mice. Toxicology letters. 2015;232:68–78. doi: 10.1016/j.toxlet.2014.09.022. [DOI] [PubMed] [Google Scholar]
- Mouret S, Wartelle J, Emorine S, Bertoni M, Nguon N, Clery-Barraud C, Dorandeu F, Boudry I. Topical efficacy of dimercapto-chelating agents against lewisite-induced skin lesions in SKH-1 hairless mice. Toxicology and applied pharmacology. 2013;272:291–298. doi: 10.1016/j.taap.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Nebehay S. Syrian government forces used chemical weapons more than two dozen times: U.N. Reuters. 2017. [Google Scholar]
- Nelson P, Hancock JR, Sawyer TW. Therapeutic effects of hypothermia on Lewisite toxicity. Toxicology. 2006;222:8–16. doi: 10.1016/j.tox.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Nguon N, Clery-Barraud C, Vallet V, Elbakdouri N, Wartelle J, Mouret S, Bertoni M, Dorandeu F, Boudry I. Time course of lewisite-induced skin lesions and inflammatory response in the SKH-1 hairless mouse model. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2014;22:272–280. doi: 10.1111/wrr.12147. [DOI] [PubMed] [Google Scholar]
- O’Neill HC, Orlicky DJ, Hendry-Hofer TB, Loader JE, Day BJ, White CW. Role of reactive oxygen and nitrogen species in olfactory epithelial injury by the sulfur mustard analogue 2-chloroethyl ethyl sulfide. American journal of respiratory cell and molecular biology. 2011;45:323–331. doi: 10.1165/rcmb.2010-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajos EJ, Olson CT, Salem H, Singer AW, Hayes TL, Menton RG, Miller TL, Rosso T, MacIver B. Evaluation of neutralized chemical agent identification sets (CAIS) for skin injury with an overview of the vesicant potential of agent degradation products. Journal of applied toxicology: JAT. 1998;18:409–420. doi: 10.1002/(sici)1099-1263(199811/12)18:6<409::aid-jat515>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Panahi Y, Ghanei M, Ghabili K, Ansarin K, Aslanabadi S, Poursaleh Z, Golzari SE, Etemadi J, Khalili M, Shoja MM. Acute and chronic pathological effects of sulfur mustard on genitourinary system and male fertility. Urology journal. 2013;10:837–846. [PubMed] [Google Scholar]
- Paromov V, Suntres Z, Smith M, Stone WL. Sulfur mustard toxicity following dermal exposure: role of oxidative stress, and antioxidant therapy. Journal of burns and wounds. 2007;7:e7. [PMC free article] [PubMed] [Google Scholar]
- Patocka JaK, Kamil Phosgene Oxime-Forgotten Chemical Weapon. Military Medical Science Letters. 2011;80:38–41. [Google Scholar]
- Petrali JP, Dick EJ, Brozetti JJ, Hamilton TA, Finger AV. Acute ocular effects of mustard gas: ultrastructural pathology and immunohistopathology of exposed rabbit cornea. Journal of applied toxicology: JAT. 2000;20(Suppl 1):S173–175. doi: 10.1002/1099-1263(200012)20:1+<::aid-jat679>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Prevention, C.f.D.C.a. PHOSGENE OXIME (CX): Blister Agent. 2011. [Google Scholar]
- Rancourt RC, Veress LA, Guo X, Jones TN, Hendry-Hofer TB, White CW. Airway tissue factor-dependent coagulation activity in response to sulfur mustard analog 2-chloroethyl ethyl sulfide. American journal of physiology Lung cellular and molecular physiology. 2012;302:L82–92. doi: 10.1152/ajplung.00306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin CL, Danne MM, Buxton KL, Casillas RP, Schlager JJ. Cytokine, chemokine, and matrix metalloproteinase response after sulfur mustard injury to weanling pig skin. Journal of biochemical and molecular toxicology. 2002;16:263–272. doi: 10.1002/jbt.10050. [DOI] [PubMed] [Google Scholar]
- Saladi RN, Smith E, Persaud AN. Mustard: a potential agent of chemical warfare and terrorism. Clinical and experimental dermatology. 2006;31:1–5. doi: 10.1111/j.1365-2230.2005.01945.x. [DOI] [PubMed] [Google Scholar]
- SAMSFoundation. SAMS Hospital Sees Mustard Gas Victims in Mare’e, Aleppo. 2015. [Google Scholar]
- Schraga ED, Pennardt A. Phosgene Oxime Exposure. Drugs and diseases: Emergency medicine. Vol. 2017. Medscape; 2016. [Google Scholar]
- Shakarjian MP, Bhatt P, Gordon MK, Chang YC, Casbohm SL, Rudge TL, Kiser RC, Sabourin CL, Casillas RP, Ohman-Strickland P, Riley DJ, Gerecke DR. Preferential expression of matrix metalloproteinase-9 in mouse skin after sulfur mustard exposure. Journal of applied toxicology: JAT. 2006;26:239–246. doi: 10.1002/jat.1134. [DOI] [PubMed] [Google Scholar]
- Shakarjian MP, Heck DE, Gray JP, Sinko PJ, Gordon MK, Casillas RP, Heindel ND, Gerecke DR, Laskin DL, Laskin JD. Mechanisms mediating the vesicant actions of sulfur mustard after cutaneous exposure. Toxicological sciences: an official journal of the Society of Toxicology. 2010;114:5–19. doi: 10.1093/toxsci/kfp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohrati M, Peyman M, Peyman A, Davoudi M, Ghanei M. Cutaneous and ocular late complications of sulfur mustard in Iranian veterans. Cutaneous and ocular toxicology. 2007;26:73–81. doi: 10.1080/15569520701212399. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Casillas R, Graham J, Skelton HG, Stemler F, Hackley BE., Jr Histopathologic features seen with different animal models following cutaneous sulfur mustard exposure. Journal of dermatological science. 1997;14:126–135. doi: 10.1016/s0923-1811(96)00560-9. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Graham JS, Moeller RB, Okerberg CV, Skelton H, Hurst CG. Histopathologic features seen in sulfur mustard induced cutaneous lesions in hairless guinea pigs. J Cutan Pathol. 1995;22:260–268. doi: 10.1111/j.1600-0560.1995.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Smith WJ. Therapeutic options to treat sulfur mustard poisoning--the road ahead. Toxicology. 2009;263:70–73. doi: 10.1016/j.tox.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Srivastava RK, Li C, Chaudhary SC, Ballestas ME, Elmets CA, Robbins DJ, Matalon S, Deshane JS, Afaq F, Bickers DR, Athar M. Unfolded protein response (UPR) signaling regulates arsenic trioxide-mediated macrophage innate immune function disruption. Toxicology and applied pharmacology. 2013;272:879–887. doi: 10.1016/j.taap.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RK, Li C, Weng Z, Agarwal A, Elmets CA, Afaq F, Athar M. Defining cutaneous molecular pathobiology of arsenicals using phenylarsine oxide as a prototype. Scientific reports. 2016;6:34865. doi: 10.1038/srep34865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann RL, Laskowski DA, McCall PJ, Vander Kuy K, Dishburger HJ. A rapid method for the estimation of the environmental parameters octanol/water partition coefficient, soil sorption constant, water to air ratio, and water solubility. In: Gunther FA, Gunther JD, editors. Residue Reviews: Residues of Pesticides and Other Contaminants in the Total Environment. Springer New York; New York, NY: 1983. pp. 17–28. [Google Scholar]
- Tewari-Singh N, Agarwal C, Huang J, Day BJ, White CW, Agarwal R. Efficacy of glutathione in ameliorating sulfur mustard analog-induced toxicity in cultured skin epidermal cells and in SKH-1 mouse skin in vivo. The Journal of pharmacology and experimental therapeutics. 2011;336:450–459. doi: 10.1124/jpet.110.173708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Agarwal R. Mustard vesicating agent-induced toxicity in the skin tissue and silibinin as a potential countermeasure. Annals of the New York Academy of Sciences. 2016;1374:184–192. doi: 10.1111/nyas.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Croutch CR, Tuttle R, Goswami DG, Kant R, Peters E, Culley T, Ammar DA, Enzenauer RW, Petrash JM, Casillas RP, Agarwal R. Clinical progression of ocular injury following arsenical vesicant lewisite exposure. Cutaneous and ocular toxicology. 2016:1–10. doi: 10.3109/15569527.2015.1127255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Goswami DG, Kant R, Croutch CR, Casillas RP, Orlicky DJ, Agarwal R. Cutaneous exposure to vesicant phosgene oxime: Acute effects on the skin and systemic toxicity. Toxicology and applied pharmacology. 2017;317:25–32. doi: 10.1016/j.taap.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Tewari-Singh N, Gu M, Agarwal C, White CW, Agarwal R. Biological and molecular mechanisms of sulfur mustard analogue-induced toxicity in JB6 and HaCaT cells: possible role of ataxia telangiectasia-mutated/ataxia telangiectasia-Rad3-related cell cycle checkpoint pathway. Chemical research in toxicology. 2010;23:1034–1044. doi: 10.1021/tx100038b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Inturi S, Jain AK, Agarwal C, Orlicky DJ, White CW, Agarwal R, Day BJ. Catalytic antioxidant AEOL 10150 treatment ameliorates sulfur mustard analog 2-chloroethyl ethyl sulfide-associated cutaneous toxic effects. Free radical biology & medicine. 2014;72:285–295. doi: 10.1016/j.freeradbiomed.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, Ammar DA, Agarwal C, Tyagi P, Kompella UB, Enzenauer RW, Petrash JM, Agarwal R. Silibinin, dexamethasone, and doxycycline as potential therapeutic agents for treating vesicant-inflicted ocular injuries. Toxicology and applied pharmacology. 2012;264:23–31. doi: 10.1016/j.taap.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Jain AK, Inturi S, White CW, Agarwal R. Clinically-relevant cutaneous lesions by nitrogen mustard: useful biomarkers of vesicants skin injury in SKH-1 hairless and C57BL/6 mice. PloS one. 2013;8:e67557. doi: 10.1371/journal.pone.0067557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari-Singh N, Rana S, Gu M, Pal A, Orlicky DJ, White CW, Agarwal R. Inflammatory biomarkers of sulfur mustard analog 2-chloroethyl ethyl sulfide-induced skin injury in SKH-1 hairless mice. Toxicological sciences: an official journal of the Society of Toxicology. 2009;108:194–206. doi: 10.1093/toxsci/kfn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOXNET, Phosgene Oxime.
- Watson AP, Griffin GD. Toxicity of vesicant agents scheduled for destruction by the Chemical Stockpile Disposal Program. Environmental health perspectives. 1992;98:259–280. doi: 10.1289/ehp.9298259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger B, Malaviya R, Sunil VR, Venosa A, Heck DE, Laskin JD, Laskin DL. Mustard vesicant-induced lung injury: Advances in therapy. Toxicology and applied pharmacology. 2016;305:1–11. doi: 10.1016/j.taap.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CW, Rancourt RC, Veress LA. Sulfur mustard inhalation: mechanisms of injury, alteration of coagulation, and fibrinolytic therapy. Annals of the New York Academy of Sciences. 2016;1378:87–95. doi: 10.1111/nyas.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismer T. Handbook of Toxicology of Chemical Warfare Agents. Academic Press; San Diego: 2009. CHAPTER 47 - Chemical Warfare Agents and Risks to Animal Health A2 - Gupta, Ramesh C; pp. 721–738. [Google Scholar]
- Wormser U, Brodsky B, Proscura E, Foley JF, Jones T, Nyska A. Involvement of tumor necrosis factor-alpha in sulfur mustard-induced skin lesion; effect of topical iodine. Archives of toxicology. 2005;79:660–670. doi: 10.1007/s00204-005-0681-5. [DOI] [PubMed] [Google Scholar]