Abstract
Chemokines, which have chemotactic abilities, are comprised of a family of small cytokines with 8–10 kilodaltons. Chemokines work in immune cells by trafficking and regulating cell proliferation, migration, activation, differentiation, and homing. CXCR-4 is an alpha-chemokine receptor specific for stromal-derived-factor-1 (SDF-1, also known as CXCL12), which has been found to be expressed in more than 23 different types of cancers. Recently, the SDF-1/CXCR-4 signaling pathway has emerged as a potential therapeutic target for human tumor because of its critical role in tumor initiation and progression by activating multiple signaling pathways, such as ERK1/2, ras, p38 MAPK, PLC/ MAPK, and SAPK/ JNK, as well as regulating cancer stem cells. CXCL12/CXCR4 antagonists have been produced, which have shown encouraging results in anti-cancer activity. Here, we provide a brief overview of the CXCL12/CXCR4 axis as a molecular target for cancer treatment. We also review the potential utility of targeting CXCL12/CXCR4 axis in combination of immunotherapy and/or chemotherapy based on up-to-date literature and ongoing research progress.
Keywords: Cancer, cancer stem cell, immunotherapy, CXCR4, CXCL12, chemokine
1. INTRODUCTION
Chemokines play active roles in embryogenesis, hematopoiesis, mitogenicity, and innate and adaptive immunity [1–6]. Chemokines consist of CXC, CC, C or CX3C subtypes depending on different cysteine residues at the N-terminus [7–9]. Chemokines bind and subsequently activate receptors such as G-protein-coupled receptors (GPCR), chemotactically guide immune cells to specific locations [4, 10–11]. Over 50 individual chemokines [4] and their corresponding receptors [10] have been identified. Recently, the alpha-chemokine receptor, C-X-C chemokine receptor type 4 (CXCR4) and its ligand, the alpha-chemokine CXCL12, have been found as the most widely expressed in tumors and implicated in cell proliferation, migration, and tumor metastasis. Importantly, the CXCL12/CXCR4 axis has emerged as a drug target for human tumor owing to its crucial role in promoting and maintaining cancer stem cells (CSC). In this review, our current understanding of the oncogenic roles of CXCL12/CXCR4 will be summarized and discussed with regards to its therapeutic potential as drug target.
CXCL12 is widely expressed in various human tissues, including liver, lungs, bone marrow, lymph nodes, stromal and endothelial cells [12–14]. Besides CXCR4, CXCR7 has also been found to bind to CXCL12 with high affinity [15–16]. CXCR7 is now classified as a chemokine co-receptor, together with CXCR4, for CXCL12 and C-X-C motif chemokine I-TAC (CXCL11)[17–18]. Similar to CXCL12, CXCR4 is also widely detected in the central nervous systems, neural stem cells, liver oval/stem cells, CD34+ hematopoietic progenitor cells, white blood cells [19], primordial germ cells, skeletal muscle satellite progenitor cells, as well as intestinal epithelium [20]. The ubiquitously present CXCL12/CXCR4 axis highlights its essential roles in various physiological processes [21], homeostasis and trafficking of immune cells [22].
CXCL12/CXCR4 axis activates various signaling pathways that promote chemotaxis, adhesion and migration, cell proliferation and survival [23]. PI3 kinase, Ras, stress-activated protein kinase (SAPK)/c-Jun N-terminal kinase (JNK), phospholipase C (PLC)/mitogen-activated protein kinase (MAPK), p38 MAPK and AKT are all downstream effectors of CXCL12/CXCR4 axis, through which tumor cell growth, dissemination and migration are facilitated [24–29].
CXCR4 can be activated in different ways in tumor cells. First, hypoxia can up-regulate CXCR4 signaling [30]. Second, Wnt/beta-catenin can also positively regulate CXCR4 expression [31]. Third, NF-κB can also activate CXCR4 expression. Upon ligand induction, NF-κB subunits of p50 and p65 bind to the CXCR4 promoter, where a NF-κB binding site, transcriptionally activating CXCR4 and stimulating tumor invasion [32–37].
CXCR4 pathway has been implicated in the SHH-GLI1-NANOG network [38], Janus kinase (JAK)/ signal transducer and activator of transcription (STAT) [39–40], phosphoinositide 3-kinase (PI3K)/AKT [41–42] and NF-κB57 [37, 43]. In turn, the CXCL12/ CXCR4 signaling activates MAPKs signaling which stimulates chemotaxis and cell proliferation [41, 44], induces PLC/PKC-Ca2+ signaling and affects PI3K/AKT, and promotes cell migration and survival [41–42]. This phenomenon suggests a feedback loop between CXCR4 and the other signaling pathways. CXCL12/CXCR4 signaling pathway may transactivate HER2-neu to stimulate invasive and metastatic signals of breast [45–46], esophageal [47], lung [48], prostate [49], as well as ovarian cancers [50].
CXCR4 protein is also activated through several post-transcriptional levels. Sustained exposure of cells to CXCL12 desensitizes them to CXCL12 through the mechanism of endocytosis of CXCR4. The receptor is ubiquitinated and simulates the CXCR4 to endosome and degrades it in lysosomes [51]. Activated YY1, a transcription factor, can inhibit expression of CXCR4 through the phosphorylation of carboxyl-terminal Src kinase homologous kinase in breast cancer cells [52–53]. Histone deacetylase 3-interacting protein CREB3 and Kruppel-like factor 2 can inactivate CXCR4, thereby repressing cell migration [54–55]. The oncogene Her2 can block the ubiquitination process and the degradation of CXCR4 after CXCL12 binds in breast cancer cells [46, 56]. CXCR4 expression is significantly associated with vascular endothelial growth factor (VEGF) production [57] and worse prognosis [58] in cancers. Resveratrol reduces VEGF secretion, via the inhibition of CXCR4 production due to the inactivation of NF-κB [59].
2. CXCR4 /CXCL12 AXIS IN CANCER AND CANCER STEM CELLS
So far, sixteen out of nineteen human chemokine receptors have been detected in cancer cells [60–63]. CXCR4 is frequently over-expressed in malignant cells, including those with the highest incidence, such as cancers of the brain, breast, colorectal, lung, pancreas, prostate, and ovarian, leukemia, and melanomas [10, 64–67]. CXCR4 mediates epithelial cell migration via the activation of Rac1, matrix metalloproteinases MMP-14 and MMP-2 [68], and increases motility of cancer cells through the up-regulation of NF-κB and ERK dependent pathway [69]. The CXCL12/CXCR4 axis regulates angiogenesis [70–72], induces epithelial-mesenchymal transition (EMT) [73–74], and promotes cancer progression and metastasis [75–78]. Prior to the metastatic process, the cancer cells must interact with a variety of stromal cells [79–82]. Therefore, blocking the CXCL12/CXCR4 axis by IL-24 may inhibit cancer cell migration, metastasis and induce apoptosis [83–84]. Chemotaxis of cancer cells, adhesion between cancer and endothelial cells, degradation of extracellular matrix are all necessary steps for cancer cells to survive in circulation, migration and proliferate in targeting organs and tissues [85]. Decreased CXCR4 production by genetic knockdowns of CXCR4 significantly inhibits cancer cells’ ability to distant invasion [75].
The chemokine CXCL12 is detected in common sites of tumor metastasis, including lungs, lymph nodes, bone marrow, liver, as well as in animal models, and expressed in circulating cancer cells [12, 86]. CXCR4 stimulates cancer metastasis to organs where its ligand, CXCL12, is produced in large quantity. The interaction between CXCL12 and CXCR4 causes tumor cells to form metastatic tumors [12]. In addition, CXCL12 hypermethylation was reported in number of cancers, such as gastric cancer [87], breast cancer [88–89], colon cancer [90], lung cancer [91], as well as prostate cancer [92] indicating that CXCL12 may have a role in carcinogenesis.
CSCs are a small population of tumor cells that possess the stem cell property and initiate, drive carcinogenesis contributing to tumor cellular heterogeneity [93–95]. Many cancers possess an enhanced tumor-initiating capacity and generally observed to be more resistant to conventional anticancer therapeutics than other cancer cells [96–99]. There are a number of cellular molecules that may contribute to CSC properties, including Aldehyde dehydrogenase (ALDH) [100–101], Stem cell factor receptor (CD117) [102–103], Pgp-1 (CD44) [104–106], Prominin-1(CD133) [107–108], Urokinase plasminogen activator (CD87) [109], Side population (SP) [110–112], et al. There are also a number of pathways, such as Notch [100, 113–114], Hedgehog (Hh) [115–116], Wnt [117–118], JNK [119–120], IL-6/STAT3[121–122], as well as CXCR4 [74] that were identified in the self-renewal of CSC. The increased CXCL12 and CXCR4 mRNAs were recognized in stem cell marker, ALDH positive populations in human H1299 and H460 lung cancer cells and murine LLC [123]. The combined use of Lgr5 and CXCR4 may facilitate the enrichment of CSCs in colorectal cancer, and that treating Lgr5+/CXCR4+ colorectal cancer cells may improve the outcome of colorectal cancer therapy [124]. Recent report also supports that CXCR4 is potentially an ideal target for lung CSC [125]. CD133(+)/CXCR4(+) CSC from patient-derived xenografts (PDX) of non-small cell lung cancer cancer (NSCLC) are associated with initiate metastasis at distant organs and poor clinical outcome [74]. Therefore, CD133/CXCR4 axis may provide novel targets for combinational therapies, as well as prognostic markers in the treatment of NSCLC [126–127].
3. CXCR4 /CXCL12 AXIS AS A THERAPEUTIC TARGET FOR CANCER
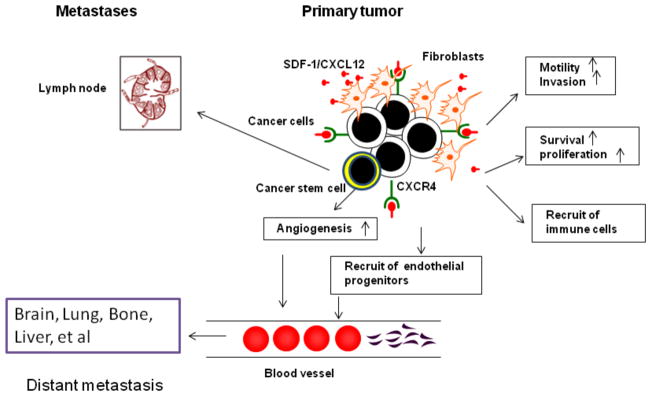
As discussed above, the communication between CXCR4 and CXCL12 contributes to the evolution and progression of cancer cells by activating multiple signaling pathways to enhance tumor cell invasion and distant metastasis [61, 128–130]. CXCR4 also regulates tumor vascularization and EMT, further strengthening the interaction between tumor cells and stromal cells [131]. CXCR4 cooperates with other transcriptional factors, such as NF-κB, Nanog, and Bmi-1 and contributes to the maintenance of stemness and induction of metastasis behavior in CSC [132–135]. It is proposed that therapies for cancer patients that specifically target CSC signaling pathways could be valuable in combating this disease [96, 136–138]. Without question, the CXCL12/CXCR4 axis is believed to be a novel drug target for cancer therapy. A schematic depiction of the effects of CXCL12/CXCR4 in cancer are shown in Fig. (1).
Fig. 1. Potential Roles for CXCL12/CXCR4 in Cancer and Cancer Stem Cells.
Cancer cells and cancer stem cells express CXCR4, and both cancer cells and fibroblasts produce SDF-1(CXCL12). Tumor cells expressing CXCR4 directs metastasis to sites such as liver, bone, pericardium,adrenal glands, spinal cord and brain. SDF-1/CXCR4 functions locally in autocrine and paracrine ways to increase tumor growth in primary locations. Tumor and tumor microenvironment secreted SDF-1 promote tumor cell survival, growth and also the recruitment of bone marrow derived cells and immune cells into the tumor environment.
In the past ten years, a number of inhibitors of CXCL12/CXCR4 which are able to attenuate the growth of tumor cells in vivo and in vitro have been reported We summarize the effects of various CXCR4 inhibitors on tumor in Table 1. So far, CXCR4 antagonists are developed by a number of programs, including five major classes: (1) small modified peptides, including BKT140 [139], FC131 [140–141], T140 [142], POL6326 [143], TF14016 [144]; (2) small-molecules, including the bicyclam AMD070 [145–146], AMD3100 [130, 147–149], AMD11070 [150], MSX-122 [151], GSK812397 [152–153], KRH-3955 [154–155]; (3) antibodies, such as MDX-1338/BMS 93656 [156]; (4) modified agonists and antagonists for CXCL12 such as CTCE-9908 [157–158] ; (5) microRNAs, such as miR-302a [159], miR-9 [160], miR-204-5p[161] and miR-126 [162].
Table 1.
The effects of various CXCR4 inhibitors on tumor.
| Compound | Type of Studies | Biological Function on Cancer | References |
|---|---|---|---|
| T140 | In vitro, in vivo | Inhibition of SDF-1 induced cancer cell migration | [142, 196] |
| TN14003 | In vivo | Limiting metastases of breast cancer, a radiotracer to detect CXCR4- positive cells; inhibiting leukemia and multiple myeloma tumor growth | [163, 170, 197] |
| AMD3100 | In vivo | Reduce lung metastases of breast cancer; sensitizing prostate cancer to docetaxel chemotherapy | [198–200] |
| GST-NT21MP | In vivo | Decrease in SDF-1-induced cell growth, adhesion, migration | [201] |
| CTCE-9908 | In vivo | Anti-tumor growth and anti-metastatic effects | [158, 202–204] |
| ALX40-4C | In vitro | Prevent breast cancer spread | [205] |
| Baohuoside | In vitro, in vivo | Antiproliferative and antimetastatic effect on cancer through the downregulation of CXCR4 expression | [206–207] |
| Ginsenoside Rg3 | In vitro | Reduce CXCR4 expression and migration and invasion | [208] |
| AKBA | In vitro, in vivo | Abolish tumor cell invasion and metastasis | [209–210] |
| Butein | In vitro, | Reduce SDF-1-induced migration and invasion of breast cancer | [211] |
| WZ811 | In vitro, in vivo | Inhibition of Matrigel invasion; suppression of chronic lymphocytic leukemia progression | [165, 212] |
| MSX-122 | In vitro, in vivo | Inhibition of cAMP and Matrigel invasion | [151] |
| AMD3465 | In vitro, in vivo | Reduction of cancer growth and metastasis | [213–214] |
| 4EGI-1 | In vitro | Selectively inhibits translation of mRNAs of CXCR4, targeting cancer stem cell | [215] |
| 508MCl | In vivo | Interfering with CXCR4 function with high potency and specificity | [216] |
| Celastrol | In vitro | Downregulation of CXCR4 expression | [217] |
| Thymoquinone | In vitro | Downregulation of CXCR4 expression | [218] |
| Benzenesulfonamides | In vitro | Inhibition of CXCR4 | [219] |
T140 analogs were previously developed as anti-HIV agents [142]. Liang et al further increased the potency of T140 to generate a synthetic antagonist 14-mer peptide compound, TN14003 (BKT140) [163]. A strong CXCR4 inhibitor, TN14003 is as an adjuvant treatment for traditional anticancer therapies against human NSCLC [132, 164]. Using TN14003 as a template, a first nitrogen atom substitution of the terminal aromatic rings was named as WZ811[165], while a second nitrogen atom substitution was named as MSX-122. Both MSX-122 and WZ811 inhibit lung metastasis of breast cancer.
AMD3100, also known as plerixafor, is able to inhibit the binding of CXCL12 to CXCR4[166]. AMD3100 inhibited CXCR4 internalization and chemotaxis of acute lymphoblastic leukemia (ALL) cells. AMD3100 was demonstrated to prevent relapse of extramedullary ALL cells after chemotherapy [167]. A phase I/II study of 52 patients with relapsed or refractory acute myelogenous leukemia (AML) using AMD3100 showed encouraging rates of remission with correlative in vivo evidence of the CXCR4/CXCL12 axis disruption [168]. These encouraging data led to randomized phase III clinical trials of AMD3100 in patients with relapsed AML. AMD3100 is currently tested in the treatment of solid tumors. AMD3100 was demonstrated to reduce the growth of the primary small cell lung cancer by 61% (P<0.05) and additionally suppress metastasis formation by 43% [169]. AMD3100 was also reported to efficiently impair tumor growth and metastasis dissemination in both Herceptin-sensitive and Herceptin-resistant HER2 breast cancer [170].A phase I/II study of AMD3100 in breast cancer patients indicated preliminary signs of efficacy [171].
Antibodies have also been used to disrupt the CXCR4 pathway. BMS-936564/MDX-1338, a fully human IgG(4) monoclonal antibody can specifically recognize human CXCR4 [156]. MDX-1338 has shown antitumor effects in established tumors, such as AML, NHL, and multiple myeloma xenograft models [156].
CTCE-9908 is a modified peptide antagonist for CXCL12 corresponding to the N-terminal region of CXCL12 chemokine. It was reported to decrease expression levels of VEGF and slow the rate of primary tumor growth. CTCE-9908 administration in combination with docetaxel reduced tumor volume than that with docetaxel alone. CTCE-9908, in combination with DC101, an anti-angiogenic agent, also reduced primary tumor volume and distant metastasis than DC101 alone [172].
MicroRNAs have been reported to play critical roles in regulating tumor progression through CXCL12/ CXCR4 axis [159]. MiR-302a decreased the invasion and metastasis of breast cancer cells by reducing CXCR4 production [173]. MiR-9 reduced the proliferation of oral squamous cell carcinoma cells by the inhibition of CXCR4 via the Wnt/β-catenin signaling pathway [160]. MiR-146a downmodulated CXCR4 production in target cells [174]. CXCR4 was inhibited upon miR-451 treatment in lung cancer cells [175]. MiR-204-5p may function as an inhibitory RNA molecule in oral squamous cell carcinoma by targeting CXCR4 [161]. Artificial microRNA was demonstrated to effectively block invasion and metastasis of breast cancer cells by targeting CXCR4 [176]. MiR-126 may also act as a tumor suppressor by inactivating RhoA signaling via CXCR4 in colon cancer [162].In addition, miR-101 was recently discovered to directly target CXCL12 in lung cancer cells [177].
4. TARGETING CXCL12/CXCR4 AXIS AND IMMUNOTHERAPY
Tumor immunotherapy has entered a phase of rapid development, based on the notion that the immune system is the best tool humans have for fighting disease, and that the immune system is capable of recognizing tumors and eliminating malignant cells. Treatment with anti-PD ligand-1 (anti–PD-L1) [178–180], anti-programmed death-receptor 1 (anti–PD-1) [181–182], checkpoint antagonists including anti-cytotoxic T lymphocyte antigen-4 (anti–CTLA-4) [183–184], as well as engineered CAR T cells [185–187], has induced striking responses in subsets of patients with a range of solid tumors. With the FDA approval of the two anti–PD-1 antibodies, pembrolizumab (formerly MK-3475 or lambrolizumab; Merck) and nivolumab (Bristol-Myers Squibb), sipuleucel-T, ipilimumab (anti–CTLA-4; Bristol-Myers Squibb), immunotherapy has become a mainstream treatment option for some cancers [188–190]. Despite the unprecedented rates of durable clinical responses observed in patients affected by advanced solid tumors[181, 191–192], many more patients with solid tumors resistant to immunotherapy such as ovary, colon, and pancreatic cancer have not yet benefited from immunotherapeutic approaches, and the mechanism of the resistance may be related to the CXCL12/CXCR4 axis. The carcinoma-associated fibroblasts (CAF) in solid tumors was were reported to have an immunologic inhibition function in recent studies. Immune suppression by the fibroblast activation protein-α (FAP) positive CAF is regulated by CXCL12 binding to cancer cells and excluding T cells, which relies on the signaling of CXCR4. The conditional depletion of the FAP+ CAF permits immune control effects of both anti–PD-L1and anti–CTLA-4; administering AMD3100, induced rapid T-cell accumulation in this autochthonous model of pancreatic ductal adenocarcinoma (PDA), and anti-PD-L1 to synergistically diminish cancer cells. The residual tumor was only composed of inflammatory cells and premalignant epithelial cells [193–194]. These results indicate that the fibroblastic component of tumors may be critical in the adaptation of cancer to the host. Another study [195] demonstrated that the up-regulation of CXCL12 alpha in HCC models increased hypoxia, increased the recruitment of immunosuppressive cells, indicated intratumoral expression of the immune checkpoint inhibitor PD-L1, and accumulated of T-regulatory cells and M2-type macrophages after treatment of sorafenib. PD-1 blockade combined with CXCR4 inhibition and sorafenib decreased HCC growth [195]. In this study, AMD3100 inhibited the polarization toward an immunosuppressive microenvironment, reduced tumor growth, decreased lung metastasis, and improved animal survival. Thus, anti-PD-1 had additional antitumor activity upon combined with sorafenib and AMD3100 in HCC models.
CONCLUSION AND OVERALL PERSPECTIVES
Over the past ten years, despite advances in techniques and protocol of diagnosis and therapies, cancer mortality remains high. Better understanding of the disease and more efficacious treatments for cancer patients are clearly needed. CXCL12 is a member of chemokines expressed by a variety of cells in bone marrow, liver, lungs, lymph nodes, stromal cells (fibroblasts) and endothelial cells. Binding of CXCL12 to its specific G protein-coupled receptor CXCR4 induces a plethora of downstream signaling events involving ERK1/2, ras, PLC/ MAPK, p38 MAPK, and SAPK/ JNK, which in turn are responsible for various biological and pathological processes including the regulation of hematopoiesis and apoptosis, immunity and mitogenic activity, cancer cell growth, migration, dissemination, and neovascularization. Aberrant CXCR4 production was significantly higher in many tumor tissues. Thus, the CXCL12/CXCR4 axis has emerged as a novel target for cancer therapeutics. CXCL12/CXCR4 antagonists have been developed and validated, which have shown promising anti-cancer activities in several tumor cell types. Five major classes of CXCR4 antagonists have been identified: (1) small modified peptides; (2) small-molecules; (3) antibodies to CXCR4; (4) modified agonists and antagonists for CXCL12; (5) microRNAs. The mechanism underlying tumor resistance to immunotherapy may relate to the CXCL12/ CXCR4 axis. Therefore, the CXCL12/CXCR4 axis cannot only be a target for monotherapy, but also be used synergistically with immunotherapy for cancer patients.
Acknowledgments
The work is supported by the National Natural Science Foundation of China (81172509), the Research Foundation of Science and Technology Department of Liaoning Province (2014020128), the Research Foundation of Shenyang Science and Technology Bureau (F15-199-1-28), RCMI Cancer Research Center through an NIH grant 2G12MD007595.
Footnotes
DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Ono SJ, Nakamura T, Miyazaki D, Ohbayashi M, Dawson M, Toda M. Chemokines: roles in leukocyte development, trafficking, and effector function. J Allergy Clin Immunol. 2003;111(6):1185–1199. doi: 10.1067/mai.2003.1594. quiz 1200. [DOI] [PubMed] [Google Scholar]
- 2.Mukaida N, Baba T. Chemokines in tumor development and progression. Exp Cell Res. 2012;318(2):95–102. doi: 10.1016/j.yexcr.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of tumor angiogenesis and neovascularization. Exp Cell Res. 2011;317(5):685–690. doi: 10.1016/j.yexcr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber PA, Hippe A, Buhren BA, Muller A, Homey B. Chemokines in tumor-associated angiogenesis. Biol Chem. 2009;390(12):1213–1223. doi: 10.1515/BC.2009.144. [DOI] [PubMed] [Google Scholar]
- 5.Itatani Y, Kawada K, Inamoto S, Yamamoto T, Ogawa R, Taketo MM, Sakai Y. The Role of Chemokines in Promoting Colorectal Cancer Invasion/Metastasis. Int J Mol Sci. 2016;17(5) doi: 10.3390/ijms17050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massara M, Bonavita O, Mantovani A, Locati M, Bonecchi R. Atypical chemokine receptors in cancer: friends or foes? J Leukoc Biol. 2016;99(6):927–933. doi: 10.1189/jlb.3MR0915-431RR. [DOI] [PubMed] [Google Scholar]
- 7.Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17(9):2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 8.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 9.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52(1):145–176. [PubMed] [Google Scholar]
- 10.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11(9):597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 11.Wald O, Shapira OM, Izhar U. CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics. 2013;3(1):26–33. doi: 10.7150/thno.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 13.Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99(8):2703–2711. doi: 10.1182/blood.v99.8.2703. [DOI] [PubMed] [Google Scholar]
- 14.Guo F, Wang Y, Liu J, Mok SC, Xue F, Zhang W. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene. 2016;35(7):816–826. doi: 10.1038/onc.2015.139. [DOI] [PubMed] [Google Scholar]
- 15.Hou KL, Hao MG, Bo JJ, Wang JH. CXCR7 in tumorigenesis and progression. Chin J Cancer. 2010;29(4):456–459. doi: 10.5732/cjc.009.10404. [DOI] [PubMed] [Google Scholar]
- 16.Pawig L, Klasen C, Weber C, Bernhagen J, Noels H. Diversity and Inter-Connections in the CXCR4 Chemokine Receptor/Ligand Family: Molecular Perspectives. Front Immunol. 2015;6(429):429. doi: 10.3389/fimmu.2015.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 18.Tang T, Xia QJ, Qiao X, Xi M. Expression of C-X-C chemokine receptor type 7 in otorhinolaryngologic neoplasms. Singapore Med J. 2016;57(3):157–160. doi: 10.11622/smedj.2016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28(7):299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91(6):2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7):879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 22.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768(4):952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewan MZ, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60(6):273–276. doi: 10.1016/j.biopha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011;286(37):32188–32197. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh AK, Arya RK, Trivedi AK, Sanyal S, Baral R, Dormond O, Briscoe DM, Datta D. Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013;24(1):41–49. doi: 10.1016/j.cytogfr.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29(4):709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng ZJ, Zhao J, Sun Y, Hu W, Wu YL, Cen B, Wu GX, Pei G. beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J Biol Chem. 2000;275(4):2479–2485. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277(51):49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 29.Saba NF, Wang Y, Fu H, Koenig L, Khuri FR, Shin DM, Chen ZG. Association of Cytoplasmic CXCR4 With Loss of Epithelial Marker and Activation of ERK1/2 and AKT Signaling Pathways in Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2016;22(16):30381–30383. doi: 10.1016/j.cllc.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Romain B, Hachet-Haas M, Rohr S, Brigand C, Galzi JL, Gaub MP, Pencreach E, Guenot D. Hypoxia differentially regulated CXCR4 and CXCR7 signaling in colon cancer. Mol Cancer. 2014;13(58):58. doi: 10.1186/1476-4598-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choe Y, Pleasure SJ. Wnt signaling regulates intermediate precursor production in the postnatal dentate gyrus by regulating CXCR4 expression. Dev Neurosci. 2012;34(6):502–514. doi: 10.1159/000345353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esencay M, Newcomb EW, Zagzag D. HGF upregulates CXCR4 expression in gliomas via NF-kappaB: implications for glioma cell migration. J Neurooncol. 2010;99(1):33–40. doi: 10.1007/s11060-010-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maroni P, Bendinelli P, Matteucci E, Desiderio MA. HGF induces CXCR4 and CXCL12-mediated tumor invasion through Ets1 and NF-kappaB. Carcinogenesis. 2007;28(2):267–279. doi: 10.1093/carcin/bgl129. [DOI] [PubMed] [Google Scholar]
- 34.Matteucci E, Ridolfi E, Maroni P, Bendinelli P, Desiderio MA. c-Src/histone deacetylase 3 interaction is crucial for hepatocyte growth factor dependent decrease of CXCR4 expression in highly invasive breast tumor cells. Mol Cancer Res. 2007;5(8):833–845. doi: 10.1158/1541-7786.MCR-07-0054. [DOI] [PubMed] [Google Scholar]
- 35.Ridolfi E, Matteucci E, Maroni P, Desiderio MA. Inhibitory effect of HGF on invasiveness of aggressive MDA-MB231 breast carcinoma cells, and role of HDACs. Br J Cancer. 2008;99(10):1623–1634. doi: 10.1038/sj.bjc.6604726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Y, Karin M. NF-kappaB in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2003;8(2):215–223. doi: 10.1023/a:1025905008934. [DOI] [PubMed] [Google Scholar]
- 37.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278(24):21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 38.Fareh M, Turchi L, Virolle V, Debruyne D, Almairac F, de-la-Forest DS, Paquis P, Preynat-Seauve O, Krause KH, Chneiweiss H, Virolle T. The miR 302–367 cluster drastically affects self-renewal and infiltration properties of glioma-initiating cells through CXCR4 repression and consequent disruption of the SHH-GLI-NANOG network. Cell Death Differ. 2012;19(2):232–244. doi: 10.1038/cdd.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vila-Coro AJ, Rodriguez-Frade JM, Martin DAA, Moreno-Ortiz MC, Martinez-A C, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 1999;13(13):1699–1710. [PubMed] [Google Scholar]
- 40.Soldevila G, Licona I, Salgado A, Ramirez M, Chavez R, Garcia-Zepeda E. Impaired chemokine-induced migration during T-cell development in the absence of Jak 3. Immunology. 2004;112(2):191–200. doi: 10.1111/j.1365-2567.2004.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinton CV, Avraham S, Avraham HK. Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis. 2010;27(2):97–105. doi: 10.1007/s10585-008-9210-2. [DOI] [PubMed] [Google Scholar]
- 42.Peng SB, Peek V, Zhai Y, Paul DC, Lou Q, Xia X, Eessalu T, Kohn W, Tang S. Akt activation, but not extracellular signal-regulated kinase activation, is required for SDF-1alpha/CXCR4-mediated migration of epitheloid carcinoma cells. Mol Cancer Res. 2005;3(4):227–236. doi: 10.1158/1541-7786.MCR-04-0193. [DOI] [PubMed] [Google Scholar]
- 43.Han Y, He T, Huang DR, Pardo CA, Ransohoff RM. TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest. 2001;108(3):425–435. doi: 10.1172/JCI12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res. 2005;65(21):9891–9898. doi: 10.1158/0008-5472.CAN-05-1293. [DOI] [PubMed] [Google Scholar]
- 45.Cabioglu N, Summy J, Miller C, Parikh NU, Sahin AA, Tuzlali S, Pumiglia K, Gallick GE, Price JE. CXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu in breast cancer cells by a novel pathway involving Src kinase activation. Cancer Res. 2005;65(15):6493–6497. doi: 10.1158/0008-5472.CAN-04-1303. [DOI] [PubMed] [Google Scholar]
- 46.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6(5):459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 47.Gros SJ, Kurschat N, Drenckhan A, Dohrmann T, Forberich E, Effenberger K, Reichelt U, Hoffman RM, Pantel K, Kaifi JT, Izbicki JR. Involvement of CXCR4 chemokine receptor in metastastic HER2-positive esophageal cancer. Plos one. 2012;7(10):e47287. doi: 10.1371/journal.pone.0047287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al ZAA, Al OBF, Yang L, Yang C, Hui Y, Yu H, Zheng F, Yang G, Xie C, Zhou F, Zhou Y. Concomitant overexpression of EGFR and CXCR4 is associated with worse prognosis in a new molecular subtype of non-small cell lung cancer. Oncol Rep. 2013;29(4):1524–1532. doi: 10.3892/or.2013.2254. [DOI] [PubMed] [Google Scholar]
- 49.Chinni SR, Yamamoto H, Dong Z, Sabbota A, Bonfil RD, Cher ML. CXCL12/CXCR4 transactivates HER2 in lipid rafts of prostate cancer cells and promotes growth of metastatic deposits in bone. Mol Cancer Res. 2008;6(3):446–457. doi: 10.1158/1541-7786.MCR-07-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porcile C, Bajetto A, Barbieri F, Barbero S, Bonavia R, Biglieri M, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res. 2005;308(2):241–253. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 51.Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5(5):709–722. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- 52.Begon DY, Delacroix L, Vernimmen D, Jackers P, Winkler R. Yin Yang 1 cooperates with activator protein 2 to stimulate ERBB2 gene expression in mammary cancer cells. J Biol Chem. 2005;280(26):24428–24434. doi: 10.1074/jbc.M503790200. [DOI] [PubMed] [Google Scholar]
- 53.Lee BC, Lee TH, Zagozdzon R, Avraham S, Usheva A, Avraham HK. Carboxyl-terminal Src kinase homologous kinase negatively regulates the chemokine receptor CXCR4 through YY1 and impairs CXCR4/CXCL12 (SDF-1alpha)-mediated breast cancer cell migration. Cancer Res. 2005;65(7):2840–2845. doi: 10.1158/0008-5472.CAN-04-3309. [DOI] [PubMed] [Google Scholar]
- 54.Kim HC, Choi KC, Choi HK, Kang HB, Kim MJ, Lee YH, Lee OH, Lee J, Kim YJ, Jun W, Jeong JW, Yoon HG. HDAC3 selectively represses CREB3-mediated transcription and migration of metastatic breast cancer cells. Cell Mol Life Sci. 2010;67(20):3499–3510. doi: 10.1007/s00018-010-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uchida D, Onoue T, Begum NM, Kuribayashi N, Tomizuka Y, Tamatani T, Nagai H, Miyamoto Y. Vesnarinone downregulates CXCR4 expression via upregulation of Kruppel-like factor 2 in oral cancer cells. Mol Cancer. 2009;8(62):62. doi: 10.1186/1476-4598-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luker KE, Luker GD. Functions of CXCL12 and CXCR4 in breast cancer. Cancer Lett. 2006;238(1):30–41. doi: 10.1016/j.canlet.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 57.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359(3):716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyoshi K, Kohashi K, Fushimi F, Yamamoto H, Kishimoto J, Taguchi T, Iwamoto Y, Oda Y. Close correlation between CXCR4 and VEGF expression and frequent CXCR7 expression in rhabdomyosarcoma. Hum Pathol. 2014;45(9):1900–1909. doi: 10.1016/j.humpath.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Seong H, Ryu J, Jeong JY, Chung IY, Han YS, Hwang SH, Park JM, Kang SS, Seo SW. Resveratrol suppresses vascular endothelial growth factor secretion via inhibition of CXC-chemokine receptor 4 expression in ARPE-19 cells. Mol Med Rep. 2015;12(1):1479–1484. doi: 10.3892/mmr.2015.3518. [DOI] [PubMed] [Google Scholar]
- 60.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 61.Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226(2):148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Sun J, Feng Y, Tian X, Wang B, Zhou Y. Oncogenic roles and drug target of CXCR4/CXCL12 axis in lung cancer and cancer stem cell. Tumour Biol. 2016;14:14. doi: 10.1007/s13277-016-5016-z. [DOI] [PubMed] [Google Scholar]
- 63.Vela M, Aris M, Llorente M, Garcia-Sanz JA, Kremer L. Chemokine receptor-specific antibodies in cancer immunotherapy: achievements and challenges. Front Immunol. 2015;6(12):12. doi: 10.3389/fimmu.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gangadhar T, Nandi S, Salgia R. The role of chemokine receptor CXCR4 in lung cancer. Cancer Biol Ther. 2010;9(6):409–416. doi: 10.4161/cbt.9.6.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu C, Zhao H, Chen H, Yao Q. CXCR4 in breast cancer: oncogenic role and therapeutic targeting. Drug Des Devel Ther. 2015;9:4953–4964. doi: 10.2147/DDDT.S84932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao H, Guo L, Zhao J, Weng H, Zhao B. CXCR4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget. 2015;6(7):5022–5040. doi: 10.18632/oncotarget.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14(3):171–179. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Ghosh MC, Makena PS, Gorantla V, Sinclair SE, Waters CM. CXCR4 regulates migration of lung alveolar epithelial cells through activation of Rac1 and matrix metalloproteinase-2. Am J Physiol Lung Cell Mol Physiol. 2012;302(9):L846–856. doi: 10.1152/ajplung.00321.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang YC, Hsiao YC, Chen YJ, Wei YY, Lai TH, Tang CH. Stromal cell-derived factor-1 enhances motility and integrin up-regulation through CXCR4, ERK and NF-kappaB-dependent pathway in human lung cancer cells. Biochem Pharmacol. 2007;74(12):1702–1712. doi: 10.1016/j.bcp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 70.Jin Z, Zhao C, Han X, Han Y. Wnt5a promotes ewing sarcoma cell migration through upregulating CXCR4 expression. BMC Cancer. 2012;12(480):480. doi: 10.1186/1471-2407-12-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang M, Chen GY, Song HT, Hong X, Yang ZY, Sui GJ. Significance of CXCR4, phosphorylated STAT3 and VEGF-A expression in resected non-small cell lung cancer. Exp Ther Med. 2011;2(3):517–522. doi: 10.3892/etm.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai X, Chen Z, Pan X, Xia L, Chen P, Yang Y, Hu H, Zhang J, Li K, Ge J, Yu K, Zhuang J. Inhibition of angiogenesis, fibrosis and thrombosis by tetramethylpyrazine: mechanisms contributing to the SDF-1/CXCR4 axis. Plos one. 2014;9(2):e88176. doi: 10.1371/journal.pone.0088176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Onoue T, Uchida D, Begum NM, Tomizuka Y, Yoshida H, Sato M. Epithelial-mesenchymal transition induced by the stromal cell-derived factor-1/CXCR4 system in oral squamous cell carcinoma cells. Int J Oncol. 2006;29(5):1133–1138. [PubMed] [Google Scholar]
- 74.Bertolini G, D’Amico L, Moro M, Landoni E, Perego P, Miceli R, Gatti L, Andriani F, Wong D, Caserini R, Tortoreto M, Milione M, Ferracini R, Mariani L, Pastorino U, Roato I, Sozzi G, Roz L. Microenvironment-Modulated Metastatic CD133+/CXCR4+/EpCAM- Lung Cancer-Initiating Cells Sustain Tumor Dissemination and Correlate with Poor Prognosis. Cancer Res. 2015;75(17):3636–3649. doi: 10.1158/0008-5472.CAN-14-3781. [DOI] [PubMed] [Google Scholar]
- 75.Su L, Zhang J, Xu H, Wang Y, Chu Y, Liu R, Xiong S. Differential expression of CXCR4 is associated with the metastatic potential of human non-small cell lung cancer cells. Clin Cancer Res. 2005;11(23):8273–8280. doi: 10.1158/1078-0432.CCR-05-0537. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Wang Z, Liu X, Liu F. High-level C-X-C chemokine receptor type 4 expression correlates with brain-specific metastasis following complete resection of non-small cell lung cancer. Oncol Lett. 2014;7(6):1871–1876. doi: 10.3892/ol.2014.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi YH, Burdick MD, Strieter BA, Mehrad B, Strieter RM. CXCR4, but not CXCR7, discriminates metastatic behavior in non-small cell lung cancer cells. Mol Cancer Res. 2014;12(1):38–47. doi: 10.1158/1541-7786.MCR-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wagner PL, Hyjek E, Vazquez MF, Meherally D, Liu YF, Chadwick PA, Rengifo T, Sica GL, Port JL, Lee PC, Paul S, Altorki NK, Saqi A. CXCL12 and CXCR4 in adenocarcinoma of the lung: association with metastasis and survival. J Thorac Cardiovasc Surg. 2009;137(3):615–621. doi: 10.1016/j.jtcvs.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 79.Singla AK, Downey CM, Bebb GD, Jirik FR. Characterization of a murine model of metastatic human non-small cell lung cancer and effect of CXCR4 inhibition on the growth of metastases. Oncoscience. 2015;2(3):263–271. doi: 10.18632/oncoscience.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25(1):30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 82.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 83.Panneerselvam J, Jin J, Shanker M, Lauderdale J, Bates J, Wang Q, Zhao YD, Archibald SJ, Hubin TJ, Ramesh R. IL-24 inhibits lung cancer cell migration and invasion by disrupting the SDF-1/CXCR4 signaling axis. Plos one. 2015;10(3):e0122439. doi: 10.1371/journal.pone.0122439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu X, Xia W, Zhang T, Wang H, Xie Y, Yang J, Miao J. Enhanced cytotoxicity of IL-24 gene-modified dendritic cells co-cultured with cytokine-induced killer cells to hepatocellular carcinoma cells. Int J Hematol. 2010;92(2):276–282. doi: 10.1007/s12185-010-0654-1. [DOI] [PubMed] [Google Scholar]
- 85.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 86.Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med. 2003;167(12):1676–1686. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- 87.Zhi Y, Chen J, Zhang S, Chang X, Ma J, Dai D. Down-regulation of CXCL12 by DNA hypermethylation and its involvement in gastric cancer metastatic progression. Dig Dis Sci. 2012;57(3):650–659. doi: 10.1007/s10620-011-1922-5. [DOI] [PubMed] [Google Scholar]
- 88.Fridrichova I, Smolkova B, Kajabova V, Zmetakova I, Krivulcik T, Mego M, Cierna Z, Karaba M, Benca J, Pindak D, Bohac M, Repiska V, Danihel L. CXCL12 and ADAM23 hypermethylation are associated with advanced breast cancers. Transl Res. 2015;165(6):717–730. doi: 10.1016/j.trsl.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Zmetakova I, Danihel L, Smolkova B, Mego M, Kajabova V, Krivulcik T, Rusnak I, Rychly B, Danis D, Repiska V, Blasko P, Karaba M, Benca J, Pechan J, Fridrichova I. Evaluation of protein expression and DNA methylation profiles detected by pyrosequencing in invasive breast cancer. Neoplasma. 2013;60(6):635–646. doi: 10.4149/neo_2013_082. [DOI] [PubMed] [Google Scholar]
- 90.Wendt MK, Johanesen PA, Kang-Decker N, Binion DG, Shah V, Dwinell MB. Silencing of epithelial CXCL12 expression by DNA hypermethylation promotes colonic carcinoma metastasis. Oncogene. 2006;25(36):4986–4997. doi: 10.1038/sj.onc.1209505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki M, Mohamed S, Nakajima T, Kubo R, Tian L, Fujiwara T, Suzuki H, Nagato K, Chiyo M, Motohashi S, Yasufuku K, Iyoda A, Yoshida S, Sekine Y, Shibuya K, Hiroshima K, Nakatani Y, Yoshino I, Fujisawa T. Aberrant methylation of CXCL12 in non-small cell lung cancer is associated with an unfavorable prognosis. Int J Oncol. 2008;33(1):113–119. [PubMed] [Google Scholar]
- 92.Goltz D, Holmes EE, Gevensleben H, Sailer V, Dietrich J, Jung M, Rohler M, Meller S, Ellinger J, Kristiansen G, Dietrich D. CXCL12 promoter methylation and PD-L1 expression as prognostic biomarkers in prostate cancer patients. Oncotarget. 2016;7(33):53309–53320. doi: 10.18632/oncotarget.10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pang LY, Argyle DJ. Using naturally occurring tumours in dogs and cats to study telomerase and cancer stem cell biology. Biochim Biophys Acta. 2009;1792(4):380–391. doi: 10.1016/j.bbadis.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 94.Chen W, Dong J, Haiech J, Kilhoffer MC, Zeniou M. Cancer Stem Cell Quiescence and Plasticity as Major Challenges in Cancer Therapy. 2016;2016:1740936. doi: 10.1155/2016/1740936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peiris-Pages M, Martinez-Outschoorn UE, Pestell RG, Sotgia F, Lisanti MP. Cancer stem cell metabolism. Breast Cancer Res. 2016;18(1):55. doi: 10.1186/s13058-016-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang J, Li ZH, White J, Zhang LB. Lung cancer stem cells and implications for future therapeutics. Cell Biochem Biophys. 2014;69(3):389–398. doi: 10.1007/s12013-014-9844-4. [DOI] [PubMed] [Google Scholar]
- 97.Alamgeer M, Peacock CD, Matsui W, Ganju V, Watkins DN. Cancer stem cells in lung cancer: Evidence and controversies. Respirology. 2013;18(5):757–764. doi: 10.1111/resp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O’Flaherty JD, Barr M, Fennell D, Richard D, Reynolds J, O’Leary J, O’Byrne K. The cancer stem-cell hypothesis: its emerging role in lung cancer biology and its relevance for future therapy. J Thorac Oncol. 2012;7(12):1880–1890. doi: 10.1097/JTO.0b013e31826bfbc6. [DOI] [PubMed] [Google Scholar]
- 99.Shi AM, Tao ZQ, Li H, Wang YQ, Zhao J. Cancer stem cells targeting agents--a review. Eur Rev Med Pharmacol Sci. 2015;19(21):4064–4067. [PubMed] [Google Scholar]
- 100.Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, Schuster K, Shao C, Larsen JE, Sullivan LA, Honorio S, Xie Y, Scaglioni PP, DiMaio JM, Gazdar AF, Shay JW, Wistuba, Minna JD. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70(23):9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang CP, Tsai MF, Chang TH, Tang WC, Chen SY, Lai HH, Lin TY, Yang JC, Yang PC, Shih JY, Lin SB. ALDH-positive lung cancer stem cells confer resistance to epidermal growth factor receptor tyrosine kinase inhibitors. Cancer Lett. 2013;328(1):144–151. doi: 10.1016/j.canlet.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 102.Gorelik E, Lokshin A, Levina V. Lung cancer stem cells as a target for therapy. Anticancer Agents Med Chem. 2010;10(2):164–171. doi: 10.2174/187152010790909308. [DOI] [PubMed] [Google Scholar]
- 103.Lennartsson J, Ronnstrand L. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiol Rev. 2012;92(4):1619–1649. doi: 10.1152/physrev.00046.2011. [DOI] [PubMed] [Google Scholar]
- 104.Yan Y, Zuo X, Wei D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl Med. 2015;4(9):1033–1043. doi: 10.5966/sctm.2015-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bourguignon LY, Shiina M, Li JJ. Hyaluronan-CD44 interaction promotes oncogenic signaling, microRNA functions, chemoresistance, and radiation resistance in cancer stem cells leading to tumor progression. Adv Cancer Res. 2014;123:255–275. doi: 10.1016/B978-0-12-800092-2.00010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Williams K, Motiani K, Giridhar PV, Kasper S. CD44 integrates signaling in normal stem cell, cancer stem cell and (pre)metastatic niches. Exp Biol Med. 2013;238(3):324–338. doi: 10.1177/1535370213480714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qu H, Li R, Liu Z, Zhang J, Luo R. Prognostic value of cancer stem cell marker CD133 expression in non-small cell lung cancer: a systematic review. Int J Clin Exp Pathol. 2013;6(11):2644–2650. [PMC free article] [PubMed] [Google Scholar]
- 108.Grosse-Gehling P, Fargeas CA, Dittfeld C, Garbe Y, Alison MR, Corbeil D, Kunz-Schughart LA. CD133 as a biomarker for putative cancer stem cells in solid tumours: limitations, problems and challenges. J Pathol. 2013;229(3):355–378. doi: 10.1002/path.4086. [DOI] [PubMed] [Google Scholar]
- 109.Kubo T, Takigawa N, Osawa M, Harada D, Ninomiya T, Ochi N, Ichihara E, Yamane H, Tanimoto M, Kiura K. Subpopulation of small-cell lung cancer cells expressing CD133 and CD87 show resistance to chemotherapy. Cancer Sci. 2013;104(1):78–84. doi: 10.1111/cas.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Y, Fan Y, Qi Y, Liu D, Wu K, Wen F, Zhao S. Side population cells separated from A549 lung cancer cell line possess cancer stem cell-like properties and inhibition of autophagy potentiates the cytotoxic effect of cisplatin. Oncol Rep. 2015;34(2):929–935. doi: 10.3892/or.2015.4057. [DOI] [PubMed] [Google Scholar]
- 111.Singh S, Trevino J, Bora-Singhal N, Coppola D, Haura E, Altiok S, Chellappan SP. EGFR/Src/Akt signaling modulates Sox2 expression and self-renewal of stem-like side-population cells in non-small cell lung cancer. Mol Cancer. 2012;11(73):73. doi: 10.1186/1476-4598-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salcido CD, Larochelle A, Taylor BJ, Dunbar CE, Varticovski L. Molecular characterisation of side population cells with cancer stem cell-like characteristics in small-cell lung cancer. Br J Cancer. 2010;102(11):1636–1644. doi: 10.1038/sj.bjc.6605668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B, Henner WR, Meisner R, Sato A, Shah J, Tang T, Wallace B, Wang M, Zhang C, Kapoun AM, Lewicki J, Gurney A, Hoey T. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin Cancer Res. 2015;21(9):2084–2095. doi: 10.1158/1078-0432.CCR-14-2808. [DOI] [PubMed] [Google Scholar]
- 114.Hassan KA, Wang L, Korkaya H, Chen G, Maillard I, Beer DG, Kalemkerian GP, Wicha MS. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin Cancer Res. 2013;19(8):1972–1980. doi: 10.1158/1078-0432.CCR-12-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang S, Wang Y, Mao JH, Hsieh D, Kim IJ, Hu LM, Xu Z, Long H, Jablons DM, You L. Inhibition of CK2alpha down-regulates Hedgehog/Gli signaling leading to a reduction of a stem-like side population in human lung cancer cells. Plos one. 2012;7(6):e38996. doi: 10.1371/journal.pone.0038996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25(2):139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yagui-Beltran A, Jablons DM. A translational approach to lung cancer research: From EGFRs to Wnt and cancer stem cells. Ann Thorac Cardiovasc Surg. 2009;15(4):213–220. [PMC free article] [PubMed] [Google Scholar]
- 118.Chang YW, Su YJ, Hsiao M, Wei KC, Lin WH, Liang CL, Chen SC, Lee JL. Diverse Targets of beta-Catenin during the Epithelial-Mesenchymal Transition Define Cancer Stem Cells and Predict Disease Relapse. Cancer Res. 2015;75(16):3398–3410. doi: 10.1158/0008-5472.CAN-14-3265. [DOI] [PubMed] [Google Scholar]
- 119.Lu C, Huang T, Chen W, Lu H. GnRH participates in the self-renewal of A549-derived lung cancer stem-like cells through upregulation of the JNK signaling pathway. Oncol Rep. 2015;34(1):244–250. doi: 10.3892/or.2015.3956. [DOI] [PubMed] [Google Scholar]
- 120.Song W, Ma Y, Wang J, Brantley-Sieders D, Chen J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 2014;74(9):2444–2454. doi: 10.1158/0008-5472.CAN-13-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee SO, Yang X, Duan S, Tsai Y, Strojny LR, Keng P, Chen Y. IL-6 promotes growth and epithelial-mesenchymal transition of CD133+ cells of non-small cell lung cancer. Oncotarget. 2015;12(10) doi: 10.18632/oncotarget.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Malanga D, De MC, Guerriero I, Colelli F, Rinaldo N, Scrima M, Mirante T, De VC, Zoppoli P, Ceccarelli M, Riccardi M, Ravo M, Weisz A, Federico A, Franco R, Rocco G, Mancini R, Rizzuto A, Gulletta E, Ciliberto G, Viglietto G. The Akt1/IL-6/STAT3 pathway regulates growth of lung tumor initiating cells. Oncotarget. 2015;6(40):42667–42686. doi: 10.18632/oncotarget.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Larzabal L, El-Nikhely N, Redrado M, Seeger W, Savai R, Calvo A. Differential effects of drugs targeting cancer stem cell (CSC) and non-CSC populations on lung primary tumors and metastasis. Plos one. 2013;8(11):e79798. doi: 10.1371/journal.pone.0079798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu W, Cao J, Ji Z, Wang J, Jiang T, Ding H. Co-expression of Lgr5 and CXCR4 characterizes cancer stem-like cells of colorectal cancer. Oncotarget. 2016;8(10):13214. doi: 10.18632/oncotarget.13214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moro M, Bertolini G, Pastorino U, Roz L, Sozzi G. Combination Treatment with All-Trans Retinoic Acid Prevents Cisplatin-Induced Enrichment of CD133+ Tumor-Initiating Cells and Reveals Heterogeneity of Cancer Stem Cell Compartment in Lung Cancer. J Thorac Oncol. 2015;10(7):1027–1036. doi: 10.1097/JTO.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tu Z, Xie S, Xiong M, Liu Y, Yang X, Tembo KM, Huang J, Hu W, Huang X, Pan S, Liu P, Altaf E, Kang G, Xiong J, Zhang Q. CXCR4 is involved in CD133-induced EMT in non-small cell lung cancer. Int J Oncol. 2016;19(10) doi: 10.3892/ijo.2016.3812. [DOI] [PubMed] [Google Scholar]
- 127.Wang Z, Sun J, Feng Y, Tian X, Wang B, Zhou Y. Oncogenic roles and drug target of CXCR4/CXCL12 axis in lung cancer and cancer stem cell. Tumour Biol. 2016;37(7):8515–8528. doi: 10.1007/s13277-016-5016-z. [DOI] [PubMed] [Google Scholar]
- 128.Mantovani A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004;14(3):147–148. doi: 10.1016/j.semcancer.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 129.Mantovani A, Allavena P, Sozzani S, Vecchi A, Locati M, Sica A. Chemokines in the recruitment and shaping of the leukocyte infiltrate of tumors. Semin Cancer Biol. 2004;14(3):155–160. doi: 10.1016/j.semcancer.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 130.Peled A, Wald O, Burger J. Development of novel CXCR4-based therapeutics. Expert Opin Investig Drugs. 2012;21(3):341–353. doi: 10.1517/13543784.2012.656197. [DOI] [PubMed] [Google Scholar]
- 131.Liekens S, Schols D, Hatse S. CXCL12-CXCR4 axis in angiogenesis, metastasis and stem cell mobilization. Curr Pharm Des. 2010;16(35):3903–3920. doi: 10.2174/138161210794455003. [DOI] [PubMed] [Google Scholar]
- 132.Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB, Ko YG, Lee JS, Lee SJ, Lee JC, Park MJ. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene. 2013;32(2):209–221. doi: 10.1038/onc.2012.37. [DOI] [PubMed] [Google Scholar]
- 133.Nian WQ, Chen FL, Ao XJ, Chen ZT. CXCR4 positive cells from Lewis lung carcinoma cell line have cancer metastatic stem cell characteristics. Mol Cell Biochem. 2011;355(1–2):241–248. doi: 10.1007/s11010-011-0860-z. [DOI] [PubMed] [Google Scholar]
- 134.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo VS, Camerini T, Mariani L, Delia D, Calabro E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106(38):16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Es-Haghi M, Soltanian S, Dehghani H. Perspective: Cooperation of Nanog, NF-kappaBeta, and CXCR4 in a regulatory network for directed migration of cancer stem cells. Tumour Biol. 2016;37(2):1559–1565. doi: 10.1007/s13277-015-4690-6. [DOI] [PubMed] [Google Scholar]
- 136.Lundin A, Driscoll B. Lung cancer stem cells: progress and prospects. Cancer Lett. 2013;338(1):89–93. doi: 10.1016/j.canlet.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marcucci F, Rumio C, Lefoulon F. Anti-Cancer Stem-like Cell Compounds in Clinical Development - An Overview and Critical Appraisal. Front Oncol. 2016;6(115):115. doi: 10.3389/fonc.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Codony-Servat J, Rosell R. Cancer stem cells and immunoresistance: clinical implications and solutions. Transl Lung Cancer Res. 2015;4(6):689–703. doi: 10.3978/j.issn.2218-6751.2015.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fahham D, Weiss ID, Abraham M, Beider K, Hanna W, Shlomai Z, Eizenberg O, Zamir G, Izhar U, Shapira OM, Peled A, Wald O. In vitro and in vivo therapeutic efficacy of CXCR4 antagonist BKT140 against human non-small cell lung cancer. J Thorac Cardiovasc Surg. 2012;144(5):1167–1175. e1161. doi: 10.1016/j.jtcvs.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 140.Tamamura H, Hiramatsu K, Ueda S, Wang Z, Kusano S, Terakubo S, Trent JO, Peiper SC, Yamamoto N, Nakashima H, Otaka A, Fujii N. Stereoselective synthesis of [L-Arg-L/D-3-(2-naphthyl)alanine]-type (E)-alkene dipeptide isosteres and its application to the synthesis and biological evaluation of pseudopeptide analogues of the CXCR4 antagonist FC131. J Med Chem. 2005;48(2):380–391. doi: 10.1021/jm049429h. [DOI] [PubMed] [Google Scholar]
- 141.Yoshikawa Y, Kobayashi K, Oishi S, Fujii N, Furuya T. Molecular modeling study of cyclic pentapeptide CXCR4 antagonists: new insight into CXCR4-FC131 interactions. Bioorg Med Chem Lett. 2012;22(6):2146–2150. doi: 10.1016/j.bmcl.2012.01.134. [DOI] [PubMed] [Google Scholar]
- 142.Tamamura H, Hori A, Kanzaki N, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, Otaka A, Fujii N. T140 analogs as CXCR4 antagonists identified as anti-metastatic agents in the treatment of breast cancer. FEBS Lett. 2003;550(1–3):79–83. doi: 10.1016/s0014-5793(03)00824-x. [DOI] [PubMed] [Google Scholar]
- 143.de NF, Schiano C, Infante T, Napoli C. CXCR4 inhibitors: tumor vasculature and therapeutic challenges. Recent Pat Anticancer Drug Discov. 2012;7(3):251–264. doi: 10.2174/157489212801820039. [DOI] [PubMed] [Google Scholar]
- 144.Otani Y, Kijima T, Kohmo S, Oishi S, Minami T, Nagatomo I, Takahashi R, Hirata H, Suzuki M, Inoue K, Takeda Y, Kida H, Tachibana I, Fujii N, Kumanogoh A. Suppression of metastases of small cell lung cancer cells in mice by a peptidic CXCR4 inhibitor TF14016. FEBS Lett. 2012;586(20):3639–3644. doi: 10.1016/j.febslet.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 145.Stone ND, Dunaway SB, Flexner C, Tierney C, Calandra GB, Becker S, Cao YJ, Wiggins IP, Conley J, MacFarland RT, Park JG, Lalama C, Snyder S, Kallungal B, Klingman KL, Hendrix CW. Multiple-dose escalation study of the safety, pharmacokinetics, and biologic activity of oral AMD070, a selective CXCR4 receptor inhibitor, in human subjects. Antimicrob Agents Chemother. 2007;51(7):2351–2358. doi: 10.1128/AAC.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nyunt MM, Becker S, MacFarland RT, Chee P, Scarborough R, Everts S, Calandra GB, Hendrix CW. Pharmacokinetic effect of AMD070, an Oral CXCR4 antagonist, on CYP3A4 and CYP2D6 substrates midazolam and dextromethorphan in healthy volunteers. J Acquir Immune Defic Syndr. 2008;47(5):559–565. doi: 10.1097/QAI.0b013e3181627566. [DOI] [PubMed] [Google Scholar]
- 147.De CE. The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil) Biochem Pharmacol. 2009;77(11):1655–1664. doi: 10.1016/j.bcp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 148.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Fruehauf S, Horwitz M, Cooper D, Bridger G, Calandra G. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 149.DiPersio JF, Uy GL, Yasothan U, Kirkpatrick P. Plerixafor. Nat Rev Drug Discov. 2009;8(2):105–106. doi: 10.1038/nrd2819. [DOI] [PubMed] [Google Scholar]
- 150.O’Boyle G, Swidenbank I, Marshall H, Barker CE, Armstrong J, White SA, Fricker SP, Plummer R, Wright M, Lovat PE. Inhibition of CXCR4-CXCL12 chemotaxis in melanoma by AMD11070. Br J Cancer. 2013;108(8):1634–1640. doi: 10.1038/bjc.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liang Z, Zhan W, Zhu A, Yoon Y, Lin S, Sasaki M, Klapproth JM, Yang H, Grossniklaus HE, Xu J, Rojas M, Voll RJ, Goodman MM, Arrendale RF, Liu J, Yun CC, Snyder JP, Liotta DC, Shim H. Development of a unique small molecule modulator of CXCR4. Plos one. 2012;7(4):e34038. doi: 10.1371/journal.pone.0034038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Planesas JM, Perez-Nueno VI, Borrell JI, Teixido J. Studying the binding interactions of allosteric agonists and antagonists of the CXCR4 receptor. J Mol Graph Model. 2015;60:1–14. doi: 10.1016/j.jmgm.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 153.Jenkinson S, Thomson M, McCoy D, Edelstein M, Danehower S, Lawrence W, Wheelan P, Spaltenstein A, Gudmundsson K. Blockade of X4-tropic HIV-1 cellular entry by GSK812397, a potent noncompetitive CXCR4 receptor antagonist. Antimicrob Agents Chemother. 2010;54(2):817–824. doi: 10.1128/AAC.01293-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Murakami T, Kumakura S, Yamazaki T, Tanaka R, Hamatake M, Okuma K, Huang W, Toma J, Komano J, Yanaka M, Tanaka Y, Yamamoto N. The novel CXCR4 antagonist KRH-3955 is an orally bioavailable and extremely potent inhibitor of human immunodeficiency virus type 1 infection: comparative studies with AMD3100. Antimicrob Agents Chemother. 2009;53(7):2940–2948. doi: 10.1128/AAC.01727-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iwanaga T, Iwasaki Y, Ohashi M, Ohinata R, Takahashi K, Yamaguchi T, Matsumoto H, Nakano D. Inhibitory effect of CXCR4 blockers on a CXCR4-expressing gastric cancer cell line in nude mice. Gan To Kagaku Ryoho. 2012;39(12):1788–1790. [PubMed] [Google Scholar]
- 156.Kuhne MR, Mulvey T, Belanger B, Chen S, Pan C, Chong C, Cao F, Niekro W, Kempe T, Henning KA, Cohen LJ, Korman AJ, Cardarelli PM. BMS-936564/MDX-1338: a fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin Cancer Res. 2013;19(2):357–366. doi: 10.1158/1078-0432.CCR-12-2333. [DOI] [PubMed] [Google Scholar]
- 157.Gil M, Seshadri M, Komorowski MP, Abrams SI, Kozbor D. Targeting CXCL12/CXCR4 signaling with oncolytic virotherapy disrupts tumor vasculature and inhibits breast cancer metastases. Proc Natl Acad Sci U S A. 2013;110(14):E1291–1300. doi: 10.1073/pnas.1220580110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Drenckhan A, Kurschat N, Dohrmann T, Raabe N, Koenig AM, Reichelt U, Kaifi JT, Izbicki JR, Gros SJ. Effective inhibition of metastases and primary tumor growth with CTCE-9908 in esophageal cancer. J Surg Res. 2013;182(2):250–256. doi: 10.1016/j.jss.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 159.Koga C, Kobayashi S, Nagano H, Tomimaru Y, Hama N, Wada H, Kawamoto K, Eguchi H, Konno M, Ishii H, Umeshita K, Doki Y, Mori M. Reprogramming using microRNA-302 improves drug sensitivity in hepatocellular carcinoma cells. Ann Surg Oncol. 2014;21(Suppl 4)(4):S591–600. doi: 10.1245/s10434-014-3705-7. [DOI] [PubMed] [Google Scholar]
- 160.Yu T, Liu K, Wu Y, Fan J, Chen J, Li C, Yang Q, Wang Z. MicroRNA-9 inhibits the proliferation of oral squamous cell carcinoma cells by suppressing expression of CXCR4 via the Wnt/beta-catenin signaling pathway. Oncogene. 2014;33(42):5017–5027. doi: 10.1038/onc.2013.448. [DOI] [PubMed] [Google Scholar]
- 161.Wang X, Li F, Zhou X. miR-204-5p regulates cell proliferation and metastasis through inhibiting CXCR4 expression in OSCC. Biomed Pharmacother. 2016;82:202–207. doi: 10.1016/j.biopha.2016.04.060. [DOI] [PubMed] [Google Scholar]
- 162.Yuan W, Guo YQ, Li XY, Deng MZ, Shen ZH, Bo CB, Dai YF, Huang MY, Yang ZY, Quan YS, Tian L, Wang X. MicroRNA-126 inhibits colon cancer cell proliferation and invasion by targeting the chemokine (C-X-C motif) receptor 4 and Ras homolog gene family, member A, signaling pathway. Oncotarget. 2016;10(10):11176. doi: 10.18632/oncotarget.11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, Nie S, Umbreit J, Shim H. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64(12):4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 164.Wald O, Izhar U, Amir G, Kirshberg S, Shlomai Z, Zamir G, Peled A, Shapira OM. Interaction between neoplastic cells and cancer-associated fibroblasts through the CXCL12/CXCR4 axis: role in non-small cell lung cancer tumor proliferation. J Thorac Cardiovasc Surg. 2011;141(6):1503–1512. doi: 10.1016/j.jtcvs.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 165.Zhan W, Liang Z, Zhu A, Kurtkaya S, Shim H, Snyder JP, Liotta DC. Discovery of small molecule CXCR4 antagonists. J Med Chem. 2007;50(23):5655–5664. doi: 10.1021/jm070679i. [DOI] [PubMed] [Google Scholar]
- 166.De CE. AMD3100/CXCR4 Inhibitor. Front Immunol. 2015;6(276):276. [Google Scholar]
- 167.Kato I, Niwa A, Heike T, Fujino H, Saito MK, Umeda K, Hiramatsu H, Ito M, Morita M, Nishinaka Y, Adachi S, Ishikawa F, Nakahata T. Identification of hepatic niche harboring human acute lymphoblastic leukemic cells via the SDF-1/CXCR4 axis. Plos one. 2011;6(11):e27042. doi: 10.1371/journal.pone.0027042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Uy GL, Rettig MP, Motabi IH, McFarland K, Trinkaus KM, Hladnik LM, Kulkarni S, Abboud CN, Cashen AF, Stockerl-Goldstein KE, Vij R, Westervelt P, DiPersio JF. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119(17):3917–3924. doi: 10.1182/blood-2011-10-383406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Taromi S, Kayser G, Catusse J, von ED, Reichardt W, Braun F, Weber WA, Zeiser R, Burger M. CXCR4 antagonists suppress small cell lung cancer progression. Oncotarget. 2016;9(10):13238. doi: 10.18632/oncotarget.13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lefort S, Thuleau A, Kieffer Y, Sirven P, Bieche I, Marangoni E, Vincent-Salomon A, Mechta-Grigoriou F. CXCR4 inhibitors could benefit to HER2 but not to triple-negative breast cancer patients. Oncogene. 2016;26:284. doi: 10.1038/onc.2016.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cavnar SP, Ray P, Moudgil P, Chang SL, Luker KE, Linderman JJ, Takayama S, Luker GD. Microfluidic source-sink model reveals effects of biophysically distinct CXCL12 isoforms in breast cancer chemotaxis. Integr Biol. 2014;6(5):564–576. doi: 10.1039/c4ib00015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hassan S, Buchanan M, Jahan K, Aguilar-Mahecha A, Gaboury L, Muller WJ, Alsawafi Y, Mourskaia AA, Siegel PM, Salvucci O, Basik M. CXCR4 peptide antagonist inhibits primary breast tumor growth, metastasis and enhances the efficacy of anti-VEGF treatment or docetaxel in a transgenic mouse model. Int J Cancer. 2011;129(1):225–232. doi: 10.1002/ijc.25665. [DOI] [PubMed] [Google Scholar]
- 173.Liang Z, Bian X, Shim H. Inhibition of breast cancer metastasis with microRNA-302a by downregulation of CXCR4 expression. Breast Cancer Res Treat. 2014;146(3):535–542. doi: 10.1007/s10549-014-3053-0. [DOI] [PubMed] [Google Scholar]
- 174.Labbaye C, Spinello I, Quaranta MT, Pelosi E, Pasquini L, Petrucci E, Biffoni M, Nuzzolo ER, Billi M, Foa R, Brunetti E, Grignani F, Testa U, Peschle C. A three-step pathway comprising PLZF/miR-146a/CXCR4 controls megakaryopoiesis. Nat Cell Biol. 2008;10(7):788–801. doi: 10.1038/ncb1741. [DOI] [PubMed] [Google Scholar]
- 175.Yin P, Peng R, Peng H, Yao L, Sun Y, Wen L, Wu T, Zhou J, Zhang Z. MiR-451 suppresses cell proliferation and metastasis in A549 lung cancer cells. Mol Biotechnol. 2015;57(1):1–11. doi: 10.1007/s12033-014-9796-3. [DOI] [PubMed] [Google Scholar]
- 176.Liang Z, Wu H, Reddy S, Zhu A, Wang S, Blevins D, Yoon Y, Zhang Y, Shim H. Blockade of invasion and metastasis of breast cancer cells via targeting CXCR4 with an artificial microRNA. Biochem Biophys Res Commun. 2007;363(3):542–546. doi: 10.1016/j.bbrc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 177.Zhang J, Liu J, Liu Y, Wu W, Li X, Wu Y, Chen H, Zhang K, Gu L. miR-101 represses lung cancer by inhibiting interaction of fibroblasts and cancer cells by down-regulating CXCL12. Biomed Pharmacother. 2015;74:215–221. doi: 10.1016/j.biopha.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 178.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gentzler R, Hall R, Kunk PR, Gaughan E, Dillon P, Slingluff CL, Jr, Rahma OE. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016;8(5):583–600. doi: 10.2217/imt-2015-0029. [DOI] [PubMed] [Google Scholar]
- 180.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Poulet FM, Wolf JJ, Herzyk DJ, DeGeorge JJ. An Evaluation of the Impact of PD-1 Pathway Blockade on Reproductive Safety of Therapeutic PD-1 Inhibitors. Birth Defects Res B Dev Reprod Toxicol. 2016;107(2):108–119. doi: 10.1002/bdrb.21176. [DOI] [PubMed] [Google Scholar]
- 183.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van dEAJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Callahan MK, Wolchok JD. Clinical Activity, Toxicity, Biomarkers, and Future Development of CTLA-4 Checkpoint Antagonists. Semin Oncol. 2015;42(4):573–586. doi: 10.1053/j.seminoncol.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 185.Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res. 2014;2(3):187–193. doi: 10.1158/2326-6066.CIR-14-0002. [DOI] [PubMed] [Google Scholar]
- 186.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Almasbak H, Aarvak T, Vemuri MC. CAR T Cell Therapy: A Game Changer in Cancer Treatment. J Immunol Res. 2016;2016(10):5474602. doi: 10.1155/2016/5474602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Byrne KT, Vonderheide RH, Jaffee EM, Armstrong TD. Special Conference on Tumor Immunology and Immunotherapy: A New Chapter. Cancer Immunol Res. 2015;12:12. doi: 10.1158/2326-6066.CIR-15-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 190.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren FO, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Calio A, Cuppone F, Sperduti I, Giannarelli D, Chilosi M, Bronte V, Scarpa A, Bria E, Tortora G. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. Plos one. 2015;10(6):e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 194.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(50):20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chen Y, Ramjiawan RR, Reiberger T, Ng MR, Hato T, Huang Y, Ochiai H, Kitahara S, Unan EC, Reddy TP, Fan C, Huang P, Bardeesy N, Zhu AX, Jain RK, Duda DG. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology. 2015;61(5):1591–1602. doi: 10.1002/hep.27665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, Kipps TJ, Burger JA. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22(50):8093–8101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 197.Beider K, Begin M, Abraham M, Wald H, Weiss ID, Wald O, Pikarsky E, Zeira E, Eizenberg O, Galun E, Hardan I, Engelhard D, Nagler A, Peled A. CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple myeloma tumor growth. Exp Hematol. 2011;39(3):282–292. doi: 10.1016/j.exphem.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 198.Muralidharan R, Panneerselvam J, Chen A, Zhao YD, Munshi A, Ramesh R. HuR-targeted nanotherapy in combination with AMD3100 suppresses CXCR4 expression, cell growth, migration and invasion in lung cancer. Cancer Gene Ther. 2015;22(12):581–590. doi: 10.1038/cgt.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64(23):8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 200.Domanska UM, Timmer-Bosscha H, Nagengast WB, Oude MTH, Kruizinga RC, Ananias HJ, Kliphuis NM, Huls G, De VEG, de JIJ, Walenkamp AM. CXCR4 inhibition with AMD3100 sensitizes prostate cancer to docetaxel chemotherapy. Neoplasia. 2012;14(8):709–718. doi: 10.1593/neo.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang Q, Zhang F, Ding Y, Huang J, Chen S, Wu Q, Wang Z, Chen C. Antitumour activity of the recombination polypeptide GST-NT21MP is mediated by inhibition of CXCR4 pathway in breast cancer. Br J Cancer. 2014;110(5):1288–1297. doi: 10.1038/bjc.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huang EH, Singh B, Cristofanilli M, Gelovani J, Wei C, Vincent L, Cook KR, Lucci A. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. J Surg Res. 2009;155(2):231–236. doi: 10.1016/j.jss.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 203.Singh B, Cook KR, Martin C, Huang EH, Mosalpuria K, Krishnamurthy S, Cristofanilli M, Lucci A. Evaluation of a CXCR4 antagonist in a xenograft mouse model of inflammatory breast cancer. Clin Exp Metastasis. 2010;27(4):233–240. doi: 10.1007/s10585-010-9321-4. [DOI] [PubMed] [Google Scholar]
- 204.Wong D, Kandagatla P, Korz W, Chinni SR. Targeting CXCR4 with CTCE-9908 inhibits prostate tumor metastasis. BMC Urol. 2014;14(12):12. doi: 10.1186/1471-2490-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62(24):7203–7206. [PubMed] [Google Scholar]
- 206.Kim B, Park B. Baohuoside I suppresses invasion of cervical and breast cancer cells through the downregulation of CXCR4 chemokine receptor expression. Biochemistry. 2014;53(48):7562–7569. doi: 10.1021/bi5011927. [DOI] [PubMed] [Google Scholar]
- 207.Yan H, Zhang Z, Jia X, Song J. d-alpha-Tocopheryl polyethylene glycol succinate/Solutol HS 15 mixed micelles for the delivery of baohuoside I against non-small-cell lung cancer: optimization and in vitro, in vivo evaluation. Int J Nanomedicine. 2016;11:4563–4571. doi: 10.2147/IJN.S112204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chen XP, Qian LL, Jiang H, Chen JH. Ginsenoside Rg3 inhibits CXCR4 expression and related migrations in a breast cancer cell line. Int J Clin Oncol. 2011;16(5):519–523. doi: 10.1007/s10147-011-0222-6. [DOI] [PubMed] [Google Scholar]
- 209.Park B, Sung B, Yadav VR, Cho SG, Liu M, Aggarwal BB. Acetyl-11-keto-beta-boswellic acid suppresses invasion of pancreatic cancer cells through the downregulation of CXCR4 chemokine receptor expression. Int J Cancer. 2011;129(1):23–33. doi: 10.1002/ijc.25966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Park B, Prasad S, Yadav V, Sung B, Aggarwal BB. Boswellic acid suppresses growth and metastasis of human pancreatic tumors in an orthotopic nude mouse model through modulation of multiple targets. Plos one. 2011;6(10):e26943. doi: 10.1371/journal.pone.0026943. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 211.Chua AW, Hay HS, Rajendran P, Shanmugam MK, Li F, Bist P, Koay ES, Lim LH, Kumar AP, Sethi G. Butein downregulates chemokine receptor CXCR4 expression and function through suppression of NF-kappaB activation in breast and pancreatic tumor cells. Biochem Pharmacol. 2010;80(10):1553–1562. doi: 10.1016/j.bcp.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 212.Li SH, Dong WC, Fan L, Wang GS. Suppression of chronic lymphocytic leukemia progression by CXCR4 inhibitor WZ811. Am J Transl Res. 2016;8(9):3812–3821. [PMC free article] [PubMed] [Google Scholar]
- 213.Hartimath SV, Khayum MA, van WA, Dierckx RA, de VEF. N-[11C]Methyl-AMD3465 PET as a Tool for In Vivo Measurement of Chemokine Receptor 4 (CXCR4) Occupancy by Therapeutic Drugs. Mol Imaging Biol. 2016;28:28. doi: 10.1007/s11307-016-1028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ling X, Spaeth E, Chen Y, Shi Y, Zhang W, Schober W, Hail N, Jr, Konopleva M, Andreeff M. The CXCR4 antagonist AMD3465 regulates oncogenic signaling and invasiveness in vitro prevents breast cancer growth metastasis in vivo. Plos one. 2013;8(3):e58426. doi: 10.1371/journal.pone.0058426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yi T, Kabha E, Papadopoulos E, Wagner G. 4EGI-1 targets breast cancer stem cells by selective inhibition of translation that persists in CSC maintenance, proliferation and metastasis. Oncotarget. 2014;5(15):6028–6037. doi: 10.18632/oncotarget.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhu A, Zhan W, Liang Z, Yoon Y, Yang H, Grossniklaus HE, Xu J, Rojas M, Lockwood M, Snyder JP, Liotta DC, Shim H. Dipyrimidine amines: a novel class of chemokine receptor type 4 antagonists with high specificity. J Med Chem. 2010;53(24):8556–8568. doi: 10.1021/jm100786g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yadav VR, Sung B, Prasad S, Kannappan R, Cho SG, Liu M, Chaturvedi MM, Aggarwal BB. Celastrol suppresses invasion of colon and pancreatic cancer cells through the downregulation of expression of CXCR4 chemokine receptor. J Mol Med. 2010;88(12):1243–1253. doi: 10.1007/s00109-010-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Badr G, Lefevre EA, Mohany M. Thymoquinone inhibits the CXCL12-induced chemotaxis of multiple myeloma cells and increases their susceptibility to Fas-mediated apoptosis. Plos one. 2011;6(9):e23741. doi: 10.1371/journal.pone.0023741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mooring SR, Liu J, Liang Z, Ahn J, Hong S, Yoon Y, Snyder JP, Shim H. Benzenesulfonamides: a unique class of chemokine receptor type 4 inhibitors. ChemMedChem. 2013;8(4):622–632. doi: 10.1002/cmdc.201200582. [DOI] [PMC free article] [PubMed] [Google Scholar]