Highlights
-
•
The lifespan trajectory of resting and motor-related beta oscillations is unknown.
-
•
These beta dynamics were examined in participants aged 9–75 years using MEG imaging.
-
•
Resting beta levels and motor-related beta oscillations follow unique trajectories.
-
•
The dynamic relationship between these two measures predicts motor performance.
Keywords: Magnetoencephalography, Motor control, Precentral gyrus, Movement, Beta ERD
Abstract
Numerous studies connect beta oscillations in the motor cortices to volitional movement, and beta is known to be aberrant in multiple movement disorders. However, the dynamic interplay between these beta oscillations, motor performance, and spontaneous beta power (e.g., during rest) in the motor cortices remains unknown. This study utilized magnetoencephalography (MEG) to investigate these three parameters and their lifespan trajectory in 57 healthy participants aged 9–75 years old. Movement-related beta activity was imaged using a beamforming approach, and voxel time series data were extracted from the peak voxels in the primary motor cortices. Our results indicated that spontaneous beta power during rest followed a quadratic lifespan trajectory, while movement-related beta oscillations linearly increased with age. Follow-on analyses showed that spontaneous beta power and the beta minima during movement, together, significantly predicted task performance above and beyond the effects of age. These data are the first to show lifespan trajectories among measures of beta activity in the motor cortices, and suggest that the healthy brain compensates for age-related increases in spontaneous beta activity by increasing the strength of beta oscillations within the motor cortices which, when successful, enables normal motor performance into later life.
1. Introduction
Transient human movement is served by a specific pattern of neural oscillatory activity, particularly in the beta band (14–30 Hz). Briefly, prior to and during movement, there is a strong decrease in beta activity relative to baseline levels, known as the peri-movement beta event-related desynchronization (ERD), which begins about 1.0 s before movement onset and dissipates shortly after movement concludes (Cheyne et al., 2006; Engel and Fries, 2010; Gaetz et al., 2010; Heinrichs-Graham and Wilson, 2015, Heinrichs-Graham and Wilson, 2016; Heinrichs-Graham et al., 2014b; Jurkiewicz et al., 2006; Pfurtscheller and Lopes da Silva, 1999; Wilson et al., 2014, Wilson et al., 2010, Wilson et al., 2011). This response has been reliably associated with movement planning and execution (Doyle et al., 2005; Grent-'t-Jong et al., 2014; Heinrichs-Graham et al., 2016; Heinrichs-Graham and Wilson, 2015; Kaiser et al., 2001; Tzagarakis et al., 2010). Following the beta ERD, there is a strong resynchronization (above baseline levels), termed the post-movement beta rebound (PMBR), which extends from approximately 0.8–2.5 s after movement has stopped (Cheyne et al., 2006; Gaetz et al., 2010; Heinrichs-Graham et al., 2014b; Jurkiewicz et al., 2006; Pfurtscheller and Lopes da Silva, 1999; Wilson et al., 2010, Wilson et al., 2011). During simple movements, these beta-band oscillations reliably peak in the precentral gyri bilaterally with stronger activity contralateral to movement, while more complex movements (and some simple movements) also induce activity in the supplementary motor area and bilateral premotor cortices, postcentral gyri, parietal cortices, and cerebellum (Cheyne et al., 2006, Cheyne et al., 2008; Fry et al., 2016; Gaetz and Cheyne, 2006; Gaetz et al., 2010; Heinrichs-Graham et al., 2016; Heinrichs-Graham and Wilson, 2015, Heinrichs-Graham and Wilson, 2016; Heinrichs-Graham et al., 2014b; Jurkiewicz et al., 2006; Muthukumaraswamy, 2010; Wilson et al., 2010).
Prior studies have shown that these movement-related oscillatory patterns change as a function of age (Gaetz et al., 2010; Rossiter et al., 2014; Wilson et al., 2010). For example, a magnetoencephalography (MEG) study of simple movements showed that the beta ERD becomes stronger (i.e., more negative relative to baseline) and the PMBR linearly increases from childhood to early adulthood, with young children (aged 4–6 years) exhibiting little-to-no PMBR response (Gaetz et al., 2010). Another MEG study showed a similar linear increase in beta ERD from early to late adulthood (Rossiter et al., 2014). Interestingly, this study also found a significant age-related linear increase in spontaneous (i.e., no task) beta activity in the motor cortices (Rossiter et al., 2014). Recently, our laboratory utilized MEG and a motor sequencing task to directly probe the relationship between spontaneous beta activity and motor-related oscillations in the context of healthy aging (Heinrichs-Graham and Wilson, 2016). Consistent with previous findings, we found that older adults exhibited an almost threefold increase in spontaneous beta power in the primary motor cortices, as well as significantly stronger beta ERD in the same regions compared to younger adults. Taken together, these studies provide substantial evidence that there are major neurophysiological changes that occur in the motor cortices throughout the lifespan.
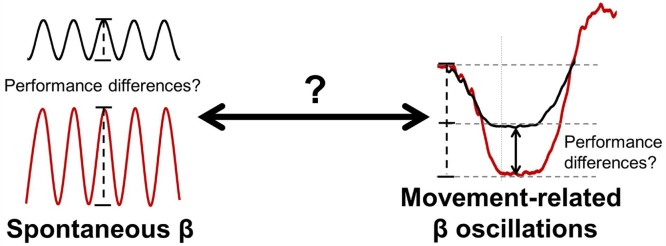
Importantly, the aforementioned study from our laboratory (Heinrichs-Graham and Wilson, 2016) also found a direct linear relationship between spontaneous beta power and peri-movement beta ERD power, such that with greater spontaneous (resting) beta, there was greater baseline-relative beta suppression (i.e., ERD) during movement. In addition, we found a significant relationship between baseline-relative beta ERD and movement duration, such that the greater the decrease in beta power relative to baseline levels, the longer the movement duration (Heinrichs-Graham and Wilson, 2016). This pattern of results suggests that spontaneous beta activity in the primary motor cortex and movement-related beta ERD power are directly related, and that the relationship between these two measures affects motor performance (see Fig. 1). This significant link between spontaneous and motor-related beta oscillatory activity also corroborated an earlier MEG study (Wilson et al., 2014), which investigated the impact of time-of-day on motor-related oscillatory activity. This study found a linear increase in peri-movement beta ERD power (i.e., more negative relative to baseline), coupled with a roughly proportional increase in spontaneous beta power, as a function of time of day (Wilson et al., 2014). While not directly investigated in this study, this pattern of results clearly suggested that the two neurophysiological measures were linked, as the stronger peri-movement beta ERD during movement appeared to be offsetting the increased spontaneous beta levels during both the baseline period and a separately-acquired resting state recording (Wilson et al., 2014).
Fig. 1.
Proposed relationship between spontaneous beta power and movement-related beta oscillations in the primary motor cortex. The central goals of this study were to identify the dynamic link between spontaneous (i.e., no task) beta power (left) and movement-related beta oscillations (right), and to determine their combined impact on motor performance throughout the lifespan. Previous studies have shown that spontaneous beta levels in the motor cortices sharply increase in later life (Heinrichs-Graham and Wilson, 2016; Rossiter et al., 2014).
While these and other studies have independently suggested that there is a unique change in beta oscillatory activity as a function of age and that these oscillatory measures are directly related, no study to date has looked at the nature of this relationship across the lifespan. Basically, studies have shown differences in movement-related beta activity from youth to adulthood (Gaetz et al., 2010), while others have shown differences in beta activity from younger to older adulthood (Heinrichs-Graham and Wilson, 2016; Rossiter et al., 2014), but critically missing is the developmental trajectory of these measures from youth to late adulthood. Such information could provide invaluable data on functional brain maturation, and serve as a baseline by which pathology could be assessed. Furthermore, examining these responses across the lifespan would provide a powerful testbed for determining whether spontaneous beta levels are directly related to the strength of the beta ERD. In the current study, we used a complex motor sequence paradigm to study the relationship between spontaneous beta activity and movement-related beta oscillations in the motor cortices from preadolescence through late adulthood. We first sought to determine how spontaneous activity in the motor cortex and movement-related beta oscillations change as a function of age. Secondly, we aimed to examine the relationship between spontaneous beta activity and movement-related oscillations in the context of development and aging (Fig. 1). We hypothesized that there would be unique developmental trajectories for both spontaneous and movement-related beta oscillatory responses. Further, we hypothesized that there would be a tight link between these responses, and that this relationship would mediate motor performance across the lifespan.
2. Material and methods
2.1. Subject selection
A total of 57 males were enrolled in the study. We focused on males in this study due to several recent reports of sex differences in the aging brain (Scheinost et al., 2015; Shaw et al., 2016). All participants were recruited from the local community. Data from the adults were included in another recent publication (Heinrichs-Graham and Wilson, 2016); however, these data were fully re-analyzed as described below. Thus, all reported results, with the exception of the adult behavioral data, are unique to this publication. Exclusionary criteria included inability to perform the task, any medical illness affecting CNS function, neurological or psychiatric disorder, history of head trauma, current substance abuse, any medication known to affect CNS function, and the MEG Laboratory’s standard exclusion criteria (e.g., dental braces, metal implants, battery operated implants, and/or any type of ferromagnetic implanted material). After complete description of the study was given to participants, written informed consent was obtained from the adult participants and parents of the youth participants, and informed assent was obtained from the youth participants, following the guidelines of the University of Nebraska Medical Center’s Institutional Review Board which approved the study protocol. Six additional youth were recruited, but excluded from analysis due to our standard MEG exclusionary criteria (e.g., movement artifacts, inability to perform the task).
2.2. Experimental paradigm and stimuli
During MEG recording, participants were seated in a nonmagnetic chair within the magnetically-shielded room, and each participant rested their right hand on a custom-made five-finger button pad. This response pad was connected such that each button sent a unique signal (i.e., TTL pulse/trigger code) to the MEG system acquisition computer, and thus behavioral responses were temporally synced with the MEG data. This allowed accuracy, reaction times (i.e., time between the cue to move and first button press), and movement durations (i.e., how long it took to complete the tapping sequence) in ms to be computed offline. Each participant first completed a motor sequencing task, during which they were instructed to complete a series of finger-tapping sequences as quickly and accurately as possible. During the motor sequencing task, participants fixated on a crosshair presented centrally. After a sufficient baseline period of 3.75 s, a series of three numbers, each corresponding to a finger on the hand, was presented on the screen in black for 0.5 s. After 0.5 s, the numbers changed color, signaling the participant to tap the fingers corresponding to the motor plan sequentially. The participant was given 2.25 s to complete the motor plan and return to rest. This series of slides constituted one trial; Fig. 2 depicts the total time course of a single trial, as well as the response pad used. A total of 10 practice trials prior to the recording (not analyzed) and 160 trials during the recording were completed. Following the motor sequencing task, participants completed six minutes of eyes-closed rest during each MEG session. Total MEG recording time was ∼22 min (including both tasks).
Fig. 2.
A) Motor sequencing task. Participants fixated on a crosshair for 3.75 s. After this baseline period, a series of three numbers (each corresponding to a digit on the finger) appeared on the screen in black and after 0.5 s the numbers changed color cueing the participant to move. The participant then had 2.25 s to complete the motor plan and return to rest. B) The button pad used during this task. Each button on the pad corresponded to a specific finger.
2.3. MEG data acquisition & coregistration with structural MRI
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using an Elekta MEG system with 306 magnetic sensors (Elekta, Helsinki, Finland). Using MaxFilter (v2.2; Elekta), MEG data from each subject were individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola, 2006; Taulu et al., 2005). For motion correction, the position of the head throughout the recording was aligned to the individual’s head position when the recording was initiated. Each participant’s MEG data were coregistered with high-resolution structural T1-weighted MRI data, prior to the application of source space analyses (i.e., beamforming), using BESA MRI (Version 2.0). These anatomic images were acquired with a Philips Achieva 3T X-series scanner using an eight-channel head coil (adult participants; TR: 8.09 ms; TE: 3.7 ms; field of view: 240 mm; slice thickness: 1 mm with no gap; in-plane resolution: 1.0 × 1.0 mm), and a 3T Siemens Skyra scanner using a 32-channel head coil (youth participants; TR: 24.0 ms; TE: 1.94 ms; field of view: 256 mm; slice thickness: 1 mm with no gap; in-plane resolution: 1.0 × 1.0 mm). The resolution and quality of the resulting images were similar across groups, and ultimately were used only for coregistration of structural and functional data. The structural volumes were aligned parallel to the anterior and posterior commissures and transformed into standardized space. After beamformer analysis, each subject’s functional images were also transformed into standardized space using the transform that was previously applied to the structural MRI volume and spatially resampled.
2.4. MEG preprocessing, time-frequency transformation, & sensor-level statistics
Prior to analysis, all MEG data were corrected for head motion, subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola, 2006), and coregistered to structural MRI. Cardio-artifacts were removed from the data using signal-space projection (SSP), which was accounted for during source reconstruction (Uusitalo and Ilmoniemi, 1997). The continuous magnetic time series was divided into epochs of 5.8 s duration, with 0.0 s defined as movement onset (i.e., first button press; −2.0 s to 3.8 s, 0.0 s = movement onset, −1.8 to −1.2 s baseline). Only correct trials were used for analysis. Epochs containing artifacts were rejected based on a fixed threshold method, supplemented with visual inspection. Following artifact rejection, the average number of trials was initially 118.39 (SD: 7.46) for youth, 131.44 (SD: 5.97) for young adults, and 130.24 (SD: 5.91) for older adults. However, this difference was significant between groups, F(1,48) = 20.061, p < 0.001, such that youth had significantly less correct, artifact-free trials than young, t(32) = 5.298, p < 0.001, and older adults, t(33) = 5.309, p < 0.001. Such differences in the number of accepted trials can cause signal-to-noise ratios to be different between groups, and thus bias later stages of the analysis (e.g., beamformer images). To ameliorate this concern, the number of accepted trials in the young and older adult groups were reduced, such that there was no longer a difference in the number of correct trials used in the subsequent analyses between groups, F(2,48) = 0.378, p = 0.687. Artifact-free epochs were then transformed into the time-frequency domain using complex demodulation (resolution: 2.0 Hz, 25 ms) and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized by dividing the power value of each time-frequency bin by the respective bin’s baseline power.
The specific time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two stage procedure was followed to control for Type 1 error. In the first stage, one-sample t-tests were conducted on each data point and the output spectrogram of t-values was thresholded at p < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also below the (p < 0.05) threshold, and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values and the significance level of the observed clusters (from stage one) was tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, at least 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, time-frequency windows that corresponded to events of a priori interest (e.g., the peri-movement beta ERD) and contained a statistically significant oscillatory event across all participants were subjected to the beamforming analysis (i.e., −0.2 to 0.4 s, 16–26 Hz, movement onset = 0.0 s).
2.5. MEG imaging & virtual sensor extraction
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Gross et al., 2001; Hillebrand et al., 2005; Van Veen et al., 1997). MEG pre-processing and imaging used the Brain Electrical Source Analysis (BESA version 6.1) software. Normalized source power was computed for the selected time-frequency bands over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. Beamformer images per time-frequency window of interest were then averaged across participants in each group individually, and coordinates corresponding to the peak responses were identified. We then extracted virtual sensors for the peak voxel of these responses, which corresponded to the left and right precentral gyri. To create the virtual sensors, we applied the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which yielded two time series for each coordinate in source space and we used the time series with the maximal response for our analyses (Gross et al., 2001). Note that this virtual sensor extraction was done per participant, once the coordinates of interest (i.e., one per cluster) were known. Virtual sensors were extracted from the eyes-closed rest data using the same coordinates and computational method. Once these virtual sensors were extracted, absolute spontaneous beta activity values (during the eyes-closed rest recording), and absolute and relative (to baseline) beta activity values during movement were computed and subject to statistical analyses.
3. Results
A total of 18 healthy youth (mean age: 11.33 (SD: 1.61) years, range: 9–14 years), 16 healthy younger adults (mean age: 28.31 (SD: 5.44) years, range 20–42 years) and 17 healthy older adults (mean age: 65.41 (SD: 7.09) years, range 54–75 years) were able to successfully complete the motor task. One-way ANOVAs probing group differences in accuracy, reaction time, and movement duration were each significant (accuracy: F(2,48) = 11.006, p < 0.001; reaction time: F(2,48) = 13.633, p < 0.001; movement duration: F(2,48) = 10.600, p < 0.001). Follow-up t-tests showed no significant difference in accuracy between young and older adults, t(31) = 0.237, p = 0.814, but youth were significantly less accurate than both young, t(32) = 3.467, p = 0.002, and older adults, t(33) = 3.909, p < 0.001. There was no difference in reaction time between young and older adults, t(31) = 0.712, p = 0.482. However, like accuracy, youth had significantly slower reaction time than young, t(32) = 4.358, p < 0.001, and older adults, t(33) = 3.401, p = 0.002. Finally, there was a difference in movement duration between young and older adults, t(31) = 4.945, p < 0.001, and younger adults and youth, t(32) = 3.537, p = 0.001, with the older adults and youth both executing sequences significantly slower than young adults. Youth and older adults did not significantly differ in movement duration, t(33) = 1.250, p = 0.220. Behavioral results are shown in Fig. 3. To control for behavioral differences between groups, MEG data analyses were synced with movement onset in each trial and all analyses were focused on the time window preceding and during the early stages of movement execution.
Fig. 3.
Motor task behavioral results. Significant differences in accuracy (percentage correct), reaction time (time between cue to move and first movement), and movement duration (time to complete the tapping sequence) between groups are denoted with an asterisk. See legend for color descriptions. Error bars denote the standard error of the mean (SEM). * = p < 0.05.
3.1. MEG sensor-level results
Sensor-level time-frequency spectrograms were statistically examined using nonparametric permutation testing to derive the precise time-frequency bins for follow up beamforming analyses. The results showed significant beta ERD in gradiometers near the left and right sensorimotor cortices in each group, which extended from approximately 0.5 s before movement onset until about 0.9 s after movement onset in the 16–26 Hz range (0.0 s = movement onset; p < 0.001; corrected). A significant resynchronization (i.e., PMBR) in the same 16–26 Hz band was detected during the 1.1–2.5 s time window (0.0 s = movement onset) in roughly the same gradiometers (p < 0.001; corrected). The time-frequency windows for the beta ERD and PMBR are in broad agreement with many previous studies (Cheyne et al., 2006; Engel and Fries, 2010; Fry et al., 2016; Gaetz et al., 2011, Gaetz et al., 2010; Heinrichs-Graham et al., 2017a; Heinrichs-Graham and Wilson, 2015, Heinrichs-Graham and Wilson, 2016; Heinrichs-Graham et al., 2014b; Houdayer et al., 2006; Jurkiewicz et al., 2006; Kaiser et al., 2001; Parkes et al., 2006; Pfurtscheller and Lopes da Silva, 1999; Reyns et al., 2008; Salenius et al., 1997; Salmelin et al., 1995; Tzagarakis et al., 2010; Wilson et al., 2014, Wilson et al., 2010, Wilson et al., 2011).
3.2. Source imaging & voxel time series results
The time window corresponding to the maximum beta ERD response (-0.2 to 0.4 s), and a window of equal bandwidth and duration from the baseline period (−1.8 to −1.2 s), was imaged using a linearly-constrained minimum variance beamformer to derive the spatial location of significant beta ERD activity for subsequent virtual-sensor analysis. We then extracted virtual sensors (voxel time series) for the peak voxel of these responses. Peak peri-movement beta ERD responses were located bilaterally within the precentral gyri in each group, and are shown with group-averaged virtual sensor spectrograms in Fig. 4.
Fig. 4.
Identification of peri-movement beta ERD and peak voxel extraction. Group mean beamformer images (pseudo-t; see color bar) of beta activity prior to and during movement (−0.2 to 0.4 s, 16–26 Hz) for each age group are shown in the top panel, with the peak voxel locations used for the virtual sensor analysis identified with a yellow dot. Note that a different pseudo-t scale is used in each group. Time-frequency spectrograms of the peak voxel time series are shown on the bottom panel. Time (in s) is denoted on the x-axis, with 0.0 s defined as movement onset. Frequency (in Hz) is shown on the y-axis. All signal power data (bottom panel) is expressed as the percent difference from baseline, with the color legend shown to the right. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.1. Spontaneous beta power
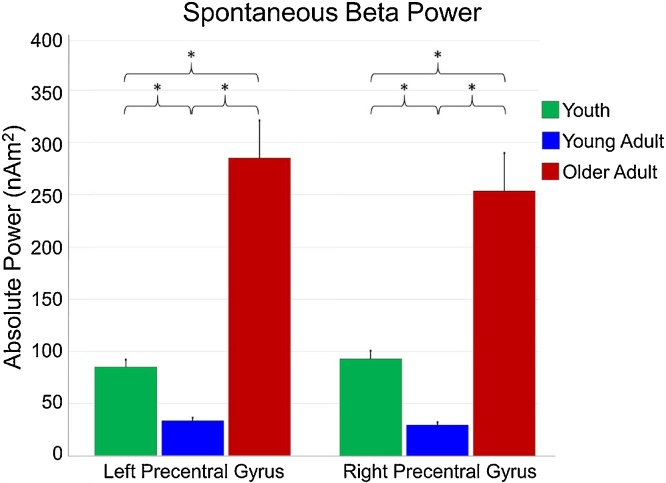
Analysis of beta power during eyes-closed rest was compared using a mixed-model ANOVA of hemisphere (left, right) as a within-subjects factor and group (youth, young adults, older adults) as a between-subjects factor. One youth participant’s data was excluded, as it was over 3 SD above the group mean. There was a significant effect of group, F(2,47) = 32.523, p < 0.001, as well as a significant hemisphere-by-group interaction, F(2,47) = 4.115, p = 0.023. There was no significant effect of hemisphere, F(1,47) = 2.401, p = 0.128. Follow-up t-tests showed that youth had significantly greater spontaneous beta power than young adults in the left, t(31) = 6.258, p < 0.001 and right precentral gyri, t(31) = 7.248, p < 0.001. On the contrary, youth had significantly less beta power than older adults in the left, t(32) = 5.329, p < 0.001, and right precentral gyri, t(32) = 4.222 p < 0.001. Young adults also showed diminished spontaneous beta power compared to older adults in the left, t(31) = 6.602, p < 0.001, and right precentral gyri, t(31) = 5.823, p < 0.001. These results are shown in Fig. 5 and Table 1.
Fig. 5.
Spontaneous beta power in the motor cortices. Beta power during eyes-closed rest was extracted from the voxels of the left and right precentral gyri that were identified in the motor task. Average power (in nAm2) is shown on the y-axis, while region is identified on the x-axis. Youth are shown in green, young adults are shown in blue, and older adults are shown in red. As shown, spontaneous beta activity was much stronger in older adults compared to youth and young adults. Youth also exhibited greater activity than young adults. Error bars denote SEM. *p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Independent t-tests between Neurophysiological Measures and Age Group.
| Response | Test measures | Direction | t | p |
|---|---|---|---|---|
| Baseline-relative beta power during movement | Total (L + R): OA and YA | OA > YA | 5.242 | < 0.001 |
| Total (L + R): OA and youth | OA > youth | 8.770 | < 0.001 | |
| Total (L + R): YA and youth | n.s. | 1.210 | 0.235 | |
| Absolute beta during movement | L precentral: OA and YA | OA > YA | 8.255 | < 0.001 |
| L precentral: OA and youth | OA > youth | 5.233 | < 0.001 | |
| L precentral: YA and youth | YA < youth | 5.836 | < 0.001 | |
| R precentral: OA and YA | OA > YA | 6.570 | < 0.001 | |
| R precentral: OA and youth | OA > youth | 3.754 | 0.001 | |
| R precentral: YA and youth | YA < youth | 7.110 | < 0.001 | |
| Spontaneous beta power | L precentral: OA and YA | OA > YA | 6.602 | < 0.001 |
| L precentral: OA and youth | OA > youth | 5.329 | < 0.001 | |
| L precentral: YA and youth | YA < youth | 6.258 | < 0.001 | |
| R precentral: OA and YA | OA > YA | 5.823 | < 0.001 | |
| R precentral: OA and youth | OA > youth | 4.222 | < 0.001 | |
| R precentral: YA and youth | YA < youth | 7.248 | < 0.001 | |
Notes: OA = older adults; YA = young adults.
For the peri-movement beta ERD, a stronger response means more negative (relative to baseline).
Beta ERD comparisons were made across hemisphere, as there was no hemisphere-by-group interaction.
In order to ensure that these baseline levels during the movement task were not artificially high due to unequal contamination by the PMBR in any group or hemisphere, we compared baseline beta power with spontaneous beta power using a mixed-model ANOVA, with task (eyes-closed rest, motor baseline) and hemisphere (left, right) as within subjects factors, and group as a between-subjects factor. This analysis can be found in the Supplementary Material (S1). In short, beta power at rest and baseline beta power during the movement task were not significantly different; thus, we are confident that differences in baseline power between groups during the task were not due to contamination by the PMBR.
3.2.2. Baseline-relative beta power during movement
Group differences in the mean peri-movement beta ERD power during the −0.2 to 0.4 s time period were assessed using the virtual sensor data from the left and right precentral gyri, and a mixed-model ANOVA with hemisphere (left, right) as a within-subjects factor, and group (youth, young adult, older adult) as a between-subjects factor. There was a significant main effect of group, F(2,48) = 29.306, p < 0.001, and hemisphere, F(1,48) = 9.234, p = 0.004. There was no significant hemisphere-by-group interaction, F(2,48) = 0.186, p = 0.831. Follow-up testing of the hemisphere effect showed greater beta ERD power (i.e., more negative relative to baseline) in the left compared to the right precentral gyrus across participants, t(50) = 3.104, p = 0.003, which was expected given that the task was performed with the right hand. Follow-up testing of the group effect revealed that older adults had significantly stronger baseline-relative beta ERD power in the left and right precentral gyri compared to younger adults, t(31) = 5.242, p < 0.001, and youth, t(33) = 8.770, p < 0.001. There was no significant difference in beta ERD power between youth and younger adults, t(32) = 1.210, p = 0.235 (Fig. 6; Table 1).
Fig. 6.
Top: Absolute and relative temporal evolution of the beta ERD response. Voxel time series were extracted from the peak voxels of the left precentral gyrus (top panel) and right precentral gyrus (bottom panel) to more precisely examine the dynamics of the beta response in youth (green line), young adults (blue line) and older adults (red line). Time (in s, movement onset = 0.0 s) is denoted on the x-axis, while power is shown on the y-axis. Note the difference in the scales of the y-axes between the left and right precentral gyri, which is sensible given that movements were performed with the right hand and thus, greater activity was found in the left (contralateral) motor cortex. The left panel shows each response as percentage relative to baseline (i.e., the ERD), while the right panel shows the absolute power (in nAm2). Shaded colored areas around each line denote the standard error of the mean (SEM). Shaded gray boxes denote time bins used for analysis of the movement period (-0.2 to 0.4 s). Bottom: Curve estimation analysis between beta activity and age. Spontaneous beta power (in nAm2) is shown in the left column, peri-movement beta ERD power (in percentage relative to baseline) is shown in the center column, and absolute beta power during movement is shown on the right column for the left (top panel) and right precentral gyri (bottom panel). Age (in years) is denoted on the x axes. Colors indicate age group (see legend). Curve estimation analysis indicated that there was a quadratic relationship between age and spontaneous beta power bilaterally, as well as age and absolute beta power during movement bilaterally, while there was a negative linear relationship between relative beta ERD power and age bilaterally. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2.3. Absolute beta power during movement
Absolute beta power during the same −0.2 to 0.4 s period was also extracted from the left and right precentral gyri, and group differences were assessed using another mixed model ANOVA with the same factors (hemisphere and group). This analysis revealed a significant effect of group, F(2,48) = 39.917, p < 0.001, as well as a significant hemisphere-by-group interaction, F(2,48) = 4.260, p = 0.020. There was no significant effect of hemisphere, F(1,48) = 0.382, p = 0.539. Follow-up t-tests showed significant differences in absolute beta power during this time period between all groups in the left precentral gyrus, youth vs. young adults: t(32) = 5.836, p < 0.001; youth vs. older adults: t(33) = 5.233, p < 0.001; young vs. older adults: t(31) = 8.255, p < 0.001. The same was true for the right precentral gyrus, youth vs. young adults: t(32) = 7.110, p < 0.001; youth vs. older adults: t(33) = 3.754, p = 0.001; young vs. older adults: t(31) = 6.570, p < 0.001 (Fig. 6; Table 1).
3.3. Relationships between measures
A curve estimation analysis was performed to determine the nature of the relationship between age and physiology. Briefly, while differences between groups were found on almost every beta metric, a curve estimation analysis provides a measure of the best-fit line from data across the lifespan. In other words, it statistically confirms and/or clarifies the trajectory of age-related differences in beta activity using age as a continuous variable. The curve estimation analysis between spontaneous beta power in the left precentral gyrus and age determined that this relationship was quadratic in nature, F(2,47) = 27.234, p < 0.001, r2 = 0.537, and a similar quadratic relationship was found between age and spontaneous beta power in the right precentral gyrus, F(2,47) = 18.862, p < 0.001, r2 = 0.445. Quadratic relationships were also found between absolute beta power during movement and age in the left and right precentral gyri, left: F(2,48) = 38.106, p < 0.001, r2 = 0.614; right: F(2,48) = 35.367, p < 0.001, r2 = 0.596. In contrast, curve estimation analysis between baseline-relative beta ERD amplitude and age was found to be negative and linear in the left, F(1,49) = 44.689, p < 0.001, r2 = 0.477, and right precentral gyrus, F(1,49) = 50.295, p < 0.001, r2 = 0.507, such that with increased age, there was a stronger (more negative relative to baseline) beta ERD response bilaterally (Fig. 6).
Next, correlations between neurophysiological and behavioral measures were performed, controlling for age. All p-values were corrected for multiple comparisons using the false-discovery rate (Benjamini and Hochberg, 1995). There was a significant relationship between movement duration and absolute beta power during movement in the left precentral gyrus, r(47) = 0.415, p = 0.012, as well as the right precentral gyrus, r(47) = 0.429, p = 0.012, such that the greater the absolute beta power during movement, the greater the movement duration, regardless of age. There was also a significant relationship between reaction time and absolute beta power during movement in the right precentral gyrus, r(47) = 0.413, p = 0.012. Finally, similar to our previous work (Heinrichs-Graham and Wilson, 2016), we found a significant linear relationship between spontaneous beta power and baseline-relative beta ERD power in the left precentral gyrus, r(47) = −0.406, p = 0.014, as well as the right precentral gyrus, r(47) = −0.476, p = 0.008, controlling for age. No other correlations were significant.
Given the unique patterns of spontaneous and baseline-relative beta power and age, coupled with the significant relationship between absolute beta power during movement, we hypothesized that movement performance may be governed by both spontaneous beta levels in the motor cortices, and the capacity of the motor cortex to compensate for differences in spontaneous beta levels by desynchronizing to a specific threshold. In order to test this hypothesis, we ran two, two-stage stepwise hierarchical regressions on the left and right precentral gyri individually, using movement duration as the dependent variable and age as a regressor at the first stage in both regressions, then adding left or right spontaneous beta power and baseline-relative beta ERD as regressors in the second stage, respectively. Interestingly, we found that left precentral spontaneous beta activity and beta ERD, together but not individually, significantly predicted movement duration, above and beyond the effects of age. This analysis can be found in the Supplementary Information (S2).
4. Discussion
The current study examined how the relationship between spontaneous beta levels and movement-related beta oscillatory activity was modulated by age in healthy participants aged 9–75 years. We found significant differences in spontaneous beta and movement-related beta oscillations between the three age groups. Interestingly, distinct patterns emerged depending on whether the analysis focused on absolute or baseline-relative beta activity during movement. Curve estimation analyses confirmed a quadratic relationship between spontaneous beta activity and age, such that spontaneous beta power in the motor cortices initially decreased from youth to early adulthood, and then drastically increased from early to late adulthood. The same pattern emerged between absolute beta activity during movement and age. In contrast, there was a linear decrease in baseline-relative beta activity during movement (i.e., stronger beta ERD) as a function of age. Moreover, we found that absolute beta power during movement uniquely correlated with movement duration, such that the higher the absolute beta power before and during movement, the longer the movement duration, controlling for age. Below, we discuss the implications of these results for understanding the functional roles and unique relationships between measures of beta oscillatory activity during motor performance, especially across the lifespan.
Perhaps our most surprising results were the differences in the lifespan trajectories of spontaneous and movement-related beta oscillatory activity, where spontaneous (resting) beta showed a quadratic relationship with age, while baseline-relative beta ERD power showed a linear relationship. We suggest that the quadratic relationship between spontaneous power and age may reflect different aspects of brain maturation. Basically, cortical thickness studies have shown that thinning in the motor cortices starts to taper-off between 9 and 10 years old (Shaw et al., 2008), with some research estimating that this process asymptotes at around 14 years old (Vandekar et al., 2015). During this time, spontaneous beta activity may also begin to become more “optimized,” such that the motor cortices become less active at rest, perhaps indicative of greater neuronal efficiency. During early adulthood, a time of optimal brain health and behavior, this spontaneous activity was at its minimum. Finally, during late adulthood, there is an decreased efficiency of neural circuits (for a review, see Antonenko and Floel (2014)), and this may result in an increase in spontaneous activity. Unfortunately, we can only speculate on the cellular physiology and micro-structural underpinnings of this pattern of motor activity, and future studies in animal models are likely needed to understand the basic mechanisms. As mentioned previously, prior work has shown a linear increase in beta ERD responses during movement as a function of age, with children exhibiting very little relative deviation from baseline levels compared to young adults (Gaetz et al., 2010), and with young adults showing a fraction of the beta suppression observed in older adults (Heinrichs-Graham and Wilson, 2016; Rossiter et al., 2014). However, this pattern of movement-related beta ERD has always been computed relative to baseline levels. Thus, the quadratic relationship that we found between spontaneous beta power and age complicates any interpretation of baseline-corrected beta ERD values between the age groups in this and other studies.
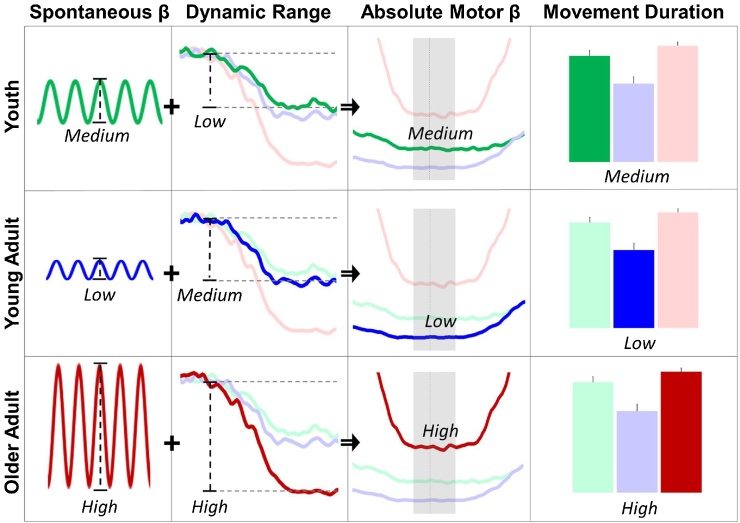
One interpretation of this distinct pattern between spontaneous and movement-related beta trajectories is that with increased age, there is an increased dynamic range of beta activity in the primary motor cortices during movement, which may be beneficial in the presence of increased, and potentially “sub-optimal” spontaneous beta levels. A schematic of this hypothesis is shown in Fig. 7 and detailed below. Basically, we posit that an elevation in spontaneous beta activity, coupled with reduced beta suppression, is related to a gross reduction in motor performance. This pattern of physiology and behavior is demonstrated in the youth data of the current study, who had significantly greater spontaneous beta activity compared to young adults, as well as less suppression of beta during movement. These participants also had significantly lower accuracy, longer reaction times, and longer movement durations compared to the young adults. During early adulthood, a reduction in spontaneous beta power coupled with increased beta suppression during movement (i.e., stronger ERD) is observed, which provides more ideal movement-related beta power levels and results in optimal motor performance, a pattern evidenced in the current study. In older adulthood, there continued to be greater beta suppression (i.e., stronger ERD), but the increase in spontaneous beta power was larger than this increased beta suppression, which resulted in elevated absolute beta power levels during movement in these participants relative to younger adults. This was coupled with in increased movement duration, although accuracy and reaction time did not differ from that of younger adults. While speculative, this hypothesis is supported by the overall pattern of movement-related and spontaneous beta levels within and between each group, as well as the fact that absolute beta power during movement was the only motor-related physiological measure to significantly correlate with movement duration in the current study, controlling for age. However, it is possible that other brain regions outside the motor cortex also mediated this relationship. In particular, youth and older adults may have reduced executive functioning ability compared to young adults (due to, for example, immature executive functioning or aging), and differences in domain-general regions may impact motor-related oscillatory activity downstream. Further investigation is needed to clarify the whole-brain dynamics serving complex motor selection throughout the lifespan.
Fig. 7.
Schematic representation of the relationship between spontaneous beta, movement-related beta oscillations, and motor performance. Dynamics of beta activity in the motor cortices (i.e., spontaneous beta, first column; dynamic range, second column; absolute beta during movement, third column) and motor performance (movement duration, fourth column) are shown for youth (top row), young adults (middle row), and older adults (bottom row). Basically, youth exhibit slightly elevated spontaneous beta, coupled with a small dynamic range of beta power during movement, which amounts to an absolute beta level that is higher than that of young adults, but lower than older adults. This results in a movement duration that is significantly higher than young adults (fourth column). Young adults exhibit low spontaneous beta and a moderate dynamic range during movement, which results in the lowest beta power during movement, and subsequent superior movement duration performance compared to youth and older adults. Finally, older adults exhibit significantly higher spontaneous beta, and while they also exhibit the largest dynamic range during movement, it is not proportional to the elevation of spontaneous levels, and results in significantly higher beta power during movement. Thus, older adults exhibit the longest movement duration of the three age groups. We propose that there is a unique combination of both optimal spontaneous beta power and dynamic range during movement which, taken together, results in optimal motor performance.
The quadratic relationship between spontaneous beta activity and age falls in line with at least some structural data, which shows that cortical gray matter most sharply decreases from childhood to adolescence, before asymptoting during early adulthood and then beginning to potentially increase again in late adulthood (Sowell et al., 2003). However, other studies have shown that gray matter volume continues to decrease as a function of age in the precentral gyri (Pfefferbaum et al., 2013). Thus, the trajectory of anatomical changes in these regions remains unclear, and very few structural studies have included participants throughout the lifespan. Previous functional studies of healthy aging (Heinrichs-Graham and Wilson, 2016; Rossiter et al., 2014) have suggested that the increase in beta power as a function of age, coupled with stronger beta suppression during movement, is due to increased γ-aminobutryic acid (GABA) transmission. The notion that GABA is important for modulating motor-related oscillatory activity is supported by a wealth of literature (Gaetz et al., 2011; Hall et al., 2011; Jensen et al., 2005; Muthukumaraswamy et al., 2013), which has generally associated higher GABA levels with increases in spontaneous sensorimotor beta power, as well as elevated motor-related oscillatory activity (Gaetz et al., 2011; Hall et al., 2011; Muthukumaraswamy et al., 2013). While this may be relevant to the maturation of the brain in later life, there seems to be a different mechanism at play in early development. Specifically, we found an elevation of spontaneous beta activity in the motor cortices in youth relative to young adults, but this was coupled with a marginal decrease (not increase), in beta ERD levels, which does not agree with prior work in adults (Heinrichs-Graham and Wilson, 2016; Rossiter et al., 2014). However, the GABA system is still under development during childhood and adolescence (Kilb, 2012), and this may differentially impact neuronal oscillatory activity. Future studies should directly investigate the distinct roles of GABA and glutamate, as well as other potentially relevant neurotransmitter systems such as dopamine, on motor-related beta dynamics.
In sum, the current study investigated the dynamics of movement-related oscillatory activity, at rest and during movement, throughout the lifespan. We found differential trajectories between absolute and baseline-relative measures of beta power in the primary motor cortices. Spontaneous beta power and beta power during movement showed a quadratic relationship with age, while baseline-relative beta power during movement was linearly related to age. Interestingly, only absolute beta power during movement significantly correlated with movement duration, and a follow-up hierarchical regression (Supplementary Material S2) suggested that spontaneous beta power and baseline-relative beta ERD power, together, significantly predicted movement duration, beyond the effects of age. Future studies should directly investigate the mechanistic roles of neurotransmitters such as GABA, glutamate, and dopamine on these movement-related dynamics. Further, it is possible that circadian rhythms are perturbed in older adults, and future work should clarify the subsequent impact on neural behavior. Nonetheless, this study was the first to show distinct trajectories of movement-related beta dynamics throughout the lifespan, and suggests that the absolute level of beta power during movement is crucial to optimal performance, and directly related to spontaneous beta levels in the motor cortices. This complex interplay between different measures of beta activity in the motor cortex holds significant promise in advancing our understanding of cortical oscillations in health and disease, especially in those with movement disorders such as Parkinson’s disease and cerebral palsy (Heinrichs-Graham et al., 2014a; Heinrichs-Graham et al., 2017b; Kurz et al., 2014).
Acknowledgements
This work was supported by the National Institutes of Health (R01 MH103220 to TWW), the National Science Foundation (#1539067 to Y-PW, JMS, VDC, and TWW), and the Shoemaker Prize from the University of Nebraska Foundation to TWW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no competing financial or non-financial interests.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2018.02.013.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Antonenko D., Floel A. Healthy aging by staying selectively connected: a mini-review. Gerontology. 2014;60:3–9. doi: 10.1159/000354376. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B. 1995;57:289–300. [Google Scholar]
- Cheyne D., Bakhtazad L., Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum. Brain Mapp. 2006;27:213–229. doi: 10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D., Bells S., Ferrari P., Gaetz W., Bostan A.C. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 2008;42:332–342. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- Doyle L.M., Yarrow K., Brown P. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin. Neurophysiol. 2005;116:1879–1888. doi: 10.1016/j.clinph.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Engel A.K., Fries P. Beta-band oscillations?signalling the status quo? Curr. Opin. Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Ernst M.D. Permutation methods: a basis for exact inference. Stat. Sci. 2004;19:676–685. [Google Scholar]
- Fry A., Mullinger K.J., O'Neill G.C., Barratt E.L., Morris P.G., Bauer M., Folland J.P., Brookes M.J. Modulation of post-movement beta rebound by contraction force and rate of force development. Hum. Brain Mapp. 2016;37:2493–2511. doi: 10.1002/hbm.23189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W., Cheyne D. Localization of sensorimotor cortical rhythms induced by tactile stimulation using spatially filtered MEG. Neuroimage. 2006;30:899–908. doi: 10.1016/j.neuroimage.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Gaetz W., Macdonald M., Cheyne D., Snead O.C. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. Neuroimage. 2010;51:792–807. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Gaetz W., Edgar J.C., Wang D.J., Roberts T.P. Relating MEG measured motor cortical oscillations to resting gamma-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55:616–621. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-'t-Jong T., Oostenveld R., Jensen O., Medendorp W.P., Praamstra P. Competitive interactions in sensorimotor cortex: oscillations express separation between alternative movement targets. J. Neurophysiol. 2014;112:224–232. doi: 10.1152/jn.00127.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Kujala J., Hamalainen M., Timmermann L., Schnitzler A., Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S.D., Stanford I.M., Yamawaki N., McAllister C.J., Ronnqvist K.C., Woodhall G.L., Furlong P.L. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage. 2011;56:1506–1510. doi: 10.1016/j.neuroimage.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Wilson T.W. Coding complexity in the human motor circuit. Hum. Brain Mapp. 2015;36:5155–5167. doi: 10.1002/hbm.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Wilson T.W. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage. 2016;134:514–521. doi: 10.1016/j.neuroimage.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Kurz M.J., Becker K.M., Santamaria P.M., Gendelman H.E., Wilson T.W. Hypersynchrony despite pathologically reduced beta oscillations in patients with Parkinson's disease: a pharmaco-magnetoencephalography study. J. Neurophysiol. 2014;112:1739–1747. doi: 10.1152/jn.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Wilson T.W., Santamaria P.M., Heithoff S.K., Torres-Russotto D., Hutter-Saunders J.A., Estes K.A., Meza J.L., Mosley R.L. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cereb. Cortex. 2014;24:2669–2678. doi: 10.1093/cercor/bht121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Arpin D.J., Wilson T.W. Cue-related temporal factors modulate movement-related beta oscillatory activity in the human motor circuit. J. Cogn. Neurosci. 2016;28:1039–1051. doi: 10.1162/jocn_a_00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Kurz M.J., Gehringer J.L., Wilson T.W. The functional role of post-movement beta oscillations in movement termination. Brain Struct. Funct. 2017;222:3075–3086. doi: 10.1007/s00429-017-1387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E., Santamaria P.M., Gendelman H.E., Wilson T.W. The cortical signature of symptom laterality in Parkinson's disease. Neuroimage Clin. 2017;14:433–440. doi: 10.1016/j.nicl.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A., Singh K.D., Holliday I.E., Furlong P.L., Barnes G.R. A new approach to neuroimaging with magnetoencephalography. Hum. Brain Mapp. 2005;25:199–211. doi: 10.1002/hbm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdayer E., Labyt E., Cassim F., Bourriez J.L., Derambure P. Relationship between event-related beta synchronization and afferent inputs: analysis of finger movement and peripheral nerve stimulations. Clin. Neurophysiol. 2006;117:628–636. doi: 10.1016/j.clinph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Jensen O., Goel P., Kopell N., Pohja M., Hari R., Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 2005;26:347–355. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz M.T., Gaetz W.C., Bostan A.C., Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 2006;32:1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kaiser J., Birbaumer N., Lutzenberger W. Event-related beta desynchronization indicates timing of response selection in a delayed-response paradigm in humans. Neurosci. Lett. 2001;312:149–152. doi: 10.1016/s0304-3940(01)02217-0. [DOI] [PubMed] [Google Scholar]
- Kilb W. Development of the GABAergic system from birth to adolescence. Neuroscientist. 2012;18:613–630. doi: 10.1177/1073858411422114. [DOI] [PubMed] [Google Scholar]
- Kurz M.J., Becker K.M., Heinrichs-Graham E., Wilson T.W. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev. Med. Child Neurol. 2014;56:1072–1077. doi: 10.1111/dmcn.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Myers J.F., Wilson S.J., Nutt D.J., Lingford-Hughes A., Singh K.D., Hamandi K. The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage. 2013;66:36–41. doi: 10.1016/j.neuroimage.2012.10.054. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D. Functional properties of human primary motor cortex gamma oscillations. J. Neurophysiol. 2010;104:2873–2885. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Parkes L.M., Bastiaansen M.C., Norris D.G. Combining EEG and fMRI to investigate the post-movement beta rebound. Neuroimage. 2006;29:685–696. doi: 10.1016/j.neuroimage.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Rohlfing T., Rosenbloom M.J., Chu W., Colrain I.M., Sullivan E.V. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. Neuroimage. 2013;65:176–193. doi: 10.1016/j.neuroimage.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G., Lopes da Silva F.H. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin. Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Reyns N., Houdayer E., Bourriez J.L., Blond S., Derambure P. Post-movement beta synchronization in subjects presenting with sensory deafferentation. Clin. Neurophysiol. 2008;119:1335–1345. doi: 10.1016/j.clinph.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Rossiter H.E., Davis E.M., Clark E.V., Boudrias M.H., Ward N.S. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage. 2014;91:360–365. doi: 10.1016/j.neuroimage.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salenius S., Schnitzler A., Salmelin R., Jousmaki V., Hari R. Modulation of human cortical rolandic rhythms during natural sensorimotor tasks. Neuroimage. 1997;5:221–228. doi: 10.1006/nimg.1997.0261. [DOI] [PubMed] [Google Scholar]
- Salmelin R., Hamalainen M., Kajola M., Hari R. Functional segregation of movement-related rhythmic activity in the human brain. Neuroimage. 1995;2:237–243. doi: 10.1006/nimg.1995.1031. [DOI] [PubMed] [Google Scholar]
- Scheinost D., Finn E.S., Tokoglu F., Shen X., Papademetris X., Hampson M., Constable R.T. Sex differences in normal age trajectories of functional brain networks. Hum. Brain Mapp. 2015;36:1524–1535. doi: 10.1002/hbm.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M.E., Sachdev P.S., Anstey K.J., Cherbuin N. Age-related cortical thinning in cognitively healthy individuals in their 60s: the PATH Through Life study. Neurobiol. Aging. 2016;39:202–209. doi: 10.1016/j.neurobiolaging.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Thompson P.M., Welcome S.E., Henkenius A.L., Toga A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Taulu S., Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S., Simola J., Kajola M. Applications of the signal space separation method (SSS) IEEE Trans. Signal Process. 2005;53:3359–3372. [Google Scholar]
- Tzagarakis C., Ince N.F., Leuthold A.C., Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J. Neurosci. 2010;30:11270–11277. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo M.A., Ilmoniemi R.J. Signal-space projection method for separating MEG or EEG into components. Med. Biol. Eng. Comput. 1997;35:135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Van Veen B.D., van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44:867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Vandekar S.N., Shinohara R.T., Raznahan A., Roalf D.R., Ross M., DeLeo N., Ruparel K., Verma R., Wolf D.H., Gur R.C., Gur R.E., Satterthwaite T.D. Topologically dissociable patterns of development of the human cerebral cortex. J. Neurosci. 2015;35:599–609. doi: 10.1523/JNEUROSCI.3628-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.W., Slason E., Asherin R., Kronberg E., Reite M.L., Teale P.D., Rojas D.C. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010;73:75–84. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.W., Slason E., Asherin R., Kronberg E., Teale P.D., Reite M.L., Rojas D.C. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev. Neuropsychol. 2011;36:596–613. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T.W., Heinrichs-Graham E., Becker K.M. Circadian modulation of motor-related beta oscillatory responses. Neuroimage. 2014;102(Pt 2):531–539. doi: 10.1016/j.neuroimage.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.