Abstract
Streptococcus pneumoniae as a complex relationship with its obligate human host. On the one hand, the pneumococci are highly adapted commensals, and their main reservoir on the mucosal surface of the upper airways of carriers enables transmission. On the other hand, they can cause severe disease when bacterial and host factors allow them to invade essentially sterile sites, such as the middle ear spaces, lungs, bloodstream and meninges. Transmission, colonization and invasion depend on the remarkable ability of S. pneumoniae to evade or take advantage of the host inflammatory and immune responses. The different stages of pneumococcal carriage and disease have been investigated in detail in animal models and, more recently, in experimental human infection. Furthermore, widespread vaccination and the resulting immune pressure have shed light on pneumococcal population dynamics and pathogenesis. Here, we review the mechanistic insights provided by these studies on the multiple and varied interactions of the pneumococcus and its host.
Streptococcus pneumoniae (also known as pneumococcus) is a Gram-positive, extracellular, opportunistic pathogen that colonizes the mucosal surfaces of the human upper respiratory tract (URT). Up to 27–65% of children and <10% of adults are carriers of S. pneumoniae and carriage involves a commensal relationship between the bacterium and the host1,2. Local spread, aspiration or seeding to the bloodstream results in invasive inflammatory diseases3 (Fig. 1). S. pneumoniae is a leading bacterial cause of a wide range of infections, including otitis media, community-acquired pneumonia, sepsis and meningitis. As all of these diseases are ‘dead ends’ in the life cycle of the organism, the bacterial factors that cause invasive diseases must also be adaptive for colonization and/or transmission.
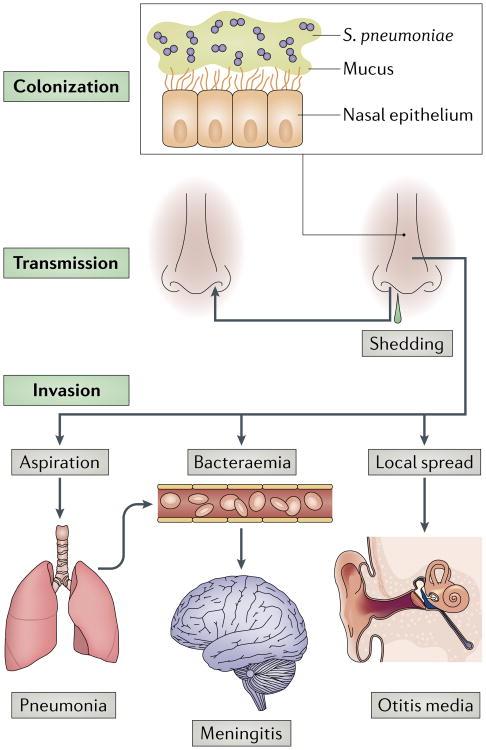
Fig. 1. The life cycle of Streptococcus pneumoniae and the pathogenesis of pneumococcal disease.
Streptococcus pneumoniae colonizes the mucosa of the upper respiratory tract (URT). This carriage is the prerequisite for both transmission to other individuals and invasive disease in the carrier. Carriers can shed S. pneumoniae in nasal secretions and thereby transmit the bacterium. Dissemination beyond its niche along the nasal epithelium, either by aspiration, bacteraemia or local spread, can lead to invasive diseases, such as pneumonia, meningitis and otitis media.
In 2017, the WHO included S. pneumoniae as one of 12 priority pathogens. The continued high burden of disease and rising rates of resistance to penicillin and other antibiotics have renewed interest in prevention. The widespread use of pneumococcal conjugate vaccines (PCVs) has reduced invasive disease of sero-types with the capsular polysaccharide (CPS) types that are included in the vaccine4 (Box 1). The remarkable capacity of S. pneumoniae to remodel its genome through the uptake and incorporation of exogenous DNA ( natural competence) from other pneumococci or closely related oral streptococci has facilitated the spread of antibiotic resistance and evasion of vaccine-induced immunity. The prominence of S. pneumoniae as a cause of disease is due to the combination of high carriage rates, its genetic adaptability and its ability to shift from a commensal to a pathogenic interaction with its host. In this Review, we discuss the bacterial, environmental and host factors that contribute to the different stages of pneumococcal disease.
Box 1. Streptococcus pneumoniae vaccination.
Streptococcus pneumoniae has a high genetic diversity, and certain lineages are particularly successful. an important source of strain-to-strain variation is the structure of the capsular polysaccharide (CPs), which is the major virulence determinant and immunodominant surface structure of S. pneumoniae. Currently, 98 immunologically and structurally distinct CPs types are recognized, but only a relatively small subset of these types is commonly found to cause carriage and disease. therefore, CPs-based vaccines target only a limited number of serotypes. when covalently conjugated to an immunogenic protein carrier, CPS is recognized as a T cell-dependent antigen, which stimulates a more effective humoral immune response (including immunoglobulin class switching, affinity maturation and memory) than polysaccharide-alone antigens, particularly in young children. since its introduction in 2000, the pneumococcal conjugate vaccine (PCv) has been highly effective in preventing invasive pneumococcal diseases. an unexpected benefit of the high levels of serotype-specific immunoglobulin G generated by PCv has been reduced rates of carriage in and transmission from immunized children, which also protects unimmunized populations (herd immunity)145. However, the protection elicited by PCv is incomplete, as current formulations contain only 10 to 13 of the 97 known CPs types146. A further issue is the rising prevalence of non-vaccine serotypes in carriage and disease (serotype replacement) as a consequence of the immune pressure from widespread use of PCv147,148. Current efforts to improve prevention through vaccination are directed at increasing the number of serotypes covered by PCv or adding conserved pneumococcal proteins that induce serotype-independent immunity.
Transmission of S. pneumoniae
Until recently, all that was known about pneumococcal contagion was that spread requires close contact with a carrier and/or carriers (especially young children), is more frequent during drier, colder months when airway secretions are more copious and is more likely to occur in conjunction with viral infections of the URT5–7. This general ignorance about transmission was a consequence of a lack of tractable animal models and an inability to study human-to-human transmission in sufficient detail. In 2010, airborne transmission among closely housed ferrets co-infected with influenza A virus (IAV) was described8. Another group modelled murine transmission from index pups colonized at 4 days of age to littermate contact pups in the setting of IAV co-infection9. Similar to human transmission, viral infection, close contact and younger age increased transmission. This infant mouse model has now enabled the study of the major steps during host-to-host spread, including exit from a colonized host (shedding), survival in the environment and acquisition by a new host.
Exit from the colonized host
IAV-induced inflammation stimulates both the expression of mucin glycoproteins and the flow of mucus10,11. There are more pneumococci in nasal secretions of pups with IAV co-infection (Fig. 2), and only young mice shed S. pneumoniae at levels permissive for transmission12. Moreover, levels of shedding correlate with the extent of URT inflammation in response to IAV infection. Toll-like receptor 2 (TLR2)-deficiency, which is associated with an increased viral load and, subsequently, greater inflammation, results in higher rates of transmission, and this effect is specific to the index mice12. Furthermore, the effect of IAV is recapitulated by intranasal treatment of the index mice with the TLR3 ligand polyiC13.
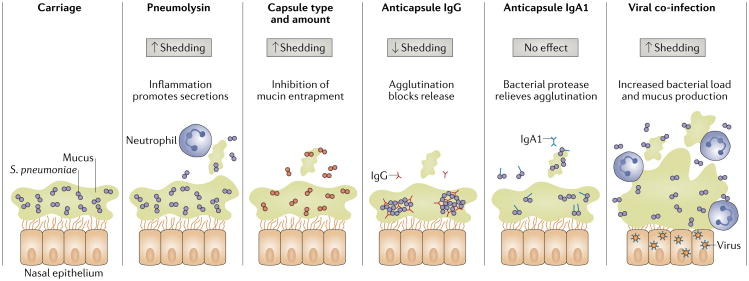
Fig. 2. bacterial and host factors affecting pneumococcal shedding from carriers.
Streptococcus pneumoniae is found predominantly in the mucus layer overlying the epithelial surface of the upper respiratory tract. Inflammation (indicated by the presence of neutrophils), which is induced by the pore-forming toxin pneumolysin or by co-infection with influenza virus or other respiratory viruses, stimulates secretions and increases shedding. By contrast, agglutinating antibodies such as anti-capsule immunoglobulin G (IgG) and IgA1 decrease shedding unless they are cleaved by the human IgA1-specific pneumococcal protease. Capsule type and amount also influence mucus association and numbers of shed bacteria.
The size of population bottlenecks in the infant mouse model during transmission was estimated by using marked isogenic bacterial strains13. In this study, all constructs colonized, shed and could be acquired in similar numbers by all pups. By contrast, after the index pup is simultaneously colonized with the marked mutants, in the majority of transmission events, only one of the mutants was successful. This tight population bottleneck during transmission would explain the need for large numbers of shed pneumococci for at least one to be successful in reaching a new host. Accordingly, increasing total shedding per cage by increasing the proportion of colonized index pups per cage to 50% made transmission to ∼30% of contacts possible without the need for IAV co-infection14.
During early childhood, rhinorrhoea is pronounced, and clinical surveys demonstrate a relationship between secretion volume and S. pneumoniae density15. In the infant mouse model, dampening inflammation by intranasal dexamethasone treatment or the use of Tlr2−/− index mice reduces shedding and transmission16. The single pneumococcal toxin, pneumolysin (Ply), has strong pro-inflammatory effects, and Ply-induced inflammation hastens clearance of bacteria from the URT17. Both a ply-knockout mutant and a point mutant, in which the toxin is unable to oligomerize to form pores after membrane insertion, reduced URT inflammation, shedding and the ability to transmit to littermates16. Additionally, intranasal administration of the purified toxin, but not the inactive toxoid (PdB), could complement the inflammation, shedding and transmission defect of the ply mutant. This is the first example of a pneumococcal factor that is specifically required for transmission. These findings with Ply also provide a link between pneumococcal virulence and transmission18, suggesting that factors such as Ply that contribute to the disease state by enhancing inflammation also promote the transmission of S. pneumoniae.
As epidemiological studies show that the prevalence of different serotypes is highly variable, the role of capsule type and amount on shedding and transmission was tested using isogenic serotype-switch and cps-promoter switch mutants19. Some serotype-switch mutants colonized at wild-type levels but were shed and transmitted poorly in infant mice. Mutants with lower expression of CPS and thinner capsules were also shed and transmitted poorly. The capsule layer shields underlying surface adhesins, and mutants with reduced shedding and transmission showed increased binding to URT mucins in an in vitro assay. Encapsulation, therefore, may facilitate shedding by allowing escape from the mucus that lines the airway surface, with a thicker capsule or capsule of certain serotypes being more effective.
Survival in the environment
The extent of airborne transmission (as demonstrated by the ferret studies) versus contact-dependent transmission (as shown by the infant mouse model) is unclear. A number of recent studies have examined factors that affect survival of S. pneumoniae outside the host. Transmission through secretions of carriers could involve direct person-to-person contact or spread involving bacteria on contaminated surfaces. As evidence of the latter, in the mouse model, the co-housed dam is not colonized but has large numbers of S. pneumoniae on her teats, and in-cage switch experiments can serve as a source of contagion9,16. S. pneumoniae can also be easily cultured from common objects, such as soft toys recently handled by colonized children20. Under ambient, nutrient-sufficient conditions, such as in ex vivo human saliva, pneumococci can survive for days21. Under nutrient-poor conditions, such as in airway surface fluid, bacterial expression of Ply increases ex vivo survival16. This effect can be explained by toxin dependent inflammation and, consequently, increased nutrients levels in secretions. Capsule expression from the cps locus increases survival in nutrient-poor environmental conditions, perhaps by providing a reserve of glycans22. Furthermore, pneumococci survive desiccation for many days, and biofilm bacteria retain viability in vitro better than planktonic bacteria20,23.
Acquisition by the new host
Given the importance of PCV in reducing transmission from immunized children, the infant mouse model has been used to explore the role of immunity in spread24. Pre-existing S. pneumoniae colonization of contact pups inhibits the acquisition of a new strain13. This bacterial interference could affect the frequency of co-colonizing strains. Passive immunization of contact pups with anti-capsular polysaccharide immunoglobulin G (IgG) is also sufficient to block acquisition, although this effect requires high levels of antibody and can be overcome by a large inoculum25. The protective activity of specific antibodies during acquisition is independent of Fc fragment-mediated effects but requires their agglutinating function, which could facilitate mechanical clearance by the mucociliary flow. However, S. pneumoniae evades clearance that is mediated by IgA1, the most abundant immunoglobulin on mucosal surfaces of the human URT26. The pneumococcal zinc metalloprotease ZmpA (also known as IgA1 protease), which cleaves the hinge region of human IgA1, eliminates the agglutinating activity of this immunoglobulin25. Thus, PCV likely is effective because it induces IgG, which is not sensitive to the protease, at levels high enough to reach the mucosal surface and block pneumococcal acquisition. In a model of experimental human colonization with S. pneumoniae, levels of CPS-specific memory B cells correlate with protection from acquisition27. Such memory B cells can quickly differentiate into antibody-secreting plasma cells following antigen exposure. Furthermore, the effectiveness of S. pneumoniae agglutination of airway secretions after PCV vaccination correlates with protection during experimental human colonization28. An additional effect of immunity demonstrated in the infant mouse model is a decrease in shedding by index pups24. Moreover, immunity in either the index or contact pups alone is sufficient to reduce rates of transmission, indicating that decreased shedding and protection from acquisition both contribute. These experiments were carried out with serotype-specific antibody. It is unclear whether immunity to other S. pneumoniae surface targets can block transmission. In this regard, immunization with Ply shows no effect on shedding and transmission, even though the toxin is required for spread between pups16. This result is not unexpected as Ply is not actively secreted, is not present on the cell surface and might be released only when pneumococci are lysed within the phagosome and therefore are not exposed to antibody29. When pneumococci are killed by lysozyme within the phagosome, the released Ply forms pores, which enable bacterial products to access the host cell cytosol and trigger the production of pro-inflammatory chemokines and cytokines30–32. In this manner, S. pneumoniae responds to an influx of professional phagocytes when it finds itself in a host that is no longer hospitable. Triggering inflammation and mucus secretions drives its transit to a new, more hospitable host.
Colonization by S. pneumoniae
Nasopharyngeal carriage is the source of S. pneumoniae spread between hosts and the first step towards invasive disease. Several bacterial factors are required for S. pneumoniae to colonize and persist on the mucosal surface at a density and duration that is sufficient for transmission to occur (Fig. 3). For example, S. pneumoniae expresses two enzymes, peptidoglycan-N-acetylglucosamine deacetylase (PgdA) and attenuator of drug resistance (Adr), that modify its peptidoglycan and render it resistant to the lytic effects of lysozyme, which is abundant on the mucosal surface of the URT33. The main features that facilitate colonization are adherence to host cells and tissues, subversion of mucosal innate and adaptive immunity, and evasion of clearance by mucociliary flow.
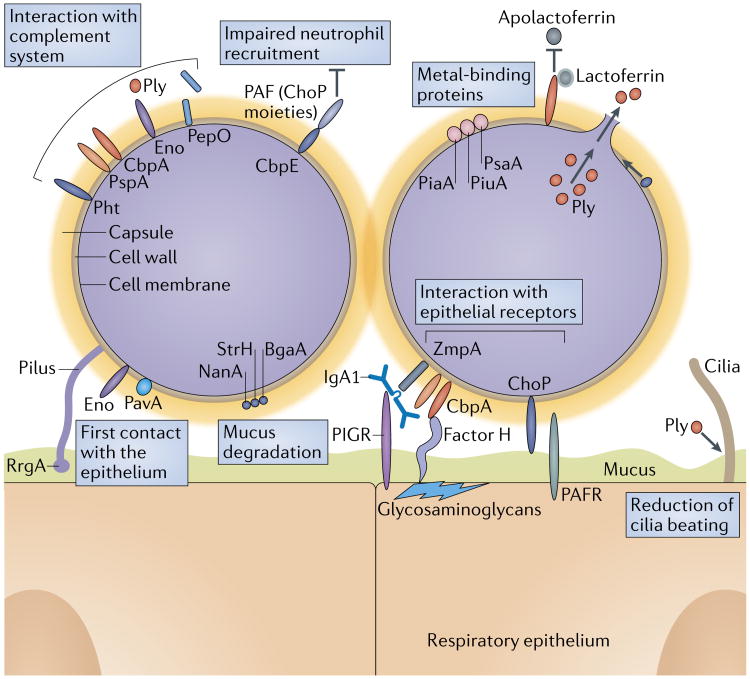
Fig. 3. Molecular mechanisms of pneumococcal colonization of host surfaces.
Key functions that enable Streptococcus pneumoniae colonization are: establishing the first contact with the epithelium and epithelial receptors, interaction with the complement system, mucus degradation, metal binding, impairment of neutrophil activity and the pro-inflammatory effects of the toxin pneumolysin (Ply). The pneumococcal enzymes Neuraminidase A (NanA), β-galactosidase (BgaA) and β-N-acetylglucosaminidase (StrH) degrade mucus and thereby inhibit mucociliary clearance. Furthermore, the LytA (autolysin)-facilitated release of Ply damages the epithelium and reduces ciliary beating. Negatively charged capsular polysaccharide (CPS) inhibits bacterial mucus entrapment. CPS and several pneumococcal proteins, including pneumococcal surface protein A (PspA), choline-binding protein A (CbpA), enolase (Eno) and pneumococcal histidine triad protein (Pht), directly and indirectly block complement deposition. PspA also binds to lactoferrin to acquire iron and blocks the antimicrobial effect of apolactoferrin. Endopeptidase (PepO), which is released from the pneumococcal surface, binds to C1q and thereby depletes complement components. Pneumococcal CbpE impairs neutrophil recruitment by degrading platelet-activating factor (PAF), a host-derived inflammatory phospholipid. CbpA interacts with factor H interactions to facilitate adherence and subsequent internalization of S. pneumoniae via cell glycosaminoglycans. CbpA also binds to polymeric immunoglobulin receptor (PIGR) to promote adherence. The zinc metalloprotease ZmpA (also known as immunoglobulin A1 protease) subverts mucosal humoral immunity by cleaving IgA1. Phosphorylcholine (ChoP) on teichoic acid mimics host PAF and allows binding to its receptor. Piliated strains express an ancillary pilus subunit tip adhesin called RrgA. Other S. pneumoniae adhesins include enolase (Eno) and adherence and virulence protein A (PavA). PAFR, platelet-activating factor receptor.
Adherence to the nasopharynx
The first defence that S. pneumoniae encounters in the nasopharynx is mucus entrapment. The glycocalyx overlying the URT epithelium is composed of gel-like mucin glycoproteins and contains antimicrobial peptides and immunoglobulins34. S. pneumoniae, like other residents of the URT, is found predominantly in and on this mucus layer35. Although the mucus layer keeps the bacteria away from the underlying cell surface, adherence to mucin glycans helps the bacteria to remain in the nasopharynx and provides a favourable niche and nutrients. On the other hand, CPSs, which are almost all negatively charged, repel the sialic acid-rich mucopolysaccha-rides in mucus36. By avoiding entrapment in the nasal mucus, S. pneumoniae might access and attach to the surface of epithelial cells. Much of our understanding of S. pneumoniae–host cell interactions comes primarily from models that use cultured human epithelial cells. S. pneumoniae uses several surface components for binding, but their relative importance in natural carriage has not been established. Examples of these adhesins are surface-located pneumococcal adherence and virulence protein A (PavA), PavB and enolase (Eno), all of which bind to the extracellular matrix proteins fibronectin and plasminogen37–39. Phosphorylcholine (ChoP) moieties on cell wall teichoic acid bind to the platelet-activating factor receptor (PAFR), and choline-binding protein A (CbpA; also known as PspC) binds the secretory component of the polymeric immunoglobulin receptor40,41. CbpA also binds the host proteins factor H and vitronectin. Other major classes of host cell receptors include carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM) and intercellular adhesion molecule 1 (ICAM1)42. S. pneumoniae increases expression of many of its epithelial surface receptors and thereby increases its adherence in response to inflammatory stimuli43. The surface-exposed lipoproteins foldase protein PrsA (also known as PpmA)44 and peptidyl-prolyl isomerase SlrA45 also contribute to adherence to epithelial cells. CbpL facilitates migration of S. pneumoniae from the nasopharynx to the lungs and blood46. S. pneumoniae encodes at least ten extracellular glycosidases, some of which have been shown to enhance adherence by modifying host glycoconjugates to reveal glycan receptors47. In addition, two of these surface glycosidases, Neuraminidase A (NanA) and the β-galactosidase BgaA, have lectin domains and seem to function as adhesins independently of their enzymatic ac tivities48,49. N-acety lglucosamine-β-(1,3)-galactose inhibits pneumococcal adherence to epithelial cells, and S. pneumoniae is one of many pathogens that bind to N-acetylglucosamine-β-(1,4)-galactose50,51. Thes e adhesive interactions with the epithelial surface may be needed for colonization but also comprise the initial step in the invasion process (see below).
Interactions with the nasopharyngeal flora
The success of S. pneumoniae as a colonizer requires interactions with the nasopharyngeal microbiota, which likely are extensive and complex. These interactions can either be cooperative or competitive52. For example, detection of Gram-negative peptidoglycan through the sensor nucleotide-binding oligomerization domain-containing protein 1 (NOD1) by neutrophils triggers killing of S. pneumoniae53. During experimental human colonization, increased diversity of the microbiota is associated with increased acquisition of S. pneumoniae following intranasal challenge54. S. pneumoniae colonization was also found to promote microbial heterogeneity in these studies. Similarly, during the first 2 years of life, S. pneumoniae colonization was associated with less stable microbiome profiles55. Co-colonizing pneumococci compete with one another through a diverse array of bacteriocins (pneumocins) and related peptides with antimicrobial activity56–58. Lysis of susceptible strains not only allows for predation but also provides a source of DNA for the adaptation of the predator.
In general, inflammatory conditions in the URT favour the presence of S. pneumoniae. A common and important example is infection with URT viruses. Nasal inflammation in response to infection with respiratory viruses, such as IAV, modulates the expression of pro-inflammatory chemokines, upregulates epithelial receptors used for S. pneumoniae adherence, compromises the integrity of the epithelium and provides a more nutrient-rich inflammatory milieu. Together, these effects of viral co-infection increase the susceptibility to acquisition and the density of colonizing S. pneumoniae59–61. Recent data from murine models and clinical studies have shown that the live attenuated influenza vaccine also increases the number of colonizing S. pneumoniae62–64. A higher pneumococcal density in the nasopharynx is likely to facilitate transmission and microaspiration to the lungs, thereby increasing the likelihood of progression to disease65.
Bacterial and host factors involved in clearance
Individual carriage episodes typically last for weeks to months. Through the use of a model for calculating the duration of carriage episodes from a longitudinal carriage study and combining these results with whole-genome sequence data, it was recently estimated that S. pneumoniae genomic variation accounts for 63% of the variation in carriage duration, whereas measured host traits (such as age and previous carriage) accounted for less than 5%. Serotype was found to have a major influence on carriage duration66. This pan-genome-wide association study also identified prophage sequences as having the greatest negative impact on carriage duration, independent of serotype.
One important characteristic that enables S. pneumoniae to successfully thrive in this competitive niche is its ability to evade and sometimes hijack host responses during colonization. In mouse models, acquisition of the organism leads to a mild acute inflammatory response in the URT that is ineffective at completely clearing the organism67. By contrast, preexisting inflammation is the most closely associated susceptibility factor in the human challenge model68. Many of the factors contributing to the eventual clearance of S. pneumoniae have been delineated. Studies in mice suggest that clearance requires TLR2-dependent responses that result in the recruitment of additional macrophages from the monocyte pool into the nasal lumen. Positive feedback and additional recruitment of macrophages are required for the gradual elimination of colonization69. The cellular immune responses to S. pneumoniae are greatly accelerated by cytosolic sensing of the pathogen, which requires the pore-forming function of Ply29,30. Accordingly, ply-deficient mutants, or mutants unable to form pores, show prolonged colonization and diminished production of key inflammatory mediators needed for clearance, including interleukin-1β (IL-1β), CXC-motif and CC-motif chemokines, and type 1 interferons29,30,32. These macrophage-dependent responses are dysfunctional in both infant and aged mice, which might explain the higher incidence of infection among the very young and old70,71. The importance of cellular clearance mechanisms is likely to be a consequence of the inability of specific antibodies that are induced during carriage to clear the organism once it is established on the mucosal surface72,73.
Immunizing effect of colonization
Colonization increases anti-capsular (serotype-specific) and anti-protein (non-serotype-specific) antibody levels74–77. Experimental data from murine models show that colonization is an immunizing event and protects against subsequent colonization and disease78,79. Experimental human carriage studies have confirmed that colonization increases nasal, lung and serum antibody levels74,80,81. Moreover, these studies corroborated observations in murine models, demonstrating the protective effect of colonization against reacquisition of the same strain up to 1 year following the first colonization episode75. Serotype-specific or strain-specific immunity seems to be required for this protection, as challenge of volunteers following a known natural carriage episode with a strain of a different serotype did not result in increased protection27. These infection studies also showed that colonization increases levels of S. pneumoniae-specific CD4+ T memory cells in the blood and lungs in humans80. In mice, anti-pneumococcal CD4+ T cells are sufficient, and the T helper 17 (TH17) cell response is necessary for efficient clearance82,83. The importance of TH17 immunity in natural colonization has yet to be confirmed, although a low ratio of TH17 to T regulatory (Treg) cells correlates with colonization in children and increases with age as colonization frequency decreases84.
Invasive pneumococcal disease
From an evolutionary perspective, stable nasopharyngeal colonization ought to be the principal modus operandi of S. pneumoniae, as this enables ready transmission to new hosts. As noted above, induction of pro-inflammatory chemokines and cytokines, upregulation of target receptors and damage to the respiratory epithelium caused by viral infection of the URT increases bacterial loads in the nasopharynx. This facilitates bacterial transmission but also increases the likelihood of penetration of host tissues and progression to localized or invasive disease. Progression to invasive disease is more likely in young children, elderly people and patients with specific lifestyle traits and comorbidities. There are also marked differences in the capacity of specific S. pneumoniae strains to cause invasive disease, which is unsurprising given the vast genetic and phenotypic heterogeneity of this bacterium. S. pneumoniae factors and pathways that contribute to tissue adherence and invasion are outlined in Fig. 4.
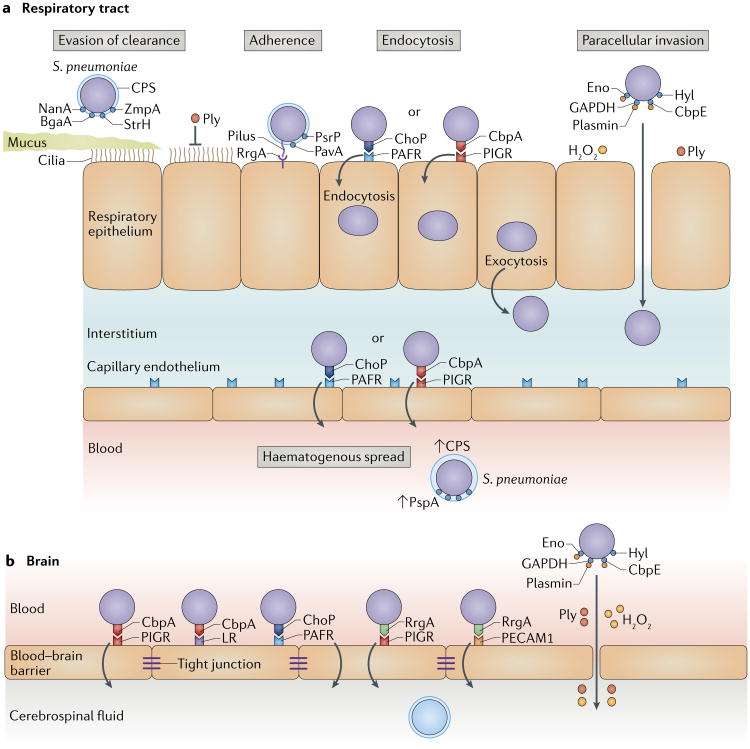
Fig. 4. Stages in pneumococcal adherence and invasion.
a | Several steps are required for invasion of the respiratory tract. Streptococcus pneumoniae evades entrapment in mucus and mucociliary clearance by negatively charged capsular polysaccharide (CPS) and proteolytic degradation of secretory immunoglobulin A1 (IgA1) by the zinc metalloprotease ZmpA (also known as IgA1 protease). Neuraminidase A (NanA), β-galactosidase (BgaA) and β-N-acetylglucosaminidase (StrH) deglycosylate mucus and unmask glycan targets for adhesins on the epithelium. Finally, pneumolysin (Ply) inhibits ciliary beating. Adherence to the apical surface of epithelial cells is mediated by diverse surface structures, including phosphorylcholine (ChoP), choline-binding protein A (CbpA), the ancillary pilus subunit RrgA at the tip of pili, adherence and virulence protein A (PavA) and large surface-exposed glycoprotein (PsrP). S. pneumoniae binds through ChoP to platelet-activating factor receptor (PAFR) and through CbpA to polymeric immunoglobulin receptor (PIGR), and by subverting the respective host receptor recycling pathways, it induces its own endocytosis, which is followed by release of pneumococci at the basolateral surface. Alternatively, Ply and hydrogen peroxide (H2O2) directly damage the epithelium, and hyaluronate lyase (Hyl) and plasmin, which is bound to the pneumococcal surface through enolase (Eno), glyceralde-hyde-3-phosphate dehydrogenase (GAPDH) or CbpE, degrade the extracellular matrix. This breaks down the epithelial barrier and provides a pathway for paracellular invasion. ChoP–PAFR and CbpA–PIGR interactions also enable pneumococci to traverse the endothelium and enter the bloodstream. Upregulation of PAFR by inflammatory cytokines amplifies ChoP–PAFR-mediated invasion. CPS and other virulence factors, including pneumococcal surface protein A (PspA), CbpA and Ply, facilitate evasion of opsonophagocytosis. b | To penetrate the blood–brain barrier, S. pneumoniae uses ChoP–PAFR, CbpA–PIGR and CbpA–laminin receptor (LR) binding. Strains that express pili also use RrgA to bind to PIGR and platelet endothelial cell adhesion molecule 1 (PECAM1). Similar to invasion of the respiratory tract, Ply, H2O2 generated by α-glycerophosphate oxidase (GlpO) and activated plasmin bound to the pneumococcal surface proteins Eno, GAPDH and CbpE can compromise the blood–brain barrier.
Niche adaptation
Translocation from the nasopharynx to deeper tissues exposes S. pneumoniae to distinct microenvironmental niches, requiring extensive adjustments to gene expression patterns. The importance of such adaptations for pathogenesis was initially suggested by genome-wide screens, for example, signature-tagged mutagenesis, which showed that in addition to known virulence genes, a large number of metabolic and transporter genes were required either for colonization or for local or invasive infections but not necessarily for growth of S. pneumoniae in vitro85–87. Subsequent studies using genomic microarray analysis identified substantial differences in expression patterns of these non-traditional virulence genes between pneumococci growing in distinct host niches (nasopharynx, lungs and blood) and compared with cells grown in vitro88,89.
Acquisition of metal ions, particularly transition metals such as iron (Fe), manganese (Mn) and zinc (Zn), is crucial for growth and survival of S. pneumoniae in multiple host niches, where availability of free ions may be restricted. These metals are essential cofactors for many metabolic and other enzymes, and in the case of Mn, they also mediate resistance to oxidative stress90. Unsurprisingly, genes encoding the metal-binding components of ATP-binding cassette (ABC) transporters responsible for uptake of Fe (piuA, piaA and pitA), Mn (psaA) and Zn (adcA and adcAII) are preferentially expressed in the host environment, and the respective S. pneumoniae knockout mutants are heavily attenuated in vivo in models of both carriage and invasive disease91–93. Indeed, the absolute requirement for psaA in vivo makes it a valid target for novel antimicrobials94. Certain metals may also be deleterious in excess, and, hence, intracellular concentrations must be strictly regulated by coordination of uptake and efflux systems90. In addition, excess Zn released into the extracellular compartment by leukocytes poses a particular problem for invading pneumococci. Zn can compete with Mn for the metal binding site in manganese ABC transporter substrate-binding lipoprotein (PsaA)95, but unlike Mn, which is passed from PsaA to the PsaBC transporter for uptake, Zn binds irreversibly to PsaA and thereby blocks the transport pathway, starving the bacterium of Mn96. Thus, host Zn release contributes to nutritional immunity and may explain why dietary Zn deficiency increases rates of pneumococcal disease97,98. Pneumococcal surface protein A (PspA) also interacts with host lactoferrin, an Fe-sequestering glycoprotein, and this protects the bacterium from killing by apolactoferrin (the Fe-free form of lactoferrin)99. Recent work has shown that the variable capacity of different S. pneumoniae strains to bind lactoferrin depends on PspA and differences in CPS100.
Optimal utilization of carbon sources available in distinct host niches is also critical for pathogenesis. S. pneumoniae is totally dependent on carbohydrates as a carbon source, and its genome encodes roughly 30 carbohydrate-specific phosphotransferase systems (PTSs) and ABC transporters capable of importing a wide range of sugars101. Many of these have previously been shown to contribute to growth and survival in vivo102. Although glucose is available in the blood, free sugars may be in low abundance at sites such as the mucosa of the upper and lower respiratory tracts. In these niches, pneumococci scavenge sugars by sequential cleavage of host cell surface N-linked glycoconjugates, which is mediated by surface-associated exoglycosidases such as NanA, BgaA and the β-N-acetylglucosaminidase StrH. The released sugars (sialic acid, galactose and N-acetylglucosamine) may then be taken up by the relevant ABC and PTS transporters and metabolized. At the same time, mannose residues are unmasked on the core gly-can structure, which may function as surface receptors for pneumococcal adherence103. S. pneumoniae also has a surface-associated endoglycosidase, EndoD104, which releases the residual mannose3-N-acetylglucosamine2 (Man3GlcNAc2) structure from host glycoconjugates. Terminal mannose can also be released from high man-nose N-glycans by an α-(1,2)-mannosidase, SpGH92, and taken up by the mannose PTS. Meanwhile, residual mannose5-N-acetylglucosamine2 (Man5Gl cNAc2) is also released from these host structures by EndoD and taken up along with Man3GlcNAc2 by an ABC transporter, with further deconstruction occurring in the pneumococcal cytoplasm104. The various released sugars can have substantial intracellular effects by regulating carbohydrate metabolism through the catabolite repressor CcpA102. Sialic acid released by NanA has been shown to act as a signal, increasing bacterial loads in the nasopharynx of mice colonized with S. pneumoniae and facilitating invasion of nasal tissue and progression to pneumonia and meningitis105,106. Such signalling involves the two- component response regulator transcriptional regulatory protein CiaR and requires sialic acid uptake by the transporter SatABC, and this results in increased pneumococcal resistance to antimicrobial reactive oxygen species107. NanA can also trigger TGFβ signalling pathways, leading to endothelial cell invasion108.
The role of biofilms in the ability of S. pneumoniae to persist at various sites of infection is not well understood, and their contribution to invasive disease remains controversial. Most studies of pneumococcal biofilms have been carried out in vitro, and in vivo data are limited. Pneumococcal biofilm structures have been detected in biopsy samples from patients with otitis media109 and in the middle ear cleft of chinchillas co-infected with S. pneumoniae and Haemophilus influenzae110. In biopsy samples from volunteers colonized in experimental human studies, S. pneumoniae was found in microcolonies68, although it has not been determined whether these have characteristics of biofilms, such as an extracellular matrix. The production of an extracellular matrix has a major impact on the ability of S. pneumoniae that has been grown in a biofilm in vitro to subsequently translocate from the nasopharynx to the lungs in a murine infection model111. A recent report has also suggested that NanA-mediated cleavage of sialic acid promotes biofilm formation in vivo and increases carbon availability during colonization112. Murine experiments suggested that the large surface-exposed glycoprotein PsrP is particularly important for bacterial attachment to lung cells and biofilm formation by intraspecies interaction113. PsrP seems to be required for bacterial persistence in the lower airway but not for nasal colonization or survival in the bloodstream during sepsis114.
Quorum sensing (QS) and phase variation also have an important role in modulating pneumococcal niche adaptations. It has been known for many years that S. pneumoniae colonies can switch between transparent and opaque phenotypes in a process known as phase variation. These variants differ in levels of expression of key virulence proteins, such as PspA and CbpA, as well as CPS and cell wall teichoic acid. The transparent phenotype is favoured in the nasopharyngeal niche, and the opaque phenotype is favoured in the blood115. A more recent study has shown that the underlying mechanism involves a type I restriction-modification system, SpnIII, within a genetic locus containing inverted repeats that enable spontaneous rearrangement of alternative specificity domain genes. This generates six different SpnIII target specificities, each with distinct genome-wide DNA methylation patterns, gene expression profiles and virulence phenotypes116. Moreover, pneumococci were shown to readily switch between SpnIII alleles during progression of disease in a murine model116. Differentially expressed genes included the CPS biosynthesis locus cps, various sugar transporters, the Mn transporter psaBCA and luxS. The luxS gene is of particular interest, as it is involved in the synthesis of the ubiquitous QS molecule autoinducer 2 (AI-2), which is an important regulator of bio-film formation and virulence in pneumococci117. Recent studies show that AI-2 accumulating in the extracellular compartment is sensed by the fructose PTS transporter subunit IIC FruA, leading to upregulation of the galactose ABC transporter and the Leloir pathway105. Galactose is an important carbon source for S. pneumoniae in the respiratory tract, and AI-2-mediated QS seems to be essential for its uptake and metabolism. Upregulation of the Leloir pathway increases the availability of activated sugar precursors, leading to increased production of CPS and a hypervirulent phenotype105.
Penetration of tissues
Invasive pneumococcal disease requires breaching of epithelial and/or endothelial barriers and penetration of tissues, ultimately providing access to the bloodstream. In the case of meningitis, this involves breaching the blood–brain barrier (BBB). Invasion involves interaction between ChoP moieties and PAFR on the surface of cytokine-activated respiratory epithelial and vascular endothelial cells, followed by hijacking of the PAFR recycling pathway to gain entry40. An alternative route involves interaction between the pneumococcal surface protein CbpA and polymeric immunoglobulin receptor (PIGR) on human respiratory epithelial cells. Subversion of the PIGR recycling pathway enables internalization and transmigration of S. pneumoniae across polarized epithelial cell monolayers41. Interestingly, another region of CbpA has been shown to bind to the laminin receptor on brain microvascular endothelium, and this facilitates penetration of the BBB during development of pneumococcal meningitis118. CbpA, as well as laminin receptor and PAFR, are also necessary for invasion of cardiomyocytes and formation of cardiac microlesions, which can occur as a complication of invasive pneumococcal disease119. Recently, the ancillary pilus sub-unit RrgA, the tip adhesin of the pneumococcal pilus 1, has also been shown to interact with PIGR and platelet endothelial cell adhesion molecule 1 (PECAM1) on brain microvascular endothelium, and antibody blockade or deletion of these two receptors reduced brain invasion in a mouse meningitis model120. Currently, the relative importance of these uptake mechanisms and the extent of cooperation between them are uncertain. Furthermore, many S. pneumoniae strains are not piliated and thus cannot use RrgA-dependent pathways. It should also be emphasized that bacteraemia is not an essential prerequisite for meningitis, as localized infections such as sinusitis or mastoiditis can also lead to meningitis. When modelled in mice, meningitis may also develop as a consequence of the interaction of pneumococci colonizing the nasopharynx with gangliosides on the surface of olfactory neurons, triggering cell invasion and direct entry of pneumococci into the central nervous system by retrograde axonal transport121. Such non-haematogenous spread is stimulated by exogenous sialic acid106.
Regardless of the mechanism or site of invasion, the pneumococcal capsule impedes adherence to and invasion of host cells because it may sterically hinder interactions between cell wall ChoP or surface proteins and their cognate host receptors122. However, pneumococci markedly reduce capsule thickness when in close contact with epithelial cells and during the invasion process123. This process of capsule shedding has recently been shown to depend on the major pneumococcal autolysin LytA and is triggered by exposure to cationic antimicrobial peptides that are released by the host cells124.
Several pneumococcal virulence factors that directly damage host tissues or induce host inflammatory responses also facilitate tissue invasion
One of the most notable examples is Ply, which, in addition to wide-ranging pro-inflammatory effects, directly lyses or induces apoptosis of diverse cell types, including lung epithelium and endothelial cells at the BBB125. Ply also inhibits mucociliary clearance in human lungs, separates tight junctions between cells (which enables tissue penetration), and exposes new sites for pneumococcal attachment126. The pneumococcal pyruvate oxidase SpxB and α-glycerophosphate oxidase GlpO produce hydrogen peroxide, which also contributes to tissue damage in the lung and at the BBB127. Surface-exposed hydrolytic enzymes, including neuraminidases, hyaluronate lyase128 and metalloproteinases129, can also directly damage host tissues. Two glycolytic enzymes, Eno and glyceraldehyde-3-phosphate dehydrogenase, are also surface-exposed and function as plasminogen-binding proteins along with CbpE (also known as Pce). They sequester and activate host plasminogen at the pneumococcal surface and facilitate adherence to and penetration of the extracellular matrix130,131. An overview of pneumococcal surface proteins and other factors contributing to adherence and invasion is provided in Table 1.
Table 1. Major pneumococcal virulence factors.
| Virulence factor | Description | Function in pathogenesis |
|---|---|---|
| CPS |
|
|
| ChoP on teichoic acid | PAFR ligand | Binds PAFR on surface of epithelial and endothelial cells, facilitating adherence and invasion |
| Lipopeptides, lipoteichoic acid and peptidoglycan fragments | Pathogen-associated molecular patterns | Promote inflammation |
| Ply |
|
|
| PspA | CBP |
|
| CbpA (also known as PspC) | CBP |
|
| LytA |
|
|
| CbpD |
|
|
| CbpE (also known as Pce) |
|
|
| CbpG | CBP serine protease |
|
| CbpL | CBP |
|
| NanA |
|
|
| BgaA |
|
Sequentially cleaves sugars from host glycoconjugates |
| StrH |
|
Sequentially cleaves sugars from host glycoconjugates |
| EndoD |
|
Sequentially cleaves sugars from host glycoconjugates |
| Hyl |
|
|
| PrtA |
|
|
| ZmpA (also known as IgA1 protease) |
|
Cleaves human IgA1 |
| ZmpB |
|
Possible adhesin |
| ZmpC |
|
Cleaves human matrix metalloproteinase 9 |
| PepO | Endopeptidase |
|
| PsrP |
|
|
| RrgA, RrgB and RrgC |
|
|
| PsaA |
|
|
| AdcA and AdcAII |
|
Zn acquisition in vivo |
| PiuA, PiaA and PitA |
|
Fe acquisition in vivo |
| SlrA and PpmA |
|
Contribute to nasopharyngeal colonization |
| PhtA, PhtB, PhtD and PhtE | Family of surface proteins with unusual His-triad motifs |
|
| PavA and PavB |
|
|
| Eno |
|
|
| GAPDH |
|
|
| SpxB | Pyruvate oxidase | Generates H2O2 |
| GlpO | α-Glycerophosphate oxidase | Generates H2O2 |
| SodA | Mn-dependent superoxide dismutase | Resistance to oxidative stress |
| Etrx1 and Etrx2 | Surface-exposed thioredoxin-family lipoproteins | Resistance to oxidative stress |
| SpMsrAB2 | Methionine sulfoxide reductase | Redox partner of Etrx1 and Etrx2 |
ABC, ATP-binding cassette; CBP, choline-binding protein; ChoP, phosphorylcholine; CPS, capsular polysaccharide; Etrx, surface-exposed thioredoxin family lipoprotein; Fe, iron; H2O2, hydrogen peroxide; iC3b, inactivated C3b; IgG, immunoglobulin G; LPXTG, sortase-anchored surface protein; MAPK, mitogen-activated protein kinase; Mn, manganese; NCSP, non-classical surface protein lacking secretion signals or anchorage motifs; NLRP3, NACHT, LRR and PYD domains-containing protein 3; PAF, platelet-activating factor ; PAFR, PAF receptor ; Pav, adherence and virulence protein; PECAM1, platelet endothelial cell adhesion molecule 1; PIGR, polymeric immunoglobulin receptor ; Ply, pneumolysin; PpmA, foldase protein PrsA ; PspA, pneumococcal surface protein A; Zn, zinc.
Evasion and subversion of host defences
S. pneumoniae expresses a plethora of factors that mediate immune evasion and subversion (Table 1). As an extracellular pathogen, S. pneumoniae must evade neutrophil-mediated killing to survive the acute inflammation that accompanies tissue invasion. Neutrophils can readily kill phagocytized pneumococci by releasing serine proteases from neutrophil granules132. One mechanism to evade neutrophil recruitment involves CbpE, which functions as a ChoP esterase. CbpE cleaves ChoP moieties on host-derived platelet-activating factor (PAF), which is a potent activator of neutrophils133. Many of the virulence determinants of S. pneumoniae target components of the complement system to minimize opsonophagocytosis and clearance of invading pneumococci (reviewed in Ref. 134). The CPS is undoubtedly the most important defence against the host immune system. For example, although non-encapsulated pneumococci can colonize the URT and cause superficial eye infections, they rarely cause invasive infection. CPS covers deeper bacterial surface structures and thereby inhibits binding of immunoglobulins, complement components and C-reactive protein. It reduces opsonization with C3b and inactivated C3b (iC3b) and physically impairs interactions between C3b, iC3b and Fc regions of immunoglobulins with their receptors on phagocytic cells135. Capsular serotypes differ in the effectiveness with which they inhibit opsonophagocytosis and the level of inhibition correlates with their ability to cause invasive disease. Studies of capsule-switch mutants have shown an inverse relationship between the amount of C3b and iC3b deposition and binding to factor H, which inhibits the alternative complement pathway136. Increased levels of factor H in nasal lavages of asymptomatic individuals infected with a URT virus predispose the host to acquisition of S. pneumoniae137. Factor H mainly binds CbpA on the pneumococcal surface. CbpA can also bind directly to C3 and, in some strains, the classical complement pathway inhibitor C4b-b inding protein (C4BP) in an interaction that is inhibited by CbpA binding to vitronectin134,138,139. Thus, CPS and CbpA on the pneumococcal surface are both important for resistance to opsonophagocytosis.
PspA also interferes with complement deposition by binding factor B and blocking formation of or accelerating the dissociation of the alternative pathway C3 convertase140. Furthermore, Ply released from the bacterium activates the classical complement pathway through a domain with structural similarity to the Fc component of IgG, thereby depleting serum opsonic activity125,141. The combined functions of PspA and Ply are essential for S. pneumoniae to successfully cause septicaemia142. Other pneumococcal proteins that interfere with opsonophagocytosis include the exoglycosidases NanA, BgaA and StrH, presumably by deglycosylating human glycoproteins that are important for complement deposition143. Eno and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) bind and activate plasminogen, and this can also contribute to immune evasion through the degradation of complement pathway components134. S. pneumoniae grows in both short-chain and long-chain forms. Short-chain forms have minimal surface area and are less likely to trigger complement activation and therefore are more likely to evade opsonophagocytic clearance during invasive disease144.
Conclusions and outlook
S. pneumoniae has proved to be a truly resilient foe. It has overcome selective pressure from multiple classes of antibiotics and now seems to be adapting to the immune pressure of widespread immunization. These developments demonstrate that we cannot be complacent, and further insights are needed to combat pneumococcal disease. This Review has highlighted the current state of our understanding of the three key stages in the pathogenesis of S. pneumoniae — transmission, colonization and invasion. In particular, our understanding has profited from progress in defining the molecular events involved in invasion and new models of transmission in infant mice and of carriage in humans. Further progress will likely come from a broader perspective that takes into account pneumococcal ecology. In this regard, there are now more than 8,000 publicly available whole-genome sequences of S. pneumoniae, which are providing a more comprehensive view of the species and the remarkable extent of its diversity. Additional insight will come from studies of the interactions of S. pneumoniae with other members of the microbiota and a better understanding of its niche in the human URT.
Acknowledgments
The authors thank J. Pagano for editorial assistance. J.N.W. is funded by grants from the United States Public Health Service (AI038446 and AI105168). Research in J.C.P.' s laboratory is supported by program grant 1071659 from the National Health and Medical Research Council of Australia (NHMRC); J.C.P. is an NHMRC Senior Principal Research Fellow. D.M.F. is supported by the Medical Research Council (grant MR/M011569/1) and the Bill and Melinda Gates Foundation (grant OPP1117728).
Glossary
- Upper respiratory tract (URT)
Includes the nasal cavity, paranasal sinuses, mouth, pharynx and larynx and forms the major passages above the trachea
- Community-acquired pneumonia
Infection of the lung acquired outside of hospitals or nursing facilities
- Natural competence
The endogenous ability of a bacterium to alter its genes by taking up extracellular DNA from its environment through transformation
- PolyIC, Polyinosinic
Polycytidylic acid is an agonist of Toll-like receptor 3 and mimics double-stranded RNA found in some viruses
- Dexamethasone
An anti-inflammatory corticosteroid
- Fc fragment
The tail region of an antibody that interacts with cell surface receptors and some proteins of the complement system
- Agglutinating function
The clumping of antigens through multivalent binding by antibodies
- Mucociliary flow
A non-immunological defence mechanism that involves ciliary action and the flow of mucus; it clears the respiratory tract of pathogens and particles
- Lectin domains
The carbohydrate-binding domains on proteins
- Bacteriocins
The proteinaceous or peptidic toxins produced by bacteria to inhibit the growth of similar or closely related bacteria
- Type 1 interferons
A group of signalling proteins expressed and released by host cells to regulate immune responses to pathogens
- Signature-tagged mutagenesis
A genetic technique using DNA signature tags (molecular barcodes) to identify mutants in mixed populations
- Two-component response regulator
The transcription factor component of a stimulus-response mechanism for bacteria to sense and respond to environmental changes
- Quorum sensing (QS)
A system of stimuli and responses that is correlated to microbial population density
- Restriction-modification system
A bacterial defence system in which restriction endonucleases cleave and inactivate specific target sequences in foreign DNA (for example, from phages); cleavage sites in host DNA are protected by methylation
- Leloir pathway
The predominant route of cellular galactose metabolism
- Opsonophagocytosis
A process by which a microorganism is labelled (opsonized) by host immune factors to facilitate uptake by phagocytic cells
Footnotes
Author contributions: All authors researched data for the article, substantially contributed to discussion of content, wrote the article and reviewed and edited the manuscript before submission.
Competing interests: The authors declare no competing interests.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information: Nature Reviews Microbiology thanks Sven Hammerschmidt and the other anonymous reviewer(s) for their contribution to the peer review of this work.
References
- 1.Abdullahi O, et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS ONE. 2012;7:e30787. doi: 10.1371/journal.pone.0030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yahiaoui RY, et al. Prevalence and antibiotic resistance of commensal Streptococcus pneumoniae in nine European countries. Future Microbiol. 2016;11:737–744. doi: 10.2217/fmb-2015-0011. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 4.Whitney CG, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 5.Musher D. How contagious are common respiratory tract infections? N Engl J Med. 2003;348:1256–1266. doi: 10.1056/NEJMra021771. [DOI] [PubMed] [Google Scholar]
- 6.Numminen E, et al. Climate induces seasonality in pneumococcal transmission. Sci Rep. 2015;5:11344. doi: 10.1038/srep11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gwaltney JJ, Sande M, Austrian R, Hendley J. Spread of Streptococcus pneumoniae in families. II. Relation of transfer of S pneumoniae to incidence of colds and serum antibody. J Infect Dis. 1975;132:62–68. doi: 10.1093/infdis/132.1.62. [DOI] [PubMed] [Google Scholar]
- 8.McCullers J, et al. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis. 2010;202:1287–1295. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diavatopoulos DA, et al. Influenza A virus facilitates Streptococcus pneumoniae and disease. FASEB J. 2010;24:1789–1798. doi: 10.1096/fj.09-146779. This study demonstrates the role of influenza virus in pneumococcal transmission in an infant mouse model. [DOI] [PubMed] [Google Scholar]
- 10.Barbier D, et al. Influenza A induces the majorsecreted airway mucin MUC5AC in a protease-EGFR-extracellular regulated kinase-Sp1-dependent pathway. Am J Respir Cell Mol Biol. 2012;47:149–157. doi: 10.1165/rcmb.2011-0405OC. [DOI] [PubMed] [Google Scholar]
- 11.Siegel S, Roche A, Weiser J. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe. 2014;16:55–67. doi: 10.1016/j.chom.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard AL, Siegel SJ, Erikson J, Weiser JN. TLR2 signaling decreases transmission of Streptococcus pneumoniae by limiting bacterial shedding in an infant mouse Influenza A co-infectionmodel. PLoS Pathog. 2014;10:e1004339. doi: 10.1371/journal.ppat.1004339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kono M, et al. Single cell bottlenecks in the pathogenesis of Streptococcus pneumoniae. PLoS Pathog. 2016;12:e1005887. doi: 10.1371/journal.ppat.1005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zafar MA, Kono M, Wang Y, Zangari T, Weiser JN. Infant mouse model for the study of shedding and transmission during Streptococcus pneumoniae monoinfection. Infect Immun. 2016;84:2714–2722. doi: 10.1128/IAI.00416-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues F, et al. Relationships between rhinitis symptoms, respiratory viral infections and nasopharyngeal colonization with Streptococcus pneumoniae. Haemophilus influenza and Staphylococcus aureus in children attending daycare Pediatr. Infect Dis J. 2013;32:227–232. doi: 10.1097/INF.0b013e31827687fc. [DOI] [PubMed] [Google Scholar]
- 16.Zafar MA, Wang Y, Hamaguchi S, Weiser JN. Host-to-host transmission of Streptococcus pneumoniae is driven by its inflammatory toxin, pneumolysin. Cell Host Microbe. 2017;21:73–83. doi: 10.1016/j.chom.2016.12.005. This study provides evidence that the toxin Ply promotes mucosal inflammation, which facilitates pneumococcal transmission in infant mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthias KA, Roche AM, Standish AJ, Shchepetov M, Weiser JN. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J Immunol. 2008;180:6246–6254. doi: 10.4049/jimmunol.180.9.6246. [DOI] [PubMed] [Google Scholar]
- 18.Lipsitch M, Moxon ER. Virulence and transmissibility of pathogens: what is the relationship? Trends Microbiol. 1997;5:31–37. doi: 10.1016/S0966-842X(97)81772-6. [DOI] [PubMed] [Google Scholar]
- 19.Zafar MA, Hamaguchi S, Zangari T, Cammer M, Weiser JN. Capsule type and amount affect shedding and transmission of Streptococcus pneumoniae. mBio. 2017;8:e00989–17. doi: 10.1128/mBio.00989-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks LR, Reddinger RM, Hakansson AP. Biofilm formation enhances fomite survival of Streptococcus pneumoniae and Streptococcus pyogenes. Infect Immun. 2014;82:1141–1146. doi: 10.1128/IAI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verhagen LM, et al. Genome-wide identification of genes essential for the survival of Streptococcus pneumoniae in human saliva. PLoS ONE. 2014;9:e89541. doi: 10.1371/journal.pone.0089541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamaguchi S, Zafar MA, Cammer M, Weiser JN. Capsule prolongs survival of Streptococcus pneumoniae during starvation. Infect Immun. 2018 doi: 10.1128/IAI.00802-17. https://doi.org/10.1128/IAI.00802-17. [DOI] [PMC free article] [PubMed]
- 23.Walsh RL, Camilli A. Streptococcus pneumoniae is desiccation tolerant and infectious upon rehydration. mBio. 2011;2:e00092–11. doi: 10.1128/mBio.00092-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zangari T, Wang Y, Weiser JN. Streptococcus pneumoniae transmission is blocked by type-specific immunity in an infant mouse model. mBio. 2017;8:e00188–17. doi: 10.1128/mBio.00188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roche AM, Richard AL, Rahkola JT, Janoff EN, Weiser JN. Antibody blocks acquisition of bacterial colonization through agglutination. Mucosal Immunol. 2015;8:176–185. doi: 10.1038/mi.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janoff EN, et al. Pneumococcal IgA1 protease subverts specific protection by human IgA1. Mucosal Immunol. 2014;7:249–256. doi: 10.1038/mi.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennington SH, et al. Polysaccharide-specific memory b cells predict protection against experimental human pneumococcal carriage. Am J Respir Crit Care Med. 2016;194:1523–1531. doi: 10.1164/rccm.201512-2467OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsi E, et al. Agglutination by anti-capsular polysaccharide antibody is associated with protection against experimental human pneumococcal carriage. Mucosal Immunol. 2017;10:385–394. doi: 10.1038/mi.2016.71. This study shows that the agglutinating activity of anticapsular antibody mediates protection from experimental pneumococcal carriage in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemon JK, Weiser JN. Degradation products of the extracellular pathogen Streptococcus pneumoniae access the cytosol via its pore-forming toxin. mBio. 2015;6:e02110–e02114. doi: 10.1128/mBio.02110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis K, Nakamura S, Weiser J. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest. 2011;121:3666–3676. doi: 10.1172/JCI57761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karmakar M, et al. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3 ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol. 2015;194:1763–1775. doi: 10.4049/jimmunol.1401624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker D, et al. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. mBio. 2011;2:e00016–11. doi: 10.1128/mBio.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis K, Akinbi H, Standish A, Weiser J. Resistance to mucosal lysozyme compensates for the fitness deficit of peptidoglycan modifications by Streptococcus pneumoniae. PLoS Pathog. 2008;4:e1000241. doi: 10.1371/journal.ppat.1000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 35.Feldman C, et al. The interaction of Streptococcus pneumoniae with intact human respiratory mucosa in vitro. Eur Respir J. 1992;5:576–583. [PubMed] [Google Scholar]
- 36.Nelson AL, et al. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun. 2007;75:83–90. doi: 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes AR, et al. The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol Microbiol. 2001;41:1395–1408. doi: 10.1046/j.1365-2958.2001.02610.x. [DOI] [PubMed] [Google Scholar]
- 38.Bergmann S, Rohde M, Chhatwal GS, Hammerschmidt S. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol Microbiol. 2001;40:1273–1287. doi: 10.1046/j.1365-2958.2001.02448.x. [DOI] [PubMed] [Google Scholar]
- 39.Jensch I, et al. PavB is a surface-exposed adhesin of Streptococcus pneumoniae contributing to nasopharyngeal colonization and airways infections. Mol Microbiol. 2010;77:22–43. doi: 10.1111/j.1365-2958.2010.07189.x. [DOI] [PubMed] [Google Scholar]
- 40.Cundell DR, Gerard NP, Gerard C, Idanpaan-Heikkila I, Tuomanen EI. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JR, et al. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell. 2000;102:827–837. doi: 10.1016/s0092-8674(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 42.Hauck CR. Cell adhesion receptors-signaling capacity and exploitation by bacterial pathogens. Med Microbiol Immunol. 2002;191:55–62. doi: 10.1007/s00430-002-0119-0. [DOI] [PubMed] [Google Scholar]
- 43.Kc R, Shukla SD, Walters EH, O'Toole RF. Temporal upregulation of host surface receptors provides a window of opportunity for bacterial adhesion and disease. Microbiology. 2017;163:421–430. doi: 10.1099/mic.0.000434. [DOI] [PubMed] [Google Scholar]
- 44.Cron LE, et al. Surface-associated lipoprotein PpmA of Streptococcus pneumoniae is involved in colonization in a strain-specific manner. Microbiology. 2009;155:2401–2410. doi: 10.1099/mic.0.026765-0. [DOI] [PubMed] [Google Scholar]
- 45.Hermans PW, et al. The streptococcal lipoprotein rotamase A (SlrA) is a functional peptidyl-prolyl isomerase involved in pneumococcal colonization. J Biol Chem. 2006;281:968–976. doi: 10.1074/jbc.M510014200. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez-Fernandez J, et al. Modular architecture and unique teichoic acid recognition features of choline-binding protein L (CbpL) contributing to pneumococcal pathogenesis. Sci Rep. 2016;6:38094. doi: 10.1038/srep38094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King SJ. Pneumococcal modification of host sugars: a major contributor to colonization of the human airway? Mol Oral Microbiol. 2010;25:15–24. doi: 10.1111/j.2041-1014.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 48.Uchiyama S, et al. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J Exp Med. 2009;206:1845–1852. doi: 10.1084/jem.20090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Limoli DH, Sladek JA, Fuller LA, Singh AK, King SJ. BgaA acts as an adhesin to mediate attachment of some pneumococcal strains to human epithelial cells. Microbiology. 2011;157:2369–2381. doi: 10.1099/mic.0.045609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersson B, et al. Identification of an active dissaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J Exp Med. 1983;158:559–570. doi: 10.1084/jem.158.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krivan HC, Roberts DD, Ginsberg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAcb1-4 Gal found in some glycolipids. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shak JR, Vidal JE, Klugman KP. Influence of bacterial interactions on pneumococcal colonization of the nasopharynx. Trends Microbiol. 2013;21:129–135. doi: 10.1016/j.tim.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lysenko ES, et al. Nod1-signaling overcomes resistance of Streptococcus pneumoniae to opsonophagocytic killing. PLoS Pathog. 2007;3:1073–1081. doi: 10.1371/journal.ppat.0030118. This study elucidates how H. influenzae signalling via NOD1 enhances neutrophil killing of S. pneumoniae, leading to bacterial clearance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cremers AJ, et al. The adult nasopharyngeal microbiome as a determinant of pneumococcal acquisition. Microbiome. 2014;2:44. doi: 10.1186/2049-2618-2-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biesbroek G, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190:1283–1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 56.Miller EL, Abrudan MI, Roberts IS, Rozen DE. Diverse ecological strategies are encoded by Streptococcus pneumoniae bacteriocin-like peptides. Genome Biol Evol. 2016;8:1072–1090. doi: 10.1093/gbe/evw055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dawid S, Roche A, Weiser J. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect Immun. 2007;75:443–451. doi: 10.1128/IAI.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bogaardt C, van Tonder AJ, Brueggemann AB. Genomic analyses of pneumococci reveal a wide diversity of bacteriocins — including pneumocyclicin, a novel circular bacteriocin. BMC Genom. 2015;16:554. doi: 10.1186/s12864-015-1729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura S, Davis K, Weiser J. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumonia e colonization in mice. This study demonstrates a mechanism by which concurrent influenza virus infection leads to increased pneumococcal carriage. J Clin Invest. 2011;121:3657–3665. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis. 2002;186:341–350. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 61.Avadhanula V, et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 2006;80:1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mina MJ, McCullers JA, Klugman KP. Live attenuated influenza vaccine enhances colonization of Streptococcus pneumoniae and Staphylococcus aureus in mice. mBio. 2014;5:e01040–13. doi: 10.1128/mBio.01040-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mina MJ. Generalized herd effects and vaccine evaluation: impact of live influenza vaccine on off-target bacterial colonisation. J Infect. 2017;74(Suppl. 1)(s107):S101. doi: 10.1016/S0163-4453(17)30199-8. [DOI] [PubMed] [Google Scholar]
- 64.Thors V, et al. The effects of live attenuated influenza vaccine on nasopharyngeal bacteria in healthy 2 to 4 year olds. A randomized controlled trial. Am J Respir Crit Care Med. 2016;193:1401–1409. doi: 10.1164/rccm.201510-2000OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCullers J. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014;12:252–262. doi: 10.1038/nrmicro3231. Provides a good overview of how influenza virus co-infection leads to bacterial superinfection in the lungs. [DOI] [PubMed] [Google Scholar]
- 66.Lees JA, et al. Genome-wide identification of lineage and locus specific variation associated with pneumococcal carriage duration. eLife. 2017;6:e26255. doi: 10.7554/eLife.26255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kadioglu A, Weiser J, Paton J, Andrew P. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 68.Jochems SP, et al. Novel analysis of immune cells from nasal microbiopsy demonstrates reliable, reproducible data for immune populations, and superior cytokine detection compared to nasal wash. PLoS ONE. 2017;12:e0169805. doi: 10.1371/journal.pone.0169805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang Z, Clarke T, Weiser J. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;11:9, 1899–1909. doi: 10.1172/JCI36731. This study shows that IL-17A by CD4+ T cells is required for the recruitment of monocytes and macrophages and effective pneumococcal clearance in unimmunized mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siegel S, Tamashiro, Weiser J. Clearance of pneumococcal colonization in infants is delayed through altered macrophage trafficking. PLoS Pathog. 2015;11:e1005004. doi: 10.1371/journal.ppat.1005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Puchta A, et al. TNF drives monocyte dysfunction with age and results in impaired anti-pneumococcal immunity. PLoS Pathog. 2016;12:e1005368. doi: 10.1371/journal.ppat.1005368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malley R, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74:2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Rossum A, Lysenko E, Weiser J. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73:7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med. 2002;195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferreira DM, et al. Controlled human infection and rechallenge with Streptococcus pneumonia e reveals the protective efficacy of carriage in healthy adults. Am J Respir Crit Care Med. 2013;187:855–864. doi: 10.1164/rccm.201212-2277OC. This study uses a human infection model to demonstrate that immunity induced by a previous colonization episode protects against reacquisition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmlund E, et al. Antibodies to pneumococcal proteins PhtD, CbpA, and LytC in Filipino pregnant women and their infants in relation to pneumococcal carriage. Clin Vaccine Immunol. 2009;16:916–923. doi: 10.1128/CVI.00050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson LA, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 78.Richards L, Ferreira DM, Miyaji EN, Andrew PW, Kadioglu A. The immunising effect of pneumococcal nasopharyngeal colonisation; protection against future colonisation and fatal invasive disease. Immunobiology. 2010;215:251–263. doi: 10.1016/j.imbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Cohen JM, Wilson R, Shah P, Baxendale HE, Brown JS. Lack of cross-protection against invasive pneumonia caused by heterologous strains following murine Streptococcus pneumoniae nasopharyngeal colonisation despite whole cell ELISAs showing significant cross-reactive IgG. Vaccine. 2013;31:2328–2332. doi: 10.1016/j.vaccine.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 80.Wright AK, et al. Experimental human pneumococcal carriage augments IL-17A dependent T-cell defence of the lung. PLoS Pathog. 2013;9:e1003274. doi: 10.1371/journal.ppat.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wright AK, et al. Human nasal challenge with Streptococcus pneumoniae is immunising in the absence of carriage. PLoS Pathog. 2012;8:e1002622. doi: 10.1371/journal.ppat.1002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malley R, et al. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci USA. 2005;102:4848–4853. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trzcinski K, et al. Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect Immun. 2008;76:2678–2684. doi: 10.1128/IAI.00141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mubarak A, et al. A dynamic relationship between mucosal T helper type 17 and regulatory T-cell populations in nasopharynx evolves with age and associates with the clearance of pneumococcal carriage in humans. Clin Microbiol Infect. 2016;22:736.e1–736.e7. doi: 10.1016/j.cmi.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 85.Polissi A, et al. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lau GW, et al. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol Microbiol. 2001;40:555–571. doi: 10.1046/j.1365-2958.2001.02335.x. [DOI] [PubMed] [Google Scholar]
- 87.Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol. 2002;45:1389–1406. [PMC free article] [PubMed] [Google Scholar]
- 88.Orihuela CJ, et al. Microarray analysis of pneumococcal gene expression during invasive disease. Infect Immun. 2004;72:5582–5596. doi: 10.1128/IAI.72.10.5582-5596.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ogunniyi AD, et al. Identification of genes that contribute to the pathogenesis of invasive pneumococcal disease by in vitro transcriptomic analysis. Infect Immun. 2012;80:3268–3278. doi: 10.1128/IAI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Honsa ES, Johnson MD, Rosch JW. The roles of transition metals in the physiology and pathogenesis of Streptococcus pneumoniae. Front Cell Infect Microbiol. 2013;3:92. doi: 10.3389/fcimb.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown JS, Gilliland SM, Holden DWA. Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol Microbiol. 2001;40:572–585. doi: 10.1046/j.1365-2958.2001.02414.x. [DOI] [PubMed] [Google Scholar]
- 92.McAllister LJ, et al. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol Microbiol. 2004;53:889–901. doi: 10.1111/j.1365-2958.2004.04164.x. [DOI] [PubMed] [Google Scholar]
- 93.Plumptre CD, et al. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol Microbiol. 2014;91:834–851. doi: 10.1111/mmi.12504. [DOI] [PubMed] [Google Scholar]
- 94.Bajaj M, et al. Discovery of novel pneumococcal surface antigen A (PsaA) inhibitors using a fragment-based drug design approach. ACS Chem Biol. 2015;10:1511–1520. doi: 10.1021/cb501032x. [DOI] [PubMed] [Google Scholar]
- 95.McDevitt CA, et al. A molecular mechanism for bacterial susceptibility to zinc. PLoS Pathog. 2011;7:e1002357. doi: 10.1371/journal.ppat.1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Counago RM, et al. Imperfect coordination chemistry facilitates metal ion release in the Psa permease. Nat Chem Biol. 2014;10:35–41. doi: 10.1038/nchembio.1382. [DOI] [PubMed] [Google Scholar]
- 97.Kumar S, Awasthi S, Jain A, Srivastava RC. Blood zinc levels in children hospitalized with severe pneumonia: a case control study. Indian Pediatr. 2004;41:486–491. [PubMed] [Google Scholar]
- 98.Coles CL, et al. Zinc modifies the association between nasopharyngeal Streptococcus pneumoniae carriage and risk of acute lower respiratory infection among young children in rural Nepal. J Nutr. 2008;138:2462–2467. doi: 10.3945/jn.108.095422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hakansson A, et al. Characterization of binding of human lactoferrin to pneumococcal surface protein A. Infect Immun. 2001;69:3372–3381. doi: 10.1128/IAI.69.5.3372-3381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mirza S, et al. The effects of differences in pspA alleles and capsular types on the resistance of Streptococcus pneumoniae to killing by apolactoferrin. Microb Pathog. 2016;99:209–219. doi: 10.1016/j.micpath.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 101.Bidossi A, et al. A functional genomics approach to establish the complement of carbohydrate transporters in Streptococcus pneumoniae. PLoS ONE. 2012;7:e33320. doi: 10.1371/journal.pone.0033320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buckwalter CM, King SJ. Pneumococcal carbohydrate transport: food for thought. Trends Microbiol. 2012;20:517–522. doi: 10.1016/j.tim.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.King SJ, Hippe KR, Weiser JN. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol Microbiol. 2006;59:961–974. doi: 10.1111/j.1365-2958.2005.04984.x. [DOI] [PubMed] [Google Scholar]
- 104.Robb M, et al. Molecular characterization of N-glycan degradation and transport in Streptococcus pneumoniae and its contribution to virulence. PLoS Pathog. 2017;13:e1006090. doi: 10.1371/journal.ppat.1006090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trappetti C, et al. Autoinducer 2 signaling via the phosphotransferase frua drives galactose utilization by Streptococcus pneumoniae, resulting in hypervirulence. mBio. 2017;8:e02269–16. doi: 10.1128/mBio.02269-16. This study was the first to identify an AI-2 receptor in Gram-positive bacteria and describe a mechanism whereby quorum sensing of AI-2 promotes invasive disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hatcher BL, Hale JY, Briles DE. Free sialic acid acts as a signal that promotes Streptococcus pneumoniae invasion of nasal tissue and nonhematogenous invasion of the central nervous system. Infect Immun. 2016;84:2607–2615. doi: 10.1128/IAI.01514-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hentrich K, et al. Streptococcus pneumoniae senses a human-like sialic acid profile via the response regulator ciaR. Cell Host Microbe. 2016;20:307–317. doi: 10.1016/j.chom.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gratz N, et al. Pneumococcal neuraminidase activates TGF-beta signalling. Microbiology. 2017;163:1198–1207. doi: 10.1099/mic.0.000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hall-Stoodley L, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Weimer KE, et al. Coinfection with Haemophilus influenzae promotes pneumococcal biofilm formation during experimental otitis media and impedes the progression of pneumococcal disease. J Infect Dis. 2010;202:1068–1075. doi: 10.1086/656046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trappetti C, Ogunniyi AD, Oggioni MR, Paton JC. Extracellular matrix formation enhances the ability of Streptococcus pneumoniae to cause invasive disease. PLoS ONE. 2011;6:e19844. doi: 10.1371/journal.pone.0019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blanchette KA, et al. Neuraminidase A-exposed galactose promotes Streptococcus pneumoniae biofilm formation during colonization. Infect Immun. 2016;84:2922–2932. doi: 10.1128/IAI.00277-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanchez CJ, et al. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog. 2010;6:e1001044. doi: 10.1371/journal.ppat.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rose L, et al. Antibodies against PsrP, a novel Streptococcus pneumoniae adhesin, block adhesion and protect mice against pneumococcal challenge. J Infect Dis. 2008;198:375–383. doi: 10.1086/589775. [DOI] [PubMed] [Google Scholar]
- 115.Kim JO, Weiser JN. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 116.Manso AS, et al. A random six-phase switch regulates pneumococcal virulence via global epigenetic changes. Nat Commun. 2014;5:5055. doi: 10.1038/ncomms6055. This study elucidates the mechanism underlying the phenomenon of colony opacity phase variation in S. pneumoniae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Trappetti C, Potter AJ, Paton AW, Oggioni MR, Paton JC. LuxS mediates iron-dependent biofilm formation, competence, and fratricide in Streptococcus pneumoniae. Infect Immun. 2011;79:4550–4558. doi: 10.1128/IAI.05644-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Orihuela CJ, et al. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest. 2009;119:1638–1646. doi: 10.1172/JCI36759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brown AO, et al. Streptococcus pneumoniae translocates into the myocardium and forms unique microlesions that disrupt cardiac function. PLoS Pathog. 2014;10:e1004383. doi: 10.1371/journal.ppat.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iovino F, et al. pIgR and PECAM-1 bind to pneumococcal adhesins RrgA and PspC mediating bacterial brain invasion. J Exp Med. 2017;214:1619–1630. doi: 10.1084/jem.20161668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Ginkel FW, et al. Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. Proc Natl Acad Sci USA. 2003;100:14363–14367. doi: 10.1073/pnas.2235844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Talbot UM, Paton AW, Paton JC. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect Immun. 1996;64:3772–3777. doi: 10.1128/iai.64.9.3772-3777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hammerschmidt S, et al. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun. 2005;73:4653–4667. doi: 10.1128/IAI.73.8.4653-4667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kietzman CC, Gao G, Mann B, Myers L, Tuomanen EI. Dynamic capsule restructuring by the main pneumococcal autolysin LytA in response to the epithelium. Nat Commun. 2016;7:10859. doi: 10.1038/ncomms10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mitchell TJ, Dalziel CE. The biology of pneumolysin. Subcell Biochem. 2014;80:145–160. doi: 10.1007/978-94-017-8881-6_8. [DOI] [PubMed] [Google Scholar]
- 126.Rayner CF, et al. Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect Immun. 1995;63:442–447. doi: 10.1128/iai.63.2.442-447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mahdi LK, Wang H, Van der Hoek MB, Paton JC, Ogunniyi AD. Identification of a novel pneumococcal vaccine antigen preferentially expressed during meningitis in mice. J Clin Invest. 2012;122:2208–2220. doi: 10.1172/JCI45850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Berry AM, Paton JC. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun. 2000;68:133–140. doi: 10.1128/iai.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chiavolini D, et al. The three extra-cellular zinc metalloproteinases of Streptococcus pneumoniae have a different impact on virulence in mice. BMC Microbiol. 2003;3:14. doi: 10.1186/1471-2180-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Attali C, Durmort C, Vernet T, Di Guilmi AM. The interaction of Streptococcus pneumoniae with plasmin mediates transmigration across endothelial and epithelial monolayers by intercellular junction cleavage. Infect Immun. 2008;76:5350–5356. doi: 10.1128/IAI.00184-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bergmann S, Rohde M, Preissner KT, Hammerschmidt S. The nine residue plasminogen-binding motif of the pneumococcal enolase is the major cofactor of plasmin-mediated degradation of extracellular matrix, dissolution of fibrin and transmigration. Thromb Haemostasis. 2005;94:304–311. doi: 10.1160/TH05-05-0369. [DOI] [PubMed] [Google Scholar]
- 132.Standish A, Weiser J. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol. 2009;183:2602–2609. doi: 10.4049/jimmunol.0900688. [DOI] [PubMed] [Google Scholar]
- 133.Hergott CB, et al. Bacterial exploitation of phosphorylcholine mimicry suppresses inflammation to promote airway infection. J Clin Invest. 2015;125:3878–3890. doi: 10.1172/JCI81888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Andre GO, et al. Role of Streptococcus pneumoniae proteins in evasion of complement-mediated immunity. Front Microbiol. 2017;8:224. doi: 10.3389/fmicb.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010;78:704–715. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hyams C, et al. Streptococcus pneumoniae capsular serotype invasiveness correlates with the degree of factor H binding and opsonization with C3b/iC3b. Infect Immun. 2013;81:354–363. doi: 10.1128/IAI.00862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hammerschmidt S, Talay SR, Brandtzaeg P, Chhatwal GS. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–1124. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 138.Dieudonne-Vatran A, et al. Clinical isolates of Streptococcus pneumoniae bind the complement inhibitor C4b-binding protein in a PspC allele-dependent fashion. J Immunol. 2009;182:7865–7877. doi: 10.4049/jimmunol.0802376. [DOI] [PubMed] [Google Scholar]
- 139.Kohler S, et al. Binding of vitronectin and Factor H to Hic contributes to immune evasion of Streptococcus pneumoniae serotype 3. Thromb Haemostasis. 2015;113:125–142. doi: 10.1160/TH14-06-0561. [DOI] [PubMed] [Google Scholar]
- 140.Tu AH, Fulgham RL, McCrory MA, Briles DE, Szalai AJ. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immun. 1999;67:4720–4724. doi: 10.1128/iai.67.9.4720-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Paton JC, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984;43:1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yuste J, Botto M, Paton JC, Holden DW, Brown JS. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J Immunol. 2005;175:1813–1819. doi: 10.4049/jimmunol.175.3.1813. [DOI] [PubMed] [Google Scholar]
- 143.Dalia A, Standish A, Weiser J. Three surface exoglycosidases from Streptococcus pneumoniae, NanA, BgaA, and StrH, promote resistance to opsonophagocytic killing by human neutrophils. Infect Immun. 2010;78:2108–2116. doi: 10.1128/IAI.01125-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dalia A, Weiser J. Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell Host Microbe. 2011;10:486–496. doi: 10.1016/j.chom.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O'Brien KL, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–1220. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 146.Geno KA, et al. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 2015;28:871–899. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Klugman K. The significance of serotype replacement for pneumococcal disease and antibiotic resistance. Adv Exp Med Biol. 2009;634:121–128. doi: 10.1007/978-0-387-79838-7_11. [DOI] [PubMed] [Google Scholar]
- 148.von Gottberg A, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371:1889–1899. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]