Abstract
Metabotropic glutamate receptors (mGluRs) are heavily expressed throughout the basal ganglia (BG), where they modulate neuronal excitability, transmitter release and long term synaptic plasticity. Therefore, targeting specific mGluR subtypes by means of selective drugs could be a possible strategy for restoring normal synaptic function and neuronal activity of the BG in Parkinson disease (PD). Preclinical studies have revealed that specific mGluR subtypes mediate significant neuroprotective effects that reduce toxin-induced midbrain dopaminergic neuronal death in animal models of PD. Although the underlying mechanisms of these effects must be further studied, there is evidence that intracellular calcium regulation, anti-inflammatory effects, and glutamatergic network modulation contribute to some of these neuroprotective properties. It is noteworthy that these protective effects extend beyond midbrain dopaminergic neurons to include other monoaminergic cell groups for some mGluRs. In this review, we discuss evidence for mGluR-mediated neuroprotection in PD and highlight the challenges to translate these findings into human trials.
Keywords: Parkinson disease, Degeneration, Basal ganglia, Substantia nigra, Dopamine, Norepinephrine, Serotonin, MPTP, Excitotoxicity, Inflammation
Introduction
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disorder clinically characterized by motor disturbances such as resting tremor, slowness of movement (bradykinesia), difficulty in initiating movements (akinesia), rigidity and postural instability. The motor symptoms largely arise through the progressive degeneration of dopamine (DA) neurons in the substantia nigra pars compacta (SNC). These DA neurons are a key component of the basal ganglia (BG), a highly organized network of brain nuclei implicated in motor, limbic and cognitive functions that receive massive extrinsic glutamatergic projections from the cortex and thalamus (Fig. 1). The onset of parkinsonian motor symptoms appears only after a critical threshold of 50–60% DA neurons loss in SNC and 70–80% degeneration of striatal DA terminals has been reached [1]. This lag between the development of motor deficits and the protracted extent of the nigrostriatal degenerative process provides an opportunity for neuroprotective intervention that could slow down the degeneration of the DA system, thereby delay or prevent the development of parkinsonian motor symptoms.
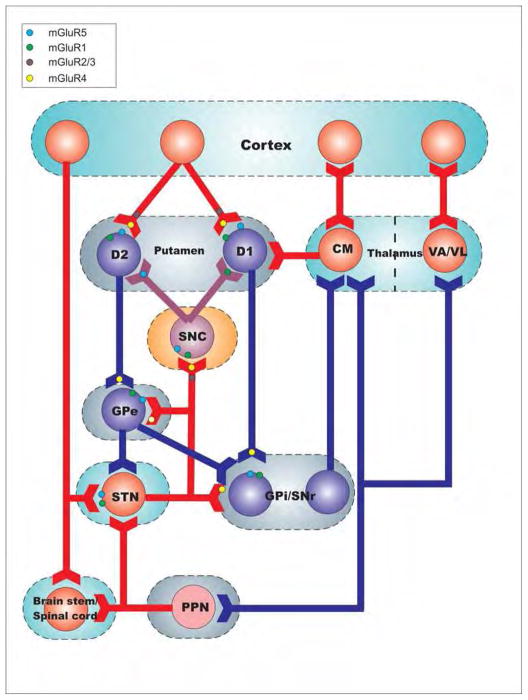
Figure 1.
Localization of metabotropic glutamate receptors (mGluR) subtypes in the basal ganglia motor circuit. Eight mGluR subtypes (mGluR1–mGluR8) have been cloned and divided into three groups (Groups I–III). Group I mGluRs (mGluR1 and 5) are predominantly expressed post-synaptically in dendrites and spines. Pre-synaptic expression in nigrostriatal dopamine terminals has also been reported in the primate striatum. Group II (mGluRs 2 and 3) and group III (mGluRs 4, 6, 7 and 8) are mainly localized pre-synaptically in glutamatergic and non-glutamatergic terminals, where they act as auto- or hetero-receptors that reduce neurotransmitter release. The blockade of group I receptors and activation of groups II and III mGluRs can reduce glutamatergic signalling and dampen neuronal excitability giving them potential neuroprotective properties.
It is well recognized that PD symptoms extend beyond motor deficits, and include cognitive, psychiatric and autonomic dysfunctions. These non-motor signs are increasingly recognized as being part of the wider clinical syndrome of PD and a major source of decreased quality of life for PD patients [2]. There is compelling evidence that some of these non-motor deficits result from the degeneration of noradrenergic neurons in the locus coeruleus (LC) and adjoining areas, a pathological feature described in the post-mortem brain of PD patients [3,4]. To date, the most effective treatment for parkinsonian motor symptoms relies on DA replacement therapy. However, as the disease advances, there is an emergence of complications related to long-term symptomatic treatment, including motor and non-motor fluctuations, dyskinesia, and psychosis [5]. Thus, there is an urgent need to develop therapies that slow down the progression of neurodegeneration in PD.
The development of therapeutic approaches that could slow down the death of midbrain DAergic neurons has been of great interest during the past decades. Although there is preclinical evidence that blockade of AMPA and NMDA ionotropic glutamate receptors protects midbrain dopaminergic neurons from toxin-induced degeneration in rodent and monkey models of PD [6], chronic administration of ionotropic glutamate receptor antagonists elicits unwanted side effects in humans, thereby limiting their usefulness as therapeutics [6].
Because of their modulatory effects, localization specificity and potential for drug targeting at allosteric modulatory sites, the G protein-coupled metabotropic glutamate receptors have generated significant interest as new therapeutic targets for brain diseases, including PD [6,7].
In this review, we will discuss evidence for neuroprotective properties of different subtypes of mGluRs in PD and the challenges that need to be overcome to translate the findings of preclinical neuroprotection studies in animal models to the human diseased condition.
Anatomical localization and function of mGluRs in the basal ganglia circuitry
The mGluRs family comprises eight receptor subtypes classified into three groups: group I (mGluR1 and 5), group II (mGluR2 and 3), and group III (mGluR4, 6, 7 and 8), based on their sequence homology, signal transduction mechanisms and pharmacological profiles [7].
The group I mGluRs, mGluR1 and mGluR5, are widely expressed in basal ganglia nuclei, including DAergic neurons of the SNC, striatal projection neurons and interneurons [7–9], globus pallidus (GP) [10] and subthalamic nucleus (STN) [9] (Fig. 1). The mGluR5 is also expressed in astrocytes and microglia in rats [7,10]. At the subcellular level, the two receptor subtypes are mainly found post-synaptically in dendritic spines and shafts, where they enhance excitability through various post-synaptic mechanisms that involve regulation of intracellular calcium, functional interactions with NMDA receptors and modulation of voltage-gated calcium channels [7,11,12]. In addition, these receptors are coupled to Gq proteins and positively modulate neuronal excitability through activation of phospholipase C leading to an increase in intracellular Ca2+ and protein kinase C [7,12].
Group II mGluRs (mGluR2 and mGluR3) are differentially expressed within BG nuclei (Fig. 1), the striatum being the most enriched nucleus, where they are found in glutamatergic axons and terminals, cholinergic interneurons and astrocytes [7–9]. Functionally, striatal group II mGluRs regulate glutamate release from the cortex and thalamus and dopamine release from the ventral midbrain, through direct or indirect pre-synaptic mechanisms [8,13]. Other BG nuclei display low to moderate levels of neuronal group II mGluRs mRNA [9] and protein immunoreactivity [8]. Electrophysiological studies have shown that activation of group II mGluRs reduces glutamatergic transmission in the STN [14], the GP and the SN [7]. Thus, group II mGluR agonists could help restore BG circuitry imbalance in the parkinsonian state by reducing the output of glutamatergic corticostriatal and subthalamofugal synapses and by inhibiting cholinergic neurons in the striatum (Fig. 1)
The Group III mGluRs, mGluR4, mGluR7 and mGluR8, are expressed throughout the BG circuitry (Fig. 1), but mGluR8 is expressed at a lower level than mGluR4, and mGluR7. In the striatum, mGluR4 and mGluR7 are mainly located pre-synaptically in glutamatergic corticostriatal terminals (Fig. 1). At the level of GPe, GPi, and SNr, mGluR4 and mGluR7 are abundantly expressed in striatal GABAergic terminals [15,16]. In addition, significant presynaptic mGluR4 expression has been reported on putative STN glutamatergic terminals in the GP and SNr of both rodents and nonhuman primates [15,16] (Fig. 1).
Activation of group III mGluRs reduces the excitatory glutamatergic drive to the STN and the SNC through presynaptic mechanisms [17]. It has been suggested that this downregulation of glutamatergic transmission upon SNC neurons may contribute to the neuroprotective effects of mGluR4 orthosteric agonists (or positive allosteric modulators-PAM) against toxin-induced degeneration of midbrain DAergic neurons in models of PD [18].
Thus, because of their pre-synaptic localization and Gi/o-mediated dampening effects upon glutamate release, the potential neuroprotective properties of group II (mGluRs 2 and 3) and group III (mGluRs 4, 6, 7 and 8) mGluRs have generated significant interest [19,20].
Neuroprotective effects of group I mGluRs
Battaglia et al. first showed that systemic administration of the mGluR5 antagonist, 2-methyl-6-(phenylethynyl) pyridine (MPEP), protected striatal dopaminergic terminals against toxicity caused by methamphetamine [21], a psychostimulant that also leads to nigro-striatal degeneration in humans. Further evidence for mGluR5-mediated neuroprotection of the nigrostriatal dopaminergic system came from MPEP-treated or mGluR5 knockout mice which both displayed increased survival of nigrostriatal DA neurons after administration of the dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [22]. Recently, we have shown that systemic administration of the mGluR5 negative allosteric modulator, 3-[(2-methyl-1,3- thiazol-4-yl) ethynyl] pyridine (MTEP), is highly protective against MPTP-induced neurodegeneration in non-human primates [23]. Furthermore, these data also showed that MTEP treatment protects noradrenergic and serotonergic neurons of the locus coeruleus (LC) and dorsal raphe (DR) against MPTP toxicity [23] (Figs 2 and 3). These observations are particularly relevant because degeneration of the LC and DR has been associated with non-motor symptoms of PD, such as depression, REM sleep behavior disorder, autonomic dysfunction, and pain [3]. Altogether, these rodent and monkey data suggest that the use of mGluR5 antagonists may be a useful strategy to reduce degeneration of monoaminergic neurons in PD. Taking into consideration evidence that mGluR5 antagonists also have significant anti-dyskinetic effects in rodent and non-human primate models of PD [24,25] and some anti-parkinsonian effects in 6-OHDA-treated rats [26], these findings provide additional support for the potential use of mGluR5 antagonist as PD-relevant therapeutic.
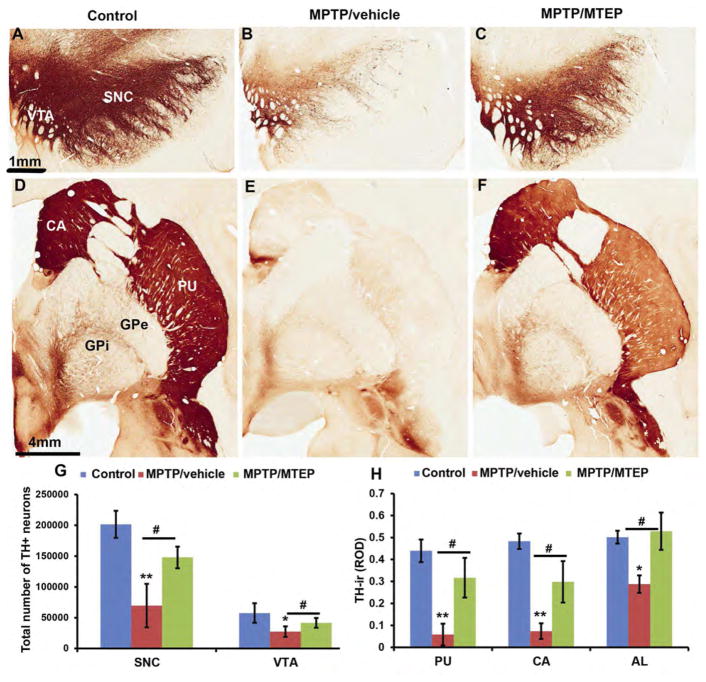
Figure 2.
Photomicrographs of TH-immunostained coronal sections at the level of the midbrain dopaminergic cell groups (A–C) and post-commissural striatum (D–F) of a control (left column), MPTP/vehicle-treated (middle column) and a MPTP/MTEP-treated (right column) monkey. Abbreviations: SNC: substantia nigra compacta, Th: thalamus; VTA: ventral tegmental area. CA: caudate nucleus; PU: putamen GPe: globus pallidus, external segment; GPi: globus pallidus, internal segment. Scale bars: A–C = 1 mm, D–F = 4 mm. G. Stereological estimate of the total number of TH-positive neurons (means ± SD) and H: Densitometry analysis in SNC and VTA regions of control, MPTP/vehicle and MPTP/MTEP treated monkeys. **, p < 0.001 and *, p< 0.05 for differences from control and MPTP/vehicle. #, p < 0.05 for differences between the vehicle and MTEP-treated animals. There were no significant difference found in the VTA between control and MPTP/MTEP treated monkeys. (See Masilamoni et al., 2011 for additional details)
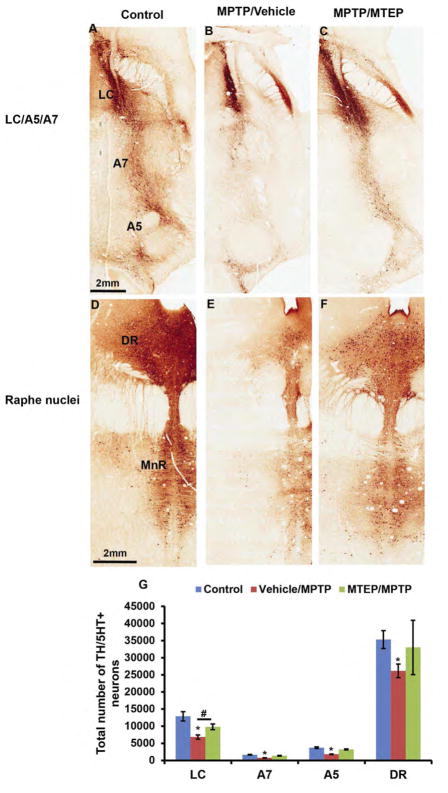
Figure 3.
Photomicrographs of TH- and 5HT-immunostained coronal sections at the level of the noradrenergic cells groups (A–C) and serotonergic cell groups (D–F) of a control (left column), MPTP/vehicle treated (middle column) and MPTP/MTEP treated (right column) monkey. Abbreviations: A5, A7: Noradrenergic cell groups A5 and A7; DR: dorsal raphe; MnR: Scale bars: A–F = 2 mm. (G) Stereological estimate of the total number of tyrosine hydroxylase-positive (TH+) and (5 HT+) neurons (means ± SD) in the LC, A7, A5 and DR regions of control, MPTP/vehicle and MPTP/MTEP-treated monkeys. *P < 0.001; **P < 0.05 for differences between control, MPTP/vehicle and MPTP/MTEP-treated animals by using one-way ANOVA with Tukey’s post hoc test. (See Masilamoni et al., 2011, 2016 for additional details)
The evidence that various mGluR5 antagonists (AFQ056-mavoglurant; ADX-48621-dipraglurant) currently being tested in human trials as anti-dyskinetic drugs are well tolerated and do not worsen PD motor symptoms [27] is encouraging, and provides further support towards the chronic use of mGluR5-related compounds as potential neuroprotective drug in PD.
Although the exact mechanisms by which mGluR5 antagonists mediate their neuroprotective effects upon catecholaminergic neurons in toxin-based models of PD remain to be established, some interesting possibilities have been raised. In light of evidence that calcium dysregulation, mitochondrial respiration impairment and excitotoxic insults contribute to the degeneration of nigral dopaminergic neurons in PD [7,20,23], the blockade of mGluR5 may provide its protective effects through reduction of intracellular calcium levels in dopaminergic neurons via decreased mGluR5-mediated activation of intracellular IP3 receptors [7,11,12,20] and/or reduction of the potentiating effects of mGluR5 upon NMDA receptor function [7,11,20]. An alternative mechanism could involve the reduction of overactive glutamatergic inputs from the STN to SNC neurons in the PD state [131,134–139]. Finally, because glial mGluR5 expression is up-regulated in some inflammatory processes [7,20], the blockade of these receptors may reduce these harmful effects and attenuate MPTP-induced toxicity towards midbrain dopaminergic neurons.
Neuroprotective effects of Group II mGluRs in PD
It has been suggested that selective Group II mGluR agonists might be a good target for PD therapy because they can pre-synaptically reduce excitatory glutamatergic neurotransmission at cortico-striatal synapses, known to be overactive in models of PD [28]. Pharmacological activation of mGluR2/3, indeed, reduces akinesia in reserpine-treated animal models of PD [29]. Furthermore, activation of group II mGluRs at the glutamatergic STN-SNC synapses reduces the amplitude of excitatory postsynaptic currents in rat SNC neurons [30]. In line with these observations, systemic treatment or intranigral administration of the group II mGluR agonists LY379268 and (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (2R,4R-APDC) reduces the extent of 6-OHDA-induced toxicity of dopaminergic neurons in the rat SNC [31]. In addition, activation of group II mGluRs by dual mGluR2/3 receptor agonist LY379268 or DCG-IV reduces SNC degeneration in mice after intrastriatal MPP+ or systemic MPTP administration, further supporting a role for group II mGluR agonists as disease-modifying agents in PD. The exact role played by mGluR2 vs mGluR3 in the neuroprotective benefits of group II mGluRs activation is not clear. The development of subtype selective agonists may help further address this issue. Through the use of the mGluR2/3 receptor agonist LY379268 in mGluR2 and mGluR3 receptor knockout mice, Corti et al. suggested that the neuroprotective properties of LY379268 in MPTP-treated mice were entirely mediated by activation of the astrocytic mGluR3 receptor and that these effects were amplified in the absence of neuronal mGluR2 receptors, suggesting that mGluR2 activation might be harmful to toxin exposure. Additional studies are needed to assess the neuroprotective properties of these specific mGluR2-related compounds in animal models of PD.
Studies of the neuroprotective properties of the selective mGluR3 receptor agonist, N-acetylaspartylglutamate (NAAG) [32], have been limited by the poor blood-brain barrier permeability of this compound [33]. There is evidence that activation of mGluR3 enhances the production of several neurotrophic factors, including TGF-β and glial derived nerve growth factor (GDNF) [34], which is highly relevant because GDNF has potent neurotrophic and neuroprotective effects upon nigral dopaminergic neurons in a variety of preclinical models of parkinsonism [35]. Future studies using mGluR3-related drugs with better brain access are warranted to assess the relevance of mGluR3 as a potential target for neuroprotection of dopaminergic neurons in PD.
Neuroprotective effects of Group III mGluRs in PD
The recent development of highly specific and potent group III mGluR-related allosteric modulators and orthosteric agonists has enabled the testing of the neuroprotective properties of specific group III mGluR subtypes in various PD models [7,36]. There is evidence that the pharmacological activation of group III mGluRs protects against NMDA-induced toxicity in cultured neurons and in vivo, an effect that is absent in mice lacking mGluR4, suggesting that mGluR4 is an important mediator of neuroprotection against excitotoxic insult [37]. Consistent with the prediction that group III mGluRs activation has neuroprotective effects in PD, both acute and subchronic intranigral infusion of the group III mGluRs agonist L-AP4 reduced the extent of 6-OHDA toxicity in the rat SNC [31,36]. Furthermore, systemic or intrapallidal administration of the mGluR4 allosteric potentiator, PHCCC (Phenyl-7-(hydroxyimino) cyclopropopa[b] chromen-1a-carboxamide), reduced the extent of nigrostriatal MPTP toxicity in wild type mice, but not in mice lacking mGluR4, further supporting the selective activation of mGluR4 as a therapeutic strategy for neuroprotection in PD [18].
Various potential mechanisms have been suggested through which group III mGluRs activation could mediate their neuroprotective effects in PD models. The anatomical, electrophysiological and pharmacological data certainly point towards direct or indirect inhibition of glutamate release in the SNC [7,20,36,37]. Activation of mGluR4 inhibits GABA release at the striatopallidal synapse, an effect that likely underlies some of the antiparkinsonian effects of mGluR4 PAM on bradykinesia and other motor symptoms associated with PD [7]. Because the over-activity of the indirect pathway is implicated not only in the pathophysiology of motor symptoms, but also in the ongoing degeneration of nigral dopaminergic neurons induced by the possible excitotoxic effects of over-active glutamatergic projections from the STN [7,36], the downregulation of GABAergic striatopallidal transmission may be another means through which mGluR4 PAM could mediate their neuroprotective effects upon SNC neurons in PD.
Activation of group III mGluRs also mediates anti-inflammatory effects [38]. For example, lipopolysaccharide (LPS)-induced microglial activation in vitro is attenuated by the group III mGluR agonists L-AP4 and RS-PPG [38]. Activation of glial mGluR4 reduces the production of RANTES, a chemokine involved in neuro-inflammation that circulates at higher levels in humans with PD compared with age- and sex-matched controls [39]. L-AP4 is similarly protective against neuronal death induced by myelin-exposed microglia, a finding attributable to the mGluR-mediated inhibition of soluble toxin production by activated microglia [40]. Similarly, oligodendrocyte precursor cells found in lesion sites are particularly vulnerable to cytotoxic and pro-inflammatory factors released by activated microglia, and stimulation of group III mGluRs by L-AP4 prevents microglial-induced inhibition of oligodendrocyte precursor cell proliferation [41]. Although these mechanisms have yet to be explored in animal models of PD, given that gliosis and inflammation are increasingly recognized as features of PD pathology [42], it is very likely that some component derived from activation of astroglial group III mGluRs contributes to the overall protective potential of targeting these receptors in PD.
Furthermore, studies using group III mGluR-selective agonists (ACPT-I, L-AP4) and mGluR4 orthosteric agonists and PAMs (PHCCC, VU0155041) demonstrated their antiparkinsonian effects in various rodent models of PD, which provide additional support for the potential use of mGluR4 potentiators as PD-relevant therapeutic [43,44].
In contrast to mGluR4, much less is known about the anti-parkinsonian and neuroprotective properties of other group III mGluR subtypes (mGluR7 and mGluR8). [45,] [46].
Pre-clinical Neuroprotection data to Clinical Trials: Challenges and Hopes
Efforts to translate these preclinical data about mGluR-mediated neuroprotection to the human trials face some challenges because of the constant failure of previous neuroprotective trials in PD [47]. Although the failure of these trials is not fully explained, various suggestions have been made including the choice of patients with profound dopaminergic cell loss [48], lack of precise knowledge of the mechanisms responsible for cell death, lack of relevant animal models, uncertainty as to drug dosing, and the lack of a clinical trial methodology that can unequivocally define a disease-modifying therapy which is not confounded by symptomatic or pharmacological effects of the study intervention [49].
Future trials of any putative neuroprotective drugs (including mGluR-related compounds) should be aimed at patients chosen at an early stage of the disease, prior to the appearance of motor symptoms and severe dopaminergic depletion. Because of the lack of PD biomarkers, the recruitment of such patients faces challenges. However, current efforts at identifying PD biomarkers such as the Parkinson’s Progression Marker Initiative (PPMI) hold promise for future PD neuroprotective trials [50]. The use of animal models that more faithfully mimic PD pathology to test potential neuroprotective drugs is also warranted. The current efforts at developing alpha-synuclein-based nonhuman primate models of PD may provide the necessary tools to achieve this goal [51].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114( Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 2.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 3**.Fornai F, di Poggio AB, Pellegrini A, Ruggieri S, Paparelli A. Noradrenaline in Parkinson’s disease: from disease progression to current therapeutics. Curr Med Chem. 2007;14:2330–2334. doi: 10.2174/092986707781745550. This is a concise review of evidence for early degeneration of noradrenergic neurons in PD and its consequences on the subsequent loss of midbrain dopaminergic neurons and the developmnet of motor and non-motor PD symptoms. [DOI] [PubMed] [Google Scholar]
- 4.Vermeiren Y, De Deyn PP. Targeting the norepinephrinergic system in Parkinson’s disease and related disorders: The locus coeruleus story. Neurochem Int. 2017;102:22–32. doi: 10.1016/j.neuint.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Obeso JA, Olanow CW, Nutt JG. Levodopa motor complications in Parkinson’s disease. Trends Neurosci. 2000;23:S2–7. doi: 10.1016/s1471-1931(00)00031-8. [DOI] [PubMed] [Google Scholar]
- 6.Johnson KA, Conn PJ, Niswender CM. Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS Neurol Disord Drug Targets. 2009;8:475–491. doi: 10.2174/187152709789824606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. This is a comprehensive review of the localization and function of mGluRs in the basal ganglia circuitry. [DOI] [PubMed] [Google Scholar]
- 8.Testa CM, Friberg IK, Weiss SW, Standaert DG. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J Comp Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- 9**.Testa CM, Standaert DG, Young AB, Penney JB., Jr Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. One of the pioneering manuscript that provides high quality maps of the localization of various mGluR mRNA subtypes in the rodent brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- 11.Alagarsamy S, Marino MJ, Rouse ST, Gereau RWt, Heinemann SF, Conn PJ. Activation of NMDA receptors reverses desensitization of mGluR5 in native and recombinant systems. Nat Neurosci. 1999;2:234–240. doi: 10.1038/6338. [DOI] [PubMed] [Google Scholar]
- 12.Sala C, Roussignol G, Meldolesi J, Fagni L. Key role of the postsynaptic density scaffold proteins Shank and Homer in the functional architecture of Ca2+ homeostasis at dendritic spines in hippocampal neurons. J Neurosci. 2005;25:4587–4592. doi: 10.1523/JNEUROSCI.4822-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson KA, Mateo Y, Lovinger DM. Metabotropic glutamate receptor 2 inhibits thalamically-driven glutamate and dopamine release in the dorsal striatum. Neuropharmacology. 2017;117:114–123. doi: 10.1016/j.neuropharm.2017.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Shen KZ, Johnson SW. Group II metabotropic glutamate receptor modulation of excitatory transmission in rat subthalamic nucleus. J Physiol. 2003;553:489–496. doi: 10.1113/jphysiol.2003.052209. One of the key evidence that group II mGluRs pre-synapticcally regulate glutamate release in the subthalamic nucleus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogenpohl J, Galvan A, Hu X, Wichmann T, Smith Y. Metabotropic glutamate receptor 4 in the basal ganglia of parkinsonian monkeys: ultrastructural localization and electrophysiological effects of activation in the striatopallidal complex. Neuropharmacology. 2013;66:242–252. doi: 10.1016/j.neuropharm.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16**.Kinoshita A, Shigemoto R, Ohishi H, van der Putten H, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: a light and electron microscopic study. J Comp Neurol. 1998;393:332–352. One of the first detailed maps of mGluR7 in the rodent brain, including the basal ganglia. [PubMed] [Google Scholar]
- 17.Valenti O, Mannaioni G, Seabrook GR, Conn PJ, Marino MJ. Group III metabotropic glutamate-receptor-mediated modulation of excitatory transmission in rodent substantia nigra pars compacta dopamine neurons. J Pharmacol Exp Ther. 2005;313:1296–1304. doi: 10.1124/jpet.104.080481. [DOI] [PubMed] [Google Scholar]
- 18**.Battaglia G, Busceti CL, Molinaro G, Biagioni F, Traficante A, Nicoletti F, Bruno V. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurosci. 2006;26:7222–7229. doi: 10.1523/JNEUROSCI.1595-06.2006. These data provide strong evidence that mGluR4 activation can reduce degeneration of midbrain dopaminergic neurons in MPTP-treated mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duty S. Therapeutic potential of targeting group III metabotropic glutamate receptors in the treatment of Parkinson’s disease. Br J Pharmacol. 2010;161:271–287. doi: 10.1111/j.1476-5381.2010.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masilamoni GJ, Smith Y. Neuroprotective Properties of Glutamate Metabotropic Glutamate Receptors in Parkinson’s Disease and Other Brain Disorders. In: Ngomba RT, Di Giovanni G, Battaglia G, Nicoletti F, editors. mGLU Receptors. Springer International Publishing; 2017. pp. 103–127. [Google Scholar]
- 21**.Battaglia G, Fornai F, Busceti CL, Aloisi G, Cerrito F, De Blasi A, Melchiorri D, Nicoletti F. Selective blockade of mGlu5 metabotropic glutamate receptors is protective against methamphetamine neurotoxicity. J Neurosci. 2002;22:2135–2141. doi: 10.1523/JNEUROSCI.22-06-02135.2002. One of the early evidence for the neuroprotective effects of mGluR5 antagonist against neuronal loss induced by dopamine neurotoxin in the rodent brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22**.Battaglia G, Busceti CL, Molinaro G, Biagioni F, Storto M, Fornai F, Nicoletti F, Bruno V. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. J Neurosci. 2004;24:828–835. doi: 10.1523/JNEUROSCI.3831-03.2004. This study provides clear evidence that the mGluR5 antagonist protects midbrain dopaminergic neurons from MPTP-induced degeneration in mice. The results of this manuscript served as the foundation for further neuroprotective studies of mGluR5 antagonists in primates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Masilamoni GJ, Bogenpohl JW, Alagille D, Delevich K, Tamagnan G, Votaw JR, Wichmann T, Smith Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain. 2011;134:2057–2073. doi: 10.1093/brain/awr137. This study shows that the chronic exposure to the mGluR5 antagonist MTEP has neuroprotective effects against MPTP-induced neurodegeneration of midbrain dopaminergic neurons and noradrenergic neurons in the locus coeruleus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res Bull. 2006;69:318–326. doi: 10.1016/j.brainresbull.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM. Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther. 2010;333:865–873. doi: 10.1124/jpet.110.166629. [DOI] [PubMed] [Google Scholar]
- 26**.Breysse N, Amalric M, Salin P. Metabotropic glutamate 5 receptor blockade alleviates akinesia by normalizing activity of selective basal-ganglia structures in parkinsonian rats. J Neurosci. 2003;23:8302–8309. doi: 10.1523/JNEUROSCI.23-23-08302.2003. This study provides strong evidence that the chronic administration of the mGluR5 antagonist, MPEP, reverses motor deficits in a rat model of PD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stocchi F, Rascol O, Destee A, Hattori N, Hauser RA, Lang AE, Poewe W, Stacy M, Tolosa E, Gao H, et al. AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Mov Disord. 2013;28:1838–1846. doi: 10.1002/mds.25561. [DOI] [PubMed] [Google Scholar]
- 28**.Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. A comprehensive overview of electrophysiological and neurochemical evidence that the corticostriatal glutamatergic system is overactive in animal models of parkinsonism. [DOI] [PubMed] [Google Scholar]
- 29.Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Kitai ST, Xiang Z. Modulation of excitatory synaptic transmission by endogenous glutamate acting on presynaptic group II mGluRs in rat substantia nigra compacta. J Neurosci Res. 2005;82:778–787. doi: 10.1002/jnr.20694. [DOI] [PubMed] [Google Scholar]
- 31.Vernon AC, Palmer S, Datla KP, Zbarsky V, Croucher MJ, Dexter DT. Neuroprotective effects of metabotropic glutamate receptor ligands in a 6-hydroxydopamine rodent model of Parkinson’s disease. Eur J Neurosci. 2005;22:1799–1806. doi: 10.1111/j.1460-9568.2005.04362.x. [DOI] [PubMed] [Google Scholar]
- 32.Bergeron R, Coyle JT, Tsai G, Greene RW. NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharmacology. 2005;30:7–16. doi: 10.1038/sj.npp.1300559. [DOI] [PubMed] [Google Scholar]
- 33.Westbrook GL, Mayer ML, Namboodiri MA, Neale JH. High concentrations of N-acetylaspartylglutamate (NAAG) selectively activate NMDA receptors on mouse spinal cord neurons in cell culture. J Neurosci. 1986;6:3385–3392. doi: 10.1523/JNEUROSCI.06-11-03385.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Onofrio M, Cuomo L, Battaglia G, Ngomba RT, Storto M, Kingston AE, Orzi F, De Blasi A, Di Iorio P, Nicoletti F, et al. Neuroprotection mediated by glial group-II metabotropic glutamate receptors requires the activation of the MAP kinase and the phosphatidylinositol-3-kinase pathways. J Neurochem. 2001;78:435–445. doi: 10.1046/j.1471-4159.2001.00435.x. [DOI] [PubMed] [Google Scholar]
- 35.d’Anglemont de Tassigny X, Pascual A, Lopez-Barneo J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front Neuroanat. 2015;9:10. doi: 10.3389/fnana.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Austin PJ, Betts MJ, Broadstock M, O’Neill MJ, Mitchell SN, Duty S. Symptomatic and neuroprotective effects following activation of nigral group III metabotropic glutamate receptors in rodent models of Parkinson’s disease. Br J Pharmacol. 2010;160:1741–1753. doi: 10.1111/j.1476-5381.2010.00820.x. This study demonstrates that group III mGluRs activation is neuroprotective against midbrain dopaminergic degeneration in rodent models of Parkinson disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Bruno V, Battaglia G, Ksiazek I, van der Putten H, Catania MV, Giuffrida R, Lukic S, Leonhardt T, Inderbitzin W, Gasparini F, et al. Selective activation of mGlu4 metabotropic glutamate receptors is protective against excitotoxic neuronal death. J Neurosci. 2000;20:6413–6420. doi: 10.1523/JNEUROSCI.20-17-06413.2000. A strong evidence that mGluR4 activation can protect midbrain dopaminergic neurons from excitotoxic death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor DL, Diemel LT, Pocock JM. Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci. 2003;23:2150–2160. doi: 10.1523/JNEUROSCI.23-06-02150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Besong G, Battaglia G, D’Onofrio M, Di Marco R, Ngomba RT, Storto M, Castiglione M, Mangano K, Busceti CL, Nicoletti FR, et al. Activation of group III metabotropic glutamate receptors inhibits the production of RANTES in glial cell cultures. J Neurosci. 2002;22:5403–5411. doi: 10.1523/JNEUROSCI.22-13-05403.2002. This manuscript demonstrates that group III mGluRs activation can attenuate the level of a chemokine involved in neuro-inflammation that circulates at higher levels in humans with PD compared with age- and sex-matched controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinteaux-Jones F, Sevastou IG, Fry VA, Heales S, Baker D, Pocock JM. Myelin-induced microglial neurotoxicity can be controlled by microglial metabotropic glutamate receptors. J Neurochem. 2008;106:442–454. doi: 10.1111/j.1471-4159.2008.05426.x. [DOI] [PubMed] [Google Scholar]
- 41.Taylor DL, Pirianov G, Holland S, McGinnity CJ, Norman AL, Reali C, Diemel LT, Gveric D, Yeung D, Mehmet H. Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. J Neurosci Res. 2010;88:1632–1644. doi: 10.1002/jnr.22335. [DOI] [PubMed] [Google Scholar]
- 42.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43**.Niswender CM, Johnson KA, Weaver CD, Jones CK, Xiang Z, Luo Q, Rodriguez AL, Marlo JE, de Paulis T, Thompson AD, et al. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008;74:1345–1358. doi: 10.1124/mol.108.049551. This study provides evidence for anti-parkinsonisn effects of newly developed mGluR4-related positive allosteric modulators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuomo D, Martella G, Barabino E, Platania P, Vita D, Madeo G, Selvam C, Goudet C, Oueslati N, Pin JP, et al. Metabotropic glutamate receptor subtype 4 selectively modulates both glutamate and GABA transmission in the striatum: implications for Parkinson’s disease treatment. J Neurochem. 2009;109:1096–1105. doi: 10.1111/j.1471-4159.2009.06036.x. [DOI] [PubMed] [Google Scholar]
- 45.Johnson KA, Jones CK, Tantawy MN, Bubser M, Marvanova M, Ansari MS, Baldwin RM, Conn PJ, Niswender CM. The metabotropic glutamate receptor 8 agonist (S)-3,4-DCPG reverses motor deficits in prolonged but not acute models of Parkinson’s disease. Neuropharmacology. 2013;66:187–195. doi: 10.1016/j.neuropharm.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jantas D, Greda A, Golda S, Korostynski M, Lason W. The neuroprotective effects of orthosteric agonists of group II and III mGluRs in primary neuronal cell cultures are dependent on developmental stage. Neuropharmacology. 2016;111:195–211. doi: 10.1016/j.neuropharm.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 47**.Olanow CW. Can we achieve neuroprotection with currently available anti-parkinsonian interventions? Neurology. 2009;72:S59–64. doi: 10.1212/WNL.0b013e318199068b. A comprehensive assessment of the potential shortcomings that have led to the failure of neuroprotective trials in Parkinson’s disease. [DOI] [PubMed] [Google Scholar]
- 48.Schapira AH, Obeso J. Timing of treatment initiation in Parkinson’s disease: a need for reappraisal? Ann Neurol. 2006;59:559–562. doi: 10.1002/ana.20789. [DOI] [PubMed] [Google Scholar]
- 49.Kieburtz K, Olanow CW. Translational experimental therapeutics: The translation of laboratory-based discovery into disease-related therapy. Mt Sinai J Med. 2007;74:7–14. doi: 10.1002/msj.20006. [DOI] [PubMed] [Google Scholar]
- 50**.Parkinson Progression Marker I. The Parkinson Progression Marker Initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. PPMI is an international, multi-center study to identify PD progression biomarkers that will increase understanding of disease etiology and course and help collect tools to enhance the likelihood of success of neuroprotective PD trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Marmion DJ, Kordower JH. alpha-Synuclein nonhuman primate models of Parkinson’s disease. J Neural Transm (Vienna) 2017 doi: 10.1007/s00702-017-1720-0. A recent review of the current state of development of alpha-synuclein-based non-human primate models of Parkinson’s disease. [DOI] [PubMed] [Google Scholar]