Abstract
Objective
Dyslipidemia (DL) is closely related to osteoporosis (OP) while the exact common genetic mechanisms are still largely unknown. We proposed to use novel genetic analysis methods with pleiotropic information to identify potentially novel and/or common genes for the potential shared pathogenesis associated with OP and/or DL.
Methods
We assessed the pleiotropy between PL (plasma lipid) and FNK BMD (femoral neck bone mineral density). We jointly applied the conditional false discovery rate (cFDR) method and the genetic analysis incorporating pleiotropy and annotation (GPA) method to the summary statistics provided by GWASs (genome-wide association studies) of FNK BMD (n = 49,988) and PL (n = 188,577) to identify potentially novel and/or common genes for BMD/PL.
Results
We found strong pleiotropic enrichment between PL and FNK BMD. 245 PL SNPs were identified as potentially novel SNPs by cFDR and GPA. The corresponding genes were enriched in GO (gene ontology) terms “phospholipid homeostasis” and “chylomicron remnant clearance“. Three SNPs (rs2178950, rs9939318 and rs9368716) might be the pleiotropic ones and the corresponding genes NLRC5 (rs2178950) and TRPS1 (rs9939318) were involved in NF-κB signaling pathway and Wnt signaling pathway as well as inflammation and innate immune processes.
Conclusion
Our study validated the pleiotropy between PL and FNK BMD and corroborated the reliability and high-efficiency of cFDR and GPA methods in further analyses of existing GWASs with summary statistics. We identified potentially common and/or novel genes for PL and/or FNK BMD, which may provide new insight and direction for further research.
Keywords: dyslipidemia, osteoporosis, pleiotropy, cFDR, GPA
Introduction
Osteoporosis (OP) is the most common metabolic bone disease, mainly characterized by low bone mineral density (BMD) and deterioration of bone microstructure (Philip and Cyrus 2006). It is a major cause of osteoporotic fracture (OF) in the elderly (Philip and Cyrus 2006). OF may occur with slight collision or even happen spontaneously and lead to severe decrease of life quality, with increased morbidity, mortality, and disability (Porter and Bhimji 2017). Only 33% of elderly women who suffered hip OF will be able to return to independence (Porter and Bhimji 2017). OP is highly prevalent worldwide, over 200 million people suffer from OP (Sözen et al. 2017). In USA, about 8 million women and 2 million men had OP in 2010 (Willson et al. 2015). In China, the prevalence of OP ranged from 6.6% to 19.3% (Wang et al. 2009).
Dyslipidemia (DL) is a condition with abnormal levels of plasma lipid (PL), including abnormally elevated levels of triglyceride (TG), total cholesterol (TC) and low density lipoprotein cholesterol (LDL), and descended levels of high density lipoprotein cholesterol (HDL) (Giner-Galvan et al. 2016). Presence of DL increases the risk of suffering coronary heart disease (CHD) and future cardiovascular (CV) diseases (Fox et al. 2016), which are ranked as the top two causes of premature death and decreased disability-adjusted life-years (DALYs) worldwide (Murray et al. 2015). In US, 36.7% adults were on or suit for lipid-lowing therapy during 2005–2012 (Mercado et al. 2015). In China, the prevalence of DL was 41.9% among adults (Huang et al. 2014).
Experimental and clinical studies have demonstrated that DL is closely related to OP (Wong et al. 2016). In clinic, patients with DL are often diagnosed with OP (Ibrahim et al. 2013). A study with 279 either pre- or post-menopausal women demonstrated that high TC level was associated with low BMD (Jeong et al. 2014). High cholesterol might influence cellular functions of bone tissue, such as increased osteoclast activity and decreased osteoblast function (Mandal 2015). Bone metabolism and lipid metabolism might interact with each other by some molecules, such as osteoprotegerin (OPG) (Maser et al. 2015), apolipoprotein E (APOE) (Singh et al. 2011), peroxisome proliferators-activated receptor γ (PPARγ) (Ren et al. 2016) and vitamin D receptor (VDR) (Hajj et al. 2016). For example, OPG can reduce the production of osteoclasts and regulate the resorption of osteoclasts. Meanwhile, elevated OPG levels have been reported in heart diseases, which may be a link between bone and atherosclerosis - a main complication of DL (Maser et al. 2015). Furthermore, medication for DL therapy, such as statins, can decrease PL and increase BMD simultaneously (Gotoh et al. 2011).
OP and DL both are complex human diseases, which are affected by multiple genes. Both BMD and PL are highly heritable. For BMD, its heritability was about 50%–80% (Videman et al. 2007), and for PL it was 40–60% (Asselbergs et al. 2012). Previous GWASs (Genome wide Association Studies) have identified about 200 loci associated with BMD and about 500 loci associated with PL (https://www.ebi.ac.uk/gwas, Aug 1, 2017). However, these identified SNPs (single nucleotide polymorphisms) only explained a small part of the heritability. Further explorations were required for the missing heritability (Asselbergs et al. 2012; Richards et al. 2012). Considering the multiple genes which might affect OP and DL as well as the high missing heritability, we would like to carry out more studies to explore the potentially novel genetic mechanisms and the potentially shared genetic relationship for OP and/or DL.
Novel pleiotropy informed analytical methods have emerged in recent years, such as conditional false discovery rate (cFDR), ccFDR (conjunction cFDR) (Andreassen et al. 2013) and genetic analysis incorporating pleiotropy and annotation (GPA) (Chung et al. 2014). These methods could incorporate summary statistics from independent GWASs to capture polygenic effects and identify more potentially novel loci for the interested phenotypes. Recently, Andreassen et al. have identified pleiotropy between BMD and PL and found some novel BMD-associated SNPs conditioned on PL by cFDR method (Reppe et al. 2015). Encouraged by their initial study, in this study, we will apply not only the cFDR method but also the complementary GPA method to larger and newer GWAS datasets of PL (Willer et al. 2013) and BMD (Zheng et al. 2015). We hope to validate the pleiotropy between BMD and PL and identify potential common genes as well as potentially novel DL-associated genetic variants, with the results to be obtained from newer and larger datasets and with different analytical approaches for robustness. In our previous studies, we have successfully implemented cFDR analyses on GWAS datasets of femoral neck (FNK) BMD, height, birth weight, type 2 diabetes and coronary artery disease (CAD) and identified potentially novel and pleiotropic genetic variants for these phenotypes simultaneously and respectively (Zeng et al. 2016; Greenbaum et al. 2017; Peng et al. 2017). These experiences would contribute to better implementation of this study.
Materials and Methods
GWAS datasets
We obtained GWAS results of FNK BMD and PL (HDL, LDL, TC, TG) in the form of summary statistic p-values. FNK BMD GWAS dataset contained more than 10 million SNPs from 49,988 subjects, and PL GWAS recruited 188,577 subjects and used GWAS array and Metabochip array to genotype/impute more than 2 million SNPs. Both datasets are the largest for FNK BMD and PL so far. FNK BMD dataset was published online by Genetic Factors for Osteoporosis (GEFOS) (http://www.gefos.org/), and PL dataset was published by Global Lipids Genetics Consortium (GLGC) (http://csg.sph.umich.edu/abecasis/public/lipids2013/). Detailed inclusion criteria and phenotype characteristics for the two GWASs were demonstrated in the original respective papers (Willer et al. 2013; Zheng et al. 2015).
SNP pruning
We performed SNP pruning on FNK BMD and PL datasets respectively before further analysis. Since most of the individuals of the original GWASs were European ancestry, we used the CEU HapMap 3 genotype data for the SNP pruning. First, we merged common SNPs between FNK BMD and each kind of PL. After merging, there were about 2 million SNPs remained in each PL type. According to HapMap3 information, we calculated LD (linkage disequilibrium) values between each pair of SNPs by PLINK 1.9 software. We selected default value of the software (50, 5, 0.2) as parameters, which meant the calculation of LD was performed in a window containing 50 SNPs. And the SNP in each pair with smaller minor allele frequency (MAF) was removed when the LD value (r2) was greater than 0.2. Then the calculation window slid forward with 5 SNPs and repeated the above process until no pairs of SNPs that were in high LD. After SNP merging and pruning, the remaining SNPs were prepared for subsequent analysis.
Statistical analysis
Pleiotropic enrichment estimation
We used “ggplot2” package in R software to construct fold-enrichment plots to estimate the pleiotropic enrichment between FNK BMD and PL. The plots were formed by nominal −log10(p) values at different stratifications. Stratification was divided by p-value of conditional phenotype with the cut-offs as p < 1 (expected base line, all SNPs), p < 0.1, p < 0.01 and p < 0.001. Nominal p-values (−log10 (p)) were plotted on x-axis and fold enrichments were plotted on y-axis. In each cutoff group, for all possible -log10(p-values) on the x axis (between 0 and 10), we compute the fold enrichment values,
where En is the enrichment values. Ni is the proportion of SNPs with −log10(p-values) ≧ x. N0 is the number of all SNPs in each cutoff group, and the i is from 1 to N0. Presence of pleiotropy can be visually observed as an upward shift from the expected base line. And there would be separation between different stratification. The greater separation indicated the stronger pleiotropy.
As a complement for pleiotropic enrichment estimation, we performed a hypothesis testing procedure by GPA. In this testing, likelihood ratio test (LRT) was used to assess statistical significance and show statistical evidence for pleiotropic enrichment. Here we set threshold as p value = 0.05. We downloaded and ran “GPA” package in R software (http://dongjunchung.github.io/GPA/), then we fit GPA model to test the hypothesis for pleiotropy.
Calculation of cFDR, ccFDR and GPA
We set PL as the principal phenotype and FNK BMD as the conditional phenotype. Summary statistic p-values of PL and FNK BMD GWAS datasets were incorporated by common SNPs for calculation of cFDR, ccFDR and GPA. Detailed procedures and formulas were described by Greenbaum and Chung D respectively (Chung et al. 2014; Greenbaum et al. 2017).
The cFDR method (Andreassen et al. 2013) was developed from standard FDR framework (Benjamini et al. 2001). The cFDR value represented a random SNP associated with PL conditioned on FNK BMD. And ccFDR value referred to the possibility of a given SNP that had association with both FNK BMD and PL simultaneously. Both cFDR and ccFDR thresholds were set to 0.05, which meant that the SNP reached this threshold achieved significant association with the corresponding phenotype and the false discovery rate was 5%.
GPA method was performed by “GPA” package in R software. We fit a GPA model with summary statistic p-values of SNPs and performed the analysis. fdr.GPA was used to represent the SNP that was associated with one of the two phenotypes, and fdr11.GPA was used to represent the SNP that was associated with both phenotypes. According to the criterion set in the original paper (Chung et al. 2014), we adopted the same significance threshold of GPA to 0.2. Over this threshold meant that a SNP might not be associated with the corresponding phenotype.
Annotation of potentially novel SNPs for PL
If a SNP met the thresholds (cFDR < 0.05 and fdr.GPA < 0.2) for PL, we conferred it as a significant one. To verify whether the identified PL significant SNPs with p-values > 5E-08 were potentially novel ones, we compared these SNPs to the other previous GWAS results by querying GWAS web site (https://www.ebi.ac.uk/gwas, Aug 1, 2017). After comparison, we input non-repeat SNPs (including the significant SNPs with cFDR < 0.05, fdr.GPA < 0.2 and p values > 5E-8 in the original PL GWAS dataset (Willer et al. 2013), and the previously confirmed SNPs which were reported in the other PL GWASs) into the SNP Annotation and Proxy Search (SNAP, http://archive.broadinstitute.org/mpg/snap/) for LD analysis. In the search options of the website, we chose suitable SNP data set and population panel based on the original GWASs (SNP data set was HapMap3 and population panel was CEU) for pairwise LD calculation. Then we used the default values (r2 threshold = 0.8, distance limit = 500, where distance means the maximum number of kilobase between query and proxy SNP (Johnson et al. 2008)) as the criteria to determine whether the identified novel SNPs were in same LD block with previous GWAS signals. Among these SNPs, only the SNP that had not been identified in other previous GWASs and was not clustered in the same LD block with those previously GWAS confirmed SNPs would be regarded as a potentially novel SNP, otherwise it was regarded as a replication of the previous GWAS results.
Conditional and conjunction Manhattan plots
We used information including SNP number, chromosome position and cFDR or ccFDR value to construct Manhattan plots by R software to visualize the localization of the significant SNPs (Fig. 2 and Fig. 4). The Manhattan plots consist of conditional and conjunction Manhattan plots, marking the significant SNPs and their chromosomal locations. In the conjunction Manhattan plot (Fig. 2), the SNPs with significant −log10(ccFDR) values more than 1.3 (corresponding to a ccFDR value less than 0.05) were shown in the plot. Only those SNPs with fdr11.GPA less than 0.2 were highlighted with SNP numbers. These SNPs were defined as being associated with both phenotypes in the conjunction Manhattan plot. In the conditional Manhattan plot (Fig. 4), the SNPs with −log10(cFDR) values more than 1.3 were determined as being associated with the principal phenotype.
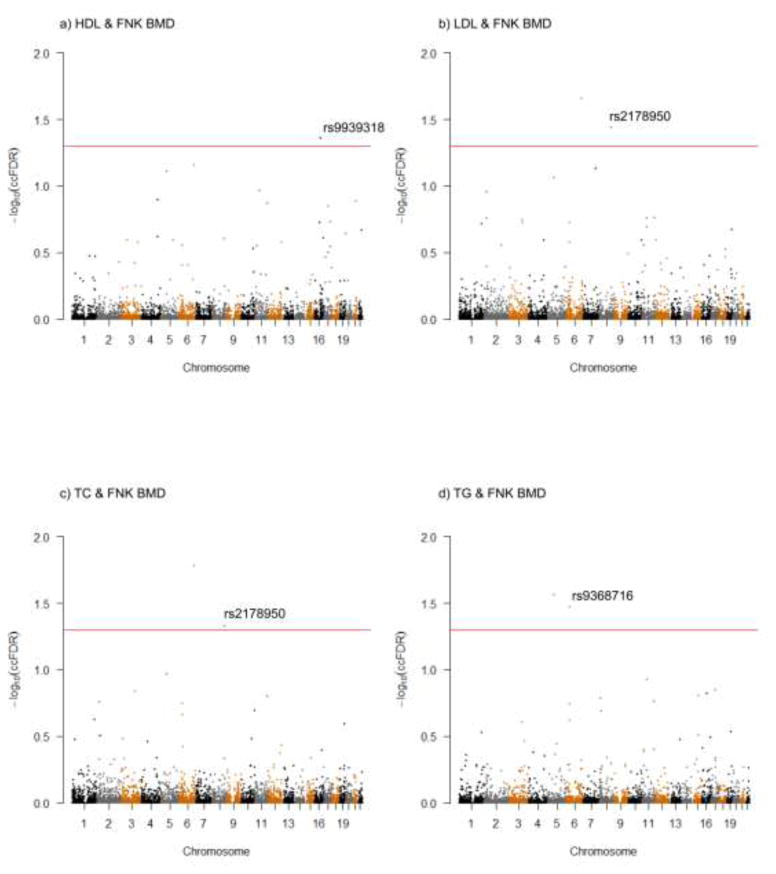
Fig. 2. Conjunction Manhattan plot of −log10(ccFDR) values for each type of PL and FNK BMD.
The black line marking the −log10(ccFDR) value of 1.3 corresponded to a ccFDR < 0.05. The figure showed the genomic locations of pleiotropic SNPs. Only the significant SNP (ccFDR < 0.05 and fdr11.GPA < 0.2) was highlighted with SNP number. Further details were presented in Table 2.
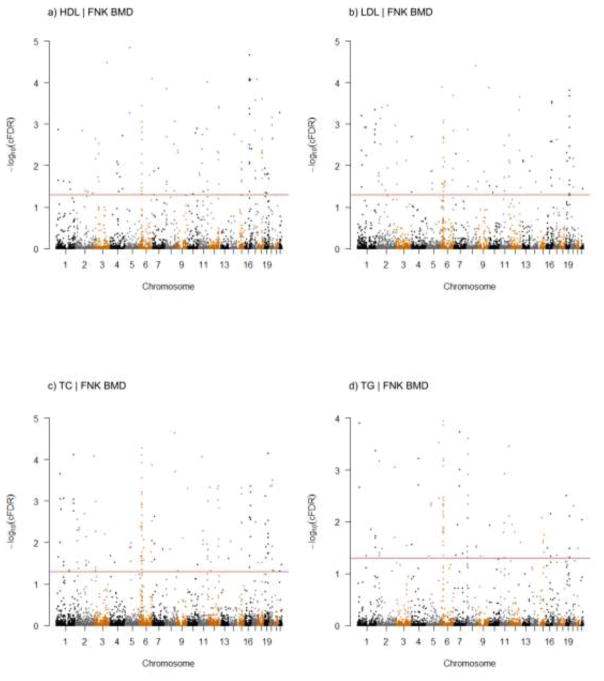
Fig. 4. Conditional Manhattan plot of −log10(cFDR) values for each type of PL conditioned on FNK BMD.
The black line marking the −log10(cFDR) value of 1.3 corresponded to a cFDR < 0.05. The figure marked the chromosomal locations of potentially novel SNPs. Details about all significant SNPs were offered in Supplemental Table 1.
Gene ontology enrichment analysis and protein-protein interaction analysis of the identified SNPs and genes
We mapped the identified SNPs to corresponding genes by the online tool SNP and CNV Annotation Database (SCAN, http://scandb.org/newinterface/about.html). To evaluate the function of these genes and to partially validate our results, we performed Gene Ontology (GO) Enrichment analysis (http://geneontology.org/page/go-enrichment-analysis). Meanwhile, using the online tool STRING 10.0 (http://string-db.org/), we further explored the functional interaction between the proteins produced by the corresponding genes. Protein-protein interaction analysis enabled us to identify the potential associations between the corresponding genes.
Results
Pleiotropy between FNK BMD and PL
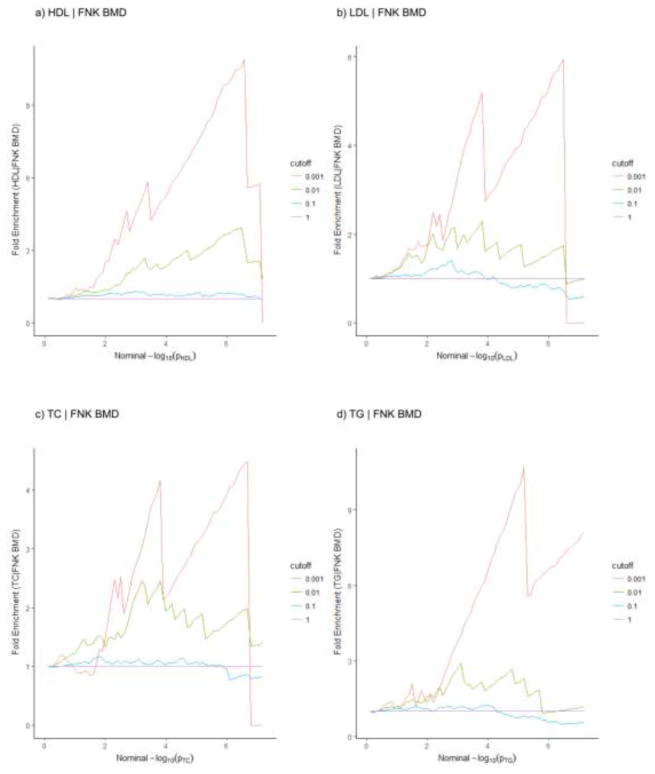
As shown in fold-enrichment plots (Fig. 1), each type of PL was the principal trait while FNK BMD was the conditional trait. When restricting the subset of SNPs with a stronger level of association in the conditional trait, we observed an obvious upward shift from the expected base line. It well demonstrated the pleiotropy between FNK BMD and each type of PL (HDL | FNK BMD, LDL | FNK BMD, TC | FNK BMD and TG | FNK BMD). Among the four plots, HDL conditioned on FNK BMD (HDL | FNK BMD) achieved the most significant pleiotropic enrichment, as a 9.5 fold enrichment was observed in Y axis while comparing the most stringent subset to the all SNPs subset (Fig. 1 a). LRT-p values acted as statistical evidence for pleiotropic enrichment through hypothesis testing of GPA and the results were presented in Table 1. The pleiotropy between FNK BMD and HDL with a LRT-pvalue of 5.92E-03 was still the strongest, suggesting the highest level of pleiotropy between FNK BMD and HDL among all the PL traits.
Fig. 1. Fold-enrichment plots of enrichment versus nominal −log10(p-values) in each type of PL conditioned on FNK BMD.
Each type of PL was as a function of significance of association with FNK BMD. Stratifications were divided by p-values of FNK BMD with the cut-offs as p < 1 (expected base line, all SNPs), p < 0.1, p < 0.01 and p < 0.001. Nominal PL p-values (−log10 (p)) were plotted on x-axis and fold enrichments were plotted on y-axis.
Table 1.
Pleiotropy estimated between FNK BMD and PL by GPA
| LRT | FNK BMD & HDL | FNK BMD & LDL | FNK BMD & TC | FNK BMD & TG |
|---|---|---|---|---|
| test statistics | 7.573 | 2.382 | 2.595 | 1.869 |
| p value | 5.92E-03 | 1.23E-01 | 1.07E-01 | 1.72E-01 |
| π00 | 9.82E-01 (2.5E-03) | 9.82E-01 (2.4E-03) | 9.81E-01 (2.4E-03) | 9.83E-01 (2.4E-03) |
| π01 | 1.55E-02 (2.5E-03) | 1.52E-02 (2.4E-03) | 1.48E-02 (2.4E-03) | 1.49E-02 (2.4E-03) |
| π10 | 2.33E-03 (2.8E-04) | 2.45E-03 (2.6E-04) | 3.38E-03 (3.1E-04) | 1.99E-03 (2.3E-04) |
| π11 | 4.73E-04 (2.0E-04) | 2.45E-04 (1.6E-04) | 2.88E-04 (1.7E-04) | 1.71E-04 (1.3E-04) |
Column definition: LRT - likelihood ratio test; FNK BMD - femoral neck bone mineral density; HDL - high density lipoprotein cholesterol; LDL - low density lipoprotein cholesterol; TC - total cholesterol; TG - triglyceride. The two rows provided LRT statistics and p-values of hypothesis testing respectively; π00 indicates the proportion of the SNP associated with neither of the two phenotypes; π01 and π10 indicate the proportion of the SNP associated with only one of the two phenotypes; π11 indicates the proportion of the SNP associated with both of the two phenotypes; The values in the brackets are standard errors of the estimates.
Potentially pleiotropic SNPs/genes for both FNK BMD and PL
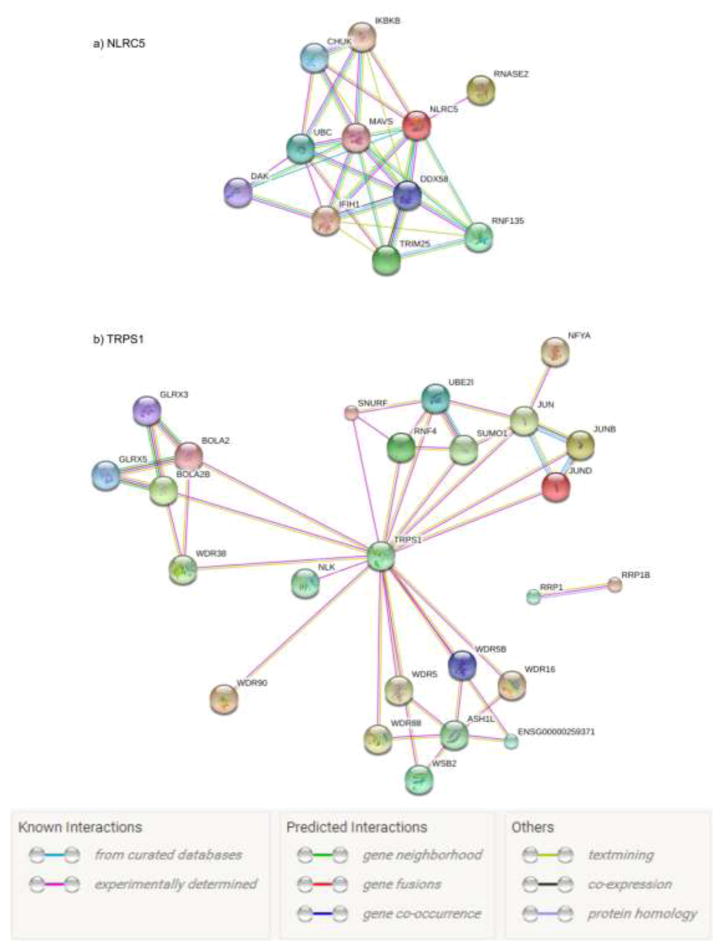
According to the thresholds of ccFDR < 0.05 and fdr11.GPA<0.2, three SNPs (rs2178950, rs9939318 and rs9368716) were identified to be associated with both FNK BMD and PL (Fig. 2). All of the three SNPs’ p-values were higher than 5E-08 in the original FNK BMD and PL GWAS datasets. They were mapped to chromosomes 6, 8, 16 and corresponded to C6orf10/LOC101929163, TRPS1 and NLRC5 genes respectively (Table 2). TRPS1 (rs2178950) gene was involved in autosomal dominant skeletal disorder, encoding a GATA-type transcription factor (Gai et al. 2011). TRPS1 was associated with HDL and CAD in a meta-analysis of 46 GWASs of lipids (Teslovich et al. 2010). NLRC5 (rs9939318) was a member of the NLR family. It acted as a transcriptional activator of MHC class I genes. Meanwhile, it contributed to inflammatory and type I interferon responses in vitro (Benko et al. 2017). According to protein-protein interaction information from “STRING Interaction Network” (Fig. 3), we found these pleiotropic genes interacted with many other bone and/or PL associated genes indirectly. For example, NLRC5 interacted with IKBKB, CHUK, DDX58, IFIH1 and RNF135, while TRPS1 interacted with JUN family and WDR family.
Table 2.
Pleiotropic loci in each type of PL and FNK BMD by ccFDR and fdr11. GPA
| rs_number | CHR | position | p value.FN | p value.PL | ccFDR | fdr11.GPA.noAnn | phenotype | Gene |
|---|---|---|---|---|---|---|---|---|
| rs9368716 | 6 | 32306090 | 0.00033 | 5.68E-06 | 0.03366 | 0.195415481 | TG | C6orf10 LOC101929163 |
| rs2178950 | 8 | 116653018 | 0.000395 | 2.96E-07 (LDL), 1.65E-07 (TC) | 0.035945 | 0.169010177 | LDL,TC | TRPS1 |
| rs9939318 | 16 | 57055184 | 0.000908 | 2.01E-07 | 0.043584 | 0.147473949 | HDL | NLRC5 |
Column definition: rs_number – formal number of single SNP; CHR – chromosome; position – chromosomal position of SNP; p value.FN - summary statistic p value of FNK BMD SNP; p value.PL - summary statistic p value of PL SNP; ccFDR - conjunction conditional false discovery rate; fdr11.GPA.noAnn - false discovery rate of GPA when one SNP was associated with both phenotypes without annotation information; phenotype - principal phenotype of the SNP; Gene - genes corresponding to the significant SNPs.
Fig. 3. Protein-protein interaction networks for significant genes NLRC5 and TRPS1.
a) Genes interacted with NLRC5. b) Genes interacted with TRPS1. Connections were based on evidence with “STRING Interaction Network Preview”. Network nodes represented proteins produced by the corresponding genes. Edges between nodes indicated protein-protein associations. Edge color indicated the type of interaction and was specified on the bottom of the figure.
Potentially novel PL loci identified by cFDR and GPA
According to the thresholds of cFDR < 0.05 and fdr.GPA < 0.2, we identified totally 395 significant SNPs associated with PL variation conditioned on FNK BMD (Supplemental Table 1). Among the 395 SNPs, 144 SNPs achieved p values lower than 5E-8 in the original PL GWAS datasets (Willer et al. 2013), 1 SNP (rs12708980) in HDL was confirmed before to be GWAS-associated with PL (Kim et al. 2011), and 5 SNPs had high LD values (according to the default value of the SNAP website, r2 > 0.8) with previous PL GWAS findings (Teslovich et al. 2010; Willer et al. 2013), including 2 SNPs (rs5754467 and rs9930506) in HDL (Teslovich et al. 2010; Willer et al. 2013), 1 SNP (rs2699429) in LDL (Willer et al. 2013), 1 SNP (rs2178950) in TC (Teslovich et al. 2010) and 2 SNPs (rs12751742 and rs9930506) in TG (Willer et al. 2013) (Table 3). These SNPs were regarded as replications of the original PL GWAS. The remaining 245 SNPs with p-values higher than genome-wide significance threshold of 5E-08 were potentially novel SNPs for PL, among which 71 SNPs were identified for HDL, 67 SNPs for LDL, 92 SNPs for TC and 54 SNPs for TG (Table 3). These significant SNPs were mapped to 21 chromosomes (1–20, 22) and the positions were showed in the conditional Manhattan plots (Fig. 4).
Table 3.
The number of SNPs with different criterions
| HDL|FNK BMD | LDL|FNK BMD | TC|FNK BMD | TG|FNK BMD | |
|---|---|---|---|---|
| cFDR < 0.05 and fdr. GPA < 0.2 | 160 | 148 | 202 | 122 |
| P > 5E-08 | 74 | 68 | 93 | 56 |
| Repeat with previous GWAS findings | rs12708980 | — | — | — |
| Had high LD with previous GWAS findings | rs5754467 | rs12751742 | ||
| (r2 = 0.807 with rs181362) | rs2699429 | rs2178950 | (r2 = 0.892 with rs12748152) | |
| rs9930506 | (r2 = 0.965 with rs6831256) | (r2 = 0.815 with rs2737229) | rs9930506 | |
| (r2 = 0.868 with rs1121980) | (r2 = 0.868 with rs1121980) |
Column definition: HDL | FNK BMD - high density lipoprotein cholesterol conditioned on femoral neck bone mineral density; LDL | FNK BMD - low density lipoprotein cholesterol conditioned on femoral neck bone mineral density; TC | FNK BMD - total cholesterol conditioned on femoral neck bone mineral density; TG | FNK BMD - triglyceride conditioned on femoral neck bone mineral density;
Row definition: cFDR - conditional false discovery rate; fdr. GPA - false discovery rate of GPA when one SNP was associated with PL conditioned on FNK BMD; P - summary statistic p value of original GWAS; LD - linkage disequilibrium; r2 - linkage disequilibrium value.
Gene annotation and function enrichment analysis for potentially novel PL SNPs
We mapped the 245 potentially novel SNPs to their corresponding genes by SCAN and performed the GO term enrichment analysis. The strongest enriched GO term and associated genes/SNPs for PL were listed in Table 4. For HDL, the most enriched GO term was “phospholipid homeostasis”, with a fold enrichment value over 100 (p-value = 6.53E-05). The significant genes in this GO term included HNF4A (rs2071197), LIPG (rs2097055, rs883218 and rs4556888), CETP (rs17369163) and GPAM (rs10787429). For TC, the most enriched GO term was “chylomicron remnant clearance“, with fold enrichment as over 100 (p-value = 2.12E-02). The significant genes in this GO term included LDLR (rs2738456), APOB (rs6733447) and LIPC (rs792902, rs1652519, rs6494007, rs4775046 and rs12324517). Both of the most enriched GO terms play key roles in PL metabolism (Cabezas et al. 1993; Lim et al. 2011). No enriched GO terms were found for LDL and TG. The other enriched GO terms were presented in Supplemental Table 2.
Table 4.
Functional term enrichment analysis about PL by GO
| GO | Genes | SNPs | Fold enrichment | +/− | P value | Phenotype |
|---|---|---|---|---|---|---|
| phospholipid homeostasis | HNF4A LIPG CETP GPAM |
rs2071197 rs2097055 rs883218 rs4556888 rs17369163 rs10787429 |
>100 | + | 6.53E-05 | HDL | FNK BMD |
| chylomicron remnant clearance | LDLR APOB LIPC |
rs2738456 rs6733447 rs792902 rs1652519 rs6494007 rs4775046 rs12324517 |
>100 | + | 2.12E-02 | TC | FNK BMD |
Column definition: GO – gene ontology about biological process; Genes - genes corresponding to significant SNPs; SNPs - significant SNPs involved in GO terms; fold enrichment - degree of enrichment on gene sets; + and − indicated over or under-representation of a term; P value - probability of genes annotated to a particular GO term; Phenotype - principal phenotype and conditional phenotype.
Discussion
In this study, we combined the summary statistics from two independent GWAS datasets and jointly implemented cFDR and GPA methods to validate the pleiotropy between FNK BMD and PL (HDL, LDL, TC, and TG). Potentially common genes were identified between FNK BMD and PL, which could lead to a further and novel understanding of the shared genetic mechanisms for both OP and DL, and potentially have a positive impact on future clinical treatment and prevention. A number of potentially novel SNPs associated with PL were also identified, which provided new directions for future studies of molecular pathogenesis for DL.
To explore more missing heritability, traditional ideas included recruiting more participants and genotyping larger samples, but these were difficult and impractical in many cases and costly. The advantages of cFDR and GPA methods were that they could leverage the power of pleiotropy by using current GWAS datasets, they virtually increased the existing sample size and enhanced statistical power to explore more potential genetic variants. They also lessened the burden of multiple testing by controlling the false discovery rate, which meant that the cFDR and GPA methods could leverage pleiotropy information and also provide less conservative control of type I errors compared to Bonferroni correction (Shaffer 1995; Benjamini et al. 2001). cFDR method was the first approach which statistically addressed the issue of pleiotropy between GWASs of two different traits based on Bayesian formula. GPA method could systematically integrate pleiotropy and annotate information based on LRT. In this study, if a SNP was identified by the two methods simultaneously, it meant that the SNP was validated virtually by two different approaches, which might further reduce false positive findings for more robust results. It should be noted that GPA method could be used to analyze GWAS results with or without annotation information. Meanwhile, summary statistic p-values of GWAS was more important than functional annotation information in GPA (Chung et al. 2014). Therefore, in this study we chose not to use annotation information, which not only rendered efficient analysis in terms of computation but more importantly also matched the cFDR results better since cFDR method could use only summary statistics information.
Andreassen et al. had identified pleiotropy between FNK BMD and PL by cFDR method in a previous study (Reppe et al. 2015). They had identified 65 novel BMD loci by conditioning on cardiovascular disease (CVD) related phenotypes, including PL (HDL, LDL, TC and TG). Unlike their initial study, we hope to explore more relationships between FNK BMD and PL from different/novel aspects, such as to identify common genes to both PL and BMD, and novel PL-associated SNPs. These study aspects were not covered in the Andreassen’s findings (Reppe et al. 2015). Hence, on one hand, we validated the pleiotropy first. In our pleiotropic enrichment analysis, we used GPA method together with cFDR method based on larger and newer GWAS datasets and successfully validated the pleiotropy. We further quantified the degree of pleiotropy by enrichment plot and LRT. On the other hand, based on the validated pleiotropy, we focused on identifying potentially novel SNPs for PL conditioned on FNK BMD and identified common genes for FNK BMD and PL. These findings were novel compared to the Andreassen’s results (Reppe et al. 2015).
By leveraging the pleiotropy, we could integrate PL and FNK BMD GWAS datasets and virtually increase the existing sample size. Then we could use cFDR method and GPA method to enhance the statistical power to explore potentially novel PL associated SNPs. In this study, we identified 395 significant SNPs for PL, of which 150 SNPs had reached p < 5E-8 in the original GWAS or were identified in other previous PL GWASs. These results reflected the reliability of the cFDR method and GPA method. Meanwhile, we identified 245 potentially novel SNPs for PL. Several genes corresponding to these potentially novel SNPs were enriched in plasma metabolism related GO terms, such as “phospholipid homeostasis” and “chylomicron remnant clearance“. This functional enrichment analysis result suggested that these potentially novel SNPs might be associated with PL metabolism.
We identified 3 pleiotropic SNPs (rs2178950, rs9939318 and rs9368716) that were associated with both FNK BMD and PL. rs2178950 was located in TRPS1. TRPS1 gene was confirmed to be associated with HDL in a previous GWAS meta-analysis (Teslovich et al. 2010). Meanwhile, TRPS1 was associated with BMD (Gai et al. 2011) and Wnt signaling pathway (Fantauzzo and Christiano 2012). Protein produced by TRPS1 was a transcription factor and played an important role in skeletal development by influencing osteoblast cell differentiation and osteocalcin expression (Piscopo et al. 2009). Deletion of TPRS1 could cause skeletal abnormalities called skeletal abnormalities of tricho-rhino-phalangeal syndrome type I, characterized by craniofacial abnormalities and disturbances in formation and maturation of bone matrix (de Barros and Kakehasi 2016).
The pleiotropic SNP rs9939318 was located in NLRC5, which was related to inflammation and immunity (Benko et al. 2017). Interestingly, PL was also involved in the pathological procedure of inflammation and immunity (Rao et al. 2015). Cholesterol could induce immune response and inflammation during the progression of atherosclerosis (Rao et al. 2015), and impact on the circulating monocytes and bone marrow (Bernelot Moens et al. 2017). So we inferred that NLRC5 might be involved in DL through the process of inflammation and immunity. Meanwhile, NLRC5 could regulate bone mineralization by stimulating Wnt signal pathway to promote osteoblast differentiation (Peng et al. 2016), and it could also inhibit nuclear factor kappa B (NF-κB) (Benko et al. 2017) to decrease osteoclast. Wnt signaling could stimulate generation of osteoblasts by promoting mesenchymal stem cells (MSCs) towards osteoblast lineage, and decrease osteoclast differentiation by inducing OPG secretion and production (Manolagas 2014). NF-κB is a transcriptional factor which regulates the bone remodeling processes and inflammatory response in both bone resorption cells and bone forming (Benko et al. 2017). As mentioned above, NLRC5 was associated with OP through Wnt signal pathway (Peng et al. 2016) and NF-κB (Benko et al. 2017).
“STRING Interaction Network Analysis” offered information about protein-protein interaction and allowed us to explore the indirect interactions between genes. As shown in Fig. 3, TRPS1 and NLRC5 were found to interact with many genes, such as IKBKB, CHUK, DDX58, IFIH1, RNF135, JUN family and WDR family. Among them, IKBKB and CHUK were related to NF-κB, which was a regulating factor to the inflammatory response and bone-remodeling processes (Benko et al. 2017). CHUK was an important paralog of IKBKB. The protein encoded by IKBKB phosphorylated the inhibitor in the NF-κB complex, causing activation of NF-κB. The encoded protein of CHUK was an inhibitor of the essential transcription factor NF-κB complex (Schmid and Birbach 2008; Solt and May 2008). DDX58, IFIH1 and RNF135 were involved in pro-inflammatory cytokines and/or immune system (Oshiumi et al. 2009; Ovsyannikova et al. 2010; Smyth et al. 2006). These information indicated that TRPS1 and NLRC5 might be the potential common genes associated with both OP and DL. NF-κB signaling pathway, Wnt signaling pathway, inflammation and innate immune might be the shared mechanisms underlying the relationship between OP and DL.
Although we successfully improved the identification of potentially novel SNPs for PL and pleiotropic SNPs for PL and FNK BMD, there were still some limitations in our study. First, the contribution of our findings to the proportion of the phenotypes’ variability could not be evaluated, since we only had access to summary statistics of GWAS without raw data to analyse. If we could get the raw genotype data in future, we are willing to perform linear regression with the novel SNPs and those earlier identified SNPs so that we could analyse how much our findings would contribute to the whole heritability. Second, neither cFDR method nor GPA method had the ability to identify causal variants for the interested phenotype. The aim of our study was to identify more potentially novel SNPs and provide a new direction for further functional studies to be performed by molecular and cellular biologists, thus follow-up studies should be conducted for replication and biological functional validation, and further elucidate the overall genetic mechanisms. However, under the experimental conditions of our group and time restriction, we could not carry out the functional validation experiments at present. We hope that this limitation could be partially addressed in the future by follow-up fine mapping studies and functional mechanistic studies of the GWAS associated regions by our own and/or other groups which are more specialized in functional studies. Meanwhile, considering the racial and population differences of heritability (Musani et al. 2017), in the future we could study other populations in the same way to identify the relationship between FNK BMD and PL among different populations.
In summary, by performing cFDR and GPA methods on current GWAS datasets, we validated pleiotropy between PL and FNK BMD and identified more potentially novel SNPs for PL. NLRC5 and TRPS1 might be the potentially common genes for PL and FNK BMD. NF-κB, Wnt, inflammation and immune may be involved in the common pathogenesis of DL and OP.
Supplementary Material
Detailed information of SNPs associated with different types of PL conditioned on FNK BMD through cFDR <0.05 and fdr.GPA <0.2
All of the functional term enrichment analysis about PL by GO
Acknowledgments
Hong-Wen Deng was partially supported by grants from the National Institutes of Health [U19AG05537301, R01AR057049, R01AR059781, D43TW009107, P20GM109036, R01MH107354, R01MH104680, R01GM109068], the Edward G. Schlieder Endowment fund to Tulane University. We acknowledged Genetic Factors for Osteoporosis (GEFOS-seq Consortium, http://www.gefos.org/) and Global Lipids Genetics Consortium (GLGC, http://csg.sph.umich.edu) for their GWAS summary statistics posted online. We acknowledged Chun-Ping Zeng for his useful suggestions for this study.
Footnotes
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Andreassen OA, Thompson WK, Schork AJ, Ripke S, Mattingsdal M, Kelsoe JR, Kendler KS, O’Donovan MC, Rujescu D, Werge T, Sklar P, Roddey JC, Chen CH, McEvoy L, Desikan RS, Djurovic S, Dale AM. Improved detection of common variants associated with schizophrenia and bipolar disorder using pleiotropy-informed conditional false discovery rate. PLoS Genet. 2013;9:e1003455. doi: 10.1371/journal.pgen.1003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergs FW, Guo Y, van Iperen EP, Sivapalaratnam S, Tragante V, Lanktree MB, Lange LA, Almoguera B, Appelman YE, Barnard J, Baumert J, Beitelshees AL, Bhangale TR, Chen YD, Gaunt TR, Gong Y, Hopewell JC, Johnson T, Kleber ME, Langaee TY, Li M, Li YR, Liu K, McDonough CW, Meijs MF, Middelberg RP, Musunuru K, Nelson CP, O’Connell JR, Padmanabhan S, Pankow JS, Pankratz N, Rafelt S, Rajagopalan R, Romaine SP, Schork NJ, Shaffer J, Shen H, Smith EN, Tischfield SE, van der Most PJ, van Vliet-Ostaptchouk JV, Verweij N, Volcik KA, Zhang L, Bailey KR, Bailey KM, Bauer F, Boer JM, Braund PS, Burt A, Burton PR, Buxbaum SG, Chen W, Cooper-Dehoff RM, Cupples LA, DeJong JS, Delles C, Duggan D, Fornage M, Furlong CE, Glazer N, Gums JG, Hastie C, Holmes MV, Illig T, Kirkland SA, Kivimaki M, Klein R, Klein BE, Kooperberg C, Kottke-Marchant K, Kumari M, LaCroix AZ, Mallela L, Murugesan G, Ordovas J, Ouwehand WH, Post WS, Saxena R, Scharnagl H, Schreiner PJ, Shah T, Shields DC, Shimbo D, Srinivasan SR, Stolk RP, Swerdlow DI, Taylor HJ, Topol EJ, Toskala E, van Pelt JL, van Setten J, Yusuf S, Whittaker JC, Zwinderman AH, Anand SS, Balmforth AJ, Berenson GS, Bezzina CR, et al. Large-scale gene-centric meta-analysis across 32 studies identifies multiple lipid loci. Am J Hum Genet. 2012;91:823–38. doi: 10.1016/j.ajhg.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Benko S, Kovacs EG, Hezel F, Kufer TA. NLRC5 Functions beyond MHC I Regulation-What Do We Know So Far? Front Immunol. 2017;8:150. doi: 10.3389/fimmu.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernelot Moens SJ, Verweij SL, Schnitzler JG, Stiekema LC, Bos M, Langsted A, Kuijk C, Bekkering S, Voermans C, Verberne HJ. Remnant Cholesterol Elicits Arterial Wall Inflammation and a Multilevel Cellular Immune Response in Humans. Arteriosclerosis Thrombosis & Vascular Biology. 2017 doi: 10.1161/ATVBAHA.116.308834. [DOI] [PubMed] [Google Scholar]
- Cabezas MC, de Bruin TW, Jansen H, Kock LA, Kortlandt W, Erkelens DW. Impaired chylomicron remnant clearance in familial combined hyperlipidemia. Arterioscler Thromb. 1993;13:804–14. doi: 10.1161/01.atv.13.6.804. [DOI] [PubMed] [Google Scholar]
- Chung D, Yang C, Li C, Gelernter J, Zhao H. GPA: a statistical approach to prioritizing GWAS results by integrating pleiotropy and annotation. PLoS Genet. 2014;10:e1004787. doi: 10.1371/journal.pgen.1004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barros GM, Kakehasi AM. Skeletal abnormalities of tricho-rhino-phalangeal syndrome type I. Rev Bras Reumatol Engl Ed. 2016;56:86–9. doi: 10.1016/j.rbre.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Fantauzzo KA, Christiano AM. Trps1 activates a network of secreted Wnt inhibitors and transcription factors crucial to vibrissa follicle morphogenesis. Development. 2012;139:203–14. doi: 10.1242/dev.069971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KM, Wang L, Gandra SR, Quek RG, Li L, Baser O. Clinical and economic burden associated with cardiovascular events among patients with hyperlipidemia: a retrospective cohort study. BMC Cardiovasc Disord. 2016;16:13. doi: 10.1186/s12872-016-0190-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai Z, Gui T, Muragaki Y. The function of TRPS1 in the development and differentiation of bone, kidney, and hair follicles. Histol Histopathol. 2011;26:915–21. doi: 10.14670/HH-26.915. [DOI] [PubMed] [Google Scholar]
- Giner-Galvan V, Esteban-Giner MJ, Pallares-Carratala V. Overview of guidelines for the management of dyslipidemia: EU perspectives. Vasc Health Risk Manag. 2016;12:357–369. doi: 10.2147/VHRM.S89038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh M, Mizuno K, Ono Y, Takahashi M. Fluvastatin increases bone mineral density in postmenopausal women. Fukushima J Med Sci. 2011;57:19–27. doi: 10.5387/fms.57.19. [DOI] [PubMed] [Google Scholar]
- Greenbaum J, Wu K, Zhang L, Shen H, Zhang J, Deng HW. Increased detection of genetic loci associated with risk predictors of osteoporotic fracture using a pleiotropic cFDR method. Bone. 2017;99:62–68. doi: 10.1016/j.bone.2017.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj A, Chedid R, Chouery E, Megarbane A, Gannage-Yared MH. Relationship between vitamin D receptor gene polymorphisms, cardiovascular risk factors and adiponectin in a healthy young population. Pharmacogenomics. 2016;17:1675–1686. doi: 10.2217/pgs-2016-0045. [DOI] [PubMed] [Google Scholar]
- Huang Y, Gao L, Xie X, Tan SC. Epidemiology of dyslipidemia in Chinese adults: meta-analysis of prevalence, awareness, treatment, and control. Popul Health Metr. 2014;12:28. doi: 10.1186/s12963-014-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N, Mohamed N, Shuid AN. Update on statins: hope for osteoporotic fracture healing treatment. Curr Drug Targets. 2013;14:1524–32. doi: 10.2174/13894501113149990195. [DOI] [PubMed] [Google Scholar]
- Jeong TD, Lee W, Choi SE, Kim JS, Kim HK, Bae SJ, Chun S, Min WK. Relationship between serum total cholesterol level and serum biochemical bone turnover markers in healthy pre- and postmenopausal women. Biomed Res Int. 2014;2014:398397. doi: 10.1155/2014/398397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, de Bakker PI. SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics. 2008;24:2938–9. doi: 10.1093/bioinformatics/btn564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Go MJ, Hu C, Hong CB, Kim YK, Lee JY, Hwang JY, Oh JH, Kim DJ, Kim NH, Kim S, Hong EJ, Kim JH, Min H, Kim Y, Zhang R, Jia W, Okada Y, Takahashi A, Kubo M, Tanaka T, Kamatani N, Matsuda K, Park T, Oh B, Kimm K, Kang D, Shin C, Cho NH, Kim HL, Han BG, Lee JY, Cho YS. Large-scale genome-wide association studies in East Asians identify new genetic loci influencing metabolic traits. Nat Genet. 2011;43:990–5. doi: 10.1038/ng.939. [DOI] [PubMed] [Google Scholar]
- Lim HY, Wang W, Wessells RJ, Ocorr K, Bodmer R. Phospholipid homeostasis regulates lipid metabolism and cardiac function through SREBP signaling in Drosophila. Genes Dev. 2011;25:189–200. doi: 10.1101/gad.1992411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal CC. High Cholesterol Deteriorates Bone Health: New Insights into Molecular Mechanisms. Front Endocrinol (Lausanne) 2015;6:165. doi: 10.3389/fendo.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC. Wnt signaling and osteoporosis. Maturitas. 2014;78:233–7. doi: 10.1016/j.maturitas.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RE, Lenhard MJ, Sneider MB, Pohlig RT. Osteoprotegerin is a Better Serum Biomarker of Coronary Artery Calcification than Osteocalcin in Type 2 Diabetes. Endocr Pract. 2015;21:14–22. doi: 10.4158/EP14229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado C, DeSimone AK, Odom E, Gillespie C, Ayala C, Loustalot F. Prevalence of Cholesterol Treatment Eligibility and Medication Use Among Adults--United States, 2005–2012. MMWR Morb Mortal Wkly Rep. 2015;64:1305–11. doi: 10.15585/mmwr.mm6447a1. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Barber RM, Foreman KJ, Abbasoglu OA, Abd-Allah F, Abera SF, Aboyans V, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Achoki T, Ackerman IN, Ademi Z, Adou AK, Adsuar JC, Afshin A, Agardh EE, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allebeck P, Almazroa MA, Alsharif U, Alvarez E, Alvis-Guzman N, Amare AT, Ameh EA, Amini H, Ammar W, Anderson HR, Anderson BO, Antonio CA, Anwari P, Arnlov J, Arsic AV, Artaman A, Asghar RJ, Assadi R, Atkins LS, Avila MA, Awuah B, Bachman VF, Badawi A, Bahit MC, Balakrishnan K, Banerjee A, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Basu A, Basu S, Basulaiman MO, Beardsley J, Bedi N, Beghi E, Bekele T, Bell ML, Benjet C, Bennett DA, Bensenor IM, Benzian H, Bernabe E, Bertozzi-Villa A, Beyene TJ, Bhala N, Bhalla A, Bhutta ZA, Bienhoff K, Bikbov B, Biryukov S, Blore JD, Blosser CD, Blyth FM, Bohensky MA, Bolliger IW, Bora BB, Bornstein NM, Bose D, Boufous S, Bourne RR, Boyers LN, Brainin M, Brayne CE, Brazinova A, Breitborde NJ, Brenner H, Briggs AD, Brooks PM, Brown JC, Brugha TS, Buchbinder R, Buckle GC, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–91. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musani SK, Martin LJ, Woo JG, Olivier M, Gurka MJ, DeBoer MD. Heritability of the Severity of the Metabolic Syndrome in Whites and Blacks in 3 Large Cohorts. Circ Cardiovasc Genet. 2017:10. doi: 10.1161/CIRCGENETICS.116.001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Hatakeyama S, Seya T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J Biol Chem. 2009;284:807–17. doi: 10.1074/jbc.M804259200. [DOI] [PubMed] [Google Scholar]
- Ovsyannikova IG, Dhiman N, Haralambieva IH, Vierkant RA, O’Byrne MM, Jacobson RM, Poland GA. Rubella vaccine-induced cellular immunity: evidence of associations with polymorphisms in the Toll-like, vitamin A and D receptors, and innate immune response genes. Human Genetics. 2010;127:207–21. doi: 10.1007/s00439-009-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Shen J, Lin X, Su KJ, Greenbaum J, Zhu W, Lou HL, Liu F, Zeng CP, Deng WF, Deng HW. Genetic sharing with coronary artery disease identifies potential novel loci for bone mineral density. Bone. 2017;103:70–77. doi: 10.1016/j.bone.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YY, He YH, Chen C, Xu T, Li L, Ni MM, Meng XM, Huang C, Li J. NLRC5 regulates cell proliferation, migration and invasion in hepatocellular carcinoma by targeting the Wnt/beta-catenin signaling pathway. Cancer Lett. 2016;376:10–21. doi: 10.1016/j.canlet.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Philip S, Cyrus C. Osteoporosis. Lancet. 2006;367:2010–8. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- Piscopo DM, Johansen EB, Derynck R. Identification of the GATA factor TRPS1 as a repressor of the osteocalcin promoter. J Biol Chem. 2009;284:31690–703. doi: 10.1074/jbc.M109.052316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JL, Bhimji SS. Osteoporosis. 2017. [Google Scholar]
- Rao LN, Ponnusamy T, Philip S, Mukhopadhyay R, Kakkar VV, Mundkur L. Hypercholesterolemia Induced Immune Response and Inflammation on Progression of Atherosclerosis in Apob(tm2Sgy) Ldlr(tm1Her)/J Mice. Lipids. 2015;50:785–97. doi: 10.1007/s11745-015-4046-4. [DOI] [PubMed] [Google Scholar]
- Ren R, Chen Z, Zhao X, Sun T, Zhang Y, Chen J, Lu S, Ma W. A possible regulatory link between Twist 1 and PPARgamma gene regulation in 3T3-L1 adipocytes. Lipids Health Dis. 2016;15:189. doi: 10.1186/s12944-016-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppe S, Wang Y, Thompson WK, McEvoy LK, Schork AJ, Zuber V, LeBlanc M, Bettella F, Mills IG, Desikan RS, Djurovic S, Gautvik KM, Dale AM, Andreassen OA. Genetic Sharing with Cardiovascular Disease Risk Factors and Diabetes Reveals Novel Bone Mineral Density Loci. PLoS One. 2015;10:e0144531. doi: 10.1371/journal.pone.0144531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zheng HF, Spector TD. Genetics of osteoporosis from genome-wide association studies: advances and challenges. Nat Rev Genet. 2012;13:576–88. doi: 10.1038/nrg3228. [DOI] [PubMed] [Google Scholar]
- Schmid JA, Birbach A. IkappaB kinase beta (IKKbeta/IKK2/IKBKB)--a key molecule in signaling to the transcription factor NF-kappaB. Cytokine Growth Factor Rev. 2008;19:157–65. doi: 10.1016/j.cytogfr.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Shaffer JP. Multiple Hypothesis Testing - Annual Review of Psychology. 1995;46(1):561. [Google Scholar]
- Singh M, Singh P, Juneja PK, Singh S, Kaur T. SNP-SNP interactions within APOE gene influence plasma lipids in postmenopausal osteoporosis. Rheumatol Int. 2011;31:421–3. doi: 10.1007/s00296-010-1449-7. [DOI] [PubMed] [Google Scholar]
- Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB, Savage DA, Walker NM, Clayton DG, Todd JA. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38:617–9. doi: 10.1038/ng1800. [DOI] [PubMed] [Google Scholar]
- Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sözen TLÖ, Başaran NÇ. An overview and management of osteoporosis. European Journal of Rheumatology. 2017;4:46. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin CY, Jin GM, Jin KY, Lee JY, Park T, Kim K, Sim X, Twee-Hee OR, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua ZJ, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videman T, Levalahti E, Battie MC, Simonen R, Vanninen E, Kaprio J. Heritability of BMD of femoral neck and lumbar spine: a multivariate twin study of Finnish men. J Bone Miner Res. 2007;22:1455–62. doi: 10.1359/jbmr.070606. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tao Y, Hyman ME, Li J, Chen Y. Osteoporosis in China. Osteoporosis International. 2009;20:1651–1662. doi: 10.1007/s00198-009-0925-y. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkila K, Hypponen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikainen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Muller-Nurasyid M, Nolte IM, O’Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Doring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45:1274–83. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson T, Nelson SD, Newbold J, Nelson RE, LaFleur J. The clinical epidemiology of male osteoporosis: a review of the recent literature. Clin Epidemiol. 2015;7:65–76. doi: 10.2147/CLEP.S40966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SK, Chin KY, Suhaimi FH, Ahmad F, Ima-Nirwana S. The Relationship between Metabolic Syndrome and Osteoporosis: A Review. Nutrients. 2016:8. doi: 10.3390/nu8060347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng CP, Chen YC, Lin X, Greenbaum J, Chen YP, Peng C, Wang XF, Zhou R, Deng WM, Shen J, Deng HW. Increased identification of novel variants in type 2 diabetes, birth weight and their pleiotropic loci. J Diabetes. 2016 doi: 10.1111/1753-0407.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ, Dahia CL, Park-Min KH, Tobias JH, Kooperberg C, Kleinman A, Styrkarsdottir U, Liu CT, Uggla C, Evans DS, Nielson CM, Walter K, Pettersson-Kymmer U, McCarthy S, Eriksson J, Kwan T, Jhamai M, Trajanoska K, Memari Y, Min J, Huang J, Danecek P, Wilmot B, Li R, Chou WC, Mokry LE, Moayyeri A, Claussnitzer M, Cheng CH, Cheung W, Medina-Gomez C, Ge B, Chen SH, Choi K, Oei L, Fraser J, Kraaij R, Hibbs MA, Gregson CL, Paquette D, Hofman A, Wibom C, Tranah GJ, Marshall M, Gardiner BB, Cremin K, Auer P, Hsu L, Ring S, Tung JY, Thorleifsson G, Enneman AW, van Schoor NM, de Groot LC, van der Velde N, Melin B, Kemp JP, Christiansen C, Sayers A, Zhou Y, Calderari S, van Rooij J, Carlson C, Peters U, Berlivet S, Dostie J, Uitterlinden AG, Williams SR, Farber C, Grinberg D, LaCroix AZ, Haessler J, Chasman DI, Giulianini F, Rose LM, Ridker PM, Eisman JA, Nguyen TV, Center JR, Nogues X, Garcia-Giralt N, Launer LL, Gudnason V, Mellstrom D, Vandenput L, Amin N, van Duijn CM, Karlsson MK, Ljunggren O, Svensson O, Hallmans G, Rousseau F, Giroux S, Bussiere J, Arp PP, et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526:112–7. doi: 10.1038/nature14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed information of SNPs associated with different types of PL conditioned on FNK BMD through cFDR <0.05 and fdr.GPA <0.2
All of the functional term enrichment analysis about PL by GO