Abstract
Objectives
Cortical abnormalities in prenatal alcohol exposure (PAE) are known, including in gyrification (LGI), thickness (CT), volume (CV), and surface area (CS). This study provides longitudinal and developmental context to the PAE cortical development literature.
Experimental design
Included: 58 children with PAE and 52 controls, ages 6–17 at enrollment, from four Collaborative Initiative on FASD (CIFASD) sites. Participants underwent a formal evaluation of physical anomalies and dysmorphic facial features associated with PAE. MRI data were collected on three platforms (Siemens, GE, and Philips) at four sites. Scans were spaced two years apart. Change in LGI, CT, CS, and CV were examined.
Principal observations
Several significant regional age-by-diagnosis linear and quadratic interaction effects in LGI, CT, and CV were found, indicating atypical developmental trajectories in PAE. No significant correlations were observed between cortical measures and IQ.
Conclusions
Regional differences were seen longitudinally in CT, CV, and LGI in those with PAE. The findings represent important insights into developmental trajectories and may have implications for the timing of assessments and interventions in this population. It is noteworthy that cortical metrics did not correlate with IQ, suggesting that more specific aspects of cognitive development may need to be explored to provide further context.
Keywords: Cerebral cortex, Fetal alcohol spectrum disorder, Longitudinal MRI, Neuropsychology, Pediatric
1. Introduction
Fetal Alcohol Spectrum Disorders (FASD) is a clinical umbrella term encapsulating fetal alcohol syndrome (FAS), partial fetal alcohol syndrome (pFAS), alcohol-related neurodevelopment disorder (ARND), and alcohol-related birth defects (ARBD); all of which are caused by prenatal alcohol exposure (PAE). The effects of PAE include observable abnormalities such as facial dysmorphology, growth deficiency, and neurodevelopmental disruption, leading to serious impact on quality of life. Neuroimaging has helped highlight the neurodevelopmental effects, reliably demonstrating both macrostructural and microstructural brain abnormalities (Archibald et al., 2001; Nardelli et al., 2011; Roussotte et al., 2012; Sowell et al., 2008, Sowell et al., 2002; Swayze et al., 1997; Wozniak et al., 2009) for reviews, see (Lebel et al., 2011; Moore et al., 2014). More subtle disruptions in functional connectivity have also been observed (Roussotte et al., 2011; Santhanam et al., 2011; Wozniak et al., 2013, Wozniak et al., 2011) and a few studies have begun to show atypical cortical development including abnormal gyrification (De Guio et al., 2014; Hendrickson et al., 2017; Infante et al., 2015), cortical thickness (Fernández-Jaén et al., 2011; Robertson et al., 2016; Sowell et al., 2008; Treit et al., 2014; Yang et al., 2012; Zhou et al., 2011), and cortical surface area and volume (Lebel et al., 2012; Leigland et al., 2013; Migliorini et al., 2015; Rajaprakash et al., 2014; Roussotte et al., 2012; Treit et al., 2013).
Much of what is known about cortical development in PAE has come from cross-sectional studies. Longitudinal studies have the potential to illustrate patterns of disrupted development that may not always be apparent in a cross-sectional “snapshot”. The existing small literature on longitudinal cortical development in PAE suggests differences in cortical volume trajectories between individuals with PAE and controls in posterior brain regions, particularly in parietal cortex (Lebel et al., 2012). Atypical longitudinal cortical thinning has also been observed in children and adolescents with PAE, especially within medial frontal and parietal brain regions (Treit et al., 2014).
The current study sought to add to this literature with a two-year longitudinal examination of change across multiple metrics of cortical development as part of the Collaborative Initiative on FASD (CIFASD) multi-site study. We applied methods that have not previously been used in this context, including longitudinal analyses of cortical change at the vertex-wise level. Probing for differences at this level has the potential to reveal patterns not previously seen using larger regions of interest (ROIs).
The purpose of the study, therefore, was to test for potential differences in developmental trajectories in children and adolescents with PAE compared to controls to better characterize the long-term neurodevelopment “cascades” that are initiated by early insults to the brain in PAE. In addition, we also sought to examine relationships between important clinical characteristics (such as diagnostic characteristics and cognitive functioning) and cortical development, knowing that cognition is tied closely to cortical development in PAE (Dubois et al., 2008; Hendrickson et al., 2017; Shaw et al., 2006). Lastly, we sought to use this multi-site project to evaluate the robustness of structural cortical measures across multiple clinical samples evaluated on different MRI scanners.
2. Methods
2.1. Participants
Participants were enrolled in the study as part of CIFASD. Details about the CIFASD project are available in a separate publication (Mattson et al., 2010) and at www.cifasd.org. For the current study, participants were recruited from four CIFASD sites (University of Minnesota, University of Southern California/Children’s Hospital of Los Angeles, San Diego State University, and Emory University School of Medicine) and scanned between 2012 and 2014. Follow-up scans took place between 2014 and 2017 approximately 2 years after the initial scan. Prenatal alcohol exposure histories were obtained through retrospective maternal report, social service, legal, and medical records. Participants were included in the PAE group if there was a history of heavy PAE (>13 drinks/week or >4 drinks per occasion at least once per week during pregnancy) or when such exposure was suspected in a child with a FAS diagnosis based on dysmorphology. In some cases, detailed history about exposure amounts was unattainable and decisions about inclusion or exclusion were made on the available evidence. For example, PAE was inferred if the mother was known to have had alcoholism and had contact with the police or social services during the pregnancy. In all cases, alcohol was the predominant substance of abuse. Participants were included in the non-exposed control group if there was a reliable history of minimal (<1 drink/week, never >2 drinks on any one occasion) or no reported exposure during pregnancy.
Control participants were recruited with flyers, mailings to control participants of previous non-CIFASD studies, online advertisements, and referrals from participants with PAE. Advertisements and flyers were placed in neighborhoods and online locations chosen to maximize the ethnic, racial, and socioeconomic diversity of the control participants so as to best match the participants with PAE. Control participants were screened by telephone, as were participants with PAE.
Participants (PAE and controls) were evaluated using a standardized examination conducted by a member of the CIFASD Dysmorphology Core (KLJ) who had not previously met the child and was not told the child’s status (PAE or control). The dysmorphologist made a determination of FAS based on two or more of the following key facial features: thin vermillion border, smooth philtrum, and short palpebral fissure length − together with either microcephaly (occipital-frontal circumference ≤10%ile) or growth deficiency (height or weight ≤10%ile) or both.
Additional exclusion criteria for all subjects included another developmental disorder (ex. Autism), very low birthweight (<1500 g), traumatic brain injury (including head injury with loss of consciousness >2 min), other medical condition affecting the brain (ex. Epilepsy), severe psychiatric disability that would prevent participation (ex. psychosis or mania), substance use by the participant, English as a second language, international adoption after age 5, or contraindications to MRI scanning.
Control participants were excluded for parent-reported history of prenatal substance exposure (other than tobacco and caffeine exposure) and for diagnosed psychiatric conditions. Parents or caregivers of all participants were administered the Diagnostic Interview Schedule for Children-IV (C-DISC-IV; (Shaffer et al., 2000)). Because pre-screening was utilized during recruitment, very few enrolled control participants had any psychiatric symptoms. The C-DISC-IV data revealed the following: 1 control had ADHD symptoms, 3 had Oppositional Defiant symptoms, 2 had Conduct Disorder symptoms, none had depressive symptoms; none had anxiety symptoms. Psychiatric co-morbidity was not an exclusion criterion for participants with PAE because it is well-recognized that co-morbidity is a common feature of FASD (Streissguth and O’Malley, 2000). Based on the C-DISC-IV data, 31 participants in the PAE group had ADHD symptoms, 19 had Oppositional Defiant symptoms, 8 had Conduct Disorder symptoms, 2 had anxiety symptoms, and 3 had depressive symptoms.
Participants were ages 6–17 at the time of the first MRI scan. The majority of participants completed the neurocognitive evaluation and MRI on the same day. In a few cases, they were separated by a few days or weeks. A total of 110 participants (58 with PAE & 52 Controls) met inclusion criteria, had baseline and follow up scans, and were included in this analysis. Table 1 contains the demographics for the participants who were included in the analyses after eliminating those with excessive movement and aberrant image processing (see Results section for complete description).
Table 1.
Demographic characteristics of participants included in analyses.
| PAE (n = 58) | Control (n = 52) | Statistical Test | |
|---|---|---|---|
| Age [M(SD)] | 12.44 (2.74) | 13.71 (2.29) | t(108) = −2.08, p < 0.01 |
| Total Intracranial Volume cm3 [M(SD)] | 1409 (202) | 1433 (225) | t(108) = −0.59, p = 0.56 |
| Intelligence Quotient [M(SD)] | 89.45 (13.03) | 102.88 (16.24) | t(108) = −4.67, p < 0.00001 |
| SES [M(SD)] | 45.42 (12.91) | 46.07 (12.23) | t(103) = 0.26, p = 0.79 |
| Sex [n (%Female)] | 22 (38%) | 29 (56%) | χ2 = 3.51, p = 0.06 |
| Race | |||
| [n(%American Indian/Alaska Native)] | 4 (7%) | 2 (4%) | χ2 = 0.49, p = 0.48 |
| [n(%Asian)] | 1 (2%) | 4 (8%) | χ2 = 2.25, p = 0.13 |
| [n(%Native Hawaiian or other Pacific Islander)] | 0 | 1 (2%) | χ2 = 1.13, p = 0.29 |
| [n(%Black or African American)] | 17 (29%) | 14 (27%) | χ2 = 0.08, p = 0.78 |
| [n(%White)] | 34 (59%) | 31 (60%) | χ2 = 0.01, p = 0.92 |
| Ethnicity [n(%Hispanic)] | 10 (17%) | 7 (14%) | χ2 = 2.22, p = 0.33 |
| Handedness [n(%Right)] | 50 (86%) | 46 (88%) | χ2 = 1.66, p = 0.65 |
| Physical characteristics | |||
| aGrowth Deficiency | 14 (24%) | 6 (12%) | χ2 = 2.92, p = 0.09 |
| bMicrocephaly | 10 (17%) | 0 (0%) | χ2 = 9.86, p < 0.01 |
| cDysmorphic Face | 25 (43%) | 11 (21%) | χ2 = 6.04, p = 0.01 |
| Site [n] | χ2 = 2.23, p = 0.53 | ||
| Atlanta | 12 (21%) | 12 (23%) | |
| Los Angeles | 15 (26%) | 12 (23%) | |
| San Diego | 12 (21%) | 6 (12%) | |
| Minnesota | 19 (33%) | 22 (42%) | |
NOTE: Demographics are from the 110 participants included in the analyses. Age range at time point 1 for inclusion in the analyses was from 6.5 to 17.5 years. Participants were excluded from this analysis if any examination or other assessment data were not collected. Of the initial eligible pool of 122 participants, 5 participants were eliminated for excessive motion during MRI, 1 participant was eliminated due to missing data, and 6 were eliminated for FreeSurfer processing errors (described in the text).
PAE = Prenatal Alcohol Exposure group
Age = age at time point 1 scan
TIV = average total intracranial volume between time point 1 and time point 2
SES = Socioeconomic Status; via the Hollingshead Score (if two caretakers take average)
Height or weight ≤ 10%ile.
Head circumference ≤ 10%ile.
At least two of the following: Palpebral Fissure Length ≤ 10%ile, thin vermillion border, smooth philtrum (4 or 5 on lipometer scale).
All participants underwent an Institutional Review Board (IRB)-approved informed consent process involving a parent or guardian as well as a separate assent process with the child. All study procedures were approved by the IRBs at each of the four sites. Participants were compensated for their time.
2.2. Evaluations
Neuropsychological testing was conducted during one or (occasionally) two sessions by trained research assistants who were blind to participant group. Quality control methods included a video review of test administration procedures and a detailed scoring check for every 10th administration. From a larger battery of neuropsychological measures administered in CIFASD, only IQ is examined here (Differential Ability Scales − Second Edition (DAS-II) (Elliott, 2007)). Demographic and historical data were acquired on all CIFASD participants. Substance exposure histories, racial and ethnic background, and socioeconomic status (SES) via the Hollingshead Four Factor Index of Social Status (Hollingshead, 1975) are examined here. These data are contained in Table 1 and/or Results Section 3.1.
2.3. MRI acquisition procedure
MRI data were acquired at four sites on scanners from three vendors: Children’s Hospital of Los Angeles (Philips Achieva); University of California – San Diego (General Electric MR750); University of Minnesota and Emory University (both Siemens Tim Trio). The acquisition sequence was modeled after protocols developed for multi-site imaging by the Pediatric Imaging Neurocognition and Genetics (PING) group (Table 2) (http://ping.chd.ucsd.edu). The protocol included several scans, however, only the high-resolution T1-weighted scan was used in this analysis. Acquisition parameters are shown in Table 2. Participants were not sedated for the MRI scan nor were their usual medications modified.
Table 2.
MRI sequence and parameters.
| Platform | Sequence | Imaging Parameters | Purpose |
|---|---|---|---|
| Phillips (Los Angeles) | T1-weighted MPRAGE | TR = 6.8 ms, TE = 3.2 ms, TI = 845 ms, 170 slices, voxel size = 1 × 1 × 1.2 mm, FOV = 256 mm, flip angle = 8° | Cortical segmentation, & parcellation, & gray matter-white matter contrast |
| General Electric (San Diego) | T1-weighted IRSPGR | TR = 7.38 ms, TE = 2.984 ms, TI = 640 ms, 166 slices, voxel size = 0.94 × 0.94 × 1.2 mm, FOV = 240 mm, flip angle = 8° | Cortical segmentation & parcellation, & gray matter-white matter contrast |
| Siemens (Minnesota & Atlanta) | T1-weighted MPRAGE | TR = 2170 ms, TE = 4.33 ms, TI = 1100 ms, 192 slices, voxel size = 1 × 1 × 1 mm, FOV = 256 mm, flip angle = 7° | Cortical segmentation & parcellation, & gray matter-white matter contrast |
2.4. MRI processing
2.4.1. T1 cortical parcellation
Cortical parcellation of the T1 volume was performed using FreeSurfer version 5.3.0 (surfer.nmr.mgh.harvard.edu) (Dale et al., 1999). Processing included removal of non-brain tissue, automated Talairach transformation, segmentation, intensity normalization, tessellation of the grey matter/white matter boundary, topology correction, and surface deformation. Data were visually inspected by a trained operator to ensure accuracy. In the case of significantly aberrant FreeSurfer processing (such as failed boundary identification), participant data were not manually edited; instead, the data were excluded from the analyses.
2.4.2. Local gyrification index
The Local Gyrification Index (LGI) is a validated add-on metric to FreeSurfer (Schaer et al., 2008). Briefly, a smoothed outer brain surface map that does not follow the convexities of the cortex is first defined by FreeSurfer. A second map of the pial surface closely follows the folds of the cortex. Overlapping 25 mm circular regions of interest (ROIs) are then defined on the smoothed outer surface map. These ROIs are then paired with matching 25 mm pial surface ROIs. LGI is computed at each vertex as the ratio of “buried” cortex to the smoothed outer surface (Schaer et al., 2008). LGI can range between 1 and 5. An LGI of 5 indicates that there is 5 times more cortex contained within the sulci than the amount of cortex on the outer surface (representing a deeply folded region); in contrast, an LGI of 1 represents a totally smooth region of cortex (Schaer et al., 2012). LGI is an improvement over two-dimensional cortical folding models such as the Gyrification Index (GI) (simply the ratio between the white matter/gray matter boundary and the pial boundary of the brain determined by 2-D coronal sections (Zilles et al., 1988)) because it takes into account the three-dimensional nature of the cortical surface (Schaer et al., 2008) and allows for regionally-specific measurements.
2.4.3. Longitudinal processing pipeline
Individual participant T1 cortical parcellations from the two-time points were processed with the automated longitudinal processing stream in FreeSurfer to extract volumetric, area, and thickness estimates in the cortex (Reuter et al., 2012). This methodology uses an unbiased, within-subject template space and creates an image using robust, inverse consistent registration (Reuter et al., 2010). Several processing steps, such as skull stripping, Talairach transforms, atlas registration, as well as spherical surface maps and parcellations, are then initialized with common information from the within-subject template, significantly increasing reliability and statistical power (Reuter et al., 2012). After processing, the data from both scans were visually compared to ensure correspondent alignment between the two scans.
2.5. Statistical analysis
Statistical analyses were carried out with the MATLAB Statistics Toolbox (MATLAB, 2016), IBM SPSS Version 22 and FreeSurfer version 5.3.0. Subject characteristics were analyzed using chi-square or independent samples t-tests.
Longitudinal analyses were performed with FreeSurfer. First, symmetrized percent change (SPC), a dimensionless measure of change, was computed at each vertex from the participant data from the two time points (Berry and Ayers, 2006; Reuter et al., 2012). Symmetrized percent change is defined as:
where V1 is the vertex-wise brain measure at baseline and V2 is the measure at two-year follow-up. More simply, SPC is a single metric that represents the percent change across the two time points at each vertex for each participant. SPC maps of cortical thickness (CT), LGI, cortical surface area, and cortical volume were computed for each subject.
Group comparisons of whole brain SPC in CT, LGI, cortical surface area, and cortical volume were performed in FreeSurfer using the general linear model (GLM). A 10 mm FWHM Gaussian smoothing kernel was applied to CT, cortical surface area, and cortical volume. Because LGI is already relatively smooth, no additional smoothing was applied (Schaer et al., 2012). Separate GLM analyses were used for right and left hemispheres.
To determine whether particular variables [gender, study site, total intracranial volume (TIV), and age] were potential confounding factors, variables were tested individually with each dependent measure (SPC in LGI, CT, cortical surface area, and cortical volume). Prior to analysis, TIV and age were normalized by subtracting each value by the mean and dividing by the standard deviation (creating z-scores). Because there were two time points, the variables age and TIV were entered into the models as averages of the baseline and follow-up values. Variables that showed a significant relationship by a dependent measure (10% or more of the vertices involved) were considered as confounding variables and were included in subsequent models as covariates.
These pre-analysis data-checking steps revealed that age and study site were likely confounders of the four SPC measures (Table 3). Therefore, group difference tests were performed using normalized age and dummy coded study site as covariates. These analyses use the SPC – which represents change across two time-points; therefore, continuous co-variates (such as age) included in these models were included as mean values across both time points. For example see Fig. 4. Group (PAE vs. control) main effect and interaction effects of group × linear age and group x quadratic age were tested.
Table 3.
Summary of tests for potential confounding variables.
| With LGI as the dependent measure | |||
|---|---|---|---|
| Variable or Covariate | Size (mm2) | Number of vertices | Vertex Coverage Percentage |
| Study Site | 19,782 | 40,730 | 14.3% |
| TIV | 0 | 0 | 0 |
| Gender | 5533 | 11,667 | 4.1% |
| Age | 22,195 | 47,165 | 17.0% |
| With CT as the dependent measure | |||
|---|---|---|---|
| Variable or Covariate | Size (mm2) | Number of vertices | Vertex Coverage Percentage |
| Study Site | 56,376 | 106,188 | 37.4% |
| TIV | 2269 | 2881 | 1.0% |
| Gender | 0 | 0 | 0 |
| Age | 17,254 | 31,082 | 11.0% |
| With Cortical Volume as the dependent measure | |||
|---|---|---|---|
| Variable or Covariate | Size (mm2) | Number of vertices | Vertex Coverage Percentage |
| Study Site | 84,368 | 162,971 | 57.3% |
| TIV | 937 | 2185 | 0.7% |
| Gender | 0 | 0 | 0 |
| Age | 53,653 | 99,261 | 35% |
| With Cortical Surface Area as the dependent measure | |||
|---|---|---|---|
| Variable or Covariate | Size (mm2) | Number of vertices | Vertex Coverage Percentage |
| Study Site | 61,853 | 119,425 | 42% |
| TIV | 0 | 0 | 0 |
| Gender | 0 | 0 | 0 |
| Age | 40,326 | 72,779 | 25.4% |
NOTE: Monte Carlo Z Simulation test was applied for multiple comparisons. Confidence interval was 90% for all clusters.
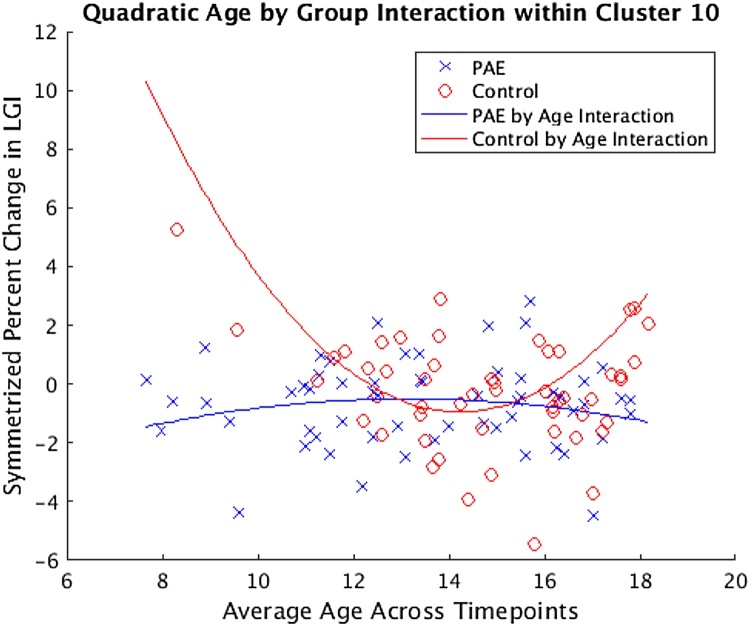
Fig. 4.
Representative scatterplot of cluster 10 corresponding to that found in Table 5B. The participants with PAE and controls are represented by blue “X” and red “O” symbols respectively. Nonlinear curves represent the quadratic function fit to the data. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The design matrix used for the analyses was the Different Offset – Different Slope (DODS) automated model option in FreeSurfer. Results from each GLM analysis were corrected for multiple comparisons with a two tailed Monte Carlo simulation implemented in FreeSurfer (Hagler et al., 2006) using a cluster-wise forming threshold of p < 0.05 and 10,000 random permutations. Results were visualized by overlaying significant clusters on top of an inflated cortical surface in the visualization tool Freeview using FreeSurfer 6.0.
Finally, IQ was evaluated as a rough “proxy” measure for overall level of neurodevelopmental status. It was hypothesized that trajectories of cortical development might be related to IQ (i.e. individuals with significant effects from PAE might have low IQ and, perhaps, an altered trajectory of cortical development over two years). To examine the spatial pattern of the relationship between IQ and SPC in cortical measures, the Query Design Estimate Contrast (QDEC) FreeSurfer interface tool, was used to compute Pearson product-moment correlations between IQ and SPC in cortical measures at each vertex. GLMs were run separately for right and left hemispheres. IQ was normalized by transforming to a z-score and entered as a covariate. Two analyses were performed: 1) including IQ as the sole covariate and 2) including normalized age as a nuisance factor in addition to IQ. To control for multiple comparisons, a two-tailed false discovery rate (FDR) was implemented (Genovese et al., 2002) to limit false positives to a corrected p-value of q = 0.05. Results were visualized in QDEC.
3. Results
3.1. Subject characteristics
As shown in Table 1, the PAE and control groups did not differ on gender, study site distribution, socioeconomic status (SES), race, ethnicity, handedness, growth deficiency, or TIV. Of note, many children in the PAE group are in adoptive families with SES that is likely higher than the biological families of origin, resulting in the uniformity in SES across groups.
By chance, the mean age of the control group was approximately one year older than the PAE group at baseline, so all subsequent longitudinal analyses controlled for age (averaged across baseline and follow up scans) in addition to other potential confounds. As expected, compared to controls, those with PAE had significantly higher occurrences of FAS diagnoses, microcephaly, and dysmorphic facial features. Additionally, the PAE group had significantly lower IQ scores compared to controls (14 points, or approximately one standard deviation lower). In addition to alcohol, prenatal exposure information for other substances was acquired. Exposure was reported as follows: amphetamines (0 controls, 8 PAE), cocaine (1 control, 16 PAE), marijuana (1 control, 18 PAE), tobacco (2 controls, 27 PAE), caffeine (25 controls, 17 PAE), hallucinogen (0 controls, 0 PAE), heroin (0 controls, 1 PAE), painkillers/opioids (0 controls, 2 PAE), and tranquilizers (0 controls, 4 PAE).
3.2. MRI data quality
All Freesurfer automated segmentation and parcellation results were visually inspected for accuracy/artifact. A total of 12 participants were excluded from the analysis due to aberrant FreeSurfer processing in one or both time points; mostly because of excessive motion during scanning. The excluded participants were as follows: 8 PAE and 4 Controls; 9 males and 3 females; 2 from Atlanta; 2 from Los Angeles; 5 from Minnesota; and 3 from San Diego. The included and excluded participants were compared on demographic characteristics. No significant differences were found between the included and excluded participants in terms study site [χ2 = 0.94, p = 0.82], sex [χ2 = 2.00, p = 0.16], handedness [χ2 = 1.73, p = 0.63], race/ethnicity [χ2 = 0.03, p = 0.98], history of prenatal alcohol exposure [χ2 = 5.622, p = 0.132], FAS Diagnosis [χ2 = 4.69, p = 0.10], microcephaly [χ2 = 0.015, p = 0.90], dysmorphic face [χ2 = 0.39, p = 0.53], growth deficiency [χ2 = 0.82, p = 0.37], age [t(120) = 1.91, p = 0.06], TIV [t(120) = 0.71, p = 0.48], or IQ [t(119) = −0.56, p = 0.57].
3.3. Cortical surface area not included in subsequent analyses
The analyses of the SPC for cortical surface area found no significant differences between the PAE and control groups. This metric was dropped from further analyses.
3.4. Diagnostic group by linear age interaction effects for the cortical measures
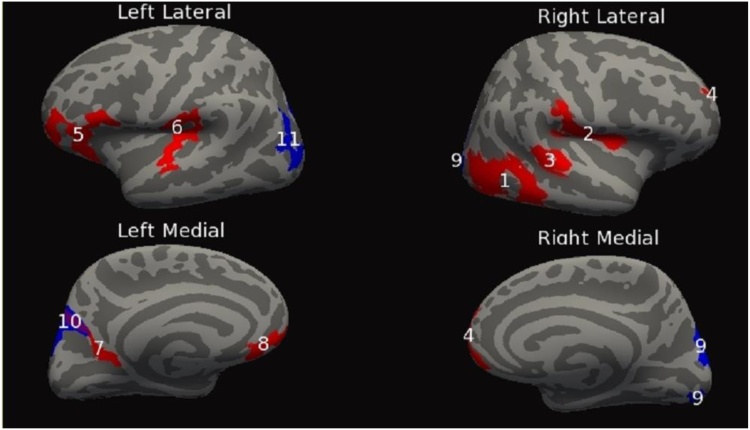
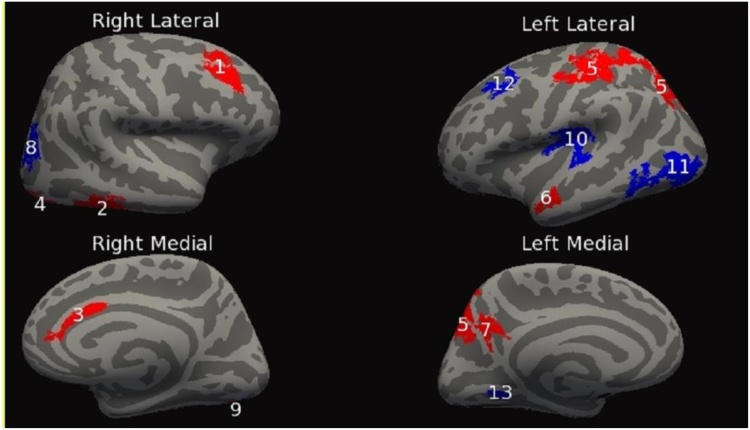
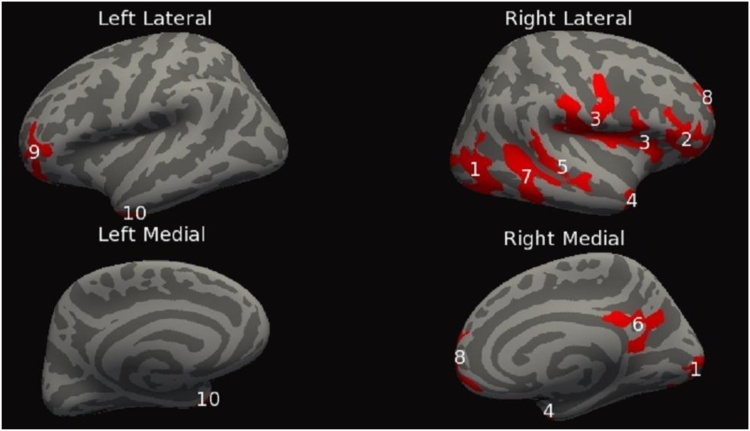
This set of analyses tested for significant group (PAE vs. control) x linear age interaction effects for each of the cortical measures. For the dependent measure SPC in CT, a group x age interaction revealed eight clusters bilaterally, four on each hemisphere. The clusters ranged in size from 661 mm2 to 3404 mm2. Additional cluster information can be found in Table 4A and Fig. 1. Second, the dependent measure SPC in LGI exhibited seven bilateral clusters, four on the right hemisphere and three on the left hemisphere. The clusters ranged in size from 300 mm2 to 4351 mm2. For additional cluster information see Table 4B and Fig. 2. Finally, the dependent measure SPC in cortical volume had twelve significant clusters, eight on the right hemisphere and four on the left hemisphere. The clusters ranged in size from 698 mm2 to 3185 mm2. Additional clusters can be found in Table 4C and Fig. 3.
Table 4.
Linear change cluster summary. Clusters (cluster forming threshold, p < 0.05; clusters for multiple comparisons, p < 0.05) showing PAE vs. Control by linear age interactions on% change in dependent measure controlling for study site.
| A. CT | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Number | Cerebral Hemisphere | Anatomical Location | Size (mm2) | Number of vertices | Peak vertex MNI (x,y,z) | Clusterwise P-value | Findings |
| 1 | Right | Inferiorparietal | 3403.62 | 5357 | (44.0, −62.9, 6.1) | 0.00020 | Con > PAE |
| 2 | Right | Insula | 1409.80 | 3893 | (35.7, −3.6, 15.7) | 0.00020 | Con > PAE |
| 3 | Right | BankSTS | 713.82 | 1697 | (58.1, −36.2, 6.8) | 0.038 | Con > PAE |
| 4 | Right | Superiorfrontal | 1026.46 | 1458 | (12.8, 60.8, 16.6) | 0.0016 | Con > PAE |
| 5 | Left | Pars Opercularis | 2338.26 | 3959 | (−54.0, 19.3, 15.0) | 0.00020 | Con > PAE |
| 6 | Left | Transversetemporal | 1362.45 | 3340 | (−34.3, −32.4, 14.7) | 0.00020 | Con > PAE |
| 7 | Left | Isthmuscingulate | 660.61 | 1377 | (−17.1, −54.3, 7.0) | 0.049 | Con > PAE |
| 8 | Left | Frontalpole | 682.94 | 1072 | (−5.6, 59.9, −12.0) | 0.041 | Con > PAE |
| B. LGI | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Number | Cerebral Hemisphere | Anatomical Location | Size (mm2) | Number of vertices | Peak vertex MNI (x,y,z) | Clusterwise P-value | Findings |
| 1 | Right | Rostralmiddlefrontal | 933.25 | 1717 | (37.9, 32.4, 31.1) | 0.00020 | Con > PAE |
| 2 | Right | Inferiortemporal | 1235.72 | 2075 | (56.3, −46.9, −20.0) | 0.00020 | Con > PAE |
| 3 | Right | Caudalanteriorcingulate | 300.17 | 673 | (7.3, 24.9, 21.0) | 0.038 | Con > PAE |
| 4 | Right | Lateraloccipital | 602.26 | 798 | (38.0, −86.3, −13.8) | 0.00020 | Con > PAE |
| 5 | Left | Superiorparietal | 4350.63 | 9296 | (−22.4, −62.1, 54.2) | 0.00020 | Con < PAE |
| 6 | Left | Superiortemporal | 394.19 | 574 | (−50.6, −8.9, −22.6) | 0.0046 | Con < PAE |
| 7 | Left | Precuneus | 386.49 | 758 | (−5.2, −64.2, 29.7) | 0.0052 | Con < PAE |
| C. Cortical volume | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Number | Cerebral Hemisphere | Anatomical Location | Size (mm2) | Number of vertices | Peak vertex MNI (x,y,z) | Clusterwise P-value | Findings |
| 1 | Right | Lateraloccipital | 3184.85 | 4430 | (41.1, −66.7, −3.1) | 0.00020 | Con > PAE |
| 2 | Right | Pars Orbitalis | 1418.77 | 2185 | (34.0, 40.8, −8.5) | 0.00020 | Con > PAE |
| 3 | Right | Precentral | 3070.70 | 7514 | (58.8, −3.2, 15.6) | 0.00020 | Con > PAE |
| 4 | Right | Inferiortemporal | 784.06 | 1286 | (37.9, 1.8, −36.9) | 0.01851 | Con > PAE |
| 5 | Right | Superiortemporal | 697.52 | 1656 | (48.1, −33.5, 2.4) | 0.04371 | Con > PAE |
| 6 | Right | Isthmuscingulate | 801.40 | 1757 | (4.8, −43.8, 27.2) | 0.01653 | Con > PAE |
| 7 | Right | Inferiortemporal | 1637 | 3084 | (47.8, −40.6,−19.2) | 0.00020 | Con > PAE |
| 8 | Right | Superiorfrontal | 1145.34 | 1511 | (11.0, 65.2, 1.6) | 0.00020 | Con > PAE |
| 9 | Left | Lateralorbitofrontal | 992.30 | 1389 | (−27.7, 39.9, −9.6) | 0.0026 | Con > PAE |
| 10 | Left | Temporalpole | 1035.55 | 1686 | (−35.4, 6.1, −37.1) | 0.0020 | Con > PAE |
NOTE: Con = Control group, PAE = Prenatal Alcohol Exposure group, STS = Superior Temporal Sulcus. Monte Carlo Z Simulation test was applied for multiple comparisons. Confidence interval was 90% for all clusters.
Fig. 1.
Inflated cortical convolution maps showing clusters after thresholding the uncorrected data and correcting for multiple comparisons, (cluster form threshold, p < 0.05; clusters for multiple comparisons, p < 0.05) showing significant% change in cortical thickness differences across those with PAE relative to controls. Linear age by patient group interaction effects are shown in red and quadratic age by patient group interaction effects are shown in blue. Locations in which red and blue clusters overlap are shown in purple. Numbers found on red cluster correspond to those found in Table 4A, while numbers found on blue clusters correspond to those found in Table 5A. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Inflated cortical convolution maps showing clusters after thresholding the uncorrected data and correcting for multiple comparisons, (cluster form threshold, p < 0.05; clusters for multiple comparisons, p < 0.05) showing significant% change in cortical gyrification (LGI) differences across those with PAE relative to controls. Linear age by patient group interaction effects are shown in red and quadratic age by patient group interaction effects are shown in blue. Locations in which red and blue clusters overlap are shown in purple. Numbers found on red cluster correspond to those found in Table 4B, while numbers found on blue clusters correspond to those found in Table 5B. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Inflated cortical convolution maps showing clusters after thresholding the uncorrected data and correcting for multiple comparisons, (cluster form threshold, p < 0.05; clusters for multiple comparisons, p < 0.05) showing significant% change in cortical volume differences across those with PAE relative to controls. Linear age by patient group interaction effects are shown in red. Numbers found on red cluster correspond to those found in Table 4C. As opposed to Fig. 1, Fig. 2 there are not quadratic age by patient group interaction effects, so there are no blue clusters. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Diagnosis group by quadratic age interaction effects across dependent measures
Because the shape of the trajectories in cortical development is not entirely known (especially in PAE), we tested for curvilinear quadratic effects in addition to the linear effects examined above. This set of analyses tested for significant diagnostic group x age quadratic interaction effects for each of the cortical measures. First, SPC in CT revealed three significant clusters, one on the right hemisphere and two on the left. The clusters ranged in size from 1139 mm2 to 2509 mm2. For more complete cluster information see Table 5A and Fig. 1. Next, SPC in LGI had six significant clusters, two on the right hemisphere and four on the left. The significant clusters ranged in size from 304 mm2 to 1826 mm2. For detailed cluster information see Table 5B and Fig. 2. Finally, SPC in cortical volume was measured, however, it did not reveal significant diagnosis group x quadratic age interaction effects.
Table 5.
Quadratic change cluster summary. Clusters (cluster forming threshold, p < 0.05; clusters for multiple comparisons, p < 0.05) showing PAE vs. Control by quadratic age interactions% change in dependent measure controlling for study site.
| A. CT | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Number | Cerebral Hemisphere | Anatomical Location | Size (mm2) | Number of vertices | Peak vertex MNI (x,y,z) | Clusterwise P-value | Findings |
| 9 | Right | Superiorparietal | 2509.18 | 3338 | (14.8, −87.2, 35.1) | 0.00020 | Con < PAE |
| 10 | Left | Precuneus | 1291.62 | 2161 | (−11.4, −71.4, 39.4) | 0.00020 | Con < PAE |
| 11 | Left | Inferiorparietal | 1138.88 | 1772 | (−33.9, −81.4, 29.2) | 0.00040 | Con < PAE |
| B. LGI | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Number | Cerebral Hemisphere | Anatomical Location | Size (mm2) | Number of vertices | Peak vertex MNI (x,y,z) | Clusterwise P-value | Findings |
| 8 | Right | Lateraloccipital | 1027.72 | 1712 | (28.3, −80.3, 14.1) | 0.00020 | Con > PAE |
| 9 | Right | Lateraloccipital | 304.10 | 380 | (23.8, −87.6, −7.1) | 0.039 | Con > PAE |
| 10 | Left | Supramarginal | 1282.00 | 3342 | (−39.7, −27.8, 22.7) | 0.00020 | Con > PAE |
| 11 | Left | Lateraloccipital | 1825.92 | 2810 | (−38.8, −87.3, −4.0) | 0.00020 | Con > PAE |
| 12 | Left | Caudalmiddlefrontal | 632.08 | 1071 | (−37.6, 21.5, 43.8) | 0.00020 | Con > PAE |
| 13 | Left | Lingual | 224.22 | 374 | (−20.5, −59.1, −5.3) | 0.05 | Con > PAE |
| C. Cortical volume | |||||||
|---|---|---|---|---|---|---|---|
| Cluster Number | Cerebral Hemisphere | Anatomical Location | Size (mm2) | Number of vertices | Peak vertex MNI (x,y,z) | Clusterwise P-value | Findings |
| N/A | Right | None | None | None | None | None | None |
| N/A | Left | None | None | None | None | None | None |
NOTE: Con = Control group, PAE = Prenatal Alcohol Exposure group. Monte Carlo Z Simulation test was applied for multiple comparisons. Confidence interval was 90% for all clusters.
For illustration, a representative scatter plot corresponding to cluster 10 from Table 5B is included to provide visualization of the quadratic function that these data fit (Fig. 4). Across the age range, control participants showed a curvilinear pattern when modeled as a quadratic function (individual change in LGI over two years was greatest in the youngest controls but there was also an increase in the rate of change in the 16–18-year-old controls). In contrast, participants with PAE showed a relatively flat pattern (with individual change in LGI over two years not varying much across different age participants).
3.6. Vertex-wise cortical measures by IQ correlation analyses
The correlational analyses conducted did not reveal cortical areas that showed a significant dependent measure by IQ correlation following FDR correction of q = 0.05.
3.7. Post-hoc diagnostic group analysis
As reported in results Section 3.1, some participants within the PAE group were also exposed to other substances of abuse. Participants with PAE and other substance exposure were grouped and compared to participants with only PAE. The groups were compared on SPC in cortical thickness, cortical volume, and cortical gyrification and Monte Carlo z-simulation was applied to correct for multiple comparisons. No statistical differences were found and, therefore, other substance use was not considered as a confound for these particular brain measures in this sample.
4. Discussion
This longitudinal study examined structural MRI scans across two years in children with and without PAE. We applied three-dimensional surface MRI techniques to determine whether individuals with PAE have altered cortical development over time compared to controls. We tested for differences between controls and individuals with PAE in symmetrized percent change (SPC) over two years in three different cortical metrics: CT, LGI, and CV. In addition, we tested for group differences in the interaction between SPC and age – effectively asking whether alcohol exposure was associated with an atypical distribution of two-year SPC over the course of development (covering 6–17 years of age). In nearly every significant regional cluster, at older ages, those with PAE showed either a flatter percent change or a more pronounced decrease in percent change in comparison to controls. In other words, although the overall pooled two-year longitudinal change was similar for the two groups, those with PAE did show an abnormal trajectory suggesting more of a “gap” between the groups at older ages. The exceptions, as found in Tables 5A and 4B, were: 1) Modeling the diagnostic group by age interaction as a quadratic, with SPC in CT as the dependent measure, controls showed a flatter change or more pronounced decrease with an increase in participant age as compared to the PAE group in the right superior parietal gyrus, left precuneus, and left inferior parietal gyrus; and 2) Modeling the diagnostic group by age interaction as a linear function, with SPC in LGI as the dependent measure, controls showed a flatter change or more pronounced decrease with an increase in participant age as compared to the PAE group in the superior parietal gyrus, superior temporal gyrus, and precuneus.
Correlations between IQ and cortical SPC were computed with and without z-scored age as a nuisance factor. These analyses sought to determine if the overall level of cognitive impairment (as measured by IQ) was associated with rates of cortical development. The correlational analysis did not reveal brain regions with significant associations following FDR correction between IQ and cortical SPC, highlighting that cognitive effects measured at one time point may not necessarily be associated with nor predictive of overall cortical change across development.
For context, it is worth noting that the cortical measurements used here are the result of developmental processes that begin very early in gestation, continue into the second and third trimesters, and in some cases continue into the third decade of life (Raznahan et al., 2011). Early cortical development can be broken into three overlapping but distinct stages: cellular proliferation (Bystron et al., 2008; Marsh et al., 2008), migration and synaptogenesis (Garel et al., 2003), and cortical myelination (Kinney et al., 2001, Kinney et al., 1988); see (Fogliarini et al., 2005) for review. Although it is not possible to pin down the timing of the specific cortical anomalies observed in PAE in the current study, these data highlight the downstream effects of early insults in a general sense and reiterate the vulnerability of the brain during all three trimesters.
Based on prior studies of typical development, it is clear that the cortex continues to undergo substantial changes throughout childhood and into adolescence. Previous research indicated that cortical gray matter volume follows an “inverted U” developmental course with volumes peaking between 10 and 12 years of age in the parietal lobe, between 11 and 13 in the frontal lobe, and 16 and 17 in the temporal lobe whereas cortical gray matter volume continued to increase in the occipital lobe through age 20 (Giedd et al., 1999; Lenroot and Giedd, 2006). The general trend observed has been that areas sub-serving primary sensory functions mature earliest whereas association areas (such as those involved in memory and executive function) mature later until cortical gray matter volume changes conclude during the third decade of life. Cortical gyrification and cortical thickness are two aspects that contribute to cortical volume that follow similar curvilinear developmental courses – with cortical gyrification showing a peak at age 4 years (both sexes) and cortical thickness showing peaks at 8.1 years (females) or 9.7 years (males) (Raznahan et al., 2011).
The regional differences in cortical trajectories between those with PAE and controls shown here could result from altered neurodevelopmental cascades leading to later cortical anomalies. Individuals in the PAE group could be showing altered patterns of change regionally in cortical volume, CT, and LGI over two years because the developmental trajectory is different (steeper or flatter overall) or because there is a difference in the timing of the change (such as a delay in initiating a period of more active change). The observed quadratic regional differences in rates of change are intriguing because these are not typically observed with LGI or CT. Instead, linear differences would be the expected pattern based on the average age of the control and PAE groups (Raznahan et al., 2011). The fact that there are longitudinal differences between controls and those with PAE in this age range, especially in high association areas such as the temporal lobe and frontal lobe, may represent useful insight for further study and also potentially for interventions and for understanding lifespan issues in FASD (Treit et al., 2014). Future studies extending further into the young adult years will help clarify the nature of the developmental trajectory alterations that result from early alcohol-related insults.
The findings presented here provide some direction for future research. FASD is a heterogeneous condition, comprised of varying symptoms across individuals (i.e. various combinations of cognitive deficits, facial dysmorphology, brain size differences, white matter anomalies, cortical abnormalities, etc.). The current data demonstrate that for cortical measures, there may be individual variance in the level of effect and there may also be different patterns that become apparent only with multiple measurements across development. In turn, it is possible that different patterns of prenatal alcohol exposure (trimester of exposure, etc.) could be associated with differential effects on the various aspects of brain development and these may each undergo different developmental trajectories. Thus, beginning to explore multiple modalities of brain development together over time will allow the field to move toward a more complete clinical understanding of the varied presentations of FASD.
5. Limitations
There are limitations of this study to be acknowledged. First, usable data cannot be obtained from every participant. In some cases, detailed cortical measurements were not obtained because of processing difficulties – most often because of motion artifact (which occurs in both PAE and control groups). Additionally, a challenge faced by many studies of FASD is the lack of detail regarding the exact timing and level of alcohol exposure. Without this level of detail, we could not conduct analyses examining the relationship between exposure amounts/timing and the trajectories of cortical development.
Individuals with FASD sometimes have been exposed to other substances of abuse that could theoretically alter neurodevelopment. To address this potential confound to the interpretation of our data, we conducted a set of post-hoc analyses and found no evidence that other substance exposure contributed significantly to the outcomes observed. Therefore, we determined that including the multiple-exposed individuals in the sample was statistically appropriate and we believe that this adds to the generalizability of the data to the population as it actually exists.
By chance, the study concluded with a small gender imbalance across groups (PAE and controls). Within the age range of the sample, it is known that males and females have differential cortical development trajectories; for example see (Koolschijn and Crone, 2013; Raznahan et al., 2011; Wierenga et al., 2014). Therefore, the impact of gender was statistically evaluated across all brain metrics but did not show a significant relationship with any metric.
The study was limited to a two-timepoint repeated-measure design; therefore a linear mixed model (LME) could not be applied to the data since it requires three or more time points. The LME would be a more powerful longitudinal analysis approach since participants with missing data, even single time point data, can be included in the model. Future studies with several time points may be able to detect significant effects of PAE on the differential rates of cortical change over time.
A third limitation is that, by chance, the PAE group was significantly (more than 1 year) younger than the control group. Although we evaluated the impact of age and included age as a factor in all our statistical models, this statistical control is not a perfect substitute for precisely matched groups; however, our analyses do suggest that these statistical controls allow us to interpret our results with confidence in this case.
A fourth potential limitation is derived from the multi-site nature of this study and the inevitability of measurement differences across sites. Although we collected human phantom data at all four sites prior to the study in order to adjust the protocols for the best possible match across sites, we did not collect additional phantom data during the study. We concluded that statistically controlling for site variation would be a more complete and parsimonious approach than attempting to apply calibration corrections to the data itself. We did observe site differences in all cortical measures (i.e. CT, LGI, cortical surface area, and cortical volume), which could be due to hardware differences, minor differences in acquisition sequences, or population differences across the sites. To address this potential confound, each site matched demographic factors for the control and PAE groups. In addition, we took a conservative approach and controlled for site in all our statistical models.
6. Conclusion
Regional brain differences were seen longitudinally in participants with PAE compared to controls in percent change in cortical thickness, cortical surface area, and gyrification by age interactions across two years of development, especially in high association areas. These findings may represent important insights when considering the timing of assessments and interventions for those with PAE. Trajectories in cortical change did not relate with IQ, suggesting that additional examinations of specific aspects of neurocognitive functioning may be warranted.
Conflict of interest
None of the authors has a relevant conflict of interest to disclose.
Funding
This study was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA): (U01AA017122 [PI: ERS], U01AA14834 [PI: SNM], U01AA026102 [PI: JRW], U24AA014811 [EPR], U24AA014815 [PI: KLJ], U24AA014818 [PI: Barnett]), and the Minnesota Supercomputing Institute.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
Acknowledgements
This work was performed in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2018.02.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Archibald S.L., Fennema-Notestine C., Gamst A., Riley E.P., Mattson S.N., Jernigan T.L. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev. Med. Child Neurol. 2001;43:148–154. [PubMed] [Google Scholar]
- Berry D.A., Ayers G.D. Symmetrized percent change for treatment comparisons. Am. Stat. 2006;60:27–31. [Google Scholar]
- Bystron I., Blakemore C., Rakic P. Development of the human cerebral cortex: boulder Committee revisited. Nat. Rev. Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-Based analysis. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- De Guio F., Mangin J.-F., Rivière D., Perrot M., Molteno C.D., Jacobson S.W., Meintjes E.M., Jacobson J.L. A study of cortical morphology in children with fetal alcohol spectrum disorders. Hum. Brain Mapp. 2014;35:2285–2296. doi: 10.1002/hbm.22327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Benders M., Borradori-Tolsa C., Cachia A., Lazeyras F., Ha-Vinh Leuchter R., Sizonenko S.V., Warfield S.K., Mangin J.F., Hüppi P.S. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131:2028–2041. doi: 10.1093/brain/awn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott C.D. 2nd ed. PsychCorp; San Antonio, TX: 2007. Differential Ability Scales, : Administration and Scoring Manual. [Google Scholar]
- Fernández-Jaén A., Fernández-Mayoralas D.M., Quiñones Tapia D., Calleja-Pérez B., García-Segura J.M., Arribas S.L., Muñoz Jareño N. Cortical thickness in fetal alcohol syndrome and attention deficit disorder. Pediatr. Neurol. 2011;45:387–391. doi: 10.1016/j.pediatrneurol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Fogliarini C., Chaumoitre K., Chapon F., Fernandez C., Lévrier O., Figarella-Branger D., Girard N. Assessment of cortical maturation with prenatal MRI. Part I: normal cortical maturation. Eur. Radiol. 2005;15:1671–1685. doi: 10.1007/s00330-005-2782-1. [DOI] [PubMed] [Google Scholar]
- Garel C., Chantrel E., Elmaleh M., Brisse H., Sebag G. Fetal MRI: Normal gestational landmarks for cerebral biometry, gyration and myelination. Child’s Nerv. Syst. 2003;19:422–425. doi: 10.1007/s00381-003-0767-4. [DOI] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Hagler D.J., Saygin A.P., Sereno M.I. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson T.J., Mueller B.A., Sowell E.R., Mattson S.N., Coles C.D., Kable J.A., Jones K.L., Boys C.J., Lim K.O., Riley E.P., Wozniak J.R. Cortical gyrification is abnormal in children with prenatal alcohol exposure. NeuroImage Clin. 2017;15:391–400. doi: 10.1016/j.nicl.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A.B. 1975. Four Factor Index of Social Status. (Unpubl. Manuscr.) [Google Scholar]
- Infante M.A., Moore E.M., Bischoff-Grethe A., Migliorini R., Mattson S.N., Riley E.P. Atypical cortical gyrification in adolescents with histories of heavy prenatal alcohol exposure. Brain Res. 2015;1624:446–454. doi: 10.1016/j.brainres.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney H.C., Brody B.A., Kloman A.S., Gilles F.H. Sequence of central nervous system myelination in human infancy. II. Patterns of myelinationin autopsied infants. J. Neuropathol. Exp. Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Kinney H.C., Karthigasan J., Borenshteyn N.I., Flax J.D., Kirschner D.A. Myelination in the developing human brain. Biochem. Correlates. Neurochem. Res. 2001;19:983–996. doi: 10.1007/BF00968708. [DOI] [PubMed] [Google Scholar]
- Koolschijn P.C.M.P., Crone E.A. Sex differences and structural brain maturation from childhood to early adulthood. Dev. Cogn. Neurosci. 2013;5:106–118. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Roussotte F., Sowell E.R. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol. Rev. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., Bookheimer S.Y., O’Connor M.J., Narr K.L., Kan E., Abaryan Z., Sowell E.R. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy In utero alcohol exposure disrupts the normal processes of brain development. J. Neurosci. 2012;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigland L.A., Ford M.M., Lerch J.P., Kroenke C.D. The influence of fetal ethanol exposure on subsequent development of the cerebral cortex as revealed by magnetic resonance imaging. Alcohol. Clin. Exp. Res. 2013;37 doi: 10.1111/acer.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- MATLAB . The MathWorks Inc.; Natick, Massachusetts: 2016. Version 9.0.0.341360 (R2016a) [Google Scholar]
- Marsh R., Gerber A.J., Peterson B.S. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1233–1251. doi: 10.1097/CHI.0b013e318185e703. (Neuroimaging) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson S.N., Foroud T., Sowell E.R., Jones K.L., Coles C.D., Fagerlund Å., Autti-Rämö I., May P.A., Adnams C.M., Konovalova V., Wetherill L., Arenson A.D., Barnett W.K., Riley E.P. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44:635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini R., Moore E.M., Glass L., Infante M.A., Tapert S.F., Jones K.L., Mattson S.N., Riley E.P. Anterior cingulate cortex surface area relates to behavioral inhibition in adolescents with and without heavy prenatal alcohol exposure. Behav. Brain Res. 2015;292:26–35. doi: 10.1016/j.bbr.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E.M., Migliorini R., Infante M.A., Riley E.P. Fetal alcohol spectrum disorders: recent neuroimaging findings. Curr. Dev. Disord. Rep. 2014;1:161–172. doi: 10.1007/s40474-014-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli A., Lebel C., Rasmussen C., Andrew G., Beaulieu C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2011;35:1404–1417. doi: 10.1111/j.1530-0277.2011.01476.x. [DOI] [PubMed] [Google Scholar]
- Rajaprakash M., Chakravarty M.M., Lerch J.P., Rovet J. Cortical morphology in children with alcohol-related neurodevelopmental disorder. Brain Behav. 2014;4:41–50. doi: 10.1002/brb3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. How does your cortex grow? J. Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Rosas H.D., Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson F.C., Narr K.L., Molteno C.D., Jacobson J.L., Jacobson S.W., Meintjes E.M. Prenatal alcohol exposure is associated with regionally thinner cortex during the preadolescent period. Cereb. Cortex. 2016;26:3083–3095. doi: 10.1093/cercor/bhv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte F.F., Bramen J.E., Nunez S.C., Quandt L.C., Smith L., O’Connor M.J., Bookheimer S.Y., Sowell E.R. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: the effects of methamphetamine, alcohol, and polydrug exposure. Neuroimage. 2011;54:3067–3075. doi: 10.1016/j.neuroimage.2010.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte F.F., Sulik K.K., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., O’Connor M.J., Narr K.L., Sowell E.R. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum. Brain Mapp. 2012;33:920–937. doi: 10.1002/hbm.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhanam P., Coles C.D., Li Z., Li L., Lynch M.E., Hu X. Default mode network dysfunction in adults with prenatal alcohol exposure. Psychiatry Res. Neuroimaging. 2011;194:354–362. doi: 10.1016/j.pscychresns.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaer M., Cuadra M.B., Tamarit L., Lazeyras F., Eliez S., Thiran J.-P. A Surface-based approach to quantify local cortical gyrification. IEEE Trans. Med. Imaging. 2008;27:161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Schaer M., Cuadra M.B., Schmansky N., Fischl B., Thiran J.-P., Eliez S. How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. J. Vis. Exp. 2012:1–8. doi: 10.3791/3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D., Fisher P., Lucas C.P., Dulcan M.K., Schwab-Stone M.E. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J., Clasen L., Lenroot R., Gogtay N., Evans A., Rapoport J., Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Trauner D.A., Gamst A., Jernigan T.L. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev. Med. Child Neurol. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Mattson S.N., Kan E., Thompson P.M., Riley E.P., Toga A.W. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb. Cortex. 2008;18:136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth A.P., O’Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin. Clin. Neuropsychiatry. 2000;5:177–190. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Swayze V.W., Johnson V.P., Hanson J.W., Piven J., Sato Y., Giedd J.N., Mosnik D., Andreasen N.C. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- Treit S., Lebel C., Baugh L., Rasmussen C., Andrew G., Beaulieu C. Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J. Neurosci. 2013;33:10098–11009. doi: 10.1523/JNEUROSCI.5004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit S., Zhou D., Lebel C., Rasmussen C., Andrew G., Beaulieu C. Longitudinal MRI reveals impaired cortical thinning in children and adolescents prenatally exposed to alcohol. Hum. Brain Mapp. 2014;35 doi: 10.1002/hbm.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Wozniak J.R., Muetzel R.L., Mueller B.A., McGee C.L., Freerks M.A., Ward E.E., Nelson M.L., Chang P.N., Lim K.O. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol. Clin. Exp. Res. 2009;33:1825–1835. doi: 10.1111/j.1530-0277.2009.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Muetzel R.L., Bell C.J., Hoecker H.L., Nelson M.L., Chang P.N., Lim K.O. Inter-Hemispheric functional connectivity disruption in children with prenatal alcohol exposure. Alcohol. Clin. Exp. Res. 2011;35:849–861. doi: 10.1111/j.1530-0277.2010.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Mueller B.A., Bell C.J., Muetzel R.L., Hoecker H.L., Boys C.J., Lim K.O. Global functional connectivity abnormalities in children with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2013;37:748–756. doi: 10.1111/acer.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Roussotte F., Kan E., Sulik K.K., Mattson S.N., Riley E.P., Jones K.L., Adnams C.M., May P.A., O’Connor M.J., Narr K.L., Sowell E.R. Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology. Cereb. Cortex. 2012;22:1170–1179. doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Lebel C., Lepage C., Rasmussen C., Evans A., Wyper K., Pei J., Andrew G., Massey A., Massey D., Beaulieu C. Developmental cortical thinning in fetal alcohol spectrum disorders. Neuroimage. 2011;58:16–25. doi: 10.1016/j.neuroimage.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Zilles K., Armstrong E., Schleicher A., Kretschmann H.J. The human pattern of gyrification in the cerebral cortex. Anat. Embryol. (Berl.) 1988;179:173–179. doi: 10.1007/BF00304699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.