Abstract
Purpose
The aim of this prospective randomized control trial was to evaluate if the use of two different volumes (20–25 vs 40–45 μl) of media used for embryo transfer affects the clinical outcomes in fresh in vitro fertilization (IVF) cycles.
Methods
In total, 236 patients were randomized in two groups, i.e., “low volume” group (n = 118) transferring the embryos with 20–25 μl of medium and “high volume” group (n = 118) transferring the embryos with 40–45 μl of medium. The clinical pregnancy, implantation, and ongoing pregnancy rates were compared between the two groups.
Results
No statistically significant differences were observed in clinical pregnancy (46.8 vs 54.3%, p = 0.27), implantation (23.7 vs 27.8%, p = 0.30), and ongoing pregnancy (33.3 vs 40.0%, p = 0.31) rates between low and high volume group, respectively.
Conclusion
Higher volume of culture medium to load the embryo into the catheter during embryo transfer does not influence the clinical outcome in fresh IVF cycles.
Trial registration number: NCT03350646
Keywords: Embryo transfer, Volume, Culture medium, Loading the catheter, Clinical pregnancy rate
Introduction
An effective embryo transfer is the final and probably the most crucial step for a successful in vitro fertilization (IVF) attempt. Several clinical or practical details on the technique of embryo transfer, such as the type of the catheter, the composition of the culture medium used for the transfer, or even the catheter loading technique, may affect implantation and subsequent pregnancy rates [1–5].
The volume of culture medium used for the transfer is another variable that has been speculated to affect the IVF outcome. Some authors have suggested that a large volume of fluid may result in embryo expulsion out of the uterus [6, 7] while extra low volumes (< 10 μl) may result in implantation failure [8]. Others have reported that higher volumes of culture medium (35–40 vs 15–20 μl) may favor the embryo implantation [9]. In the majority of the studies, however, the volume of culture medium used to load the embryos is in the range of 20 to 30 μl [10, 11], albeit there is not a consensus on the volume of media that should be used during the transfer. Interestingly, according to a recent web-based survey, the fluid volume loaded during the embryo transfer by the embryologist/technician showed great heterogeneity [12]. However, it is not clear which is the exact volume of the transferred medium during embryo transfer. Since the evidence for optimal transfer volume is lacking, the objective of this study was to determine if a low vs high volume of transfer medium influenced cycle outcomes.
Material and methods
Patients undergoing embryo transfers in our center during the period between November 2014 to December 2016 were evaluated for possible inclusion in the study. The inclusion criteria were as follows: age ≤ 45 years old, BMI ≤ 36 kg/m2, baseline FSH concentration < 15 IU/l, ovarian stimulation with the same GnRH antagonist protocol, normal uterine cavity with endometrium thickness > 7 mm, and trilaminar morphology at the time of transfer and semen parameters of > 1 × 106/ml motile spermatozoa with > 4% physiological morphology (according to 5th edition of WHO laboratory manual for the examination and processing of human semen) [13]. PGD, frozen–thaw, natural cycles or patients following different ovarian stimulation protocol were excluded from the study. Patients who fulfilled the inclusion criteria and agreed to participate in the study were randomized, on the day of the embryo transfer, into group A, low volume (20–25 μl), and group B, high volume (40–45 μl).
The randomization was accomplished with the use of an online research randomizer. To avoid potential bias, the embryo assessment and embryo loading on the transfer catheter were performed by the same experienced embryologist. Both patients and physician performing the transfer were unaware of the volume of the culture medium used in each case.
Cases requiring a rigid catheter, the presence of retained embryos, or excessive presence of mucus or blood on the catheter tip or cases where extensive manipulations during catheter insertion were documented, were excluded from further analysis. The study was approved by the Aretaieion University Hospital ethics committee (School of Medicine, National and Kapodistrian University of Athens, registration no. B-74/30-10-2014). Informed consent form was obtained from all patients.
Ovarian stimulation, fertilization, and embryo culture
Ovarian stimulation was achieved according to a short GnRH antagonist protocol with the use of human menopausal gonadotropins (hMG) (Merional, IBSA or Menopur, Ferring) followed by the administration of GnRh antagonist (Cetrorelix, Merck Serono Europe or Ganirelix, Merck Sharp & Dohme) starting on day 6 of gonadotropin stimulation. The final maturation of follicles was induced by administration of 10.000 IU hCG (Pregnyl, Merck Sharp &Dohme) when at least 2 follicles reached a mean diameter of 18 mm. Oocytes were aspirated under ultrasound guidance 34–36 h after the hCG administration.
Oocytes were inseminated either by conventional IVF or by ICSI, 4 h after oocyte retrieval. Embryos were cultured in sequential medium (Origio Sequential Series, Denmark) at 37 °C, 6% CO2. 17 ± 1 h post fertilization oocytes were evaluated for the appearance of two pronuclei and two polar bodies. Embryo morphology was assessed 68 ± 1 h post insemination according to the Istanbul consensus workshop [14]. The embryos were rated as good (GI) when six to eight cells were present, the cell size was stage specific with no multinucleation, and the fragmentation was less than 10%. The embryos were rated as average (GII) when six to eight cells were present, the majority of the cells had normal size with no evidence of multinucleation, and the fragmentation was less than 10–25%. The embryos that did not fulfill these criteria were rated as poor (GIII). After morphological evaluation, the selected embryos were placed in a center well culture dish filled with sequential Blast medium (Origio Sequential Series, Denmark) at 37 °C, 6% CO2 until embryo catheter loading. According to the manufacturer, the protein and macromolecule supplementation of the transferring medium are human serum albumin (HSA), hyaluronan, and synthetic serum replacement (SSR); however, the ratio of each component is unknown. All embryo transfers were performed on day 3 and surplus embryos, when available, were vitrified for future transfers. The number of the transferred embryos varied from one to four according to the national regulating law.
Luteal phase support was initiated the day after oocyte retrieval, with 200 mg of progesterone three times a day vaginally (Utrogestan; Besins Healthcare, Brussels, Belgium). Serum hCG levels were measured 12 days after the embryo transfer. In the case of a positive hCG, a vaginal ultrasound was performed 2 weeks later to confirm a clinical pregnancy with positive fetal heart rate. The follow-up of clinical pregnancies continued up to the 20th week of gestation to ensure the ongoing pregnancy rate.
Embryo transfer technique
For all embryo transfers, an ultrasoft catheter with an echogenic tip (Ultrasoft Frydman Set Echo, CCD, France) was used in conjunction with a sterile, disposable insulin syringe (as recommended from ESHRE guideline group on good practice in IVF) [15].
Since no disposable syringe with scale < 100 μl is available and to assure that the loading volume is accurate, the volume was pre-measured based on Frydman’s catheter guide marks and a 100-μl (P100) Gilson pipette (Pipetman®, Classic, Gilson Inc). A drop of 5, 20, and 40 μl was accurately measured with the Gilson pipette and the drop was immediately loaded into the catheter to match each volume with catheter’s guide marks. The measurements were repeated (× 10) to assure accuracy. Pre-marked catheters were used to load the embryos with low or high volume of transferring medium at the time of the actual transfer.
To avoid any drop in the temperature of the culture medium during loading, all catheters were kept in the incubator for 20 min at 37.0 °C prior to loading. The warm catheter was pre-washed with culture medium and loaded by the same experienced embryologist (G.S.). The three-drop method was used to load the catheter [16] with two different volumes of culture medium (low volume, 20–25 μl, vs high volume, 40–45 μl).
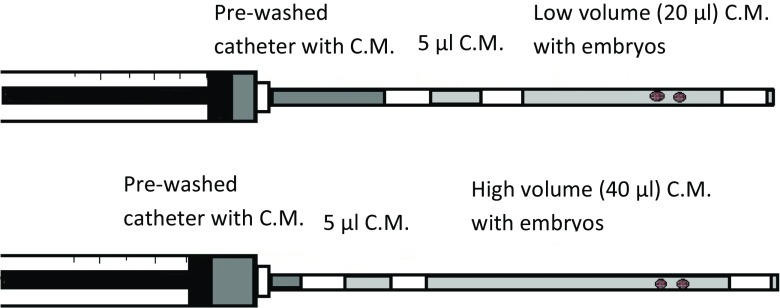
Initially, the catheter was loaded with 5 μl of culture medium separated with an air bubble from the main (high or low volume) drop of medium containing the embryos. An air bubble followed the main drop and at the end of the catheter, another 2–3 μl of culture medium was loaded. In all cases, special attention was paid to avoid formation of small air bubbles within the drop containing the embryos (Fig. 1).
Fig. 1.
Loading the catheter with the three-drop method. C.M. culture medium
All transfers were performed by a single highly experienced doctor (A.K.) under transabdominal ultrasound guidance. Before loading the embryos, any cervical mucus was removed with a sterile cotton swab. The catheter containing the embryos was inserted atraumatically through the cervical canal and advanced up to 15–20 mm from the fundus. The position of the catheter was confirmed via ultrasound and the embryos were smoothly expelled. After the expulsion of the embryos, the catheter was slowly withdrawn with the plunger continuously compressed to avoid the re-suction of the embryos, and returned to the embryologist to evaluate if there were retained embryos or excessive amount of blood or mucus.
Statistical analysis
The main outcome measured was clinical pregnancy rate (CPR) and secondary outcomes were implantation rate (IR) and ongoing pregnancy rate (OPR). The sample size was calculated based on an expected 48% clinical pregnancy rate (the overall pregnancy rate of the clinic) and the findings of the retrospective study from Montag et al. [9]. Based on a power of 80% and an alpha error of 5%, a study population of 117 patients per arm was required to indicate a minimum increase of 10% in clinical pregnancy rate between the low- and the high-medium volume groups. The chi-square test (Fisher’s correction, where applicable) was used to check for statistically significant differences. Statistical analyses were conducted with Statistical Package for Social Sciences (SPSS) version 23 (IBM Corp., Armonk, NY, USA).
Regarding CPR, IR, and OPR, in order to test for confounders among BMI, patient age and the number of fertilized oocytes, we used logistic regression (or Poisson regression, where appropriate) analysis. The multivariate regression models included the volume of culture medium used for transfer as well as the aforementioned parameters.
Results
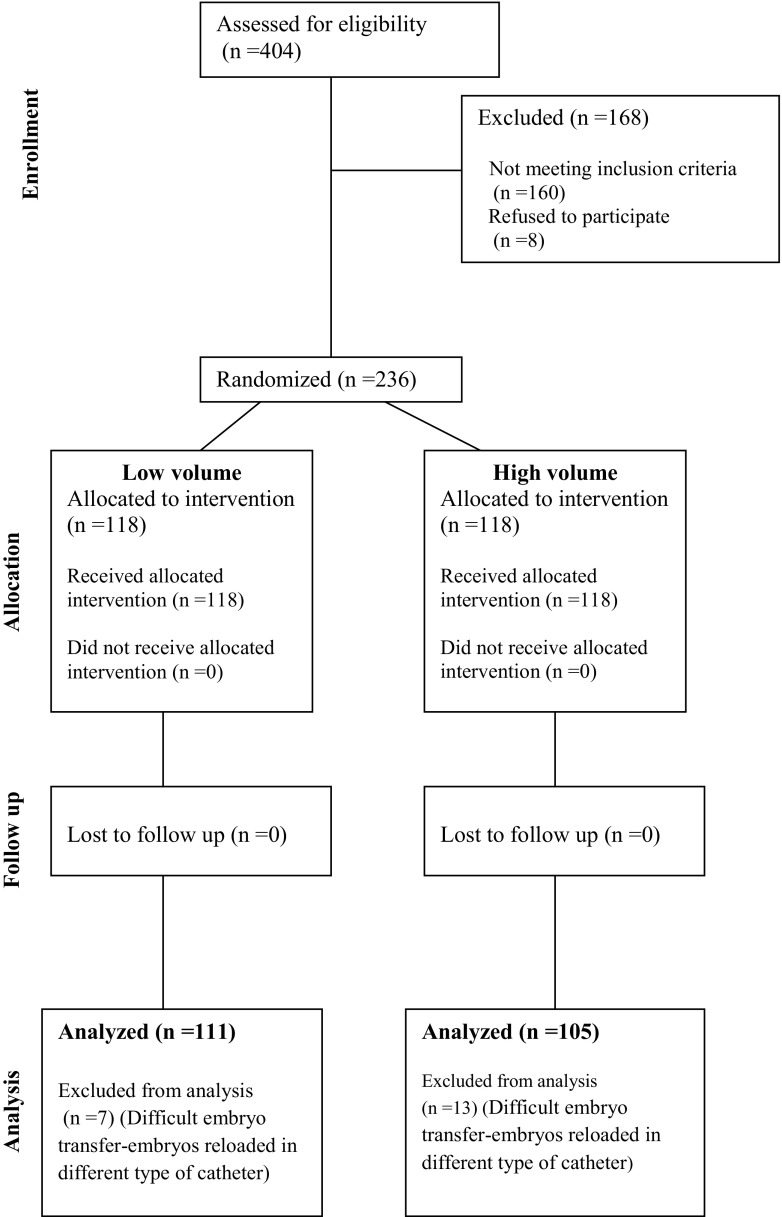
A total of 404 patients undergoing fresh IVF cycles/embryo transfer were assessed for eligibility. Eventually, 236 patients were randomized in two groups: 118 patients in group A (20–25 μl total volume of transfer medium) and 118 patients in group B (40–45 μl total volume of transfer medium). Twenty patients were excluded from the analysis due to the difficulty in embryo transfer procedure or the use of different types of catheter (Fig. 2). There were no embryos retained in both groups of patients. Patient’s demographics in terms of age, BMI, type of infertility, and baseline hormonal values are shown in Table 1. Overall, there were no differences between the two treatment groups (Table 1).
Fig. 2.
Flowchart showing the number of patients in each stage of the study
Table 1.
Demographic and baseline characteristics of the two groups
| Group A (n = 111) | Group B (n = 105) | p value | |
|---|---|---|---|
| Age (year) | 35.5 ± 4.0 | 36 ± 3.6 | 0.34 |
| BMI (kg/m2) | 24.2 ± 4.0 | 23.7 ± 3.9 | 0.35 |
| Infertility factor (%) | |||
| Unexplained | 20 (18%) | 17 (16.2%) | 0.44 |
| Male | 42 (37.8%) | 37 (35.2%) | |
| Tubal | 15 (13.5%) | 11 (10.5%) | |
| Endometriosis | 15 (13.5%) | 14 (13.3%) | |
| Pcos | 16 (14.4%) | 16 (15.2%) | |
| Combined | 3 (2.8%) | 10 (9.6%) | |
| Treatment cycle | |||
| 1 | 65 (58.6%) | 59 (56.2%) | 0.44 |
| 2 | 30 (27.0%) | 29 (27.6%) | |
| 3 | 11 (9.9%) | 7 (6.7%) | |
| ≥ 4 | 5 (4.5%) | 10 (9.5%) | |
| FSH (IU/ml)a | 7.43 ± 2.40 | 7.10 ± 2.45 | 0.32 |
| LH (IU/ml)a | 4.69 ± 2.20 | 4.99 ± 2.17 | 0.31 |
| Estradiol-E2 (pg/ml)a | 36.62 ± 10.51 | 39.38 ± 31.26 | 0.38 |
Group A = low volume, Group B = high volume
Values are mean ± standard deviation or no. (%)
BMI body mass index, Pcos polycystic ovarian syndrome
aDay 2 of the cycle, before stimulation
Similarly, there was no difference between the two groups in any of the stimulation parameters such as peak estradiol levels and total dose of gonadotropins required for the stimulation (Table 2).
Table 2.
Stimulation characteristics, embryological, and clinical outcomes according to the volume of culture medium used for transfer
| Group A (n = 111) | Group B (n = 105) | p value | |
|---|---|---|---|
| Peak estradiol-E2 on the day of hCG (pg/ml) | 2123 ± 1157 | 2203 ± 1025 | 0.59 |
| Total gonadotrophin dose (IUs) | 1924 ± 556 | 1884 ± 510 | 0.58 |
| Ooocyte retrieval (n) | 8.7 ± 5.0 (n = 809) | 9.3 ± 4.8 (n = 975) | 0.37 |
| Fertilization rate (n) | 71.1% (n = 686) | 70.5% (n = 687) | 0.92 |
| Number of embryos transferred (n) | 2.3 ± 0.6 (n = 253) | 2.3 ± 0.6 (n = 245) | 1.00 |
| Embryo quality (%) | |||
| em. Q I | 135 (53.4%) | 144 (58.8%) | 0.45 |
| em. Q II | 84 (33.2%) | 74 (30.2%) | |
| em. Q III | 34 (13.4%) | 27 (11.0%) | |
| Number of embryos vitrified (n) | 1.6 ± 2.4 (n = 179) | 1.7 ± 2.3 (n = 175) | 0.76 |
| Clinical pregnancy rate (CPR) (n) | 46.8% (n = 52) | 54.3% (n = 57) | 0.27 |
| Implantation rate (IR) (n) | 23.7% (n = 60) | 27.8% (n = 68) | 0.30 |
| Ongoing pregnancy rate (OPR) (n) | 33.3% (n = 37) | 40.0% (n = 42) | 0.31 |
Group A = low volume, Group B = high volume
Values are mean ± standard deviation or no. (%)
There was no difference between the two groups regarding the mean number of the oocytes retrieved (8.7 vs 9.3, p = 0.37), the fertilization rate (71.1 vs 70.5%, p = 0.92), the mean number of the embryos transferred (2.3 vs 2.3, p = 1.00), the mean number of embryos vitrified (1.6 vs 1.7, p = 0.76), and the embryo quality (GI 53.4 vs 58.8%, GII 33.2 vs 30.2%, GIII 13.4 vs 11.0%; p = 0.45). Finally, no significant differences were observed in CPR (46.8 vs 54.3%, p = 0.27), IR (23.7 vs 27.8%, p = 0.30), and OPR (33.3 vs 40.0%, p = 0.31) between groups A and B, respectively (Table 2).
Multivariate regression analysis showed that only patient’s age and the number of fertilized oocytes are significant parameters that can affect CPR (OR 0.9, 95% CI 0.83–0.98, p = 0.010 and OR 1.12, 95% CI 1.04–1.22, p = 0.004, respectively), IR (OR 0.93, 95% CI 0.89–0.97, p = 0.002 and OR 1.06, 95% CI 1.02–1.11, p = 0.003, respectively), and OPR (OR 0.88, 95% CI 0.80–0.94, p < 0.001 and OR 1.11, 95% CI 1.02–1.20, p = 0.012, respectively). On the other hand, higher volume of culture medium used for transfer is not significantly related to CPR (OR 1.40, 95% CI 0.80–2.46, p = 0.24), IR (OR 1.24, 95% CI 0.87–1.76, p = 0.23), and OPR (OR 1.54, 95% CI 0.85–2.78, p = 0.15).
Discussion
This prospective randomized study examined whether the volume of the media used for fresh transfers [(low (20–25 μl) or high (40–45 μl)] has any impact on pregnancy rates. Our study did not show any significant differences in pregnancy rates with the use of either low or high volume. The CPR, IR, and OPR were comparable between the two groups.
The optimum media volume used for embryo transfer is still unresolved in the literature [8, 9, 16]. While some recommend the use of low volume in order to minimize the incidence of ectopic pregnancy [7] and avoid embryo expulsion out of the uterus [17], others argue that there is a minimal volume of culture media required to fulfill the nutritional requirements of the embryo inside the endometrial cavity prior to implantation [9]. Moreover, some authors advocate that ultra low volume of transferring medium (< 10 μl) may negatively affect pregnancy and implantation rates [8].
So far, there has been no prospective randomized trial to evaluate the volume of medium used for embryo transfer. In addition, the majority of the relative studies are of limited value due to significant heterogeneity and variability regarding the operator loading the catheter or the person performing the transfer. Furthermore, in most relevant studies, the description of the transfer method is lacking the necessary details [18].
Our study was designed to detect a 10% difference in clinical pregnancy rates between the two arms because we believe that this difference is of clinical importance.
We were not able to demonstrate any difference in pregnancy rates or in any other outcome between the low and the high volume groups. Our results are in contrast with the results of the retrospective study of Montag et al. [9] which found that a high volume (40–50 vs 15–20 μl) for loading the transfer catheter resulted in significantly higher clinical pregnancy and implantation rates. In the study of Montag et al., two experienced doctors performed the embryo transfers. However, there is evidence that there may be differences in pregnancy rates even between experienced providers and their effectiveness to achieve high outcomes may change over time [19]. Additionally, differences in pregnancy rates have been observed between providers even there is no discernible difference in the embryo transfer technique. Furthermore, it is not clear if ultrasound guidance or “clinical touch” was used to transfer the embryos by the two operators. This could also introduce a potential bias in the study of Montag et al. since there is evidence to suggest that ultrasound guidance prevails over “clinical touch” in terms of live birth/ongoing and clinical pregnancy rates [20].
Our protocol was conducted based on the current knowledge on ovarian stimulation and embryo transfer. Ovarian stimulation was achieved based on the classic GnRH antagonist protocol. In order to avoid bias, the catheter was loaded by a single embryologist and embryo transfers were performed by a single experienced doctor. To avoid the possible effect of the type of catheter on the outcomes, all embryo transfers were performed with the same ultra soft type and if extra manipulations or use of more rigid catheter was required, these patients were excluded from the analysis [3, 21]. Moreover, the three-drop method was selected to load the catheter since the air brackets serve to protect the embryos till their expulsion into the uterine cavity [8] and enables the embryo position monitoring during ultrasound guidance [22]. It has been shown that the presence of air bubbles to bracket the drops do not influence the implantation and pregnancy rates when compared with the fluid-only loading technique [23]. Special attention was paid to prevent the formation of small air bubbles within the drop, containing the embryos, since this may harm the embryos [8]. To avoid an excessive decrease in medium temperature during embryo loading, the catheters were pre-warmed at least 20 min before each embryo transfer. The embryos were injected smoothly into the uterus in order to avoid embryo degeneration [24]. To achieve optimal implantation rates, the embryos were expelled 15–20 mm from the fundus [25]. The position of the embryo expulsion into the uterus was confirmed via ultrasound; however, there were no observations of air bubble movements following the removal of the catheter.
In the literature, the final transferring volume may fluctuate from 0.1 μl (media volume) [26] to 50 μl [16]. Since the volume variability is so large, we assume that the use of different ways of volume measurement may be the confound parameter. In our opinion, the volume of the transferring medium should be documented in the standard operation procedure (SOP) manual of each laboratory. It has been our experience (according to our SOPs) that a final volume up to 50 μl (40 μl culture medium, 10 μl air brackets) is large enough to load the Ultrasoft Frydman catheter with the embryos, regardless of the number of the embryos, even for trainee embryologists. Moreover, since a minority of trials present a standardized protocol or report details for the embryo transfer [18], this study provides a detailed protocol to investigate the role of culture medium volume in pregnancy rates.
To our knowledge, this is the first prospective randomized trial with adequate power to investigate the potential differences between low and high volume of transferring medium during stimulated fresh IVF cycles. Our study did not reveal any significant impact on pregnancy rates whether a low or a high volume of media is used during embryo transfer. However, these findings may apply only to the specific volumes used (20 vs 40 μl). Moreover, considering that one type of transferring medium (with a specific composition) was used in this study, it is essential to investigate if our findings apply to other types of media used for transfer. Additionally, the embryo transfer was performed with a specific type of embryo transfer catheter by a single doctor and these findings should be verified under different conditions. Consequently, larger prospective randomized control trials are needed to evaluate further these findings.
Compliance with ethical standards
The study was approved by the Aretaieion University Hospital ethics committee (School of Medicine, National and Kapodistrian University of Athens, registration no. B-74/30-10-2014). Informed consent form was obtained from all patients.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Schoolcraft WB, Surrey ES, Gardner DK. Embryo transfer: techniques and variables affecting success. Fertil Steril. 2001;76:863–870. doi: 10.1016/S0015-0282(01)02731-5. [DOI] [PubMed] [Google Scholar]
- 2.Bontekoe S, Heineman MJ, Johnson N, Blake D. Adherence compounds in embryo transfer media for assisted reproductive technologies. Cochrane Database Syst Rev. 2014;2:CD007421. doi: 10.1002/14651858.CD007421.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abou-Setta AM, Al-Inany HG, Mansour RT, Serour GI, Aboulghar MA. Soft versus firm embryo transfer catheters for assisted reproduction: a systematic review and meta-analysis. Hum Reprod. 2005;20:3114–3121. doi: 10.1093/humrep/dei198. [DOI] [PubMed] [Google Scholar]
- 4.Mansour RT, Aboulghar MA. Optimizing the embryo transfer technique. Hum Reprod. 2002;17:1149–1153. doi: 10.1093/humrep/17.5.1149. [DOI] [PubMed] [Google Scholar]
- 5.Sigalos G, Triantafyllidou O, Vlahos N. How do laboratory embryo transfer techniques affect IVF outcomes? A review of current literature. Hum Fertil (Camb) 2017;20:3–13. doi: 10.1080/14647273.2016.1255357. [DOI] [PubMed] [Google Scholar]
- 6.Poindexter AN, 3rd, Thompson DJ, Gibbons WE, Findley WE, Dodson MG, Young RL. Residual embryos in failed embryo transfer. Fertil Steril. 1986;46:262–267. doi: 10.1016/S0015-0282(16)49523-3. [DOI] [PubMed] [Google Scholar]
- 7.Leeton J, Trounson A, Jessup D, Wood C. The technique for human embryo transfer. Fertil Steril. 1982;38:156–161. doi: 10.1016/S0015-0282(16)46451-4. [DOI] [PubMed] [Google Scholar]
- 8.Ebner T, Yaman C, Moser M, Sommergruber M, Polz W, Tews G. The ineffective loading process of the embryo transfer catheter alters implantation and pregnancy rates. Fertil Steril. 2001;76:630–632. doi: 10.1016/S0015-0282(01)01980-X. [DOI] [PubMed] [Google Scholar]
- 9.Montag M, Kupka M, van der Ven K, van der Ven H. Embryo transfer on day 3 using low versus high fluid volume. Eur J Obstet Gynecol Reprod Biol. 2002;102:57–60. doi: 10.1016/S0301-2115(01)00579-6. [DOI] [PubMed] [Google Scholar]
- 10.Lee HC, Seifer DB, Shelden RM. Impact of retained embryos on the outcome of assisted reproductive technologies. Fertil Steril. 2004;82:334–337. doi: 10.1016/j.fertnstert.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Halvaei I, Khalili MA, Razi MH, Agha-Rahimi A, Nottola SA. Impact of different embryo loading techniques on pregnancy rates in in vitro fertlization/embryo transfer cycles. J Hum Reprod Sci. 2013;6:65–69. doi: 10.4103/0974-1208.112385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christianson MS, Zhao Y, Shoham G, Granot I, Safran A, Khafagy A, et al. Embryo catheter loading and embryo culture techniques: results of a worldwide web-based survey. J Assist Reprod Genet. 2014;31:1029–1036. doi: 10.1007/s10815-014-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 2010 5th edn Geneva World Health Organization.
- 14.Alpha Scientists in Reproductive M, Embryology ESIGo The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 15.De los Santos MJ, Apter S, Coticchio G, Debrock S, Lundin K, Plancha CE, et al. Revised guidelines for good practice in IVF laboratories (2015) Hum Reprod. 2016;31:685–686. doi: 10.1093/humrep/dew016. [DOI] [PubMed] [Google Scholar]
- 16.Friedman BE, Lathi RB, Henne MB, Fisher SL, Milki AA. The effect of air bubble position after blastocyst transfer on pregnancy rates in IVF cycles. Fertil Steril. 2011;95:944–947. doi: 10.1016/j.fertnstert.2010.07.1063. [DOI] [PubMed] [Google Scholar]
- 17.Schulman JD. Delayed expulsion of transfer fluid after IVF/ET. Lancet. 1986;1(8471):44. doi: 10.1016/S0140-6736(86)91925-2. [DOI] [PubMed] [Google Scholar]
- 18.Gambadauro P, Navaratnarajah R. Reporting of embryo transfer methods in IVF research: a cross-sectional study. Reprod BioMed Online. 2015;30:137–143. doi: 10.1016/j.rbmo.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Hearns-Stokes RM, Miller BT, Scott L, Creuss D, Chakraborty PK, Segars JH. Pregnancy rates after embryo transfer depend on the provider at embryo transfer. Fertil Steril. 2000;74:80–86. doi: 10.1016/S0015-0282(00)00582-3. [DOI] [PubMed] [Google Scholar]
- 20.Brown J, Buckingham K, Buckett W, Abou-Setta AM. Ultrasound versus ‘clinical touch’ for catheter guidance during embryo transfer in women. Cochrane Database Syst Rev. 2016;3:CD006107. doi: 10.1002/14651858.CD006107.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Kava-Braverman A, Martinez F, Rodriguez I, Alvarez M, Barri PN, Coroleu B. What is a difficult transfer? Analysis of 7,714 embryo transfers: the impact of maneuvers during embryo transfers on pregnancy rate and a proposal of objective assessment. Fertil Steril. 2017;107:657–63 e1. doi: 10.1016/j.fertnstert.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Krampl E, Zegermacher G, Eichler C, Obruca A, Strohmer H, Feichtinger W. Air in the uterine cavity after embryo transfer. Fertil Steril. 1995;63:366–370. doi: 10.1016/S0015-0282(16)57370-1. [DOI] [PubMed] [Google Scholar]
- 23.Abou-Setta AM. Air fluid versus fluid-only models of embryo catheter loading: a systematic review and meta-analysis. Reprod BioMed Online. 2007;14:80–84. doi: 10.1016/S1472-6483(10)60767-5. [DOI] [PubMed] [Google Scholar]
- 24.Grygoruk C, Pietrewicz P, Modlinski JA, Gajda B, Greda P, Grad I, et al. Influence of embryo transfer on embryo preimplantation development. Fertil Steril. 2012;97:1417–1421. doi: 10.1016/j.fertnstert.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Schoolcraft WB. Importance of embryo transfer technique in maximizing assisted reproductive outcomes. Fertil Steril. 2016;105:855–860. doi: 10.1016/j.fertnstert.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Bodri D, Colodron M, Garcia D, Obradors A, Vernaeve V, Coll O. Transvaginal versus transabdominal ultrasound guidance for embryo transfer in donor oocyte recipients: a randomized clinical trial. Fertil Steril. 2011;95:2263–2268. doi: 10.1016/j.fertnstert.2011.03.028. [DOI] [PubMed] [Google Scholar]