Abstract
The presence of a carbon-concentrating mechanism in the symbiotic dinoflagellate Symbiodinium sp. was investigated. Its existence was postulated to explain how these algae fix inorganic carbon (Ci) efficiently despite the presence of a form II Rubisco. When the dinoflagellates were isolated from their host, the giant clam (Tridacna gigas), CO2 uptake was found to support the majority of net photosynthesis (45%–80%) at pH 8.0; however, 2 d after isolation this decreased to 5% to 65%, with HCO3− uptake supporting 35% to 95% of net photosynthesis. Measurements of intracellular Ci concentrations showed that levels inside the cell were between two and seven times what would be expected from passive diffusion of Ci into the cell. Symbiodinium also exhibits a distinct light-activated intracellular carbonic anhydrase activity. This, coupled with elevated intracellular Ci and the ability to utilize both CO2 and HCO3− from the medium, suggests that Symbiodinium sp. does possess a carbon-concentrating mechanism. However, intracellular Ci levels are not as large as might be expected of an alga utilizing a form II Rubisco with a poor affinity for CO2.
Dinoflagellates of the genus Symbiodinium (=zooxanthellae) are known for their role in a number of symbiotic associations with mainly tropical marine invertebrates, including corals, clams, and sea anemones (Trench, 1987). The algae are either intra- or intercellular and generally associated with the digestive system of the host. The host therefore has a major influence on the supply of inorganic carbon (Ci) to the symbiont. Following carbon fixation by the zooxanthellae, much of the photosynthate is exported to the host and can contribute up to 100% of the host's energy requirements (Klumpp et al., 1992). The supply and fixation of carbon therefore has a major influence on the symbiosis.
In the giant clam (Tridacna gigas) zooxanthellae are found in tubules emanating from the stomach (Norton et al., 1992). These are in close proximity to the hemal sinuses, which contain hemolymph, the clam's blood supply. The hemolymph is the immediate source of nutrients for the zooxanthellae and its composition is affected by the photosynthetic rate of the dinoflagellates resulting in a diurnal variation in a number of parameters (Fitt et al., 1995, D. Yellowlees, personal communication). Thus, during photosynthesis the hemolymph [Ci] can drop from 1.8 to 0.8 mm, with a concomitant increase in pH from 7.3 in the dark to 8.2 at high light levels. During high rates of photosynthesis the hemolymph is supersaturated with O2, as bubbles are present in hemolymph samples removed from the sinuses. The fluctuations in Ci and pH are probably greater in the tubules themselves, but no measurements have been reported to date.
Recently, we reported that Symbiodinium sp. possesses a form II Rubisco (Whitney et al., 1995), which had previously been reported only in prokaryotic anaerobic, non-sulfur purple bacteria. Like other form II enzymes, dinoflagellate Rubisco has a relatively low discrimination ratio (Srel) between CO2 and O2 (Jordon and Ogren, 1981). Apart from dinoflagellates, all form II enzymes are found in anaerobic bacteria, in which a low Srel value has no physiological significance. Whitney and Andrews (1998) reported an Srel of approximately 35 for the form II Rubisco from Amphidinium carterae, a related free-living dinoflagellate. While this is the highest reported Srel for a form II Rubisco, it is still 40% lower than any form I enzyme. Whitney and Andrews (1998) concluded that this Srel value would allow dinoflagellates to maintain a positive photosynthetic carbon balance; however, the ratio of oxygenation to carboxylation would utilize light energy very inefficiently. This raises the question of how do dinoflagellates, with a form II Rubisco, survive in an aerobic environment and, in the case of Symbiodinium sp., export significant amounts of photosynthate? One possible mechanism for overcoming the limitations of a form II Rubisco in a potentially unfavorable CO2/O2 ratio environment would be the utilization of a carbon-concentrating mechanism (CCM). This would increase internal CO2 concentrations and minimize the effect of the oxygenation reaction of Rubisco.
A significant number of algae and cyanobacteria have been shown to actively accumulate Ci internally by utilizing a CCM (for review, see Badger et al., 1998). These elevated internal Ci levels allow algae to grow in Ci-limiting environments, produce higher carbon fixation rates, and also reduce the energetically wasteful oxygenation reaction of Rubisco.
There is circumstantial evidence that Symbiodinium sp. does possess a CCM; it has both internal and external carbonic anhydrase (CA) (Yellowlees et al., 1993) and appears capable of both HCO3− and CO2 utilization. Zooxanthellae isolated from corals utilize predominantly HCO3− (Goiran et al., 1996), while those from giant clams appear to utilize CO2 (Yellowlees et al., 1993). Whether this is due to the different environment within the host or different zooxanthellae strains is not known. Zooxanthellae also exhibit changes in photosynthetic characteristics after isolation from a host, with a decrease in both Pmax and K0.5 photosynthesis, suggesting that there might be changes in the Ci supply to Rubisco (W. Leggat, personal observation).
In addition, the fresh water dinoflagellate Peridinium gatunense has been found to acquire a CCM under Ci-limiting conditions and can maintain internal Ci concentrations between 7- and 80-fold above external levels (Berman-Frank and Erez, 1996; Berman-Frank et al., 1998).
This study was designed to determine if the symbiotic dinoflagellate possesses a CCM and whether the characteristics of the CCM change with time after isolation from the giant clam. We report here the results of our study into the uptake and accumulation of Ci by Symbiodinium sp. The results indicate that zooxanthellae do possess a CCM; however, intracellular Ci concentrations are not as high as might be expected of an alga utilizing a form II Rubisco for photosynthetic carbon fixation.
MATERIALS AND METHODS
Isolation of Zooxanthellae and Culturing of Algae
Giant clams (Tridacna gigas) were obtained from the Australian Centre for International Agricultural Research Giant Clam Project (James Cook University Orpheus Island Research Station, Queensland, Australia) and transported to the open-air aquarium at James Cook University (Townsville). Clams were acclimatized there for at least 3 weeks before experiments were commenced. Clams were then either killed at Townsville or flown to the Australian National University (Canberra, ACT, Australia) before being killed. Zooxanthellae were isolated by blending the mantle of a freshly killed clam in 0.45 μm of filtered seawater. The homogenate was then strained through two layers of cheesecloth and zooxanthellae pelleted by centrifugation at 600g for 2.5 min at 25°C. The zooxanthellae were then washed four more times in filtered seawater before they were suspended in filtered seawater at a cell density of approximately 2 × 106 cells mL−1 and cultured with a photon flux density of 100 μE m−2 s−1.
A culture of Amphidinium carterae (CS-21) was obtained from the Commonwealth Scientific and Industrial Research Organization Culture Collection of Microalgae (Hobart, Australia) and grown in G media (Loeblich, 1975) at 25°C at a photon flux density of 100 μE m−2 s−1. The algae were harvested during log-phase growth.
MS Measurements
Steady-State Photosynthesis and Ci Fluxes
All experiments were conducted at 28°C in CO2-free artificial seawater medium containing 428 mm NaCl and 25 mm 1,3-bis(Tris[hydroxymethyl]methylamino) propane (BTP) (pH 7.0 or 8.0). Experiments were conducted as previously described in Badger et al. (1994). An O2 electrode chamber was connected to a mass spectrometer via a gas-permeable membrane. The mass spectrometer was sequentially focused on masses 44 (CO2) and 32 (O2), and the changes in the concentrations recorded. Estimations were made of the HCO3− concentration by calibrating at acidic, buffered, and basic pH. Estimates of the flux of CO2, O2, and HCO3− into the cell were made using the equations of Badger et al. (1994).
Zooxanthellae were pelleted at 600g for 2.5 min, and resuspended in CO2-free artificial seawater medium, so that the Chl a concentration was between 4 and 10 μg Chl a mL−1, as determined by the method of Jeffrey and Humphrey (1975). Artificial seawater medium (5 mL, pH 7.0 or 8.0) was placed in the electrode chamber with 5 μL of acetazolamide (final concentration in cuvette of 50 μm), and 200 μL of zooxanthellae suspension was added. The cuvette was then illuminated (approximately 500 μE m−2 s−1) for 2 min to acclimatize the algae before the light was shut off and the cuvette was purged with N2 until the [O2] was approximately 100 μm. NaHCO3 was then added and dark measurements taken until equilibrium was achieved. The cells were then illuminated at a saturating light level (500 μE m−2 s−1) (Chang et al., 1983; Iglesias-Prieto and Trench, 1994) and readings taken until steady state was reached. Dark-light cycles were repeated with increasing HCO3− concentrations.
H13C18O3− Exchange
These experiments were also conducted in the mass spectrometer using methods similar to that of Palmqvist et al. (1995). This method gives qualitative information about the presence of a CCM. The loss of 18O from CO2 was measured by monitoring the CO2 masses 49 (13C18O2), 47 (13C18O16O), 45 (13C16O2), and 44 (CO2). The log enrichment of the 18O fraction in 13C18O2 was calculated using the equations of Palmqvist et al. (1995):
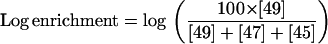 |
Experiments were conducted in 4 mL of artificial seawater medium (pH 8.0) to which was added H13C18O3− (final concentration 1 mm), and the uncatalyzed exchange was allowed to equilibrate (2 min). Zooxanthellae (100 μL) was then added (approximately 5 μg Chl a mL−1) and left in the dark for 3 min until equilibrium was established. They were then exposed to light for 3 min (500 μE m−2 s−1), followed by 5 min of darkness. In acetazolamide (AZA) and ethoxyzolamide (EZA) treatments, the inhibitor was added before the labeled bicarbonate to a final concentration of 50 and 500 μm respectively.
Photosynthetic O2 Exchange
All experiments were conducted at 28°C in CO2-free artificial seawater medium containing 428 mm NaCl and 25 mm BTP (pH 8.0). This media was maintained CO2 free by purging with CO2-free air, and was degassed under vacuum prior to use in the O2-exchange assays. Cells were maintained in a concentrated suspension at room temperature prior to use. O2-exchange assays were conducted in a 4-mL cuvette attached to a mass spectrometer via a Teflon semipermeable membrane. A similar method and calculations have been previously described (Canvin et al., 1980; Furbank et al., 1982). The assay and measurements involve the introduction of 18O2 into reaction medium that has been depleted of 16O2. This was done after the introduction of cells, using a small bubble of 18O2 above the reaction medium. During both the dark and light periods, changes in mass 32 (16O2) and mass 36 (18O2) were continuously monitored, and the rate of change in the concentration of these species was used to calculate gross O2 evolution, gross O2 uptake, and net O2 evolution. Assays were conducted with a cell density of 2 to 4 μg Chl a mL−1, and an O2 concentration of 250 to 350 μm (higher at the end of the experiment due to net O2 evolution). Light was provided at the top of the cuvette at 500 μE m−2 s−1 through a fiber-optic light source. Experiments were performed by adding cells to the cuvette and waiting for a steady-state dark value. Light was then switched on and Ci was added sequentially, allowing 4 to 7 min for a steady-state rate to be achieved at each Ci concentration.
Silicone Oil Centrifugation
This experiment used a method adapted from that of Badger et al. (1980). Freshly isolated or cultured zooxanthellae or A. carterae were pelleted (200g) and resuspended at a density of approximately 15 μg Chl a mL−1 in a solution of 25 mm BTP and 428 mm NaCl (pH 7.0 or 8.0) that had been bubbled with CO2-free air for 2 d. The zooxanthellae suspension was then placed in an O2 electrode chamber (Hansatech Instruments, King's Lynn, UK) at light levels of 500 μE m−2 s−1 until O2 production had ceased and the cells had reached their CO2 compensation point. Killing solution (20 μL of 2 n KOH and 10% MeOH) was added to 400-μL plastic microfuge tubes (Eppendorf Scientific, Westbury, NY), and 50 μL of silicone oil (approximately 2:1, 200/20, Wacker Chemie, Munich) was overlaid. The zooxanthellae suspension (200 μL) was then added.
The tubes were illuminated at a photon flux density of approximately 500 μE m−2 s−1. H14CO3− of known specific activity was added so that the final concentration was between 20 and 2,000 μm. Cells were incubated at each Ci concentration for 15 to 20 s, after which the tubes were centrifuged at 15,000g for 15 s to pellet the zooxanthellae. The tubes were snap-frozen and the bottom layer containing the zooxanthellae and the killing solution cut from the tube and resuspended in 500 μL of 0.1 n NaOH. The resuspended solution (200 μL) was added to 200 μL of 0.1 n NaOH or 200 μL of 0.2 n HCl. The acidic sample was placed in a fume hood and heated to 60°C for 1 h so that any unfixed 14C was evolved. BSC scintillation fluid (4 mL, Amersham-Pharmacia Biotech, Uppsala) was added to both the acid and basic samples and the 14C counted in a liquid scintillation counter (Wallac 1410, EG&G Wallac, Turku, Finland). The basic samples were assumed to contain both the fixed and unfixed 14C in the cell, while the acidic sample contained only the fixed Ci. Corrections were made for any extracellular 14C.
Calculation of Intracellular Volume and pH
Total extracellular and intracellular volumes were calculated using 3H2O and either [14C]mannitol or [14C]dextran (ICN, Costa Mesa, CA). Intracellular pH was measured using 5,5-dimethyl-[2-14C]oxazolidine 2,4-dione (Badger et al., 1980).
RESULTS
Measurement of Steady-State Photosynthesis and Ci Fluxes before and after Isolation
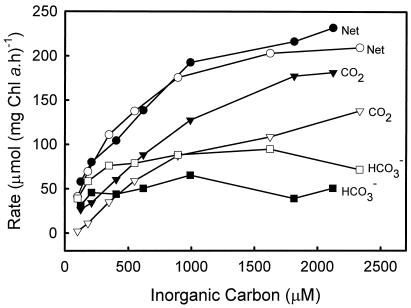
An initial characterization of Ci uptake processes in the zooxanthellae can be approached by deriving estimates for the ability of the cells to utilize both CO2 and HCO3− as carbon sources for photosynthesis. Mass spectrometry techniques (Badger et al., 1994) enable measurements of the net fluxes of CO2, HCO3−, and O2 into and out of the cell under steady-state photosynthesis conditions. This approach shows that freshly isolated zooxanthellae assayed at pH 8.0 predominantly take up CO2 from the external media but are also capable of some HCO3− uptake (Fig. 1). Net CO2 uptake is able to support between 45% and 80% of net photosynthesis, with the contribution increasing at higher levels of Ci. Conversely, the contribution of net HCO3− uptake declines from 55% to 20% over this same range.
Figure 1.
Comparison of net photosynthetic rate (●, ○), CO2 uptake (▾, ▿), and HCO3− uptake (▪, □) for Symbiodinium sp. freshly isolated from the giant clam T. gigas (black symbols) and a 2-d-old culture (white symbols) with differing Ci concentrations. Assays were conducted in 25 mm BTP (pH 8.0), 428 mm NaCl, and 50 μm AZA at 28OC with a photon flux density of 500 μE m−2 s−1. Data were collected using the mass spectrometry disequilibrium technique described in “Materials and Methods.”
After 2 d of isolation, the rates and patterns of Ci uptake changed significantly to increase the capacity for HCO3− uptake. The contribution of net CO2 uptake declined to 5% to 65%, while HCO3− increased to 95% to 35% over the same range of Ci concentrations. This change in HCO3− uptake indicates the induction of a HCO3− uptake system that allows utilization of a greater proportion of the available Ci, particularly at limiting [Ci]. Following isolation, the maximum net photosynthetic rate decreased from 300 ± 20 to 260 ± 10 μmol mg−1 Chl a h−1 (Fig. 1), while the K0.5 (Ci) was found to be 640 ± 100 and 500 ± 50 μm, respectively. A similar, although larger decrease has previously been observed after isolation (W. Leggat, personal observation).
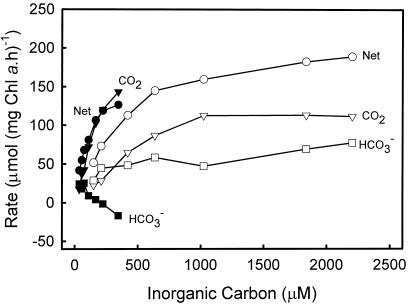
Flux measurements at pH 7.0 of zooxanthellae isolated for 1 d (Fig. 2) show that there is less HCO3− uptake capacity at this pH, where CO2 is a more dominant species. Although HCO3− does support 40% of photosynthesis at the most limiting [Ci], this rapidly declines as Ci increases. At half-saturating [Ci], the net HCO3− uptake supports only 10% of net photosynthesis. The K0.5(Ci) at pH 7.0 was 99 ± 9 μm compared with 433 ± 41 μm at pH 8.0. This indicates that K0.5(CO2) declines from around 16 μm to 9 μm from pH 7.0 to 8.0, supporting the occurrence of some increased HCO3− uptake, but clearly indicating that CO2 uptake plays a significant role in photosynthesis at both pH values. The net photosynthesis (Pmax) declined at pH 7.0 from 230 ± 10 (pH 8.0) to 160 ± 5 μmol mg−1 Chl a h−1. The negative HCO3− uptake values at pH 7.0 in this figure are most likely an artifact due to the errors involved in the calculation of this value. The main significance of this is that HCO3− uptake is low compared with CO2 uptake.
Figure 2.
Comparison of net photosynthetic rates (●, ○), CO2 uptake (▾, ▿), and HCO3− uptake (▪, □) for Symbiodinium sp. at pH 7.0 (black symbols) and pH 8.0 (white symbols) after being in culture for 1 d. Assays were conducted in 25 mm BTP and 428 mm NaCl with 50 μm AZA at 28°C with a photon flux density of 500 μE m−2 s−1. Data was collected using the mass spectrometry disequilibrium technique described in “Materials and Methods.”
Light-Stimulated CA Activity
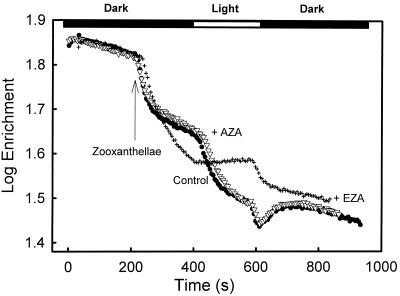
In searching for evidence for the operation of a CCM, one of the key processes common to both algae and cyanobacteria is active Ci transport, taking both CO2 and HCO3− from outside the cell and placing it in contact with localized regions of CA inside the cell. This enables the generation of CO2 from accumulated HCO3− so that [CO2] can be elevated around Rubisco (Badger and Price, 1992). A robust way that this can be measured is through monitoring light-stimulated inorganic exchange processes using 18O-enriched Ci species (Palmqvist et al., 1994). The active uptake of Ci species promotes the loss of 18O to unlabeled water due to internal CA activity, and this can be measured by examining the changes in isotopic enrichment of the CO2 species.
Figure 3 shows the results of such experiments. Control experiments and those with 50 μm AZA showed similar patterns (Fig. 3). The initial rapid decrease in enrichment with the addition of zooxanthellae is due to access of external CO2 to internal CA. This rate declines as steady-state equilibrium is reached. Upon illumination, there was a distinct light-stimulated decline in enrichment due to light-activated CA activity. AZA (50 μm), which inhibits external CA (Miyachi et al., 1983; Moroney et al., 1985), did not have any effect on the enrichment pattern in the dark or the light, suggesting that there may be little external CA activity associated with these cells, and that it does not inhibit active uptake of Ci.
Figure 3.
Effect of dark/light periods and CA inhibitors on the log enrichment (percentage 13C18O2) of Ci in the medium during assays of Symbiodinium sp. Assays were conducted in a water jacketed cuvette (28°C) connected to a mass spectrometer. Freshly isolated cells were added to the cuvette (final concentration of 0.125 μg Chl a mL−1) containing 4 mL of CO2-free media (25 mm BTP, pH 8.0, and 428 mm NaCl) and H13C18O3− (1 mm) that had achieved chemical equilibrium. After 3 min of darkness, the cuvette was illuminated at a photon flux density of 500 μE m−2 s−1 for 3 min, followed by another 5 min of darkness, as indicated by the bar at the top of the graph. Where appropriate, the CA inhibitors AZA (▿; final concentration 50 μm) or EZA (+; final concentration 500 μm) were added prior to the addition of the cells. Data are presented as log enrichment as per the formula detailed in “Materials and Methods.”
However, 500 μm EZA, which inhibits both internal and external CA, eliminated light-stimulated CA activity and active Ci uptake (Fig. 3). Patterns similar to those found with AZA and EZA have been observed in Chlamydomonas reinhardtii and Scenedesmus obliquus (Palmqvist et al., 1995). The effects of EZA are most readily interpreted as being due to an inhibition of both Ci transport processes and internal CA. This leads to a situation in which external Ci species are in passive equilibrium with the internal compartment. When light activates photosynthesis under these conditions, there is a competition between Rubisco and Ci hydration processes for CO2, which actually reduces the exchange of label from CO2, causing the enrichment to rise rather than fall. This pattern is also typical of algal species that appear to lack a CCM (Palmqvist et al., 1995). The rapid decline in the dark in the presence of EZA is somewhat anomalous and not readily explained. However, this feature was present in all experiments that were conducted using EZA as an inhibitor.
Similar experiments were also conducted on zooxanthellae that had been cultured for 2 d, and the results obtained were similar to those of freshly isolated zooxanthellae (data not shown).
Silicone Oil Centrifugation
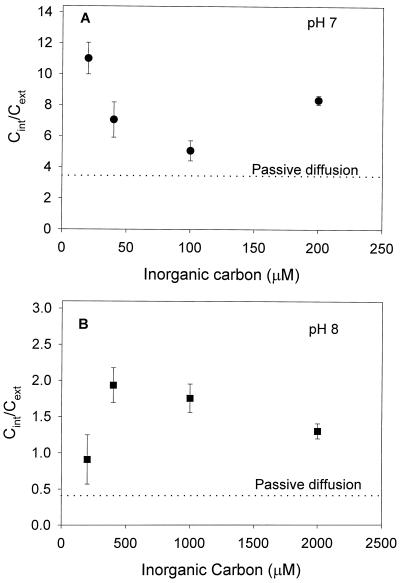
Although mass spectrometry allows estimates to be made of Ci uptake, it does not provide information about the intracellular Ci concentration (Cint). However, silicone oil centrifugation provides estimates of both the intracellular pool size and the pH, which influences the equilibrium concentration of Ci within the cell. When measurements were made of the Cint and fixed carbon at both pH 7.0 and 8.0, the Pmax at pH 7.0 and 8.0 was 144 ± 7 and 142 ± 9 μmol C fixed mg−1 Chl a h−1, respectively, while the K0.5 (Ci) was 90 ± 10 and 600 ± 100 μm (data not shown). The internal pH of the zooxanthellae was estimated as 7.62 ± 0.09, while the total intracellular volume of the cells in the assay was 0.12 ± 0.05 μL. At pH 7.0, freshly isolated zooxanthellae had an internal Ci/external Ci (Cint/Cext) between 1.5 and 3.2 times what would be expected if only passive diffusion of Ci into the cells had occurred (Fig. 4; Table I). At pH 8.0 the results were similar, with the Cint between 2.2 and 4.6 times what would be expected after passive diffusion (Fig. 4, Table I). Cint/Cext was measured for 5 d after isolation, over this period Cint/Cext increased to a maximum of 24.2 (7 times passive diffusion) after 1 d and decreased to a maximum of 14.4 (4.2 times passive diffusion) 5 d after isolation (Table I).
Figure 4.
The effect of external Ci on Cint/Cext at pH 7.0 (A) and 8.0 (B) for Symbiodinium sp. freshly isolated from T. gigas. Passive diffusion indicates the Cint/Cext expected if only passive diffusion of CO2 into the cell was occurring, assuming an intracellular pH of 7.62. Assays were conducted using silicone oil centrifugation in 25 mm BTP and 428 mm NaCl at a photon flux density of 500 μE m−2 s−1 using H14CO3− as a substrate as in “Materials and Methods.” Error bars represent ses (n = 3).
Table I.
Comparison of intracellular and extracellular inorganic carbon concentration of Symbiodinium sp. and A. carterae calculated using silicone oil centrifugation at differing pH values
| Species | Culture Conditions | External pH | Ci External | Cint/Cext | Estimated Cint/Cext for Passive Diffusiona | Cint/Cext
|
|---|---|---|---|---|---|---|
| Estimated Cint/Cext | ||||||
| μm | ||||||
| Symbiodinium sp. | Freshly isolated | 8.0 | 200 | 0.9 ± 0.3 | 0.41 | 2.2 ± 0.7 |
| 400 | 1.9 ± 0.2 | 0.41 | 4.6 ± 0.5 | |||
| 1,000 | 1.7 ± 0.2 | 0.41 | 4.1 ± 0.5 | |||
| 2,000 | 1.3 ± 0.1 | 0.41 | 3.2 ± 0.2 | |||
| Freshly isolated | 7.0 | 20 | 11.0 ± 1.0 | 3.47 | 3.2 ± 0.3 | |
| 40 | 7.1 ± 1.1 | 3.47 | 2.0 ± 0.3 | |||
| 100 | 5.1 ± 0.6 | 3.47 | 1.5 ± 0.2 | |||
| 200 | 8.4 ± 0.3 | 3.47 | 2.4 ± 0.1 | |||
| 1-d-old Cultureb | 7.0 | 20 | 24.2 ± 5.1 | 3.47 | 7.0 ± 1.5 | |
| 40 | 23.0 ± 4.5 | 3.47 | 6.6 ± 1.3 | |||
| 100 | 16.5 ± 1.4 | 3.47 | 4.8 ± 0.4 | |||
| 200 | 12.9 ± 1.2 | 3.47 | 3.7 ± 0.3 | |||
| 5-d-old Cultureb | 7.0 | 20 | 14.4 ± 2.4 | 3.47 | 4.1 ± 0.1 | |
| 40 | 11.8 ± 1.7 | 3.47 | 3.4 ± 0.5 | |||
| 100 | 9.0 ± 0.9 | 3.47 | 2.6 ± 0.3 | |||
| 200 | 6.8 ± 0.7 | 3.47 | 2.0 ± 0.2 | |||
| A. carterae | Culturedc | 7.0 | 20 | 7.3 ± 2.6 | ND | ND |
| 40 | 26.4 ± 7.7 | ND | ND | |||
| 100 | 17.4 ± 2.2 | ND | ND | |||
| 200 | 20.5 ± 5.8 | ND | ND |
Assays were conducted in 25 mm BTP and 428 mm NaCl at a photon flux density of 500 μE m−2s−1.
Estimated Cint/Cext assuming only passive diffusion of CO2 into the cell and an intracellular pH of 7.62.
Symbiodinium sp. was isolated from T. gigas and maintained in filtered seawater (0.45 μm).
A. carterae was grown in G media (Loeblich, 1975) and harvested in log phase growth.
The internal Ci was also determined for the free-living dinoflagellate A. carterae. The Cint/Cext ratio was similar to that found for Symbiodinium, between 7.3 and 26.4 (Table I).
Photosynthetic O2 Uptake
One of the key roles of the operation of a CCM in photosynthetic organisms is to suppress the oxygenase activity of Rubisco and thus reduce the deleterious effects of O2 on photosynthesis. This may be particularly so for zooxanthellae possessing a form II Rubisco with low Srel and a potentially high oxygenase activity (Whitney and Andrews, 1998). Therefore, it was of interest to examine the photosynthetic O2 uptake associated with photosynthesis and the effects of Ci limitation on potential oxygenase activity.
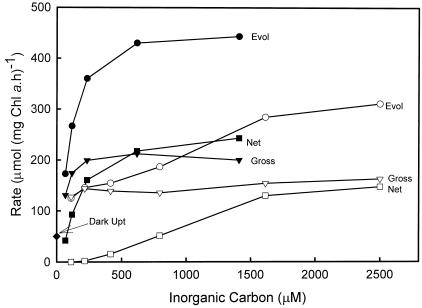
Figure 5 shows the response of both gross and net O2 fluxes to varying inorganic carbon at pH 8.0. In control cells, gross O2 uptake near the Ci compensation point represented about 30% of the maximum O2 evolution rate at saturating Ci. O2 uptake was stimulated by the light, increasing from around 50 in the dark to 130 μmol mg−1 Chl a h−1 at the lowest Ci concentration. Increasing Ci actually stimulated O2 uptake reactions up to around 0.5 mm Ci, increasing O2 uptake to 45% maximum O2 evolution. A stimulation of O2 uptake in the light by Ci has been seen in higher plants (Canvin et al., 1980) and has been interpreted as being due to an activation of Rubisco by CO2, leading to increased oxygenase activity. Cells were treated with EZA to inhibit CCM activity and induce increased Ci limitation. EZA-treated cells showed a decreased affinity for Ci, and the Pmax photosynthesis may have also been reduced. Despite an increased Ci limitation in EZA-treated cells, O2 uptake was actually decreased at limiting Ci compared with control cells, and again there was evidence for stimulation by increasing Ci. In a number of experiments with control cells, stimulation of O2 uptake by increasing Ci was always observed, and the maximum O2 uptake capacity varied between 35% to 45% of maximum O2 evolution at saturating Ci.
Figure 5.
Photosynthetic O2 exchange of freshly isolated Symbiodinium sp. in response to external Ci. The experiments were conducted as described in “Materials and Methods” at a cell density of 2.2 μg Chl a mL−1 and a photon flux density of 500 μE m−2 s−1. Shown are values for gross O2 evolution (Evol), gross O2 uptake, and net O2 evolution. Ci responses are shown for both control (black symbols) and plus 500 μm EZA (white symbols) added just before cells for each treatment, together with a value for O2 uptake measured in the dark.
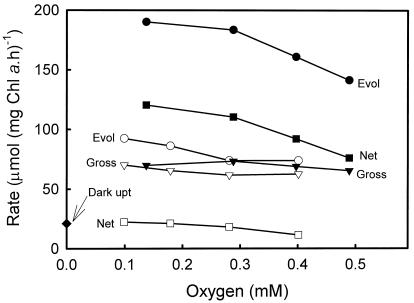
It is possible that the zooxanthellal Rubisco may have a low affinity for O2, as is the case for cyanobacterial and non-green algal form I Rubiscos (Jordon and Ogren, 1981). Thus, the response of O2 uptake to varying O2 was examined and is shown in Figure 6. Both close to the Ci compensation point and at near-saturating [Ci], there was no stimulation of O2 uptake by increasing O2 from 0.1 to 0.5 mm.
Figure 6.
Photosynthetic O2 exchange in freshly isolated Symbiodinium sp. in response to external O2. The experiments were conducted as described in Figure 6 at a cell density of 4.7 μg Chl a mL−1. Values for gross O2 evolution (Evol), gross O2 uptake, and net O2 evolution are shown for each treatment, together with a value for O2 uptake measured in the dark. O2 responses are shown both in the presence of 1 mm added Ci (black symbols) and in the absence of added Ci (white symbols). Experiments were conducted from low to high O2, and the O2 concentration was increased between points by the introduction of a small bubble of 18O2.
DISCUSSION
It has been hypothesized that as Symbiodinium sp. possess a form II Rubisco they must have a CCM that increases CO2 at the site of carbon fixation. This study was designed to examine the Ci uptake and utilization of Symbiodinium sp. after isolation to determine if this hypothesis is true. We also examined the effect of culturing freshly isolated zooxanthellae in filtered seawater. This was prompted by two previous observations: that the mantle tissue of giant clams contain high levels of CA that may assist in supplying Ci to zooxanthellae in symbiosis (Yellowlees et al., 1993; Baillie and Yellowlees, 1998), and that zooxanthellae Pmax and K0.5 both decrease by approximately one-half after isolation (W. Leggat, personal observation).
Freshly isolated zooxanthellae exhibit a number of characteristics of algae that possess a CCM: they are able to utilize HCO3− for photosynthesis (Fig. 1), they show light-stimulated CA exchange (Fig. 3), EZA decreases the affinity of photosynthesis for Ci (Figs. 3 and 5), they are able to accumulate a modest amount of internal Ci in excess of a passive accumulation (Table I), and their photosynthetic O2 uptake shows few characteristics to suggest that there is substantial Rubisco oxygenase activity at limiting Ci (Figs. 5 and 6). These features will be discussed in further detail below.
CO2 and HCO3− Uptake
Presently there is some conjecture about what form of Ci is utilized by zooxanthellae. Studies on zooxanthellae isolated from corals found that HCO3− was the Ci species taken up mostly by zooxanthellae (Goiran et al., 1996); however, studies on Symbiodinium sp. isolated from giant clams suggest that CO2 is preferentially utilized (Yellowlees et al., 1993). Different clades of Symbiodinium sp. are known to populate different hosts (Rowan and Powers, 1991) and different environments within the one host (Rowan and Knowlton, 1995). This may account for observed differences in the photosynthetic characteristics of the zooxanthellae isolated from different hosts. We found that zooxanthellae from T. gigas do utilize CO2 predominantly, with net CO2 uptake supporting 45% to 80% of net photosynthesis at pH 8.0 when first isolated (Fig. 1), and the contribution increasing at higher Ci levels. The contribution of CO2 is even more pronounced at pH 7.0 (Fig. 2). However, 2 d after isolation, net CO2 uptake decreased to 5% to 65% over the same Ci range, while net HCO3− uptake increased (Fig. 1). With isolation there was also a concomitant decrease in Pmax by 13% and K0.5 by 23% (Fig. 1). These changes in Ci uptake and utilization would appear to be the result of the changes associated with moving from a symbiotic to a free-living lifestyle.
Light-Stimulated CA Activity
When given labeled H13C18O3− as a Ci source, freshly isolated, aged, and cultured zooxanthellae displayed a light-stimulated CA activity that was inhibited by the EZA (Fig. 3). These data clearly show that light is able to stimulate the access of external Ci to internal CA activity, and is consistent with light-stimulated Ci uptake activities. Similar activities have been observed in green and non-green algae (Palmqvist et al., 1994, 1995; Badger et al., 1998) and cyanobacteria (Badger and Price, 1989) that possess CCMs.
Intracellular Ci Accumulation
The initial discovery of CCM activity in both cyanobacteria and green algae was associated with the demonstration that these cells could actively accumulate Ci in the light, and that this accumulated Ci was used to elevate internal [CO2] around Rubisco. Thus, many attempts to demonstrate a CCM have relied on the ability to measure such accumulation in the light. The experiments conducted in this study with freshly isolated and cultured zooxanthellae (Fig. 4; Table 1) found that the internal Ci was between 1.5 and 4.6 times what would be expected if only passive diffusion of Ci into the cell was occurring. This was true at both pH 7.0 and pH 8.0. Internal Ci concentration measurements were also made for A. carterae, a non-symbiotic marine dinoflagellate, as a comparison. A. carterae was found to concentrate internal Ci approximately 25-fold more than the external Ci (Table I), which is slightly more than Symbiodinium sp.. These values are similar to those found by Burns and Beardall (1987) for other marine algae, in which Cint/Cext values were between 5.5 and 8.3. Berman-Frank and associates have made the only other report of an intracellular pool for a dinoflagellate, finding that the P. gatunense have internal Ci levels between 7 and 80 times the external medium (Berman-Frank and Erez, 1996; Berman-Frank et al., 1998). However, this algae lives in freshwater and there were no estimates made of the intracellular pH, therefore, it is not possible to determine how much greater the Cint is compared with that which would be facilitated by passive diffusion.
It is surprising that marine dinoflagellates do not show larger accumulation ratios considering they possess a form II Rubisco that may have poor affinity for CO2. The levels obtained for Cint were comparable to those values previously found for other marine algae that utilize a form I Rubisco (Burns and Beardall, 1987). It may be expected that dinoflagellates would have to concentrate Ci to a greater extent than other algae to overcome the limitations of a relatively inefficient Rubisco. There may be a number of explanations for this. Recently, a thylakoid CA has been found to be central to the operation of the CCM in C. reinhardtii (Karlsson et al., 1995, 1998; Funke et al., 1997), and this CA has been hypothesized to use thylakoid protons to convert HCO3− to CO2 (Raven, 1997). If this is the case, then models of such a CCM indicate that proton-facilitated conversion of HCO3− to CO2 may actually lead to a depletion of the internal Ci pool relative to passive accumulation, although CO2 is still elevated within a localized region (Badger et al., 1998). Another possibility is that the measured accumulation of Ci may be in a specific region such as the stroma or even a subregion of the chloroplast. Thus, the actual level of Ci may be much higher in this subregion compared to the values estimated here on a whole-cell basis.
Due to the unstable nature of the dinoflagellate enzyme (Whitney and Yellowlees, 1995) the kinetic parameters of Symbiodinium sp. Rubisco are not known. It is therefore difficult to accurately model the CO2 concentrations required within the cell to achieve the photosynthetic responses observed in this report. However, some estimates can be made by taking the kinetic properties of the form II Rubisco from Rhodospirillum rubrum and adjusting the K0.5(CO2) to achieve an Srel of 37, which was the value recently obtained for A. carterae (Whitney and Andrews, 1998). This adjustment can be made by simply reducing the K0.5(CO2) to 50 to 60 μm in the presence of 21% O2. A K0.5(CO2) of 16 μm at pH 7.0 (Fig. 2) could therefore theoretically be produced by a 3- to 4-fold concentration of external CO2 within the cell, while at pH 8.0 a 6- to 7-fold concentration could produce a K0.5(CO2) of 9 μm (Fig. 2). More careful calculations will have to await a full kinetic characterization of dinoflagellate Rubisco.
Photosynthetic O2 Uptake
The photosynthetic O2 uptake displays characteristics that are not consistent with the presence of a large Rubisco oxygenase activity in these cells. Although there was considerable O2 uptake capacity, representing some 35% to 45% of maximum O2 evolution, it was not stimulated by increasing O2 and was inhibited by limiting Ci concentrations (Figs. 5 and 6). The stimulation of O2 uptake by increasing Ci may be interpreted as being due to activation of Rubisco by increasing internal CO2, as seen with higher plants (Canvin et al., 1980), but the insensitivity to O2 is not readily explained. Based on other form II enzymes and cyanobacterial form I Rubiscos with low Srel values (Badger et al., 1998), it may be expected that the oxygenase activity from zooxanthellae may display a low affinity for O2, thus exhibiting low oxygenase activity at ambient levels of O2. The saturation of O2 uptake by 0.1 mm O2 at both low and high Ci concentrations is inconsistent with this and is more similar to an O2 uptake reaction coupled to photosynthetic electron transport, such as the Mehler reaction (Badger, 1985). The photosynthetic O2 uptake process in zooxanthellae requires further study before an adequate explanation is forthcoming.
A Zooxanthella CCM
Currently, the function of the pyrenoid in eukaryotic algae is not understood, but it has been hypothesized that it may play a role in CO2 elevation, similar to carboxysomes in cyanobacteria. This hypothesis is based on changes in pyrenoid morphology when cells are transferred from high to low Ci conditions (Ramazanov et al., 1994), localization of Rubisco to the pyrenoid (Osafune et al., 1990), and a general correlation between the presence of pyrenoids and CCMs (Badger et al., 1998). All of these data suggest that the pyrenoid plays some role in Ci accumulation. Recently, electron microscopic examination has found that Symbiodinium sp. also has Rubisco localized within the pyrenoid (D. Yellowlees, personal communication); therefore, it is reasonable to hypothesis that zooxanthellal pyrenoids may also play a role in Ci accumulation.
Recent evidence and speculation suggest that there may be considerable diversity in the mechanistic operation of CCMs in algae. This diversity may include the extent to which a thylakoid-generated proton supply is coupled to the dehydration of HCO3−, as well as the need for a pyrenoid and pyrenoid-located CA to be present (see Raven, 1997; Badger et al., 1998). The evidence presented for a zooxanthella CCM in the present study indicates the presence of active Ci transport, a dependence on internal CA for efficient photosynthesis, and a suppression of photorespiratory O2 uptake, as well as a well-developed pyrenoid. However, we found no substantial Ci accumulation. Such considerations would favor the operation of a CCM in which Ci is elevated only in a very localized region and/or protons are used to aid conversion of HCO3− to CO2, leading to a depressed internal HCO3− concentration. Obviously, further research must be conducted to determine what type of CCM Symbiodinium sp. possesses. This includes localization of intracellular CA and further study on the characteristics of dinoflagellate Rubisco. This is currently hindered by the extremely unstable nature of the Rubisco after isolation (Whitney and Yellowlees, 1995). Investigations into zooxanthellae isolated from different hosts may also provide further information about zooxanthellae Ci accumulation.
ACKNOWLEDGMENT
We would like to thank G.D. Price for his expertise in analysis of data from the mass spectrometer and his helpful comments.
Footnotes
This work was supported by an Australian Research Council grant (D.Y.) including a Ph.D. scholarship (W.L.).
LITERATURE CITED
- Badger MR. Photosynthetic oxygen exchange. Annu Rev Plant Physiol. 1985;36:27–53. [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. The diversity and co-evolution of Rubisco, plastids, pyrenoids and chloroplast-based CCMs in the algae. Can J Bot. 1998;76:1052–1071. [Google Scholar]
- Badger MR, Kaplan A, Berry JA. Internal inorganic carbon pool of Chlamydomonas reinhardtii. Plant Physiol. 1980;66:407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Palmqvist K, Yu J. Measurement of CO2 and HCO3− fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Physiol Plant. 1994;90:529–536. [Google Scholar]
- Badger MR, Price GD. Carbonic anhydrase activity associated with the cyanobacterium Synechococcus PCC7942. Plant Physiol. 1989;89:51–60. doi: 10.1104/pp.89.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Price GD. The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiol Plant. 1992;84:606–615. [Google Scholar]
- Baillie B, Yellowlees D. Characterization and function of carbonic anhydrase in the zooxanthellae-giant clam symbiosis. Proc R Soc Lond B Biol Sci. 1998;265:465–473. doi: 10.1098/rspb.1998.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman-Frank I, Erez J. Inorganic carbon pools in the bloom-forming dinoflagellate Peridinium gatunense. Limnol Oceanogr. 1996;41:1780–1789. [Google Scholar]
- Berman-Frank I, Erez J, Kaplan A. Changes in inorganic carbon uptake during the progression of a dinoflagellate bloom in a lake ecosystem. Can J Bot. 1998;76:1043–1051. [Google Scholar]
- Burns BD, Beardall J. Utilization of inorganic carbon by marine microalgae. J Exp Mar Biol Ecol. 1987;107:75–86. [Google Scholar]
- Canvin DT, Berry JA, Badger MR, Fock H, Osmond CB. Oxygen exchange in leaves in the light. Plant Physiol. 1980;66:302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SS, Prezelin BB, Trench RK. Mechanisms of photoadaptation in three strains of the symbiotic dinoflagellate Symbiodinium microadriaticum. Mar Biol. 1983;76:219–229. [Google Scholar]
- Fitt WK, Rees TAV, Yellowlees D. Relationship between pH and the availability of dissolved inorganic nitrogen in the zooxanthellae-giant clam symbiosis. Limnol Oceanogr. 1995;40:976–982. [Google Scholar]
- Funke RP, Kovar JL, Weeks DP. Intracellular carbonic anhydrase is essential to photosynthesis in Chlamydomonas reinhardtii at atmospheric levels of CO2. Plant Physiol. 1997;114:237–244. doi: 10.1104/pp.114.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Badger MR, Osmond CB. Photosynthetic oxygen-exchange in isolated cells and chloroplasts of C-3 plants. Plant Physiol. 1982;70:927–931. doi: 10.1104/pp.70.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goiran C, Al-Moghrabi S, Allemand D, Jaubert J. Inorganic carbon uptake for photosynthesis by the symbiotic coral/dinoflagellate association. I. Photosynthetic performances of symbionts and dependence on sea water bicarbonate. J Exp Mar Biol Ecol. 1996;199:207–225. [Google Scholar]
- Iglesias-Prieto R, Trench RK. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Response of the photosynthetic unit to changes in photon flux density. Mar Ecol Prog Ser. 1994;113:163–175. [Google Scholar]
- Jeffrey SW, Humphrey GF. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz. 1975;107:191–194. [Google Scholar]
- Jordon DB, Ogren WL. Species variation in the specificity of ribulose biphosphate carboxylase/oxygenase. Nature. 1981;291:513–515. [Google Scholar]
- Karlsson J, Clarke AK, Chen ZY, Hugghins SY, Park YI, Husic HD, Moroney JV, Samuelsson G. A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. EMBO J. 1998;17:1208–1216. doi: 10.1093/emboj/17.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Hiltonen T, Husic HD, Ramazanov Z, Samuelsson G. Intracellular carbonic anhydrase of Chlamydomonas reinhardtii. Plant Physiol. 1995;109:533–539. doi: 10.1104/pp.109.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp DW, Bayne BL, Hawkins AJS. Nutrition of the giant clam Tridacna gigas (L.). I. Contribution of filter feeding and photosynthates to respiration and growth. J Exp Mar Biol Ecol. 1992;155:105–122. [Google Scholar]
- Loeblich AR. A seawater medium for dinoflagellates and the nutrition of Cachonina niei. J Phycol. 1975;11:80–96. [Google Scholar]
- Miyachi S, Tsuzuki M, Avramova T. Utilization modes of inorganic carbon for photosynthesis in various species of Chlorella. Plant Cell Physiol. 1983;24:441–451. [Google Scholar]
- Moroney JV, Husic HD, Tolbert NE. Effect of carbonic anhydrase inhibitors on inorganic carbon accumulation by Chlamydomonas reinhardtii. Plant Physiol. 1985;79:177–183. doi: 10.1104/pp.79.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JH, Shepherd MA, Long HM, Fitt WK. The zooxanthellal tubular system in the giant clam. Biol Bull. 1992;183:503–506. doi: 10.2307/1542028. [DOI] [PubMed] [Google Scholar]
- Osafune T, Yokato A, Sumida S, Hase E. Immunogold localization of ribulose-1,5-bisphosphate carboxylase with reference to pyrenoid morphology in chloroplasts of synchronized Euglena gracilis cells. Plant Physiol. 1990;92:802–808. doi: 10.1104/pp.92.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist K, Sultemeyer DF, Baldet P, Andrews TJ, Badger MR. Characterisation of inorganic carbon fluxes, carbonic anhydrase(s) and ribulose-1,5-biphosphate carboxylase-oxygenase in the green unicellular alga Coccomyxa. Planta. 1995;197:352–361. [Google Scholar]
- Palmqvist K, Yu J, Badger MR. Carbonic anhydrase activity and inorganic carbon fluxes in low- and high-Ci cells of Chlamydomonas reinhardtii and Scenedesmus obliquuus. Physiol Plant. 1994;90:537–547. [Google Scholar]
- Ramazanov Z, Rawat M, Henk MC, Manson CB, Matthews SW, Moroney JV. The induction of the CO2-concentrating mechanism is correlated with the formation of the starch sheath around the pyrenoid of Chlamydomonas reinhardtii. Planta. 1994;195:210–216. [Google Scholar]
- Raven JA. CO2-concentrating mechanisms: a direct role for thylakoid lumen acidification. Plant Cell Environ. 1997;20:147–154. [Google Scholar]
- Rowan R, Knowlton N. Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc Natl Acad Sci USA. 1995;92:2850–2853. doi: 10.1073/pnas.92.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan R, Powers DA. Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae) Mar Ecol Prog Ser. 1991;71:65–73. [Google Scholar]
- Trench RK. Dinoflagellates in non-parasitic symbioses. In: Taylor FJR, editor. Biology of the Dinoflagellates. London: Blackwell Scientific Publishers; 1987. pp. 530–570. [Google Scholar]
- Whitney SM, Andrews TJ. The CO2/O2 specificity of single-subunit ribulose-bisphosphate carboxylase from the dinoflagellate, Amphidinium carterae. Aust J Plant Physiol. 1998;25:131–138. [Google Scholar]
- Whitney SM, Shaw DC, Yellowlees D. Evidence that some dinoflagellates contain a ribulose-1,5-bisphosphate carboxylase/oxygenase related to that of the α-proteobacteria. Proc R Soc Lond B Biol Sci. 1995;259:271–275. doi: 10.1098/rspb.1995.0040. [DOI] [PubMed] [Google Scholar]
- Whitney SM, Yellowlees D. Preliminary investigations into the structure of ribulose bisphosphate carboxylase from two photosynthetic dinoflagellates. J Phycol. 1995;31:138–146. [Google Scholar]
- Yellowlees D, Dionisio-Sese ML, Masuda K, Maruyama T, Abe T, Baillie B, Tsuzuki M, Miyachi S. Role of carbonic anhydrase in the supply of inorganic carbon to the giant clam-zooxanthellae symbiosis. Mar Biol. 1993;115:605–611. [Google Scholar]