Abstract
Purpose
The aim of this study is to analyze clinical pregnancy rates (CPR) and ongoing pregnancy rates (OPR) for frozen embryo transfers (FET) performed with blastocysts in the cycle immediately after GnRH agonist (GnRHa) versus human chorionic gonadotropin (hCG) triggers, with outcomes of delayed FET for comparison.
Methods
Retrospective cohort study at a university-affiliated in vitro fertilization (IVF) clinic, including patients undergoing IVF between 2013-16 with a blastocyst FET performed within two menstrual cycles of a previous stimulation cycle and vaginal oocyte retrieval (VOR). FETs included programmed and natural endometrial preparation. Outcome measures were clinical and ongoing pregnancy rates.
Results
CPR and OPR for 344 FET cycles were similar when comparing immediate and delayed transfer overall (crude CPR 67.5 versus 76.5%, p = 0.11; OPR 57.5 versus 66.7%, p = 0.13), and after stratifying by cycles following hCG trigger (OPR 62.5 versus 66.3%, p = 0.61) and GnRHa trigger (OPR 55.6 versus 64.5%, p = 0.17). When considering a number of predictors for OPR, an adjusted odds ratio (OR) of 1.74 [95% CI 1.00–3.03] approached significance in favor of delayed FET.
Conclusions
Regardless of trigger modality, patients can be reassured that pregnancy rates with FET are high in immediate and delayed cycles. However, our study suggests a potential benefit in delaying a cycle before proceeding with FET.
Keywords: Frozen embryo transfer, Ovulation induction, Gonadotropin-releasing hormone/agonists
Introduction
In controlled ovarian hyperstimulation (COH), two oocyte maturation trigger modalities are available: the more commonly used human chorionic gonadotropin (hCG) and gonadotropin-releasing hormone agonists (GnRHa). GnRHa stimulates an endogenous release of gonadotropins similar to a natural surge, but the shorter duration of the luteinizing hormone (LH) surge and the pituitary downregulation properties of the drug result in a dysfunctional and shortened luteal phase [1]. Interventions to improve pregnancy rates after GnRHa trigger may be necessary, including consideration to cryopreserve all good quality embryos for subsequent frozen embryo transfers (FET) and deferral of fresh transfer (referred to as “freeze-only”). Although the effects of GnRHa trigger on the luteal phase of the cycle have been well described, much less is known about the characteristics of the subsequent menstrual cycle.
For a number of reasons, the field is experiencing increasing numbers of freeze-only cycles and FETs [2]. Many couples have good quality cryopreserved supernumerary embryos after a failed fresh embryo transfer, or after successful ongoing pregnancy and delivery. Evidence suggests that FETs, particularly after freeze-only stimulation cycles with a deferred fresh embryo transfer, can help eliminate ovarian hyperstimulation syndrome (OHSS) and address endometrial asynchrony resulting from ovarian stimulation [3–5]. Finally, studies suggest that a dysfunctional endometrium during fresh transfer could have lasting detrimental effects on perinatal and obstetric outcomes after fresh transfer compared to FET [6, 7].
With increasing FETs, the timing of transfer after the stimulated cycle has become controversial. Prior studies have evaluated the duration of time between stimulation and frozen-thawed embryo transfer [8–10], but only three specifically after GnRHa trigger [11–13]. All three showed equivalent pregnancy rates despite timing differences. Given the unsupported luteal phase after GnRHa trigger is very short and menses typically starts within 1 week, it is reasonable to expect that the subsequent cycle may have important differences compared to hCG triggered cycles that could impact the success of the subsequent FET. Therefore, additional investigation is warranted, including a qualitative evaluation of cycle dysfunction.
It is currently unclear whether the cycle immediately following COH results in the same pregnancy rates as cycles further out from exposure to stimulation and trigger medications. Whether or not there is a benefit to delaying FET and allowing the hypothalamic-pituitary-ovarian axis, dysfunctional corpora lutea, and the endometrium to “reset” after stimulation and egg retrieval, particularly after a GnRHa trigger, has not been thoroughly studied. The objective of this study was to retrospectively collect information regarding the optimal timing for FET after an ovarian stimulation cycle, focusing on the trigger modality used to achieve egg maturation. In addition, we aimed to collect information regarding the frequency of ovulatory dysfunction in the subsequent cycle following GnRHa and hCG triggers.
Materials and methods
This is a retrospective cohort study including all patients age 18–40 years undergoing IVF with stimulation and vaginal oocyte retrieval (VOR) between 2013 and 2016 with a subsequent blastocyst FET. We classified these cycles as either “immediate” or “delayed” based on menses during time lapsed from stimulation; FET within the first menstrual cycle after VOR was designated “immediate” and after at least two menses was designated “delayed.”
Generating a database from our electronic record, we included all cycles for women undergoing ovarian stimulation and IVF who either (1) had a fresh embryo transfer but failed to get pregnant and have cryopreserved blastocysts or (2) completed their stimulation cycle with “freeze-only” rather than a fresh embryo transfer. This includes patients who are recommended to forego fresh transfer due to elevated progesterone (P) levels, patients planning preimplantation genetic diagnosis/screening (PGD/PGS), patients who develop symptoms of or risk factors for OHSS, patients who do not have any suitable embryos to transfer on day 5 but cryopreserve blastocysts on day 6, and those who electively cryopreserved all good quality embryos for reasons such as planned surgery, use of medications contraindicated in pregnancy, or preferred pregnancy timing. Patients with all diagnoses were included.
We excluded patients who did not undergo a stimulation cycle prior to FET, such as those receiving donor oocytes or those who had cryopreserved embryos from a prior cycle greater than 120 days from FET. We also excluded patients who had an endometrial biopsy in the cycle prior to ET, including “endometrial scratching.”
Stimulation and FET protocols after cryopreservation have been previously described [14]. In brief, stimulation cycles included protocols utilizing either a GnRHa or GnRH antagonist administered for COH. Gonadotropins included either recombinant FSH alone or FSH in combination with hMG (Gonal-F, EMD Serono; Follistim, Merck; Menopur, Ferring Pharmaceuticals). After the three leading follicles reached 17–18 mm, oocyte maturation was triggered with either hCG (3300, 5000, or 10,000 IU based on peak estradiol (E2) levels and BMI; Pregnyl, Merck; Novarel, Ferring Pharmaceuticals) or GnRHa (leuprolide acetate 1 mg, given once), or a dual trigger comprised of GnRHa 1 mg plus a lower dose of adjuvant hCG (1000 IU) given at the time of trigger [15]. The trigger modality was determined by physician preference based on peak E2 levels and the patient’s history of hypothalamic dysfunction and/or prior cycle outcomes when applicable. Cryopreservation of good quality embryos (3BB or better) per the Gardner scoring system was performed, predominantly at the blastocyst stage [16]. Embryos that were frozen at cleavage stage were cultured to blastocyst stage after thaw (n = 3) such that all FETs were at the blastocyst stage.
FET was performed in either a natural or programmed cycle based on the patient’s ovulatory history and physician preference. Programmed cycles consisted of downregulation with GnRHa prior to administration of increasing transdermal and/or oral E2 (Vivelle-Dot, Novartis) followed by addition of intramuscular (IM) P after the endometrial thickness was confirmed to reach at least 6 mm. Transfer was performed on the sixth day of IM P. Ovulatory patients started GnRHa for a programmed FET after documenting a P suggestive of ovulation in the mid-luteal phase in the prior cycle; when ovulation does not occur, we provide P for a withdrawal bleed with GnRHa overlap. In the cases of immediate transfer with a programmed cycle, downregulation with GnRHa was started in the luteal phase of the stimulation cycle, before the first menses after VOR.
Patients undergoing a natural cycle transfer were monitored with bloodwork daily beginning on cycle day 10 and FET was performed 6 days after the LH surge. The natural cycle luteal phase was supplemented with vaginal P (Crinone, Merck Biopharma; Endometrin, Ferring Pharmaceuticals) beginning 2 days after the LH surge.
Clinical pregnancy was defined as at least one gestational sac visualized within the uterus on transvaginal ultrasound by 8 weeks of gestation, ongoing pregnancy was defined as fetal heart movement seen on ultrasound and present at the time of discharge at 10–12 weeks of gestation, and clinical pregnancy loss was defined as a pregnancy that failed to progress prior to 20 weeks of gestation after confirming presence of an intrauterine gestational sac on ultrasound.
Baseline information was compared between groups using a t test for continuous variables, chi-squared for categorical variables, and Mann-Whitney U test for non-parametric continuous variables. We used chi-squared and Fisher’s exact tests to compare cycle outcomes between ET groups. Cochran-Mantel-Haenszel test was used to compare outcomes after stratification. A p value < 0.05 was considered statistically significant. Additionally, logistic regression was performed to assess the contribution of potential clinical confounders including age at time of oocyte retrieval, whether the stimulation cycle resulted in cryopreservation of all embryos, whether the indication for a freeze-only cycle was increased risk of OHSS, trigger medication (both including and excluding dual trigger patients as these were a small number), intracytoplasmic sperm injection (ICSI) versus conventional insemination, endometrial preparation protocol, inclusion of multiple FET cycles from a single patient, and performance of PGS testing. Variables reaching significance p < 0.2 on bivariate analysis were included in the final model. IRB approval was obtained from the authors’ university.
Results
Three hundred forty-four FET cycles were analyzed, including 208 following hCG trigger, 120 after GnRHa trigger, and 16 after a dual trigger of GnRHa and hCG 1000 IU. There were 80 cycles in the immediate group and 264 in the delayed group, including 27 (33.8%) and 93 (35.2%) receiving GnRHa trigger, respectively (p = 0.81).
Table 1 provides demographic and baseline information by FET group; data presented as mean ± standard deviation unless otherwise stated. Endometrial preparation protocol differed between groups with the majority of immediate FETs performed in a natural cycle (72.5%) and the majority of delayed FETs performed in a programmed cycle (63.6%) (p < 0.01). Otherwise, FET groups were noted to be similar. All diagnoses were included and there were no significant differences between groups. There were no differences in distribution or outcomes between FET cycles utilizing embryos obtained from antagonist or agonist stimulation protocols. For the fresh cycles preceding the embryo transfers analyzed, 87.3 and 76.5% of cycles utilized a GnRH antagonist protocol in the immediate and delayed groups, respectively (p = 0.06). Data included two FET cycles analyzed separately for two patients in the immediate group (2.5%) and 14 patients in the delayed group (5.3%) (p = 0.38); all remaining patients had only one included FET cycle.
Table 1.
Baseline information and stimulation and frozen FET cycle characteristics, by timing of FET
| Immediate, n = 80 | Delayed, n = 264 | p value | |
|---|---|---|---|
| Median days between VOR and FET (range) | 37 (23–78) | 69 (51–120) | |
| BMI | 25.16 ± 5.12 | 26.41 ± 5.86 | 0.09 |
| Age at oocyte retrieval | 33.65 ± 3.78 | 33.52 ± 3.77 | 0.79 |
| Baseline FSH | 6.48 ± 2.53 | 6.00 ± 2.24 | 0.10 |
| Median AMH (IQR) | 3.94 (2.85–5.38) | 3.02 (1.60–5.18) | 0.19 |
| GnRH antagonist stimulation protocol | 70 (87.3) | 202 (76.5) | 0.06 |
| Peak E2 in fresh cycle | 2384 ± 1197 | 2606 ± 1324 | 0.18 |
| Total oocytes retrieved | 16.98 ± 8.65 | 18.33 ± 9.55 | 0.26 |
| Total embryos cryopreserved | 4.14 ± 4.10 | 4.50 ± 3.35 | 0.43 |
| PGS performed, n (%) | 24 (30.0) | 91 (34.5) | 0.46 |
| Freeze-only in fresh cycle, n (%) | 59 (73.8) | 167 (63.3) | 0.08 |
| Trigger medication, n (%) | 0.73 | ||
| hCG | 48 (60.0) | 160 (60.6) | |
| GnRH agonist | 27 (33.8) | 93 (35.2) | |
| Dual trigger | 5 (6.3) | 11 (4.2) | |
| Endometrial preparation, n (%) | < 0.01 | ||
| Natural | 58 (72.5) | 96 (36.4) | |
| Programmed | 22 (27.5) | 168 (63.6) | |
| Number embryos transferred | 1.4 ± 0.49 | 1.48 ± 0.53 | 0.23 |
Table 2 shows overall reproductive outcomes by FET group. No significant differences were noted in our primary outcome of clinical pregnancy rate.
Table 2.
Reproductive outcomes, by timing of FET
| Outcome | Immediate (n = 80) | Delayed (n = 264) | p value |
|---|---|---|---|
| Implantation rate | 61% | 68% | 0.26 |
| Clinical pregnancy, n (%) | 54 (67.5) | 202 (76.5) | 0.11 |
| Ongoing pregnancy, n (%) | 46 (57.5) | 176 (66.7) | 0.13 |
| Live birth,* n (%) | 34/68 (50.0) | 118/209 (56.5) | 0.40 |
| Multiples at delivery, n (%) | 14 (17.5) | 45 (17.0) | 0.93 |
| Clinical pregnancy loss, n (%) | 17 (21.3) | 52 (19.7) | 0.76 |
| Biochemical pregnancy, n (%) | 8 (10.0) | 25 (9.5) | 0.89 |
*Live birth data not currently available for 67 cycles at time of analysis and excluded from final count for that outcome
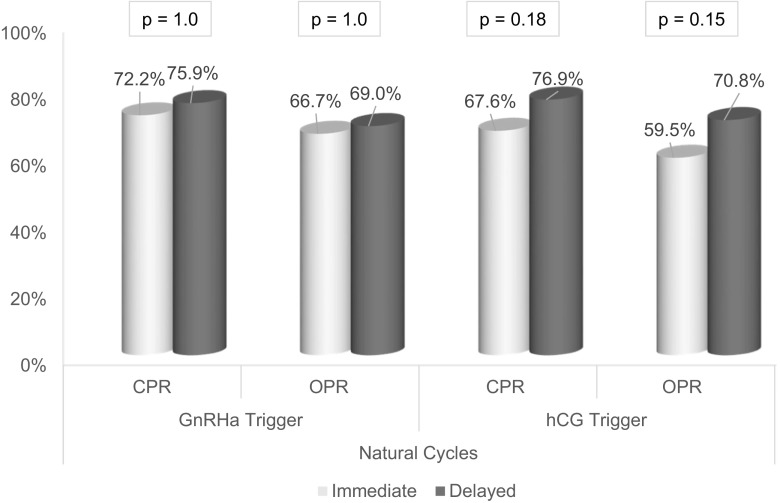
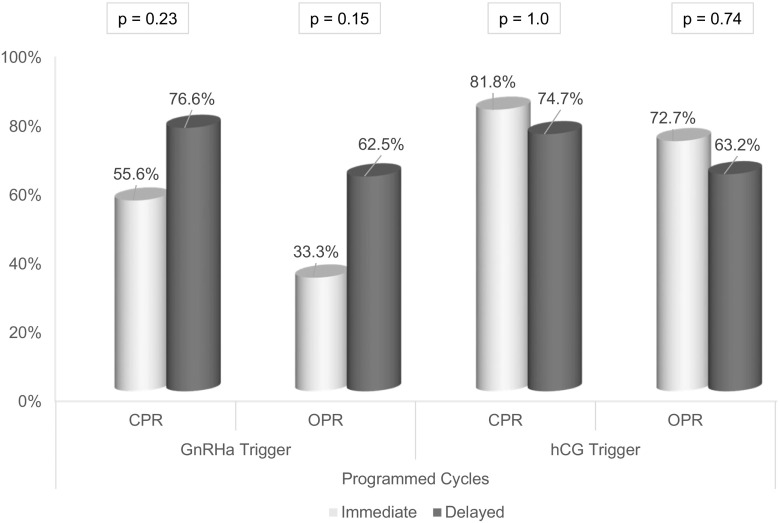
Stratification was performed to account for the numerous possible confounders in our study. Table 3 shows the odds of ongoing pregnancy for immediate and delayed groups separately given trigger modality, endometrial preparation protocol, or PGS. In patients undergoing delayed FET, the odds of ongoing pregnancy were not significantly different compared to an immediate FET, after adjusting for trigger medication (OR 1.48, p = 0.17), endometrial preparation protocol (OR 1.67, p = 0.09), or PGS (OR 1.48, p = 0.17). Figure 1 illustrates the outcomes for natural cycle FETs by trigger modality; the effect of a GnRHa trigger on the outcomes of an immediate natural cycle appears negligible. Figure 2 breaks down outcomes for programmed cycle FETs by trigger modality.
Table 3.
Odds of ongoing pregnancy by timing of FET, after stratification by trigger medication, endometrial preparation protocol, and use of PGS
| n (OPR) | Immediate | Delayed | CMH* |
|---|---|---|---|
| Trigger medication, n (%) | OR 1.48 | ||
| hCG | 30/48 (62.5) | 106/160 (66.3) | p = 0.17 |
| GnRH agonist | 15/27 (55.6) | 60/93 (64.5) | |
| Dual trigger | 1/5 (20.0) | 10/11 (90.9) | |
| Endometrial prep protocol, n (%) | OR 1.67 | ||
| Natural | 35/58 (60.3) | 68/96 (70.8) | p = 0.088 |
| Programmed | 11/22 (50.0) | 108/168 (64.3) | |
| PGS, n (%) | OR 1.48 | ||
| Yes | 13/24 (54.2) | 61/91 (67.0) | p = 0.172 |
| No | 33/56 (58.9) | 115/173 (66.5) |
CMH Cochran-Mantel-Haenszel
*Cochran-Mantel-Haenszel test of conditional independence; homogeneity across strata confirmed with non-significant Breslow-Day test for each variable
Fig. 1.
Natural FET cycle outcomes by stimulation cycle trigger medication
Fig. 2.
Programmed FET cycle outcomes by stimulation cycle trigger medication
Logistic regression for OPR was performed to evaluate additional potential confounders and determine the weight of the stratified variables above (Table 4). An unadjusted odds ratio (OR) of 1.48 in favor of delayed transfer was noted (95% CI 0.89–2.47). After initially including numerous variables in a preliminary regression model, bivariate analysis identified patient’s age at oocyte retrieval, freeze-only for OHSS in stimulation cycle, use of ICSI, and endometrial preparation protocol as potentially contributing to ongoing pregnancy (significance set at p < 0.2); these were included in the final model equation. An adjusted OR of 1.74 (95% CI 1.00–3.03) (p = 0.049) was calculated after adjusting for the above variables, suggesting a trend toward a difference overall in favor of delayed FET. However, when using the same model for analysis of clinical pregnancy rate and live birth rate, no difference was found (clinical pregnancy OR 1.63, p = 0.1; live birth OR 1.41, p = 0.26).
Table 4.
Logistic regression for ongoing pregnancy rate with significant variables (p < 0.2)
| Predictor | Regression coefficient (B) | Odds ratio | 95% CI | p value |
|---|---|---|---|---|
| Timing of FET | 0.555 | 1.74 | 1.00–3.03 | 0.05 |
| Age at retrieval | − 0.042 | 0.96 | 0.90–1.02 | 0.18 |
| Freeze-only for OHSS | − 0.644 | 0.53 | 0.22–1.28 | 0.16 |
| Endometrial preparation protocol | 0.396 | 1.49 | 0.91–2.42 | 0.11 |
| ICSI | − 0.642 | 0.53 | 0.20–1.37 | 0.19 |
Qualitative analysis of subsequent cycle dysfunction
We recorded notes regarding the FET cycle and any delays or changes in protocol that may have resulted from factors such as an unexpected anovulatory cycle, presence of new ovarian cysts at baseline, or long menstrual cycles in a patient with documented normal length cycles. We also recorded increases in our standard E2 and P supplementation in programmed cycles as a result of a lower than desired serum level or endometrial thickness less than 6 mm prior to FET.
Eighteen patients had planned transfers but experienced delays due to conversion from a natural FET to a programmed FET when ovulation failed to occur; we observed this in seven patients receiving GnRHa trigger (5.8%) and 11 patients after hCG trigger (5.3%) (p = 0.83). Others underwent their natural cycle as planned, either immediately or delayed, but experienced what was considered a “late” LH surge on cycle day 17 or later. After GnRHa trigger and dual trigger this occurred in 7.5% of FET cycles (9/120) compared to 4.3% of FET cycles after hCG trigger (9/208) (p = 0.34). Alternatively, two patients with previously regular cycles intending an immediate natural cycle FET experienced an LH surge prior to cycle day 10 in their first cycle after VOR resulting in a postponed and therefore delayed FET, both after hCG trigger.
In planned programmed cycles when ovulation does not occur as expected, we provide P for a withdrawal bleed with GnRHa overlap. This occurred in six patients expected to be ovulatory: two after GnRHa trigger, three after hCG, and one after dual trigger.
Eleven patients had a delay in their FET cycle due to the presence of at least one new ovarian cyst, representing either a residual corpus luteum cyst or a functional cyst after GnRHa suppression, four (3.3%) had cysts after GnRHa trigger, five (2.4%) after hCG, and two (12.5%) after dual trigger. All triggers experienced both types of ovarian cysts and in two cases trigger was required to expedite FET cycle restart. One patient experienced a delayed programmed FET due to a thin endometrium after GnRHa trigger. Another patient experienced a canceled programmed FET for uterine bleeding and thin endometrium after GnRHa trigger and instead underwent a delayed programmed FET in the next cycle.
In immediate FET cycles, 40.7% following GnRHa trigger (11/27) required adding E2 and/or P above the routine minimum for endometrial preparation/luteal support due to suboptimal serum levels, compared to 43.8% after hCG trigger (21/48) (p = 0.99). This was comparable to the overall rate of increased E2 and/or P in delayed cycles of 53.4%.
Discussion
IVF increasingly requires cryopreservation of embryos and cycle segmentation for various reasons, including the greater use of PGS/PGD in current practice. A number of questions in the process remain unanswered, particularly regarding the lasting effects of medications used for stimulation and trigger and the time interval required before subsequent successful treatment. Our study suggests that prior to an immediate FET cycle, there is no difference in the effect of the medication per se on reproductive outcomes. Controlling for clinical confounders, delayed FET appears to provide better results than immediate FET but outcomes were not statistically significant in nearly all comparisons. Moreover, there may be signs of menstrual cycle dysfunction in the immediate cycle after all trigger types that warrant anticipatory guidance regarding unforeseen delays for patients hoping to proceed with an FET as soon as possible.
The prioritization of time efficiency is not new in the fresh cycle setting. One study examined the efficacy of performing stimulation cycles back-to-back compared to increasing the interval of time between cycles [17]. They found no benefit in waiting for additional menstrual cycles to attempt another ovarian stimulation and transfer cycle. An additional study by Silverberg et al. showed similar findings [18]. Both suggested that medications can overtake or replace the function of the ovary but do not provide guidance in situations where a cycle relies on physiologically normal ovarian function.
Three recent studies have evaluated similar questions related to timing of FET. One large study examined the effects of duration of time after a fresh embryo transfer that did not result in pregnancy; Santos-Ribeiro et al. found that there was no difference in pregnancy outcomes, namely clinical pregnancy rates, among 1183 cycles receiving a frozen embryo transfer immediately compared to delayed [8]. In this study of cleavage stage and blastocyst FETs, including programmed and natural endometrial preparation, there was no evidence to suggest a benefit in delaying the next treatment step beyond one menstrual cycle (CPR 32.5% in immediate FET versus 31.7% in delayed) [8].
Three recent studies evaluated outcomes specifically after FETs from freeze-only stimulation cycles, reflecting a shift in the field to segmented-IVF programs. Lattes et al. reviewed live birth rates for 512 freeze-only with subsequent FET cycles including hCG and GnRHa triggers [12]. All cycles included a day 3 or 4 FET in a programmed cycle, and they found no difference in CPR or live birth rates due to timing after adjusting for numerous confounders, though crude data suggested differences in live birth rates (crude CPR 44.1% in immediate versus 36.1% in delayed, p = 0.07; crude live birth rate 37.6 versus 27.3%, p = 0.01). Similarly, Santos-Ribeiro et al. combined data from two centers conducting 333 FETs of cleavage and blastocyst stage embryos in programmed immediate and delayed cycles after exclusively GnRHa trigger and freeze-only; they noted an equivalent CPR 52.5% versus 41.8% in immediate versus delayed FETs [11]. In contrast to our study, they did not include cycles that were canceled after an attempt at immediate FET due to thin endometrium. Finally, Ozgur et al. provided live birth outcomes after 1121 freeze-only cycles with programmed blastocyst FETs and with stratification analysis by trigger (hCG, GnRHa, or both) [13]. Again, they found no differences between their immediate transfer group and their referent FET group after two menstrual cycles (live birth rate 57.8 versus 59.7%) [13].
These large studies draw consistent conclusions regarding FET after freeze-only, despite their differences in embryo stage at transfer and variation in trigger modality; unfortunately, none of them analyze outcomes from a natural cycle FET.
PGS is an emerging reason why freeze-only cycles are gaining popularity [19]. None of the above studies included patients using this screening modality in an effort to reduce confounding. Given its increasing utilization, we opted to include them in our study. Our regression model suggested that PGS/PGD was not a significantly contributing confounder (p = 0.89). Additionally, some of the above studies report lower CPRs than reported here, possibly due to our inclusion of PGS and blastocyst transfers only.
This is the first study to report findings indicative of subsequent cycle dysfunction in a qualitative manner. Additionally, only one prior study includes findings from both programmed and natural cycle FETs [8]. In contrast to prior studies, we have attempted to perform a more comprehensive analysis that acknowledges and includes the many confounding variables representing the current state of practice. We found that a possible association between FET timing and pregnancy outcomes grew stronger as we accounted for these confounders in a regression analysis. Some of the difference that we see may be attributable to differences in pregnancy rates in the immediate programmed cycles, when hCG triggered patients appear to outperform GnRHa triggered patients (Fig. 2). Our study remains limited by its retrospective approach and the uneven distribution of immediate and delayed FETs; most notably, stratification by multiple variables substantially decreases the number of immediate FETs available for analysis and results in Figs. 1 and 2, and should be interpreted as such.
After controlling for confounding variables, there may be a clinically significant, though not statistically significant advantage to delayed FET which aligns with the theoretical advantage of providing sufficient time for the endometrium to “reset.” Stratified analysis showed there were no significant differences in pregnancy rates between hCG and GnRHa triggers in the previous cycle. However, patients planning an immediate FET should be advised that a dysfunctional menstrual cycle is possible, warranting close monitoring.
Acknowledgements
We would like to thank Dr. James Grady for his consultation on the statistical analysis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Conflicts of interest
The authors declare that they have no conflict of interest.
Contributor Information
Leah Kaye, Email: lkaye@uchc.edu.
Audrey Marsidi, Email: audrey.marsidi@emory.edu.
Puja Rai, Email: prai@tuftsmedicalcenter.org.
Jeffrey Thorne, Email: jthorne@uchc.edu.
John Nulsen, Email: nulsen@uchc.edu.
Lawrence Engmann, Email: lengmann@uchc.edu.
Claudio Benadiva, Phone: 1.860.321.7082, Email: benadiva@uchc.edu.
References
- 1.Nakano R, Mizuno T, Kotsuji F, Katayama K, Wshio M, Tojo S. “Triggering” of ovulation after infusion of synthetic luteinizing hormone releasing factor (LRF) Acta Obstet Gynecol Scand. 1973;52(3):269–272. doi: 10.3109/00016347309158325. [DOI] [PubMed] [Google Scholar]
- 2.Wong KM, Mastenbroek S, Repping S. Cryopreservation of human embryos and its contribution to in vitro fertilization success rates. Fertil Steril. 2014;102(1):19–26. doi: 10.1016/j.fertnstert.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 3.Devroey P, Polyzos NP, Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod. 2011;26(10):2593–2597. doi: 10.1093/humrep/der251. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102(1):3–9. doi: 10.1016/j.fertnstert.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Roque M, Lattes K, Serra S, Solà I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99(1):156–162. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Maheshwari A, Pandey S, Shetty A, Hamilton M, Bhattacharya S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2012;98(2):368–377-9. doi: 10.1016/j.fertnstert.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Weinerman R, Mainigi M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril. 2014;102(1):10–18. doi: 10.1016/j.fertnstert.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos-Ribeiro S, Siffain J, Polyzos NP, Van De Vijver A, Van Landuyt L, Stoop D, et al. To delay or not to delay a frozen embryo transfer after a failed fresh embryo transfer attempt? Fertil Steril. 2016;105(5):1202–1207e1. doi: 10.1016/j.fertnstert.2015.12.140. [DOI] [PubMed] [Google Scholar]
- 9.Maas KH, Baker VL, Westphal LM, Lathi RB. Optimal timing of frozen embryo transfer after failed IVF attempt. Fertil Steril. 2008;90:S285. doi: 10.1016/j.fertnstert.2008.07.1101. [DOI] [Google Scholar]
- 10.Volodarsky-Perel A, Eldar-Geva T, Holzer HEG, Schonberger O, Reichman O, Gal M. Cryopreserved embryo transfer: adjacent or non-adjacent to failed fresh long GnRH-agonist protocol IVF cycle. Reprod BioMed Online. 2017;34(3):267–273. doi: 10.1016/j.rbmo.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Santos-Ribeiro S, Polyzos NP, Lan VTN, Siffain J, Mackens S, Van Landuyt L, et al. The effect of an immediate frozen embryo transfer following a freeze-all protocol: a retrospective analysis from two centres. Hum Reprod. 2016;31(11):2541–2548. doi: 10.1093/humrep/dew194. [DOI] [PubMed] [Google Scholar]
- 12.Lattes K, Checa MA, Vassena R, Brassesco M, Vernaeve V. There is no evidence that the time from egg retrieval to embryo transfer affects live birth rates in a freeze-all strategy. Hum Reprod. 2017;32(2):368–374. doi: 10.1093/humrep/dew306. [DOI] [PubMed] [Google Scholar]
- 13.Ozgur K, Bulut H, Berkkanoglu M, Humaidan P, Coetzee K. Frozen embryo transfer can be performed in the cycle immediately following the freeze-all cycle. J Assist Reprod Genet. 2017;22:1–8. doi: 10.1007/s10815-017-1048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaye L, Will EA, Bartolucci A, Nulsen J, Benadiva C, Engmann L. Pregnancy rates for single embryo transfer (SET) of day 5 and day 6 blastocysts after cryopreservation by vitrification and slow freeze. J Assist Reprod Genet. 2017;12:1–7. doi: 10.1007/s10815-017-0940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S. Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril. 2008;90(1):231–233. doi: 10.1016/j.fertnstert.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 16.Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obs Gynecol. 1999;11(3):307–311. doi: 10.1097/00001703-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Reichman DE, Chung P, Meyer L, Greenwood E, Davis O, Rosenwaks Z. Consecutive gonadotropin-releasing hormone-antagonist in vitro fertilization cycles: does the elapsed time interval between successive treatments affect outcomes? Fertil Steril. 2013;99(5):1277–1282. doi: 10.1016/j.fertnstert.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 18.Silverberg KM, Klein NA, Burns WN, Schenken RS, Olive DL. Consecutive versus alternating cycles of ovarian stimulation using human menopausal gonadotrophin*. Hum Reprod. 1992;7(7):940–944. doi: 10.1093/oxfordjournals.humrep.a137775. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Purata J, Lee J, Whitehouse M, Duke M, Grunfeld L, Sandler B, et al. Reproductive outcome is optimized by genomic embryo screening, vitrification, and subsequent transfer into a prepared synchronous endometrium. J Assist Reprod Genet. 2016;33(3):401–412. doi: 10.1007/s10815-016-0647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]