Abstract
Purpose
Endometrial receptivity issues represent a potential source of implantation failure. The aim of this study was to document our experience with the endometrial receptivity array (ERA) among patients with a history of euploid blastocyst implantation failure. We investigated whether the contribution of the endometrial factor could be identified with the ERA test and if actionable results can lead to improved outcomes.
Methods
A retrospective review was performed for 88 patients who underwent ERA testing between 2014 and 2017. Reproductive outcomes were compared for patients undergoing frozen embryo transfer (FET) using a standard progesterone protocol versus those with non-receptive results by ERA and subsequent FET according to a personalized embryo transfer (pET) protocol.
Results
Of patients with at least one previously failed euploid FET, 22.5% had a displaced WOI diagnosed by ERA and qualified for pET. After pET, we found that implantation and ongoing pregnancy rates were higher (73.7 vs. 54.2% and 63.2 vs. 41.7%, respectively) compared to patients without pET, although differences were not statistically significant.
Conclusions
Our experience demonstrates that a significant proportion of patients with a history of implantation failure of a euploid embryo have a displaced WOI as detected by the ERA. For these patients, pET using a modified progesterone protocol may improve the outcomes of subsequent euploid FET. Larger randomized studies are required to validate these results.
Keywords: Endometrial receptivity, ERA, In vitro fertilization, Recurrent implantation failure, CCS
Introduction
Human implantation is an intricate process that requires synchronous dialog between a healthy embryo and a receptive endometrium. Aneuploid embryos which lack developmental competence likely account for the most common cause of implantation failure [1]. In the context of in vitro fertilization (IVF), comprehensive chromosome screening (CCS) can be utilized to select euploid blastocysts and avoid transfer of aneuploid embryos, an approach which has been shown to significantly improve sustained implantation compared to morphologic selection alone [2]. Still, even euploid, morphologically normal blastocysts fail to implant in about 1/3 of transfers [3, 4]. Failure of a euploid embryo to implant may suggest a non-embryonic source of implantation failure, with endometrial receptivity issues representing another potential cause [1].
Endometrial receptivity is characterized by a finite and time-sensitive window of implantation (WOI) orchestrated by an incompletely defined complex of endocrine, paracrine, and autocrine factors [5]. During a typical physiologic menstrual cycle, the endometrial environment is limited to a 4–5-day period when blastocyst implantation can occur. In assisted reproduction, this process is pharmacologically mimicked through a combination of estrogen and progesterone supplementation, with monitoring for the WOI assessed by ultrasonography and blood hormone levels [6]. Unfortunately, these methods of assessing endometrial receptivity lack precision and objectivity, due in large part to inter-patient and inter-observer variability, and attempts to clinically define the WOI have had limited utility to date. Hence, a better method of objectively and reproducibly assessing endometrial receptivity is required.
Several studies have investigated the potential of proteomic analysis to characterize the expression patterns of proliferative and secretory endometrium [7, 8]. Identification of gene expression profiles has led to a differential analysis of receptive and non-receptive patterns in endometrial signaling [9, 10]. Through extensive molecular analysis of these expression patterns from endometrial tissue samples, the endometrial receptivity array (ERA) was developed as an objective molecular dating method to accurately and reproducibly identify endometrial receptivity status [9, 11]. By profiling the transcriptome of 238 genes that are expressed at different stages of the endometrial cycle, the ERA was developed as a means of personalizing embryo transfer (pET) timing, particularly in cases of recurrent implantation failure where endometrial receptivity may play a dominant factor. Interestingly, the ERA has been demonstrated to be reproducible in patients across multiple menstrual cycles and more accurate than histological analysis in defining the optimal WOI [12]. The clinical benefit of such an assay, however, is still undergoing further investigation.
Importantly, the ERA has not been specifically investigated in the context of implantation failure of embryos that were designated euploid by CCS. According to the manufacturer, the ERA is indicated for patients under 37 with at least 3 or more failed transfers of morphologically good quality embryos or 2 or more failed transfers in older patients [13]. Embryonic karyotype was not considered in prior studies that utilized ERA, but ostensibly failure of a euploid embryo to implant might justify investigation by ERA even sooner. Because embryonic aneuploidy is so common even in blastocysts of good morphology [14], controlling for a normal karyotype allows for a more meaningful interpretation of an endometrial assessment.
In the following report, we document our experience with the ERA with a particular focus on patients with a history of euploid blastocyst implantation failure. The aim of this study was to investigate if the contribution of the endometrial factor can be identified with the ERA test and if actionable results can lead to improved outcomes after frozen embryo transfer (FET).
Methods
A retrospective review was performed for all cases where the ERA was ordered between October 2014 and July 2017. The decision to perform the ERA followed a discussion between the patient and her physician. As shown in Fig. 1, most cases were indicated by at least one previous implantation failure of a euploid embryo, as determined by aCGH (array comparative genomic hybridization) or NGS (next generation sequencing) testing. However, ERA was also performed for some patients who experienced recurrent implantation failure (RIF) who did not have embryos screened by CCS. RIF was defined as ≥ 2 prior failed fresh or frozen embryo transfer cycles. The study was approved by the University of British Columbia Institutional Review Board.
Fig. 1.
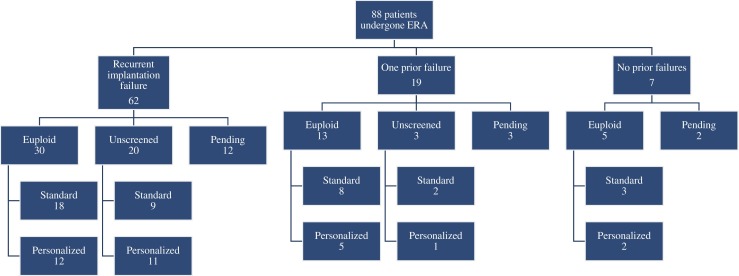
Overview of retrospective study design and distribution of included patients. N.B. ERA = endometrial receptivity array. “Pending” refers to patients who were awaiting their next cycle, those with inconclusive ERA results, or those who had an embryo transfer and were awaiting pregnancy results
Patient characteristics
Eighty-eight unique patients, with a mean age of 37.5 ± 4.8, underwent ERA testing between October 11, 2014 and July 27, 2017 at the Olive Fertility Centre in Vancouver BC, Canada. All patients were deemed eligible for IVF or donor IVF before initial treatment. A standard infertility evaluation was performed within 12 months of treatment, including transvaginal sonography and assessment of the uterine cavity via HSG (hysterosalpingogram) or hysteroscopy. TSH and thyroid peroxidase antibody screening was also performed and thyroid hormone supplementation provided when indicated.
As shown in Table 1, 70.5% of patients had RIF, 21.6% had just one prior implantation failure, and 8% had no history of prior failed cycles. The patients with no prior failed cycles underwent ERA due to findings of unfavorable endometrial proliferation on sonographic assessment, or a scarcity of viable frozen embryos. The average number of prior failed implantations was 2.7 ± 0.9. Of those who had prior implantation failures, 48 (59.3%) had at least 1 prior implantation failure of a euploid embryo, while 33 (40.7%) had failed prior transfers with unscreened fresh or frozen embryos. Patients waited an average of 78.6 ± 60.8 days between ERA biopsy and their next FET cycle, with an average of 1.07 ± 0.25 embryos transferred. Overall implantation rate was 63.3% (45/71) and ongoing pregnancy rate was 50.7% (36/71). Of 36 ongoing pregnancies, 13 have resulted in live births while the remaining 23 were still ongoing at the time of data collection.
Table 1.
Patient characteristics
| Patients | |
|---|---|
| Patients | 88 |
| RIF | 62 (70.5) |
| 1 prior failed | 19 (21.6) |
| 0 prior failed | 7 (8.0) |
| No. of euploid embryo transfers | 48 (59.3) |
| No. of unscreened embryo transfers | 33 (40.7) |
| Age | 37.5 ± 4.8 years |
| Prior implantation failures | 2.7 ± 0.9 |
| Patients requiring more than 1 ERA biopsy to determine receptivity | 32 (36.4) |
| After all biopsies, receptive on | 87 (98.9) |
| P + 4 | 3 (3.4) |
| P + 5 | 50 (57.5) |
| P + 6 | 27 (31.0) |
| P + 7 | 7 (8.0) |
| Patients awaiting next cycle/pending pregnancy results | 16 (18.1) |
| Patients with reproductive outcomes post-ERA | 71 (80.7) |
| Days from biopsy to next cycle | 78.6 ± 60.8 days |
| Embryos transferred | 1.07 ± 0.25 embryos |
| Overall implantation rate | 45/71 (63.3) |
| Overall ongoing pregnancy rate | 36/71 (50.7) |
N.B. Values expressed as N (%) and mean ± SD
RIF recurrent implantation failure
Endometrial sampling and processing
The endometrial preparation protocol that had been used for the previous failed transfer was repeated for the ERA cycle. For the patients that never had a previous transfer, the standard programmed hormone replacement cycle used in our clinic was prescribed. Generally, oral estradiol (Estrace 2 mg) was administered from day 2 of menses and escalated every 5 days at 2 mg intervals to a maximum of 6 mg daily. Transvaginal sonography (TVS) was used to assess the pattern and thickness of the endometrium approximately 14 days after menses, and progesterone (endometrin 200 mg TID) was administered when a trilaminar pattern was achieved with a thickness between 8 and 14 mm. The initial day of progesterone administration was deemed “P + 0”, and biopsy was performed with a Pipelle catheter after five full days of progesterone administration (“P + 5”). The specimen was processed and shipped according to manufacturer’s protocol.
ERA results were tabulated as reported by iGenomix and classified as receptive or non-receptive. Non-receptive results were deemed either pre- or post-receptive and details for endometrial adjustment recommendations or rebiopsy were documented. Vitrified blastocysts were re-warmed and transferred after receipt of ERA results, typically in the cycle subsequent to endometrial biopsy. Patients with a receptive endometrium underwent FET in an HRT cycle simulating the ERA cycle. In patients with a modified implantation window, FET was adjusted in subsequent cycles based on the personalized WOI identified by ERA (pET).
Implantation and clinical pregnancy rates were documented. When available, live birth rates were also reported. Outcomes were compared for patients who had an initial receptive ERA with the standard FET protocol repeated versus those who had an initial non-receptive result and subsequent transfer according to the pET protocol. Biochemical pregnancies and spontaneous abortions were included for calculating implantation rates, while ectopic pregnancies were excluded. Implantation rates were calculated based on a positive pregnancy test after embryo transfer, which included all biochemical pregnancies, spontaneous abortions, and clinical pregnancies defined by the presence of a viable fetal heart rate and crown rump length (CRL) ≥ 10 mm by U/S performed between 7 and 9 weeks’ gestation. Ongoing pregnancy rates were defined as those persisting > 12 weeks’ gestation and calculated based on the total number of live and pending births after embryo transfer. Live birth rates were calculated based on the total number of live births > 20 weeks’ gestation over the total number of ongoing pregnancies. Statistical comparisons of outcomes were performed using chi-squared test with significance determined by a p < 0.05. The Fisher’s exact test was used when the expected frequency of outcomes was less than 5 due to smaller sample size. Comparisons of patient characteristics were performed using Mann-Whitney U test for non-parametric data, with significance determined by a p < 0.05.
Results
ERA results
ERA test results were documented for 88 patients, with 71 subsequent reproductive outcomes reported, and follow-up treatment pending for 16 patients at the time of data collection. As shown in Table 2(a), the initial ERA biopsy returned with a receptive profile in 55.7% (49/88) and non-receptive in 44.3% (39/88). Of the non-receptive results, 74.3% (29/39) indicated a pre-receptive state, 10.3% (4/39) resulted in a post-receptive state, and 15.4% (6/39) were inadequate samples. There was no difference in age when patients with receptive and non-receptive ERA results were compared. Thirty-two (36.4%) required more than 1 ERA biopsy to determine endometrial receptivity.
Table 2.
(a) Receptive vs. non-receptive endometrial receptivity array (ERA) results on initial biopsy and (b) receptive vs non-receptive ERA results for patients with failed euploid transfers on initial biopsy
| (a) | |||
| Receptive | Non-receptive | p value | |
| Patients | 49 (55.7) | 39 (44.3) | 0.75 |
| RIF | 33 (67.3) | 29 (74.4) | |
| 1 prior failed | 12 (24.4) | 7 (17.9) | |
| 0 prior failed | 4 (8.2) | 3 (7.7) | |
| Age | 37.1 ± 4.3 years | 37.9 ± 5.4 years | 0.52 |
| Prior implantation failures | 2.8 ± 1.1 times | 2.5 ± 0.6 times | 0.32 |
| Receptive | 49 (100) | 0 | – |
| Pre-receptive | 0 | 29 (74.4) | |
| Post-receptive | 0 | 4 (10.3) | |
| Inadequate sampling | 0 | 6 (15.4) | |
| (b) | |||
| Euploid receptive | Euploid non-receptive | p value | |
| Patients | 30 (62.5) | 18 (37.5) | 0.21 |
| RIF | 20 (66.7) | 15 (83.3) | |
| 1 prior failed | 10 (33.3) | 3 (16.7) | |
| 0 prior failed | 0 | 0 | |
| Age | 36.9 ± 3.8 years | 38.8 ± 5.8 years | 0.97 |
| Prior implantation failures | 2.85 ± 1.3 times | 2.7 ± 0.6 times | 0.99 |
| Receptive | 30 (100) | 0 | – |
| Pre-receptive | 0 | 15 (83.3) | |
| Post-receptive | 0 | 1 (5.6) | |
| Inadequate sampling | 0 | 2 (11.1) | |
N.B. Values expressed as N (%) and mean ± SD. p < 0.05 is significant
RIF recurrent implantation failure
When patients with failed euploid transfers were further analyzed (Table 2(b)), ERA results indicated a receptive profile in 62.5% (30/48) and a non-receptive in 37.5% (18/48). Of the non-receptive results in the failed euploid transfers, a pre-receptive profile was seen in 83.3% (15/18) and a post-receptive in 5.6% (1/18). Again, no age difference between was demonstrated when receptive and non-receptive results were compared in the failed euploid transfer group.
Pregnancy outcomes after pET
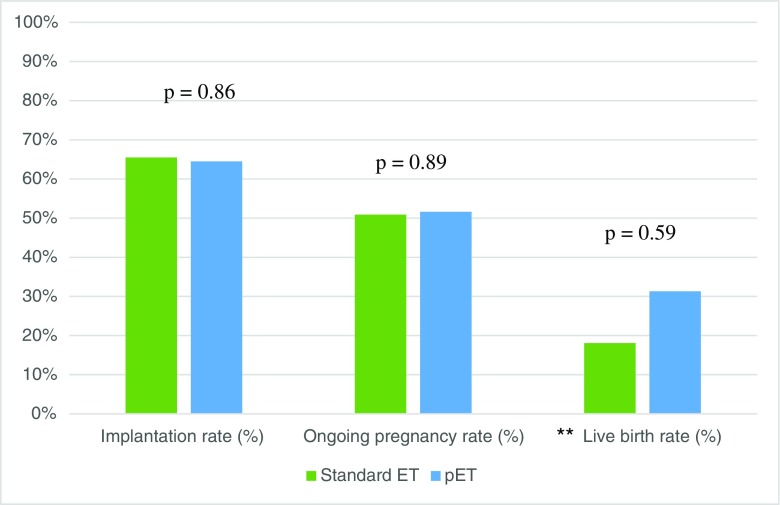
As shown in Table 3, patient profiles of those who had an initially receptive ERA and underwent standard unmodified progesterone protocols vs. pET were similar. Forty patients with a mean age of 36.8 ± 5.1 had an initially receptive endometrium by ERA and underwent standard FET. Conversely, 31 individuals with a mean age of 38.1 ± 4.8 demonstrated a non-receptive endometrium and underwent pET. There were no statistically significant differences in the biopsy-to-next-transfer times and mean embryos transferred. As shown in Fig. 2, implantation rates and ongoing pregnancy rates were largely similar between those who underwent standard protocol (65.5 and 50.9%, respectively) vs. pET (64.5 and 51.6%, respectively; p = 0.86 and 0.89, respectively). Live birth rates were also similar between the two groups (40.0 vs. 31.3%, respectively, p = 0.59).
Table 3.
Patient profiles and reproductive outcomes of patients undergoing standard ET protocol after receptive ERA vs. patients with pET protocol after non-receptive ERA
| Standard ET | pET | p value | |
|---|---|---|---|
| Patients | 40 | 31 | 0.82 |
| RIF | 27 (67.5) | 23 (74.2) | |
| 1 prior failed | 10 (25) | 6 (19.4) | |
| 0 prior failed | 3 (7.5) | 2 (6.5) | |
| Age | 36.8 ± 5.1 years | 38.1 ± 4.8 years | 0.35 |
| After all biopsies, receptive on | – | ||
| P + 4 | 0 | 3 (9.7) | |
| P + 5 | 40 (100) | 0 (0) | |
| P + 6 | 0 | 22 (71) | |
| P + 7 | 0 | 6 (19.4) | |
| Time from biopsy to next cycle | 97.2 ± 69.5 days | 58.5 ± 39.9 days | 0.0022 |
| Embryos transferred | 1.1 ± 0.30 embryos | 1.03 ± 0.26 embryos | 0.83 |
| Implantation rate | 25/40 (65.5) | 20/31 (64.5) | 0.86 |
| Ongoing pregnancy rate | 20/40 (50.9) | 16/31 (51.6) | 0.89 |
| Live birth ratea | 8/20 (40.0) | 5/16 (31.3) | 0.59 |
N.B. Values expressed as N (%) and mean ± SD. p < 0.05 is significant
ET embryo transfer, pET personalized embryo transfer, RIF recurrent implantation failure
aNot all ongoing pregnancies could be followed through to term; therefore, live birth rates would not reflect true birth outcomes
Fig. 2.
Reproductive outcomes of patients with standard ET protocol vs. patients with pET after non-receptive ERA. N.B. pET is personalized progesterone protocol. p < 0.05 is significant. **Not all ongoing pregnancies were followed through to term; therefore, live birth rates would not reflect true birth outcomes
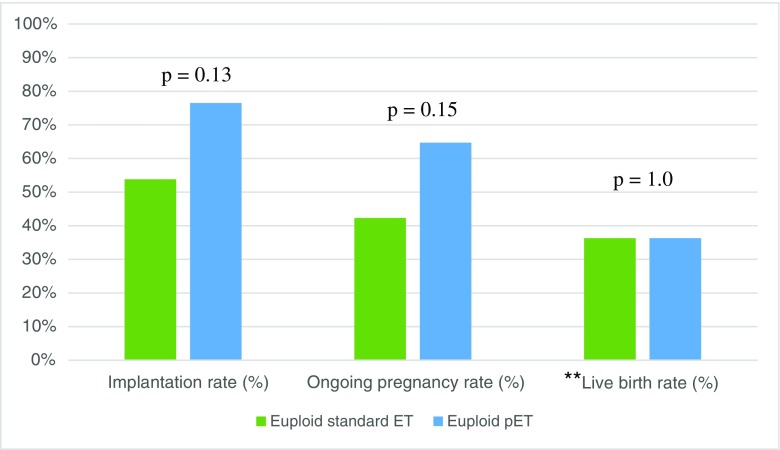
To further investigate the individual effect of pET, we performed a subgroup analysis among cases of euploid ET, as shown in Table 4. Twenty-six individuals with an initially receptive ERA underwent euploid FET with an unmodified progesterone protocol, with an average age of 36.8 ± 4.1 years and a mean number of 2.2 ± 1.4 prior failures. Conversely, 17 patients with an initially non-receptive ERA subsequently underwent pET with euploid blastocysts, with an average age of 37.6 ± 3.9 years and a mean number of 2.1 ± 0.9 prior failures. As shown in Fig. 3, implantation rates were not statistically different for patients who underwent euploid pET (76.5%) compared to those who underwent standard euploid FET (76.5 vs. 53.8%, respectively, p = 0.13). A similar trend was observed with respect to ongoing pregnancy rates (64.7 vs. 42.3%, p = 0.15). Live birth rates were similar between the two groups (36.3%, p = 1.0).
Table 4.
Patient profiles and reproductive outcomes of patients undergoing euploid embryo transfer after standard progesterone protocol vs. patients undergoing euploid embryo transfer after pET
| Euploid standard ET | Euploid pET | p value | |
|---|---|---|---|
| Patients | 26 | 17 | 1.0 |
| RIF | 18 (69.2) | 12 (68.4) | |
| 1 prior failed | 8 (30.8) | 5 (31.6) | |
| Age | 36.8 ± 4.1 years | 37.6 ± 3.9 years | 0.33 |
| Prior implantation failures | 2.2 ± 1.4 times | 2.1 ± 0.9 times | 0.79 |
| After all biopsies, receptive on | – | ||
| P + 4 | 0 (0) | 0 (0) | |
| P + 5 | 26 (100) | 0 (0) | |
| P + 6 | 0 (0) | 14 (82.3) | |
| P + 7 | 0 (0) | 3 (17.6) | |
| Time from biopsy to next cycle | 97.3 ± 51.9 days | 61.3 ± 48.2 days | 0.04 |
| Embryos transferred | 1.09 ± 0.3 embryos | 1 ± 0 embryos | 0.68 |
| Implantation rate | 14/26 (53.8) | 13/17 (76.5) | 0.13 |
| Ongoing pregnancy rate | 11/26 (42.3) | 11/17 (64.7) | 0.15 |
| Live birth ratea | 4/11 (36.3) | 4/11 (36.3) | 1.0 |
N.B. Values expressed as N (%). p < 0.05 is significant
ET embryo transfer, pET personalized embryo transfer, RIF recurrent implantation failure
aNot all ongoing pregnancies could be followed through to term; hence, live birth rates would not reflect true birth outcomes
Fig. 3.
Reproductive outcomes of patients undergoing euploid transfer with standard ET protocol vs. patients undergoing euploid transfer after pET. N.B. pET = personalized embryo transfer. p < 0.05 is significant. **Not all ongoing pregnancies were followed through to term; therefore, live birth rates would not reflect true birth outcomes
Pregnancy outcomes in RIF vs. non-RIF groups
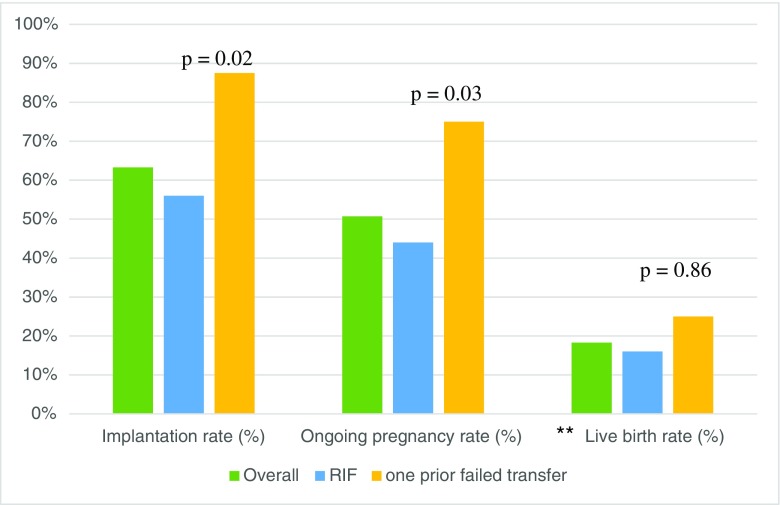
As shown in Table 5, 62 patients had a history of RIF, with a mean of 2.7 ± 0.9 prior failures and a mean age of 37.1 ± 4.9, and 19 patients who had just 1 prior IVF failure, with a mean age of 37.9 ± 4.4. Patient profiles were largely similar between the RIF and non-RIF groups. There were no statistically significant differences in the proportion of patients for whom reproductive outcomes were available (80.7% versus 84.2%). Biopsy-to-next-transfer times and mean embryos transferred were also similar, and most individuals demonstrated a receptive endometrium on initial ERA biopsy in both groups. As shown in Fig. 4, however, the non-RIF group demonstrated significantly higher implantation rates (87.5 vs. 56%, p = 0.02) and ongoing pregnancy rates (75 vs. 44%, p = 0.03) compared to the RIF group. Live birth rates were similar between the RIF and non-RIF groups (36.4 vs. 33.3%, respectively, p = 0.86).
Table 5.
Patient profiles and reproductive outcomes of RIF and non-RIF
| RIF | Non-RIF | p value | |
|---|---|---|---|
| Patients | 62 | 19 | – |
| Number of prior failures | 2.7 ± 0.9 times | – | |
| Age | 37.1 ± 4.9 years | 37.9 ± 4.4 years | 0.37 |
| Prior implantation failures | 2.7 ± 0.9 | – | – |
| After all biopsies, receptive on | – | ||
| P + 4 | 3 (6) | 0 (0) | |
| P + 5 | 27 (54) | 10 (62.5) | |
| P + 6 | 16 (32) | 5 (31.3) | |
| P + 7 | 4 (8) | 1 (6.3) | |
| Patients awaiting next cycle | 12 (19.4) | 3 (15.8) | 0.73 |
| Patients with reproductive outcomes post-ERA | 50 (80.7) | 16 (84.2) | |
| Time from biopsy to next cycle | 78.1 ± 50.2 days | 65.4 ± 47.1 days | 0.65 |
| Embryos transferred | 1.08 ± 0.27 embryos | 1.07 ± 0.26 embryos | 0.99 |
| Implantation rate | 28/50 (56.0) | 14/16 (87.5) | 0.02 |
| Ongoing pregnancy rate | 22/50 (44.0) | 12/16 (75.0) | 0.03 |
| Live birth ratea | 8/22 (36.4) | 4/12 (33.3) | 0.86 |
N.B. Values expressed as N (%) and mean ± SD. p < 0 .05 is significant
RIF recurrent implantation failure and is defined as ≥ 2 previous implantation failures; non-RIF patients have one prior implantation failure
aNot all ongoing pregnancies could be followed through to term; therefore, live birth rates would not reflect true birth outcomes
Fig. 4.
Reproductive outcomes of all patients, patients with RIF, and those with one prior failed transfer. N.B. RIF = ≥ 2 past implantation failures (recurrent implantation failure). p < 0.05 is significant. **Not all ongoing pregnancies were followed through to term; therefore, live birth rates would not reflect true birth outcomes
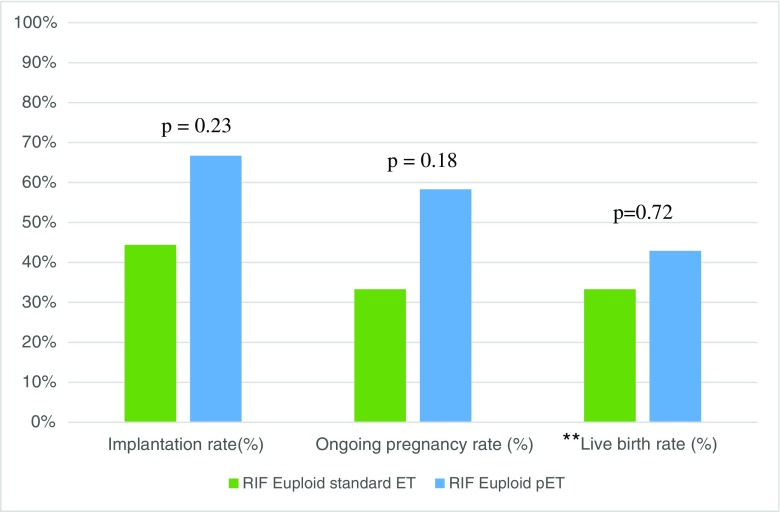
A further subgroup analysis was performed to assess whether improved pregnancy outcomes after pET were maintained in patients with euploid embryos and RIF compared to unmodified progesterone ET (Table 6). Overall, 18 RIF patients had an unmodified euploid transfer with an average age of 35.6 ± 5.1 and average of 2.7 ± 1.3 prior implantation failures. Conversely, 12 patients demonstrated a non-receptive endometrium and underwent pET in the RIF group, with an average age of 37.7 ± 4.4 and average 2.5 ± 0.7 prior implantation failures. As shown in Fig. 5, implantation rates and ongoing pregnancy rates were not statistically different in the RIF pET group (66.7 vs. 58.3%, respectively) than those who underwent unmodified euploid ET (44.4 and 33.3%, respectively; p = 0.28 and 0.26, respectively).
Table 6.
Patient profiles and reproductive outcomes of patients with RIF undergoing euploid embryo transfer after standard progesterone protocol vs. patients with RIF undergoing euploid embryo transfer after pET
| RIF euploid standard ET | RIF euploid pET | p value | |
|---|---|---|---|
| Patients | 18 | 12 | – |
| Age | 35.6 ± 5.1 years | 37.7 ± 4.4 years | 0.22 |
| Prior implantation failures | 2.7 ± 1.3 times | 2.5 ± 0.7 times | 0.73 |
| After all biopsies, receptive on | – | ||
| P + 4 | 0 (0) | 0 (0) | |
| P + 5 | 18 (100) | 0 (0) | |
| P + 6 | 0 (0) | 9 (75) | |
| P + 7 | 0 (0) | 3 (25) | |
| Time from biopsy to next cycle | 103 ± 48.7 days | 70.8 ± 53.3 days | 0.14 |
| Embryos transferred | 1.1 ± 0.3 embryos | 1 ± 0 embryos | 0.61 |
| Implantation rate | 8/18 (44.4) | 8/12 (66.7) | 0.28 |
| Ongoing pregnancy rate | 6/18 (33.3) | 7/12 (58.3) | 0.26 |
| Live birth ratea | 2/6 (33.3) | 3/7 (42.9) | 1.0 |
N.B. Values expressed as N (%). p < 0.05 is significant
RIF recurrent implantation failure
aNot all ongoing pregnancies could be followed through to term; therefore, live birth rates would not reflect true birth outcomes
Fig. 5.
Reproductive outcomes of patients with RIF undergoing euploid embryo transfer after standard progesterone protocol vs. pET. N.B. RIF = recurrent implantation failure, pET = personalized embryo transfer. p < 0.05 is significant. **Not all ongoing pregnancies were followed through to term; therefore, live birth rates would not reflect true birth outcomes
Discussion
To the best of our knowledge, this is the first report of the clinical efficacy of ERA as a diagnostic tool to characterize endometrial receptivity in the context of euploid embryo transfer. While prior studies [15, 16] have documented the utility of the ERA for RIF, results would have been confounded by the high prevalence of aneuploidy in embryos screened only by morphology. Controlling for euploid status allows for a more meaningful assessment of the endometrial contribution.
According to the ERA, 22.5% of patients who underwent testing at our fertility clinic had a displaced WOI and qualified for pET, which is relatively consistent with previous studies that report a 25–30% contribution by the endometrial factor in cases of implantation failure [14]. Among patients with a displaced WOI, the vast majority of cases were pre-receptive (88%), which is also consistent with previously reported studies [9]. After individualizing progesterone protocols from the initial ERA biopsy, 72.73% were receptive after 5 days of progesterone supplementation and 18.18% after P + 6. Interestingly, initial observational studies in the 1980s reported higher pregnancy rates from embryo transfers between P + 3 to P + 5 (48.3%) days compared to P + 6 (20.4%), but these studies utilized day 4–6 cell embryos in fresh cycles [17, 18]. To date, no prospective randomized control trials have addressed the optimal duration of progesterone administration prior to FET and its impact on implantation and pregnancy rates [19]. With more widespread use of objective and reproducible diagnostic methods such as ERA for assessing endometrial receptivity, it will be interesting to see emerging population-based trends in levels of progesterone supplementation required for achieving endometrial receptivity.
With regard to reproductive outcomes, patients waited an average of 78.6 days between their initial endometrial biopsy and subsequent IVF cycle, which is consistent with previously reported studies [15]. Furthermore, patients with recurrent implantation failure had lower overall implantation rates (56 vs. 87.50%) and ongoing pregnancy rates (44 vs. 75%) compared to patients with one prior IVF failure (Table 2 and Fig. 4), which was statistically significant and consistent with the existing body of literature reporting overall reduced reproductive outcomes among RIF patients [20, 21]. Finally, although implantation, ongoing pregnancy, and live birth rates were higher than in similar studies [20, 21], the number of prior failed IVF cycles and mean maternal age was also lower in our study group which likely contributed to more favorable pregnancy outcomes.
Regarding pregnancy outcomes after pET, patients who underwent modified personalized progesterone protocols prior to FET were found to have a similar implantation rates, ongoing pregnancy rates, and live birth rates compared to patients who underwent unmodified P + 5 progesterone protocol prior to ET (Table 3 and Fig. 2). Among patients that underwent euploid FET (Table 4 and Fig. 3), those in the pET group demonstrated higher implantation and ongoing pregnancy rates compared to those in the unmodified ET group, although observed differences were not statistically significant. Among RIF patients undergoing euploid FET (Table 6 and Fig. 5), implantation and ongoing pregnancy rates in the pET vs. unmodified ET groups were 66.7 vs. 44.4% and 58.3 vs. 33.3%, respectively; once again, however, these differences were not statistically significant.
These results demonstrate two key points: (1) The effect of pET may be masked in cases where embryos are selected based on morphology alone, underscoring the effect of aneuploidy and fundamental benefit of chromosomal screening. (2) Once aneuploidy has been addressed, the effects of the “endometrial factor” and benefits of pET on reproductive outcomes may be more impactful, although the observed differences in this study were not statistically significant and would require further evaluation using a larger sample size. Nevertheless, patients may be counseled to first pursue CCS to address the most common cause of implantation failure; however, once euploidy has been established, patients may benefit from the ERA to characterize endometrial receptivity. Patients with implantation failure in the context of both a euploid embryo and a receptive ERA may have more elusive etiologies yet to be defined.
There are several limitations to this retrospective case series, including a small sample size and lack of a control arm to compare reproductive outcomes among patients with a history of implantation failure who did not undergo pET with ERA. Indeed, based on the observed implantation rate of 76.5% (13/17) and 53.8% (14/26) in the pET and standard progesterone protocol group, respectively, our sample size yielded a 31.5% post hoc power to detect differences in implantation rates based on an alpha of 0.05. Similarly, our sample size yielded a 29.5% post hoc power to detect differences in ongoing pregnancy rates given the observed ongoing pregnancy rates of 64.7% (11/17) and 42.3% (11/26) in the pET and standard progesterone protocol groups, respectively. To achieve 80% power, we would require a sample size of 136 (68 in each group) to detect a statistically significant difference in implantation rates and a sample size of 154 (77 in each group) to detect a statistically significant difference in ongoing pregnancy rates. Similarly, randomizing non-receptive ERA patients to pET versus routine endometrial preparation would have been more meaningful for attributing the outcomes to the intervention compared to chance alone. Furthermore, reproductive outcome data was only available for the cycle immediately post-ERA diagnosis and 50% of included pregnancies were still awaiting live birth outcomes. Hence, the short follow-up period precluded our ability to compare more clinically meaningful cumulative pregnancy and live birth outcomes. Although many studies have also shown positive results from ERA, particularly among patients with pET, validated prospective studies are still required to confirm the clinical benefits of ERA for improving reproductive outcomes from ART. Ideally, a randomized control trial would be performed with a sample size adequately powered to detect differences in reproductive outcomes. Since there is also evidence that local injury induced by endometrial biopsy (i.e., endometrial scratching) might improve implantation following embryo transfer, any future prospective studies would ideally also control for this potential confounding variable [22].
Conclusion
Consistent with the modern trend to personalize medical care by leveraging genomics, the ERA has the capacity to offer an individualized approach to identification of the WOI and embryo transfer. Unlike prior attempts to characterize endometrial receptivity through histological sampling, sonography, or hormonal markers, the ERA may help identify the window of implantation with greater objectivity and less inter-cycle variability. In conjunction with CCS to select a single euploid embryo for transfer, the concept of a personalized embryo transfer (pET) offers the potential to improve reproductive outcomes through ART.
Our experience demonstrates that a significant proportion of patients with a history of implantation failure have displaced windows of implantation and may benefit from personalized adjustment of progesterone exposure, particularly in cases of euploid embryo transfer. Indeed, genetic abnormalities and aneuploidy are major contributors to poor reproductive outcomes from ART, and screening with CCS should be among the first tools employed to improve pregnancy rates in patients with a history of implantation failure. However, among patients with euploid embryos who have a non-receptive endometrium by ERA, pET using a modified progesterone protocol may improve IVF outcomes. Although larger randomized studies are required to validate these observations, our preliminary experience demonstrates that ERA may be a promising technique to help characterize endometrial receptivity and provide actionable directives to improve implantation in instances of previous failure and non-receptive endometrium, perhaps most significantly in cases of failed euploid transfer.
Acknowledgements
We would like to thank colleagues at the University of British Columbia and Olive Fertility Centre.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, et al. Uterine selection of human embryos at implantation. Sci Rep. 2014;4:3894. doi: 10.1038/srep03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahdouh EM, Balayla J, Garcia-Velasco JA. Impact of blastocyst biopsy and comprehensive chromosome screening technology on preimplantation genetic screening: a systematic review of randomized controlled trials. Reprod BioMed Online. 2015;30(3):281–289. doi: 10.1016/j.rbmo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24. doi: 10.1186/1755-8166-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100(1):100–107. doi: 10.1016/j.fertnstert.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 5.Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754–1767. doi: 10.1038/nm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remohi J, Ardiles G, Garcia-Velasco JA, Gaitan P, Simon C, Pellicer A. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod. 1997;12(10):2271–2276. doi: 10.1093/humrep/12.10.2271. [DOI] [PubMed] [Google Scholar]
- 7.Koot YE, van Hooff SR, Boomsma CM, van Leenen D, Groot Koerkamp MJ, Goddijn M, et al. An endometrial gene expression signature accurately predicts recurrent implantation failure after IVF. Sci Rep. 2016;6:19411. doi: 10.1038/srep19411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altmae S, Esteban FJ, Stavreus-Evers A, Simon C, Giudice L, Lessey BA, et al. Guidelines for the design, analysis and interpretation of 'omics' data: focus on human endometrium. Hum Reprod Update. 2014;20(1):12–28. doi: 10.1093/humupd/dmt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Gomez E, Fernandez-Sanchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100(3):818–824. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez F, Garrido-Gomez T, Lopez JA, Camafeita E, Quinonero A, Pellicer A, et al. Proteomic analysis of the human receptive versus non-receptive endometrium using differential in-gel electrophoresis and MALDI-MS unveils stathmin 1 and annexin A2 as differentially regulated. Hum Reprod. 2009;24(10):2607–2617. doi: 10.1093/humrep/dep230. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Alonso M, Galindo N, Pellicer A, Simon C. What a difference two days make: “personalized” embryo transfer (pET) paradigm: a case report and pilot study. Hum Reprod. 2014;29(6):1244–1247. doi: 10.1093/humrep/deu070. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martinez-Conejero JA, Alama P, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013;99(2):508–517. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 13.Website E. Endometrial receptivity test for implantation failure: Igenomix; 2017 [Available from: https://www.igenomix.com/tests/endometrial-receptivity-test-era/.
- 14.Fox C, Morin S, Jeong JW, Scott RT, Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertil Steril. 2016;105(4):873–884. doi: 10.1016/j.fertnstert.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto T, Koizumi M, Doshida M, Toya M, Sagara E, Oka N, et al. Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: a retrospective, two-centers study. Reprod Med Biol. 2017;16(3):290–296. doi: 10.1002/rmb2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan N. Endometrial receptivity array: clinical application. J Hum Reprod Sci. 2015;8(3):121–129. doi: 10.4103/0974-1208.165153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones EE, Prapas Y, Olive DL, Prapas N, Vlassis G, Duleba AJ, et al. The window for embryo transfer in oocyte donation cycles depends on the duration of progesterone therapy. Hum Reprod. 1998;13(3):720–723. doi: 10.1093/humrep/13.3.720. [DOI] [PubMed] [Google Scholar]
- 18.Schreiner-Engel P, Garrlsi GJ, Fox J, Williams M, Hofmann GE, Navot D, et al. An insight into early reproductive processes through the in VivoModel of ovum donation. J Clin Endocrinol Metab. 1991;72(2):408–414. doi: 10.1210/jcem-72-2-408. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Majumdar A. Determining the optimal duration of progesterone supplementation prior to transfer of cryopreserved embryos and its impact on implantation and pregnancy rates: a pilot study. Int J Reprod Med. 2016;2016(6):1–7. doi: 10.1155/2016/7128485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitri F, Casper RF, Bentov Y, Nayot D. Current tools for the optimization of embryo transfer technique for recurrent implantation failure. Minerva Ginecol. 2016;68(4):431–449. [PubMed] [Google Scholar]
- 21.Das M, Holzer HEG. Recurrent implantation failure: gamete and embryo factors. Fertil Steril. 2012;97(5):1021–1027. doi: 10.1016/j.fertnstert.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 22.El-Toukhy T, Sunkara S, Khalaf Y. Local endometrial injury and IVF outcome: a systematic review and meta-analysis. Reprod BioMed Online. 2012;25(4):345–354. doi: 10.1016/j.rbmo.2012.06.012. [DOI] [PubMed] [Google Scholar]