Abstract
Purpose
To evaluate the available randomized controlled trials (RCTs) in the literature investigating the use of gonadotropin-releasing hormone agonist (GnRHa) co-treatment for ovarian preservation in women receiving chemotherapy.
Methods
A systematic review of the literature was performed from 1960 through 2017 to identify relevant RCTs. Included patients had lymphoma, ovarian cancer, or breast cancer. The primary outcome was the proportion of women who retained ovarian function after chemotherapy. Extracted data points included study design, patient characteristics, and proportion of women who developed premature ovarian failure (POF). A risk of bias assessment was performed according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions. The pooled odds ratio was calculated, and outcomes of individual studies were compared using the random-effects model with the inverse-variance method and the DerSimonian-Laird estimator.
Results
Twenty-nine RCTs were identified, and 10 met criteria for inclusion in the meta-analysis. An analysis of patients who did not develop POF after chemotherapy revealed eight studies supporting the use of GnRHa (OR 1.83; 95% CI 1.34–2.49). The duration of benefit of GnRHa is unclear. An analysis of three studies with outcome data at 2 years revealed a non-significant OR of 0.53 (95% CI 0.22–1.30) for the preservation of ovarian function with GnRHa treatment.
Conclusion
GnRHa may have a protective effect against the development of POF after gonadotoxic chemotherapy; however, the duration of benefit is unclear and requires further study.
Keywords: Chemotherapy, Gonadotropin-releasing hormone, Ovarian function, Premature ovarian failure, Systematic review
Introduction
A cancer diagnosis for a reproductive-aged woman has unique medical and psychosocial consequences. Cancer treatments can negatively impact both ovarian reserve and function, ranging from little or no long-term effects to permanent infertility and premature ovarian failure (POF) [1, 2]. Beyond its role in the overall hormonal milieu, gonadal function affects many other organ systems including bone, the cardiovascular system, neurocognitive function, and sexuality [3, 4]. Chemotherapy-related POF can significantly impact a woman’s overall well-being and quality of life.
For women undergoing cancer treatment, the first-line options for fertility preservation include embryo and oocyte cryopreservation prior to the initiation of chemotherapy. However, these methods have no role in preserving gonadal function. Ovarian tissue cryopreservation and pharmacologic ovarian suppression with gonadotropin-releasing hormone agonists (GnRHa) are options to preserve both fertility and gonadal function. Ovarian tissue cryopreservation, while intriguing, is still considered an experimental approach.
GnRHa therapy has been associated with relatively low risk, time, and cost. GnRHa act at the anterior pituitary to interrupt endogenous GnRH pulsatility and induce a hypogonadal state [5]. There are several proposed mechanisms for chemotherapy-induced damage to the ovaries [5]. One is apoptosis of growing follicles, resulting in faster recruitment of primordial follicles and a subsequent decline in ovarian reserve [6]. Another mode of chemotherapy-induced injury is fibrosis of the stromal cells and injury to blood vessels, resulting in ischemia that further decreases ovarian reserve. GnRHa are thought to mitigate ovarian injury by preventing primordial follicle recruitment and slowing the depletion of ovarian follicles, and by reducing vascularity, subsequently decreasing the dose of chemotherapy affecting the ovary [7, 8].
In addition to protecting against ovarian failure, both retrospective and prospective data suggest that GnRHa administration concurrent with chemotherapy may also improve disease-free and overall survival among estrogen/progesterone receptor negative breast cancer patients [9, 10]. A recent expert meeting on cancer and fertility preservation deemed GnRHa “a reliable strategy to preserve ovarian function” for breast cancer patients [11]. Additionally, the American Society for Reproductive Medicine (ASRM) acknowledges that data are mixed, but supports the use of GnRHa when it is combined with other methods to preserve ovarian function [12]. Adopting GnRHa use into clinical practice has been controversial and often regarded as experimental in nature due to heterogeneity in the literature regarding its effectiveness [11, 12].
The aim of this systematic review and meta-analysis is to evaluate the efficacy of GnRHa co-treatment during gonadotoxic chemotherapy for preserving ovarian endocrine function.
Materials and methods
We performed a systematic review of prospective randomized controlled trials (RCTs) in order to evaluate the efficacy and safety of GnRHa co-treatment on ovarian function during gonadotoxic chemotherapy. A secondary review of previously published meta-analyses was also performed. The Cochrane Handbook for Systematic Reviews of Interventions (version 5.0.1) [13], and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [14] were utilized in the development and reporting of this review.
Search strategy
A search strategy similar to that previously published by Bedaiwy et al. was utilized for this study [15]. MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, Ovid Healthstar, and ClinicalTrials.gov were queried for published trials with an English language restriction. A combination of the main Medical Subject Headings (MeSH) and text words “gonadotropin-releasing hormone,” “chemotherapy,” and “premature ovarian failure” were used, and the search was performed for publications from 1960 to May 2017. A manual search of the ASRM and European Society of Human Reproduction and Embryology (ESHRE) conference abstracts was also performed. We reviewed the references of included studies, recent review articles, and other meta-analyses to identify any additional studies.
We included RCTs of women receiving GnRHa versus control during treatment with chemotherapy for cancer. Studies were excluded if women underwent bilateral oophorectomy or if different chemotherapy regimens were utilized in the GnRHa and control groups. One author (LCH) reviewed the identified reports and two authors (LNV and TF) confirmed selected studies. All disagreements were resolved by consensus.
Outcome measures
The primary outcome for this study was the proportion of women who retained ovarian function after chemotherapy, as defined by the trials’ investigators (Table 1).
Table 1.
Description of prospective, RCTs assessing the role of GnRHa treatment for preservation of gonadal function during gonadotoxic chemotherapy
| Study | Risk of bias assessment | Overall bias risk | Diagnosis | Treatment groups (#) | Age (median [range] or mean + SD) |
Interventions | GnRH agonist | Time to outcome | POF definition | Available outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Waxman et al. 1987 [16] | Random sequence generation: unclear Allocation concealment: unclear Blinding: N/A Incomplete outcome data: high Selective reporting: low Other bias: low |
High | Hodgkin lymphoma | GnRHa (8) control (10) |
GnRHa: 28.5 (17–34) control: 25.9 (17–46) |
Chemotherapy: up to 6 cycles of MVPP RT: none Surgery: N/A |
Buserelin | Up to 30 months | Cessation of menstruation | Incidence of: - spontaneous menstruation - return to spontaneous ovulation - spontaneous pregnancy |
| Gilani et al. 2007 [17] | Random sequence generation: low Allocation concealment: unclear Blinding: N/A Incomplete outcome data: low Selective reporting: low Other: low |
Low | Ovarian | GnRHa (15) control (15) |
GnRHa: 21 (13–33) control: 22 (15–35) |
Chemotherapy: up to 6 cycles of alkylating or alkylating-like MCT RT: none Surgery: conservative with ovarian preservation (≥ 1) |
Diphereline | Up to 6 months | Early, permanent cessation of menstruation after 6 months of chemo and a serum FSH > 20 mIU/ml | Incidence of: - spontaneous menstruation |
| Giuseppe et al. 2007 [18] | Random sequence generation: low Allocation concealment: unclear Blinding; N/A Incomplete outcome data: low Selective reporting: low Other bias: low |
Low | Hodgkin lymphoma | GnRHa (14) control (15) |
GnRHa: 24.3 ± 5.3 control: 24.26 + 7.92 |
Chemotherapy: ABVD; up to 6 cycles of ABVD alternating with C(M)OPP; or C(M)OPP alternating with ABV; additional DHAP in cases of incomplete remission RT: no pelvic, supra-diaphragmatic in 82.8% Surgery: N/A |
Triptorelin | Mean 4.2 ± 2.8 years | Cessation of menstruation | Incidence of: - spontaneous menstruation |
| Badawy et al. 2009 [19] | Random sequence generation: low Allocation concealment: low Blinding: N/A Incomplete outcome data: low Selective reporting: low Other bias: high |
High | Breast | GnRHa (40) control (40) |
GnRHa: 30.0 ± 3.51 control: 29.2 ± 2.93 |
Chemotherapy: up to 6 cycles of FAC RT: none Surgery: modified radical mastectomy or breast-conserving surgery plus full axillary LND |
Goserelin | Up to 8 months | Early cessation of menstruation, ovulation, and increased serum FSH level | Incidence of: - spontaneous menstruation - spontaneous ovulation |
| Sverrisdottir et al. 2009 [20] | Random sequence generation: low Allocation concealment: low Blinding: N/A Incomplete outcome data: low Selective reporting: low Other bias: low |
Low | Breast | GnRHa (66) control (57) |
GnRHa: 45 (29-54); 45 (29-54) controls: 45 (29-53); 46 (36-55) |
Chemotherapy: up to 6 cycles of CMF RT: for those with ≥ 4 + LN Surgery: breast conserving Tamoxifen given to 74 of GnRHa and 60 of controls |
Goserelin | Up to 36 months | Cessation of menstruation | Incidence of: - spontaneous menstruation |
| Gerber et al. 2011 [21] | Random sequence generation: low Allocation concealment: unclear Blinding: N/A Incomplete outcome data: low Selective reporting: low Other bias: low |
Low | Breast | GnRHa (30) control (30) |
GnRHa: 35.1 (26–44) control: 38.2 (29–47) |
Chemotherapy: up to 8 cycles of anthracycline, cyclophoshamide ± taxane RT: none Surgery none |
Goserelin | Up to 24 months | Cessation of menstruation | Incidence of: - spontaneous menstruation - spontaneous pregnancy Assessment of ovarian function reserve and fertility (via AMH, inhibin B, E2, FSH, and AFC by U/S) |
| Del Mastro et al. 2011 [22] | Random sequence generation: low Allocation concealment: low Blinding: N/A Incomplete outcome data: low Selective reporting: low Other bias: low |
Low | Breast | GnRH (148) control (133) |
GnRHa: 39 (25–45) control: 39 (24–45) |
Chemotherapy: up to 6 cycles of anthracycline-based, anthracycline + taxane-based, or CMF-based Tamoxifen for hormone receptor positive tumors RT: none Surgery: none |
Triptorelin | Up to 12 months | No resumption of menstrual activity and post-menopausal levels of both FSH and E2 for 1 year after the end of chemotherapy | Incidence of: - spontaneous menstruation - spontaneous pregnancy |
| Munster et al. 2012 [23] | Random sequence generation: low Allocation concealment: unclear Blinding: N/A Incomplete outcome data: low Selective reporting: low Other bias: low |
Low | Breast | GnRHa (26) control (21) |
GnRHa: 39 (21–44) control: 38 (36–44) |
Chemotherapy: up to 6 cycles of doxorubicin + cyclophosphamide; doxorubicin + cyclophosphamide + taxane; fluorourocil + epirubicin tamoxifen for hormone receptor positive tumors RT: none Surgery: none |
Triptorelin | Up to 30 months | Cessation of menstruation | Incidence of: - spontaneous menstruation Interval to resumption of menstrual cycles |
| El Gindy et al. 2013 [24] | Random sequence generation: low Allocation concealment: low Blinding: N/A Incomplete outcome data: low Selective reporting: low Other bias: low |
Low | Breast | GnRHa (50) control (50) |
GnRHa early: 33.3 ± 3.3 control early: 32.3 ± 4.0 GnRHa late: 33.0 ± 3.8 control late: 32.8 ± 4.3 |
Chemotherapy: up to 6 cycles of cyclophosphamide, 5-FU, doxorubicin RT: regional adjuvant therapy permitted Surgery: unclear, performed on a case-by-case basis |
Triptorelin plus GnRH antagonist cetrorelix | Up to 18 months | Cessation of menstruation | Incidence of: - spontaneous menstruation - resumption of regular menstrual cycles - Biochemical/ultrasound parameters of ovarian reserve |
| Song et al. 2013 [25] | Random sequence generation: low Allocation concealment: unclear Blinding: N/A Incomplete outcome data: low Selective reporting: low Other bias: low |
Low | Breast | GnRHa (89) control (94) |
GnRHa: 40.3 ± 6.5 control: 42.1 ± 5.9 |
Chemotherapy: up to 6 cycles of cyclophosphamide and doxorubicin ± paclitaxel; tamoxifen for hormone receptor positive tumor RT: none Surgery: modified mastectomy or breast-conserving with ipsalateral or sentinel LND |
Luprolide acetate | Up to 12 months | FSH > 40 mIU/ml and E2 < 20 pg/ml in absence of menstrual activity resumption within 12 months after end of chemotherapy |
Effective treatment: -resumption of menses OR - FSH < 40 mIU/ml and E2 > 20 pg/mL in absence of menstrual activity during the 12 month follow-up |
| Moore et al. 2015 [10] | Random sequence generation: low Allocation concealment: unclear Blinding: N/A Incomplete outcome data: low Selective reporting: low Other bias: low |
Low | Breast | GnRHa (66) control (69) |
GnRHa: 37.6 (26.1–48.6) control: 38.7 (25.1–49.9) |
Chemotherapy: up to 8 cycles of anthracycline-based or non-anthracycline-based; Tratuzumab for HER2 + RT: none Surgery: none |
Goserelin | Up to 24 months | Amenorrhea in the preceding 6 months and FSH in the post-menopausal range at 2 years | Incidence of: - spontaneous pregnancy within 5 years - ovarian dysfunction (amenorrhea in preceding 3 months and FSH, E2, or inhibin B in post-menopausal range |
| Demeestere et al. 2016 [26] | Random sequence generation: low Allocation concealment: low Blinding: N/A Incomplete outcome data: low Selective reporting: low Other bias: low |
Low | Hodgkin or non-Hodgkin lymphoma | GnRHa (32) control (35) |
GnRHa: 25.8 ± 1.0 control: 26.6 ± 0.8 |
Chemotherapy: up to 8 cycles of BEAM ACVBP or BEACOPP or (R)-CHOP or R-CHO(E)P, or ABVD, or CHLVVP/ABVVP or other RT: N/A Surgery: N/A |
Triptorelin + norethisterone | Up to 7 years | FSH ≥ 40 IU/L | Incidence of: - complete ovarian function recovery rate (FSH < 15 IU/L and AMH) - pregnancy |
AFC antral follicle count, AMH anti-mullerian hormone, FSH follicle-stimulating hormone, E2 estradiol, LND lymph node dissection, N/A not applicable, RT radiation therapy, U/S ultrasound
Secondary outcomes in this study included the proportion of women who developed POF at 2 years after completion of treatment by either amenorrhea or hormonal criteria (follicle-stimulating hormone [FSH] levels > 40 mIU/L or estradiol [E2] < 20 pg/ml) [27].
Quantitative data synthesis
A standardized data extraction sheet was developed and piloted for consistency. One review author (LCH) performed the initial data extraction, which was then confirmed by two review authors (LNV and TF). Consensus was utilized to resolve any disagreements.
Statistical analysis
For the meta-analysis, the number of individuals in each treatment group experiencing the study outcome was recorded. An intention-to-treat analysis, utilizing the denominator for all originally included patients, was utilized when possible during the data extraction. The pooled odds ratio was calculated, and outcomes of individual studies were compared using the random-effects model with the inverse-variance method and the DerSimonian-Laird estimator, which takes into account both study sample size, inter-study and intra-study variation, and gives a conservative estimate of effect. An offset value of 0.05 was used to allow the calculation of the odds ratio for studies in which non-POF was observed in the GnRHa group. Forest plots were constructed for visual display of odds ratios of individual studies. Heterogeneity between studies was evaluated with the Q statistic and quantified using the I2 statistic. Levels of the I2 statistic above 50% indicate high heterogeneity, while levels above 25% indicate moderate heterogeneity. Funnel plots were used to evaluate publication bias. All analyses were done utilizing the rmeta package in R (version 3.0.3, The R Foundation for Statistical Computing, Vienna, Austria).
Risk of bias assessment
Criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions were utilized to assess the risk of bias for each of the included studies [28]. Each RCT was assessed for seven areas that bias the estimates of treatment effect: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Risk of bias was classified by low, high, or unclear risk and assessed by two reviewers (LCH and LNV), with disagreements resolved by consensus.
Results
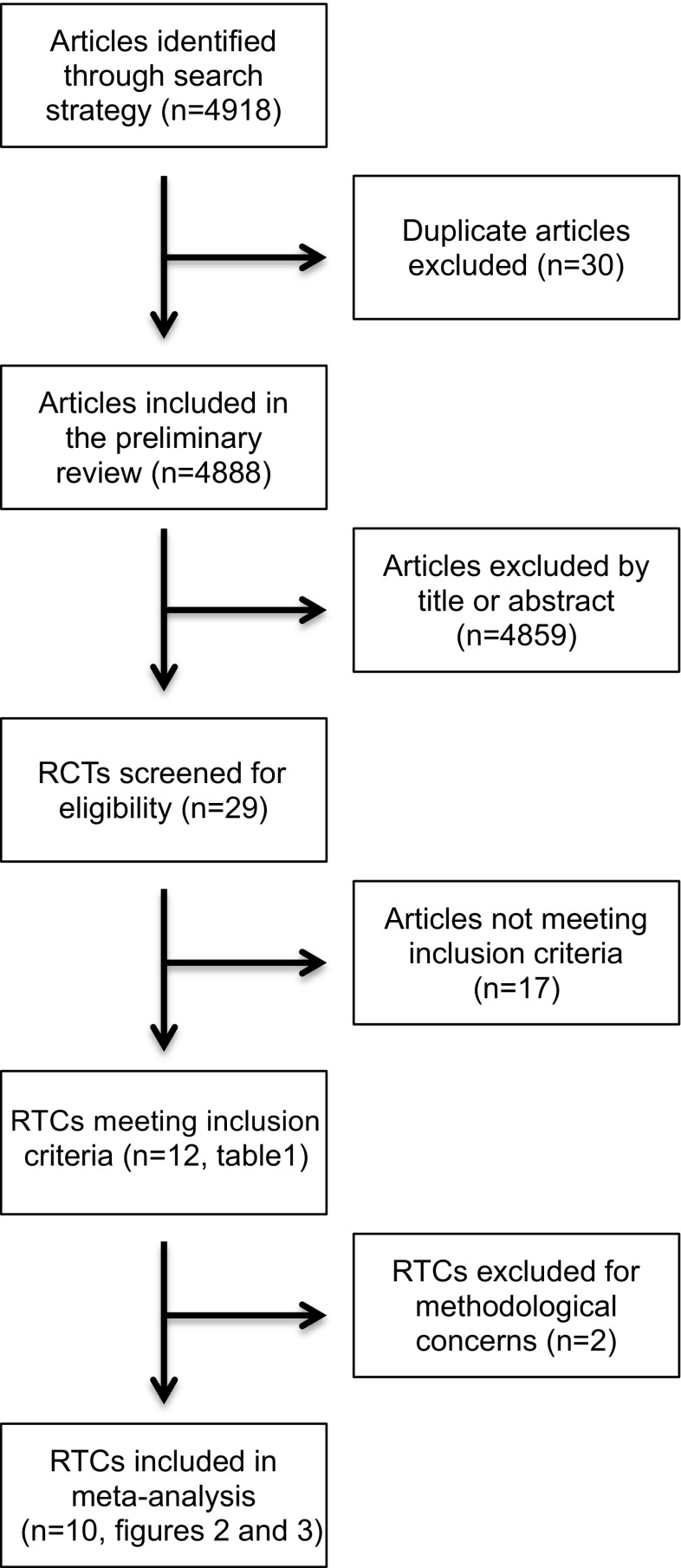
Twenty-nine RCTs examining the effect of GnRHa co-treatment during gonadotoxic chemotherapy on ovarian function were identified (Fig. 1). After further evaluation of these studies, only 12 trials met the study inclusion criteria [10, 16–26]. The methods for each trial are outlined in Table 1, including type of malignancy studied, patient characteristics, chemotherapy regimen and GnRHa utilized, and study outcomes. The risk of bias analysis for each trial using the seven previously described domains is also outlined in Table 1. After assessment of the risk of bias, two RCTS [16, 19] were designated as high-risk; therefore, they were excluded from the analysis.
Fig. 1.
Article selection strategy
Outcome measures
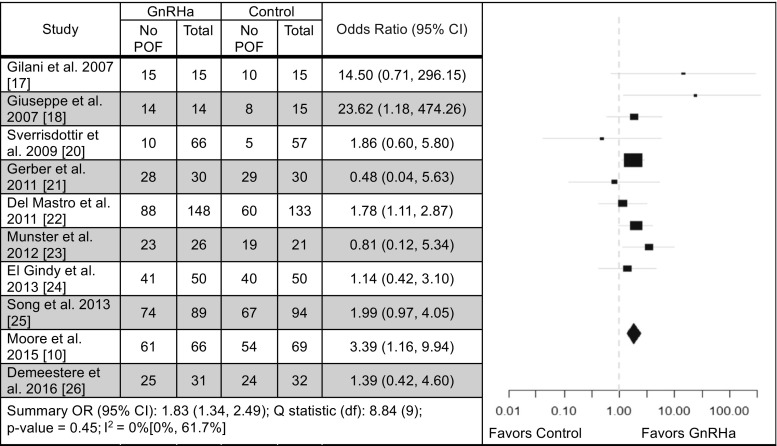
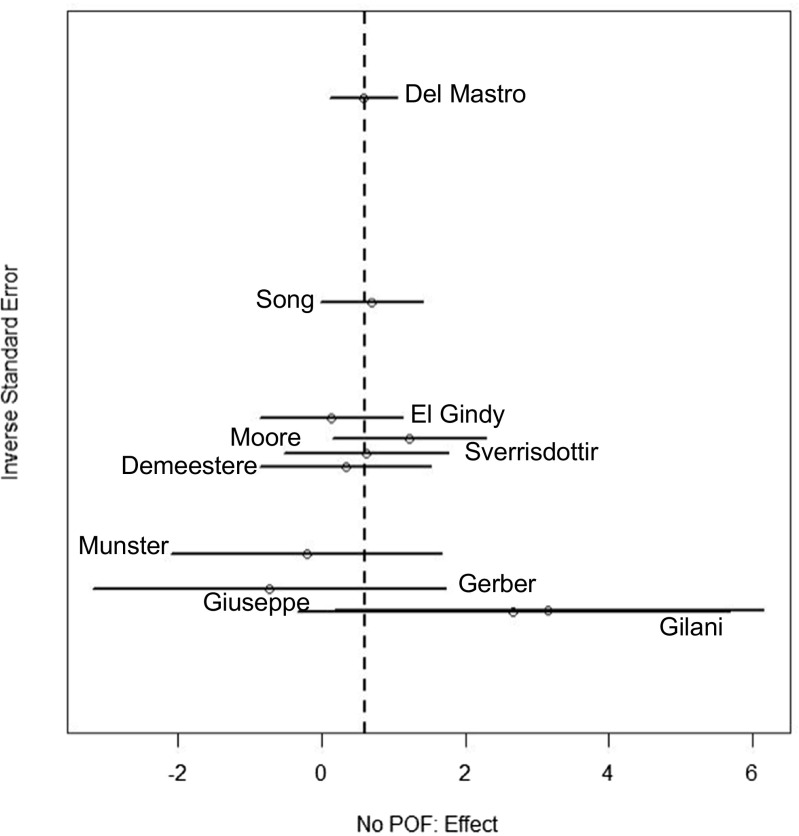
An analysis of patients who retained ovarian function after chemotherapy was performed based on the definition of POF outlined in each study’s methods. Eight studies favored a positive impact on preservation of ovarian function with GnRHa treatment compared to chemotherapy alone [10, 17, 18, 20, 22, 24–26], with three demonstrating statistical significance [10, 18, 22] and two studies showing no difference [21, 23] (OR 1.83; 95% CI 1.34–2.49, Q statistic p = 0.45) (Fig. 2). Overall this analysis favored the use of the GnRHa. A funnel plot was created based on the results of these 10 studies to evaluate publication bias (Fig. 3). The funnel plot is a scatter plot of the studies included in the meta-analysis containing the effect size on the x-axis and the inverse standard error on the y-axis. The overall funnel shape and symmetry of the plot are associated with a lack of publication bias [29].
Fig. 2.
Forest plot illustrating incidence of preservation of ovarian function. CI confidence interval, DF degrees of freedom, POF premature ovarian failure
Fig. 3.
Funnel plot illustrating publication bias for the analysis of not having premature ovarian failure
We then performed a similar analysis, including only studies of women with breast cancer to examine the impact of GnRHa in preventing POF, again using the individual study’s definition of POF [10, 20–25]. A total of seven studies were included [10, 20–25], and of these, five favored GnRHa compared to chemotherapy alone [10, 20, 22, 24, 25] (one reaching statistical significance [10]), with an OR for retaining ovarian function of 1.77 (95% CI 1.28, 2.44, Q statistic p = 0.68) in the GnRHa group. In conclusion, this approach to the analysis also favored the use of GnRHa.
The benefit of GnRHa for ovarian preservation did not persist at 2 years after chemotherapy treatment. All studies were evaluated using a strict definition of POF, based on either biochemical criteria (E2 < 20 pg/ml or FSH > 40mIU/L) or absence of menstruation at 2 years after completion of chemotherapy. This analysis included three studies [10, 21, 26] and revealed a non-significant pooled OR of 0.53 (95% CI 0.22–1.30, Q statistic p = 0.32) for the development of POF with GnRHa treatment.
For these three analyses, the estimated Q statistic for the heterogeneity ranged from 2.29 to 8.84. Heterogeneity (Q statistic p value < 0.05) was not found in any of the three approaches.
Discussion
Treatment with GnRHa during gonadotoxic chemotherapy may confer protection from the development of POF, as evidenced by the analysis of the 10 studies included in this systematic review and meta-analysis, the most comprehensive to date.
Twelve studies met inclusion criteria for the review, but two were designated as having a high risk of bias based on Cochrane Handbook criteria and were excluded [16, 19]. Waxman et al. [16] was excluded due to concerns regarding incomplete pituitary downregulation at the dose of GnRHa (busereilin) utilized in the study, which the authors themselves concede could have led to their results [15]. The dose of buserelin used in the study (200 mcg TID) is half the dose currently recommended by Sanofi-Aventis for the treatment of endometriosis (400 mcg TID). In addition, the Waxman study was underpowered, with only eight patients in the treatment group and 10 patients in the control group. Badawy et al. [19] was excluded due to methodological and data concerns, including the improbability of all patients being in the luteal phase of their cycles based on hormonal evaluations, and because a potential impact of tamoxifen on hormonal status was not accounted for in the study groups [30–32]. Other published reviews have also raised concerns regarding the validity of these studies [15, 30–32].
A pooled analysis of all included studies favored a positive impact on preservation of ovarian function with GnRHa treatment compared to chemotherapy alone with an OR of 1.83. We also evaluated the impact of GnRHa co-treatment on patients with breast cancer, as this is the most common malignancy of reproductive-aged women. Seven RCTs were analyzed, and again GnRHa treatment on the preservation of ovarian function was favored, with a pooled OR of 1.77. The benefit of GnRHa treatment did not appear to persist two years after completion of chemotherapy; however, these data must be interpreted with caution given the small number of studies (N = 3) with this data point. Variation in patient age is another factor that may affect outcomes at two years. The mean age of patients in the RCTs included in this analysis varied widely from 22 to 45 years, providing another reason to interpret these data with caution. A recent RCT of 227 breast cancer patients found a significant benefit to GnRHa 12–24 months after chemotherapy in patients < 40 years old [33]. The effect did not reach statistical significance in older patients [33]. Learning the duration of GnRHa effect is critical given that attempting pregnancy after cancer must be delayed until chemotherapy is out of the patient’s system (3–6 months), and the patient is at a low risk of recurrence. Expert opinion suggests that the timing of pregnancy should be individualized. Estrogen/progesterone receptor positive breast cancer patients are typically treated with tamoxifen for 5–10 years; however, the POSITIVE study is underway to determine the safety of an interruption in endocrine therapy to allow childbearing [11].
Varying results are reported in the literature for the impact of GnRHa on preservation of ovarian function, which has contributed to the controversy surrounding its clinical implementation. A number of study factors, including methodologic heterogeneity, sample size and power limitations, differences in the gonadotoxicity of chemotherapy regimens, and heterogeneous patient characteristics, likely contribute to the mixed findings. Among the 15 published meta-analyses [15, 30, 34–46], 13 showed a benefit of GnRHa treatment on POF, and two found no impact of GnRHa co-treatment on gonadal function [30, 34]. The El Gindy et al. meta-analysis is one of the two studies concluding that GnRHa administration during chemotherapy had no protective effect on gonadal function; however, their results appear to show a trend towards significance in favor of GnRHa with a RR of 1.12 (95% CI: 0.99–1.27, p = 0.07) for resumption of ovarian function. Additionally, the El Gindy analysis [30] was flawed in that it included the Waxman et al. study [16], which was excluded from the current analysis due to concerns regarding incomplete pituitary downregulation in the GnRHa group. Without explanation, El Gindy et al. also failed to include the Song et al. study [25], a high-quality RCT, and one of the largest RCTs published on this topic.
Regarding the outcome of post-treatment pregnancy, 9 of the 14 meta-analyses included pregnancy as an outcome measure, but of these, only three favored GnRHa for fertility preservation [35, 37, 40]. Data regarding pregnancy rates must be interpreted cautiously given the large number of variables that factor into the pursuit of fertility after cancer treatment, including age, parity, and cancer prognosis. Indeed, none of the RCTs evaluating the effects of GnRHa on ovarian function included a desire for future fertility as an eligibility criterion [47]. We did not perform an analysis of pregnancy rates in the current study because of the small number of studies reporting data related to pregnancy outcomes.
The lack of a standardized definition of chemotherapy-associated ovarian failure limits the interpretation of the literature about GnRHa. Although pregnancy may be the most specific indicator of gonadal function, infertility can be multifactorial, and inability to conceive does not necessarily indicate hypogonadism. The majority of available RCTs use either resumption of menses or FSH as surrogates of ovarian function; however, the time at which ovarian function was evaluated varies widely. Whereas Gerber et al. [21] assessed for resumption of menses at 6 months after treatment, other studies evaluated outcomes at 12 and 24 months. Drawing conclusions too early about ovarian failure may result in an underestimation of GnRHa effect [48]. Although AMH has been proposed as an alternative method of ovarian reserve assessment, its role in predicting chemotherapy-associated ovarian dysfunction is unclear. Patients who have undergone ovarian tissue cryopreservation, for instance, often have nearly undetectable AMH levels, but excellent pregnancy rates have been reported [49]. Similarly, a retrospective study showed that AMH levels did not accurately predict pregnancy in breast cancer patients who have undergone chemotherapy [50]. A recent prospective time-to-pregnancy cohort study including 750 women aged 30 to 44 found no association between AMH levels and rates of spontaneous conception [51]. Additionally, the exclusive use of biochemical markers such as AMH or FSH for evaluating ovarian function may overestimate the rate of gonadal failure, as indicated by the fact that five patients in the Demeestere et al. study (which relied only on FSH) with protocol-defined POF, became pregnant during follow-up [26, 47].
Differences in the gonadotoxicity of chemotherapy regimens may also contribute to the variability in the literature regarding GnRHa administration. A recent meta-analysis found that women exposed to taxanes demonstrated a lower rate of menses recovery than those treated with chemotherapy regimens that did not contain taxanes [42]. The same meta-analysis reported patient age as a predictor of menses recovery. Patients ≤ 40 years of age were significantly more likely to recover menses than older patients. The mean age of patients in the RCTs included in this analysis varied from 22 to 45 years [17, 20]. Despite the wide range of patient ages, the two studies with the oldest mean ages [20, 25] both showed statistically significant protective effects of GnRHa on ovarian function.
The timing of GnRHa administration relative to the initiation of chemotherapy is another factor that has not been standardized across studies. Timing may, in part, account for the difference in outcomes noted between the POEMS trial and the Demeestere study [10, 24]. The POEMs trial is a large RCT published in 2015 involving 218 hormone receptor-negative breast cancer patients that demonstrated a decreased incidence of ovarian failure and increased rates of pregnancy and live birth with co-administration of GnRHa and chemotherapy [10]. Conversely, the Demeestere trial involved 129 patients with lymphoma and reported no benefit of GnRHa for the preservation of ovarian function [26]. In the Demesteere trial, the GnRHa was started only two days before the initiation of chemotherapy, which may partially account for the observed differences between the two studies. In breast cancer, there is typically a longer interval prior to the initiation of chemotherapy as compared to treatment onset for lymphoma.
Aside from the timing of GnRHa administration, several methodological concerns have been raised regarding the Demeestere study [26], including the fact that both study arms received the hormone norethisterone acetate, which may have undermined the protective effect of GnRHa. Additionally, 46% of patients did not have documentation of hormonal contraceptive use (which could decrease FSH levels) at the time of ovarian function evaluation [47]. Finally, only about half of patients had sufficient follow-up to be included in the final analysis, and the study was underpowered.
The Demeestere trial is not the only one to suffer from small sample size and insufficient power. Munster et al. initially planned to enroll 124 patients, but the trial was “stopped for futility” after 49 patients were enrolled [23]. The rates of resumption of menstruation were remarkably high (90% in the control group and 88% in the GnRHa group). For a chemotherapy regimen with such low gonadotoxicity, much larger sample sizes would have been required to detect a difference in outcome between the two groups [48]. There is a wide range of reported rates of ovarian failure after chemotherapy, with rates ranging from 10 to 55% across the literature [22]. The variation in rates of ovarian failure is yet another factor that makes interpreting the magnitude of GnRHa effect challenging.
The main purpose of this review was to evaluate the role of GnRHa in the preservation of ovarian function in patients receiving gonadotoxic chemotherapy. The strengths of our approach include the performance of a comprehensive review of the literature, a thorough risk of bias analysis, and utilization of multiple analyses to further evaluate potential relationships among RCTs. Weaknesses of this study include methodological heterogeneity among study definitions and outcome measures assessed, small patient populations, diverse cancer diagnoses and treatment regimens, and lack of long-term follow-up, which may limit one’s ability to draw conclusions about magnitude and duration of efficacy. Other limitations may be the use of the menstrual cycle and FSH as the primary approach to assessment of gonadal function. Additionally, this study is unable to evaluate the effect of GnRHa on pregnancy rates following gonadotoxic chemotherapy.
GnRHa should be offered to all women of reproductive age, without contraindications, undergoing gonadotoxic chemotherapy. Although data regarding the impact of GnRHa on fertility preservation are mixed, the administration of GnRHa is a relatively benign intervention, and the results of this meta-analysis demonstrate that, at a minimum, GnRHa may protect against chemotherapy-induced hypogonadism. GnRHa and ovarian tissue cryopreservation are the only technologies that aim to preserve gonadal function in chemotherapy patients, and GnRHa may be used in conjunction with ovarian tissue cryopreservation, oocyte cryopreservation, or embryo cryopreservation. Given the importance of fertility and gonadal function to patient quality of life, the long-term effects of chemotherapy treatment on ovarian function must not be ignored. This systematic review, in addition to many prior studies [15, 35–46], supports the use of GnRHa during gonadotoxic chemotherapy for the preservation of ovarian function.
Conclusion
GnRHa appear to have a protective effect against the development of POF after treatment with gonadotoxic chemotherapy. Beyond fertility preservation, patients may benefit from improved bone health, sexual function, cardiovascular health, and overall quality of life.
Acknowledgements
The authors would like to acknowledge Ms. Lorelei Woody and Ms. Gretchen Hallerberg, the librarians who assisted with the literature search, as well as Mr. James Bena, a statistician who assisted with a portion of the data analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Levine JM, Kelvin JF, Quinn GP, Gracia CR. Infertility in reproductive-age female cancer survivors. Cancer. 2015;121:1532–1539. doi: 10.1002/cncr.29181. [DOI] [PubMed] [Google Scholar]
- 2.Trivers KF, Fink AK, Partridge AH, Oktay K, Ginsburg ES, Li C, Pollack LA. Estimates of young breast cancer survivors at risk for infertility in the U.S. Oncologist. 2014;19:814–822. doi: 10.1634/theoncologist.2014-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindau ST, Abramsohn EM, Matthews AC. A manifesto on the preservation of sexual function in women and girls with cancer. Am J Obstet Gynecol. 2015;213:166–174. doi: 10.1016/j.ajog.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maclaran K, Panay N. Current concepts in premature ovarian insufficiency. Women's Health (Lond Engl) 2015;11:169–182. doi: 10.2217/WHE.14.82. [DOI] [PubMed] [Google Scholar]
- 5.Hickman LC, Valentine LN, Falcone T. Preservation of gonadal function in women undergoin chemotherapy: a review of the potential role for gonadotropin-releasing hormone agonists. Am J Obstet Gynecol. 2016;215(4):415–422. doi: 10.1016/j.ajog.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 6.Roness H, Gavish Z, Cohen Y, Meirow D. Ovarian follicle burnout: a universal phenome- non? Cell Cycle. 2013;12:3245–3246. doi: 10.4161/cc.26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemothera-peutic agents damage the ovary? Hum Reprod Update. 2012;18:525–535. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 8.Hasky N, Uri-Belapolsky S, Goldberg K, et al. Gonadotropin-releasing hormone agonists for fertility preservation: unraveling the enigma? Hum Reprod. 2015;30:1089–1101. doi: 10.1093/humrep/dev037. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Yoon TI, Chae HD, Kim JE, Chae EY, Yu JH, Sohn G, Ko BS, Lee JW, Son BH, Ahn SH. Concurrent gonadotropin-releasing hormone agonist administration with chemotherapy improves neoadjuvant chemotherapy responses in young premenopausal breast cancer patients. J Breast Cancer. 2015;18:365–370. doi: 10.4048/jbc.2015.18.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore HC, Unger JM, Phillips KA, Boyle F, Hitre E, Porter D, Francis PA, Goldstein LJ, Gomez HL, Vallejos CS, Partridge AH, Dakhil SR, Garcia AA, Gralow J, Lombard JM, Forbes JF, Martino S, Barlow WE, Fabian CJ, Minasian L, Meyskens FL, Gelber RD, Hortobagyi GN, Albain KS, POEMS/S0230 Investigators Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med 2015;372:923–932; doi: 10.1056/NEJMoa1413204. [DOI] [PMC free article] [PubMed]
- 11.Lambertini M, Del Mastro L, Pescio MC, Andersen CY, Azim HA, Peccatori FA, Costa M, Revelli A, Salvagno F, Gennari A, Ubaldi FM, La Sala GB, De Stefano C, Wallace WH, Partridge AH, Anserini P. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. 2016;14:1–015. doi: 10.1186/s12916-015-0545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Practice Committee of American Society for Reproductive Medicine Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2013;100:1214–1223. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.0.2. The Cochrane Collaboration. In: 2009. http://www.mrc-bsu.cam.ac.uk/cochrane/handbook. Accessed August 2015.
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097; doi: 10.1371/journal.pmed.1000097. [PMC free article] [PubMed]
- 15.Bedaiwy MA, Abou-Setta AM, Desai N, Hurd W, Starks D, El-Nashar SA, Al-Inany HG, Falcone T. Gonadotropin-releasing hormone analog cotreatment for preservation of ovarian function during gonadotoxic chemotherapy: a systematic review and meta-analysis. Fertil Steril. 2011;95:906–14.e1. doi: 10.1016/j.fertnstert.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Waxman JH, Ahmed R, Smith D, Wrigley PF, Gregory W, Shalet S, Crowther D, Rees LH, Besser GM, Malpas JS Failure to preserve fertility in patients with Hodgkin’s disease. Cancer Chemother Pharmacol 1987;19:159–162. [DOI] [PubMed]
- 17.Gilani MM, Hasanzadeh M, Ghaemmaghami F, Ramazanzadeh F. Ovarian preservation with gonadotropin-releasing hormone analog during chemotherapy. Asia-Pacific Journal of Clinical Oncology. 2007;3:79–83. 10.1111/j.1743-7563.2007.00089.x
- 18.Giuseppe L, Attilio G, Edoardo DN, Loredana G, Cristina L, Vincenzo L. Ovarian function after cancer treatment in young women affected by Hodgkin disease (HD) Hematology. 2007;12:141–147. doi: 10.1080/10245330600954072. [DOI] [PubMed] [Google Scholar]
- 19.Badawy A, Elnashar A, El-Ashry M, Shahat M. Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: prospective randomized study. Fertil Steril. 2009;91:694–697. doi: 10.1016/j.fertnstert.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 20.Sverrisdottir A, Nystedt M, Johansson H, Fornander T. Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: results from a randomized trial. Breast Cancer Res Treat. 2009;117:561–567. doi: 10.1007/s10549-009-0313-5. [DOI] [PubMed] [Google Scholar]
- 21.Gerber B, von Minckwitz G, Stehle H, Reimer T, Felberbaum R, Maass N, Fischer D, Sommer HL, Conrad B, Ortmann O, Fehm T, Rezai M, Mehta K, Loibl S, German Breast Group Investigators Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: the GBG 37 ZORO study. J Clin Oncol. 2011;29:2334–2341. doi: 10.1200/JCO.2010.32.5704. [DOI] [PubMed] [Google Scholar]
- 22.Del Mastro L, Boni L, Michelotti A, Gamucci T, Olmeo N, Gori S, Giordano M, Garrone O, Pronzato P, Bighin C, Levaggi A, Giraudi S, Cresti N, Magnolfi E, Scotto T, Vecchio C, Venturini M. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: a randomized trial. JAMA. 2011;306:269–276. doi: 10.1001/jama.2011.991. [DOI] [PubMed] [Google Scholar]
- 23.Munster PN, Moore AP, Ismail-Khan R, Cox CE, Lacevic M, Gross-King M, Xu P, Carter WB, Minton SE. Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol. 2012;30:533–538. doi: 10.1200/JCO.2011.34.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Gindy EA, El-Haieg DO, Khorshid OM, Ismail EI, Abdelgawad M, Sallam HN, Abou-Setta AM. Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: a randomized controlled trial. Obstet Gynecol. 2013;121:78–86. doi: 10.1097/AOG.0b013e31827374e2. [DOI] [PubMed] [Google Scholar]
- 25.Song G, Gao H, Yuan Z. Effect of leuprolide acetate on ovarian function after cyclophosphamide-doxorubicin-based chemotherapy in premenopausal patients with breast cancer: results from a phase II randomized trial. Med Oncol. 2013;30:667–013. doi: 10.1007/s12032-013-0667-8. [DOI] [PubMed] [Google Scholar]
- 26.Demeestere I, Brice P, Peccatori FA, Kentos A, Dupuis J, Zachee P, et al. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J Clin Oncol. 2016. [DOI] [PubMed]
- 27.Randolph JF, Crawford S, Dennerstein L, Cain K, Harlow SD, Little R, Mitchell ES, Nan B, Taffe J, Yosef M. The value of follicle-stimulating hormone concentration and clinical findings as markers of the late menopausal transition. J Clin Endocrinol Metab. 2006;91:3034–3040. doi: 10.1210/jc.2006-0243. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.0.2. The Cochrane Collaboration 2009:2015.
- 29.Light RJ, Pillemer DB. Summing up. The science of reviewing research. Cambridge: Harvard University Press; 1984. [Google Scholar]
- 30.Elgindy E, Sibai H, Abdelghani A, Mostafa M. Protecting ovaries during chemotherapy through gonad suppression: a systematic review and meta-analysis. Obstet Gynecol. 2015;126:187–195. doi: 10.1097/AOG.0000000000000905. [DOI] [PubMed] [Google Scholar]
- 31.Oktay K, Sonmezer M. Questioning GnRH analogs for gonadal protection in cancer patients. Fertil Steril. 2009;92:e32. doi: 10.1016/j.fertnstert.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Peccatori F, Demeestere I. GnRH analogue for chemotherapy-induced ovarian damage: too early to say? Fertil Steril. 2009;92:e33. doi: 10.1016/j.fertnstert.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Leonard RCF, Adamson DJA, Bertelli G, Mansi J, Yellowlees A, Dunlop J, Thomas GA, Coleman RE, Anderson RA. GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: the Anglo Celtic Group OPTION trial. Ann Oncol. 2017;28:1811–1816. [DOI] [PubMed]
- 34.Vitek WS, Shayne M, Hoeger K, Han Y, Messing S, Fung C. Gonadotropin-releasing hormone agonists for the preservation of ovarian function among women with breast cancer who did not use tamoxifen after chemotherapy: a systematic review and meta-analysis. Fertil Steril. 2014;102:808–815.e1. doi: 10.1016/j.fertnstert.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Pharmacological interventions for fertility preservation during chemotherapy: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010;122:803–811. doi: 10.1007/s10549-010-0996-7. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Li J, Cui T, Hu L. Adjuvant gonadotropin-releasing hormone analogues for the prevention of chemotherapy induced premature ovarian failure in premenopausal women. Cochrane Database Syst Rev. 2011a:(11):CD008018. [DOI] [PubMed]
- 37.Clowse ME, Behera MA, Anders CK, Copland S, Coffman CJ, Leppert PC, Bastian LA. Ovarian preservation by GnRH agonists during chemotherapy: a meta-analysis. J Women's Health (Larchmt) 2009;18:311–319. doi: 10.1089/jwh.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Mastro L, Ceppi M, Poggio F, Bighin C, Peccatori F, Demeestere I, Levaggi A, Giraudi S, Lambertini M, D'Alonzo A, et al. Gonadotropin-releasing hormone analogues for the prevention of chemotherapy-induced premature ovarian failure in cancer women: systematic review and meta-analysis of randomized trials. Cancer Treat Rev. 2014;40:675–683. doi: 10.1016/j.ctrv.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Kim SS, Lee JR, Jee BC, Suh CS, Kim SH, Ting A, Petroff B. Use of hormonal protection for chemotherapy-induced gonadotoxicity. Clin Obstet Gynecol. 2010;53:740–752. doi: 10.1097/GRF.0b013e3181f96cb1. [DOI] [PubMed] [Google Scholar]
- 40.Lambertini M, Ceppi M, Poggio F, Peccatori FA, Azim HA, Jr, Ugolini D, Pronzato P, Loibl S, Moore HC, Partridge AH, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol. 2015;26:2408–2419. doi: 10.1093/annonc/mdv335.01. [DOI] [PubMed] [Google Scholar]
- 41.Munhoz RR, Pereira AA, Sasse AD, Hoff PM, Traina TA, Hudis CA, Marques RJ. Gonadotropin-releasing hormone agonists for ovarian function preservation in premenopausal women undergoing chemotherapy for early-stage breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:65–73. doi: 10.1001/jamaoncol.2015.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva C, Caramelo O, Almeida-Santos T, Ribeiro Rama AC. Factors associated with ovarian function recovery after chemotherapy for breast cancer: a systematic review and meta-analysis. Hum Reprod. 2016;31:2737–2749. doi: 10.1093/humrep/dew224. [DOI] [PubMed] [Google Scholar]
- 43.Sun X, Dongol S, Jiang J, Kong B. Protection of ovarian function by GnRH agonists during chemotherapy: a meta-analysis. Int J Oncol. 2014;44:1335–1340. doi: 10.3892/ijo.2014.2296. [DOI] [PubMed] [Google Scholar]
- 44.Yang B, Shi W, Yang J, Liu H, Zhao H, Li X, Jiao S. Concurrent treatment with gonadotropin-releasing hormone agonists for chemotherapy-induced ovarian damage in premenopausal women with breast cancer: a meta-analysis of randomized controlled trials. Breast. 2013;22:150–157. doi: 10.1016/j.breast.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Xiao Z, Wang Y, Luo S, Li X, Li S. Gonadotropin-releasing hormone for preservation of ovarian function during chemotherapy in lymphoma patients of reproductive age: a summary based on 434 patients. PLoS One. 2013;8:e80444. doi: 10.1371/journal.pone.0080444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Chen M, Fu F, Huang M. Gonadotropin-releasing hormone analog cotreatment for the preservation of ovarian function during gonadotoxic chemotherapy for breast cancer: a meta-analysis. PLoS One. 2013;8:e66360. doi: 10.1371/journal.pone.0066360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambertini M, Falcone T, Unger JM, Phillips KA, Del Mastro L, Moore HC. Debated role of ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in women with cancer. J Clin Oncol. 2017;35:804–805. doi: 10.1200/JCO.2016.69.2582. [DOI] [PubMed] [Google Scholar]
- 48.Blumenfeld Z, Katz G, Evron A. ‘An ounce of prevention is worth a pound of cure’: the case for and against GnRH-agonist for fertility preservation. Ann Oncol. 2014;25:1719–1728. doi: 10.1093/annonc/mdu036. [DOI] [PubMed] [Google Scholar]
- 49.Janse F, Donnez J, Anckaert E, de Jong FH, Fauser BC, Dolmans MM. Limited value of ovarian function markers following orthotopic transplantation of ovarian tissue after gonadotoxic treatment. J Clin Endocrinol Metab. 2011;96:1136–1144. doi: 10.1210/jc.2010-2188. [DOI] [PubMed] [Google Scholar]
- 50.Hamy AS, Porcher R, Eskenazi S, Cuvier C, Giacchetti S, Coussy F, Hocini H, Tournant B, Perret F, Bonfils S, et al. Anti-Mullerian hormone in breast cancer patients treated with chemotherapy: a retrospective evaluation of subsequent pregnancies. Reprod BioMed Online. 2016;32:299–307. doi: 10.1016/j.rbmo.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Steiner A, Pritrchard D, Stancyzk F. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367–1376. doi: 10.1001/jama.2017.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]