Abstract
Studying the reproductive biology of wild animal species produces knowledge beneficial to their management and conservation. However, wild species also share intriguing similarities in reproductive biology with humans, thereby offering alternative models for better understanding the etiology of infertility and developing innovative treatments. The purpose of this review is to raise awareness in different scientific communities about intriguing connections between wild animals and humans regarding infertility syndromes or improvement of fertility preservation. The objectives are to (1) highlight commonalities between wild species and human fertility, (2) demonstrate that research in wild species-assisted reproductive technologies can greatly enhance success in human reproductive medicine, and (3) recognize that human fertility preservation is highly inspiring and relevant to wild species conservation. In addition to having similar biological traits in some wild species and humans, the fact of sharing the same natural environment and the common needs for more options in fertility preservation are strong incentives to build more bridges that will eventually benefit both animal conservation and human reproductive medicine.
Keywords: Assisted reproductive technologies, Fertility, Human, Wildlife, Conservation
Introduction
Value of assisted reproduction in animal conservation and urgent needs for more options
Sustaining viable populations (genetic diversity) of any wildlife species requires a combination of adequate habitat protection as well as a good understanding of environmental and biological factors that ensure species survival. Thousands of species are under threat of extinction due to habitat loss/degradation, over-exploitation, pollution, disease, alien species invasions, and urban sprawl. This has served as incentive for intensive management of animal populations, both ex situ (in captivity) and in situ (living in their natural habitat) [1]. Understanding reproductive physiology is one of the critical efforts to ensure species survival by enhancing natural mating in breeding centers or in the wild. It also is a prerequisite to the development of assisted reproductive technologies (ART) that have a huge potential to overcome mating difficulties, address infertility issues, and maintain genetic diversity in rare and endangered animal populations [2]. Indeed, one of the highest priorities in wildlife ex situ management is sustaining all existing genetic diversity to (1) preserve heterozygosity to avoid inbreeding depression and (2) ensure species integrity and the persistence of genomic adaptability to environmental changes [2]. However, considerable physiological variations across species and lack of fundamental knowledge in reproduction for most species have limited how ART can be used to help rapidly re-build sustainable populations of endangered wildlife [3]. For instance, among the 5400 mammalian species, reproductive physiology is precisely known in less than 200 of them [2]. There is a range of reproductive strategies, from the highly seasonal, monoestrus giant panda to the aseasonal, polyestrus elephant. Some species exhibit spontaneous ovulations, while others are induced ovulators or both, with variations in ovarian cycle lengths that range from a few days to several months (Table 1). Anatomy of reproductive tracts and gonads as well as structure and biology of gametes also vary among species [2, 7]. This biological diversity therefore has to be taken into account when developing in vitro culture conditions or cryopreservation protocols for gametes and gonadal tissues [7, 8].
Table 1.
| Common and scientific names | Sexual maturity (months) | Seasonal breeder | Estrous cycle (days) | Estrus (days) | Ovulationa | Gestation (days) |
|---|---|---|---|---|---|---|
| Black-footed cat (Felis nigripes) | 12–21 | No | 5–29 | 2–9 | Ind. | 63–71 |
| Bobcat (Lynx rufus) | 10–12 | Yes | 44 | 5–10 | Ind. | 50–70 |
| Cheetah (Acinonyx jubatus) | 24–36 | No | 7–23 | 2–6 | Ind. | 90–98 |
| Clouded leopard (Neofelis nebulosa) | 20–30 | Yes | 25–30 | 3–6 | Spont. | 85–93 |
| Domestic cat (Felis catus) | 5–12 | Varies | 14–21 | 3–7 | Both | 64–67 |
| Asian elephant (Elephas maximus) | 120–140 | No | 100–110 | 2–4 | Spont. | 640–660 |
| Eld’s deer (Rucervus eldii) | 18–22 | Yes | 20–24 | 1–2 | Spont. | 230–240 |
| Giant panda (Ailuropoda melanoleuca) | 60–70 | Yes | 7–10 | 1 | Spont. | 80–180b |
aInd. induced, Spont. spontaneous
bBroad range due to delayed implantation of the embryo
Applying ARTs to support the conservation of rare and endangered species was originally explored in the 1980s based on successes of these techniques in livestock species and humans [9, 10]. More than 35 years later, healthy offspring have been produced by artificial insemination (AI) or embryo transfer in no more than 50 wild species. It has often been difficult to progress from “first-ever” births to repeated successes (for instance in cheetahs or clouded leopards). Except for a handful of mammalian species, primarily the giant panda and the black-footed ferret, ARTs are not broadly implemented and integrated into the management of rare and endangered populations. In addition to the limiting factors mentioned above, too few investigators and specialized spaces are available in the conservation breeding community and resources allocated to basic reproductive biology remain scarce [1].
Due to the few numbers of individuals available in endangered populations, it is prudent and safer to first test approaches in closely related species that are more common. The realization that little information was available on the basic reproductive physiology of any of the existing 37 species of felids resulted in developing the domestic cat as a model system. Basic studies then were followed by many fascinating discoveries on species-specific reproductive mechanisms, for example, a high rate of spontaneous ovulation in the clouded leopard (most felids are induced ovulators); resistance to exogenous gonadotropins in the ocelot; peculiar, protracted luteal function in the Iberian lynx; and ability of female cheetahs to mutually suppress their reproductive cycles, among other phenomena [4]. Besides the domestic cat as a target for felids, other valuable models include the domestic dog for wild canids, red- or white-tailed deer for wild cervids, brushtail possum for rare marsupials, or common frog for near-extinct amphibians [11]. However, many species have uniquely evolved and became so specialized that there are no closely related experimental models, for example, the two species of elephants, the five species of rhinoceroses, and the giant panda (among hundreds of others). Such cases likely will require more bold and straightforward actions directly to the target species, which is supportable if adequate fundamental reproductive knowledge is available [2].
Despite slow progress and limitations mentioned above, there still is enormous potential in applying ART to wild animals, especially novel approaches that could assist in managing or “rescuing” gametes and gonadal tissues from genetically valuable individuals. There currently are too many examples of species that have very small populations composed of acyclic females, sterile males, and aging individuals (cheetah, clouded leopards, black footed ferrets, and elephants, to name a few) and therefore are facing huge issues of sustainability. They may go extinct before “conventional” ARTs are fully developed so alternate approaches are required to preserve male and female germplasms from any living adults, protect the fertility of prepubertal individuals, extend the fertility of aging individuals, and rescue valuable germplasm postmortem (see examples below).
Status of ART in humans and the need for more animal models
Human infertility is not a trivial issue as it affects > 6.5 million people in the USA and one in every four couples worldwide [12]. In the USA, in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) with fresh or thawed spermatozoa resulted in the birth of more than 68,000 infants in 2014 alone [13]. Human infertility affects 10 to 15% of couples through ovulation/ovarian function disruptions (21% of cases), oviductal anomalies (14%), male factors (24%), or anatomical or immunological problems (13%), or for inexplicable reasons (28%) [14]. Prime causes of couple infertility originate from women (ovarian failure, tubal damage, endometriosis in 41% of the cases), men (21%), coital problems (5%), other (5%), and unexplained (28%). Treating human infertility costs more than one billion dollars annually in the USA, with an ever-increasing demand for ART that, in turn, requires major research efforts [13]. Interestingly, the vast majority of research funding goes to studies on laboratory rodents which represents 80% of the scientific production in reproduction [2].
The first human baby born after conception by IVF and autologous embryo transfer was Louise Brown, a conception credited to Drs. Patrick Steptoe and Robert Edwards in 1978. This first success was the result of many years of research where IVF was achieved with in vitro capacitated spermatozoa in several animal models—from the rabbit in 1959 [15] to the first non-human primate in 1973 [16]. Nowadays, human IVF and appending technologies like oocyte retrieval are leading the field and are inspiring to many other species in need of similar approaches. However, the fact that almost a third of human infertility issues have unknown etiology calls for more studies in animal models because, the more diversity we explore, the better we can address complex fertility conditions [2, 17]. Lessons that we learned over the last decades in wildlife reproductive biology (either from wild or captive populations) could be highly relevant to the advancement of human health and fertility. To date, there has been little advocacy for wild species, as efficacious models for addressing human infertility issues. However, morphological and mechanistic commonalities would argue that wild species have value for this purpose. One particular advantage is the ability to examine etiologies or treatments in animals that are largely heterozygotic, similar to what is observed in humans (and certainly absent in invariant, homozygous mouse and rat strains). Thus, the study of many populations and species would permit evaluating not only the influence of heterozygosity, but also the impact of genetic uniqueness of individuals, including the need to develop customized treatments. Such issues already have been raised for canids in terms of ovarian response to exogenous stimulations and gamete quality/cryo-resistance [18, 19]. Carnivores have commonalities with humans in sperm concentration, incidence of abnormal spermatozoa, cryptorchidism, time course of follicle development, oocyte size at each growth stage, acquisition of oocyte meiotic and developmental competence, luteal dysfunctions, endometrial hyperplasia, gestational failure, and age-related syndromes [2, 18]. Additionally, studies conducted at the molecular or cellular level always are related to physiological investigations in wild individuals or entire populations and take into account the interactions with the environment. This concept of reproductive fitness is impossible to measure in laboratory species.
The purpose of this review therefore is to raise awareness in different scientific communities about intriguing connections between wild species and humans that are relevant to understanding and treating infertility syndromes or improving fertility preservation. Specific objectives are to (1) highlight commonalities between wild species and human fertility, (2) demonstrate that research in wild species ART can greatly enhance success in human reproductive medicine, and (3) recognize that human fertility preservation is highly inspiring and relevant to wild species conservation.
Commonalities between wild species and human fertility issues
Efforts directed towards understanding the natural and significant variation in basic reproductive mechanism among species have generated a large set of data regarding infertility issues in wild species [2, 3]. Many challenges are common between human and wild species (Table 2). Interestingly, novel drug therapies are showing promise, such as cabergoline to treat hyperprolactinemia (elephants), domperidone to stimulate follicular activity (elephants), GnRH vaccination to suppress ovarian function and treat urogenital pathologies (elephants), and progesterone supplementation to sustain pregnancy (rhinoceroses, tigers) [5].
Table 2.
| Hot topics in human reproductive medicine | Species particularly affected by the same issues |
|---|---|
| Stress and reproduction | Felids, canids, elephants, rhinos, ungulates, birds |
| Negative social effects on ovarian activity | Cheetahs, elephants |
| Sexual incompatibility | Elephants, felids, bears |
| Increased uterine cysts and tumors related to age and parity | Cheetahs, elephants |
| Hyperprolactinemia induced infertility | Elephants |
| Unexplained periods of ovarian inactivity | Lions, cheetahs, elephants |
| Poor conception rates and pregnancy maintenance | Giant pandas |
| High incidence of stillbirths and dystocia | Elephants |
| Obesity and infertility | Felids, canids, elephants, rhinos, ungulates, birds |
| Precocious puberty | Elephants, rhinos |
| Reproductive aging | Felids, canids, elephants, rhinos |
| Oligozoospermia, teratospermia | Felids, canids |
| Ovarian hyper-stimulation | Felids |
| Inconsistent ART results | Felids, canids, elephants, rhinos, ungulates, birds |
Infertility associated with uterine pathologies (including endometrial hyperplasia) often is reported in humans [21]. Female cheetahs offer an interesting model for this pathology, with a high incidence of hyperplasia (87%) in animals of 9 years or older [22]. This condition and its prevalence contribute to failed establishment of pregnancy in ex situ cheetah populations. Interestingly, oocytes recovered from older, gonadotropin-stimulated cheetahs have the capacity to mature, fertilize, and form early-stage embryos in vitro [22]. Such findings suggest that uterine environment (more than oocyte quality) influence reproductive success in older individuals, a phenomenon that may be occurring in some women as well.
Felids also have been especially valuable in generating insights into the significance of teratospermia (ejaculation of high proportions of malformed spermatozoa), a situation also common in men [23]. More than 78% of infertile men produce ejaculates containing sperm with a preponderance of poor morphology that, in turn, may be related to low fertilizing capacity in vitro [24]. Of the 37 felid species, 28 produce unusually high proportions (> 60%) of pleiomorphic spermatozoa that are compromised at multiple levels of functionality [23]. Further studies in wild species will enrich the understanding about what regulates sperm structure and effectiveness. This also includes differentially exploring candidate causes such as age. For example, studies in another carnivore, a mustelid called the black-footed ferret, has revealed that even modest aging increases pleiomorphic sperm number while decreasing libido [25].
Reproductive aging is observed in animal species as well as humans [26]. Thus, studies to prolong reproductive life span can benefit both human and wildlife. In clinical ART, aging is the most common factor associated with infertility. In many regions of the world, women and men are delaying childbearing until later in life. Unfortunately, oocyte and sperm quality declines after the age of 35 years, more rapidly in women than in men [27, 28]. Reproductive aging ultimately leads to infertility, including embryonic aneuploidy, DNA fragmentation, a decline in the number of germ cells, and ultimately menopause. Molecular causes of reproductive aging are largely unknown and likely diverse among species. Zoo animal populations represent an ideal model to study reproductive aging process since animal life span is longer than in the wild. For instance, recent studies on cheetahs demonstrate that the age-associated decline in anti-Mullerian hormone (AMH) is variable but needs to be taken into consideration if AMH is to be used successfully to optimize breeding management decisions in exotic species [29] as it already is demonstrated in livestock species [30].
ART failure often is due to highly varied/inconsistent ovarian responses to ovulation induction with gonadotropins, inappropriate insemination timing, and poor quality of semen (fresh or frozen-thawed). For many species, however, reproductive failure is of unknown cause. Treating infertility generally requires a species-based approach, and a good knowledge of ovulatory mechanisms (induced vs. spontaneous), seasonality, and gestational aspects (e.g., delayed implantation, pseudopregnancy). This approach is critical to answer key questions and potentially address similar issues in humans.
Lastly, wild species and humans often share the same natural habitat. Monitoring the influence of environmental changes on reproductive health therefore is critical in all living species. Specifically, the release of chemicals into the environment is seriously affecting both human and wildlife reproduction and the long-term health of offspring. Of particular interest about reproduction are the class of chemicals known as endocrine disruptors. These include estrogens, androgens, steroid hormone inhibitors, or anti-thyroid substances interfering with normal hormone function in animals and humans [31]. Chemicals are everywhere in our daily lives, such as bisphenol A which leaches from plastic food containers and parabens which are found in many cosmetics, lotions, and hair care products. Both substances are recognized to have estrogen-like activities that can disrupt reproductive hormone cycles and maybe contributing to early puberty in girls and gynecomastia in boys [32, 33]. Wild species also are exposed to these chemicals when they leach from our garbage and wastewater into the environment. Endocrine disruptors have even been detected in waterways, fishes, and wildlife in rural Africa [34]. However, not all environmental estrogen exposures are caused by contamination. Many plants are naturally high in estrogenic compounds. Feeding cattle and sheep on forage that is high in phytoestrogens induces female infertility [35]. Similarly, a recent study on the effects of diet on captive white rhinoceros discovered that lowering the phytoestrogen content of their feed significantly improved breeding success for this critically endangered species [36]. These few examples highlight the fundamental need to monitor reproductive fitness and health in both wild species and humans.
Research advances in wild species and human ART that are mutually inspiring
Monitoring of physiological status
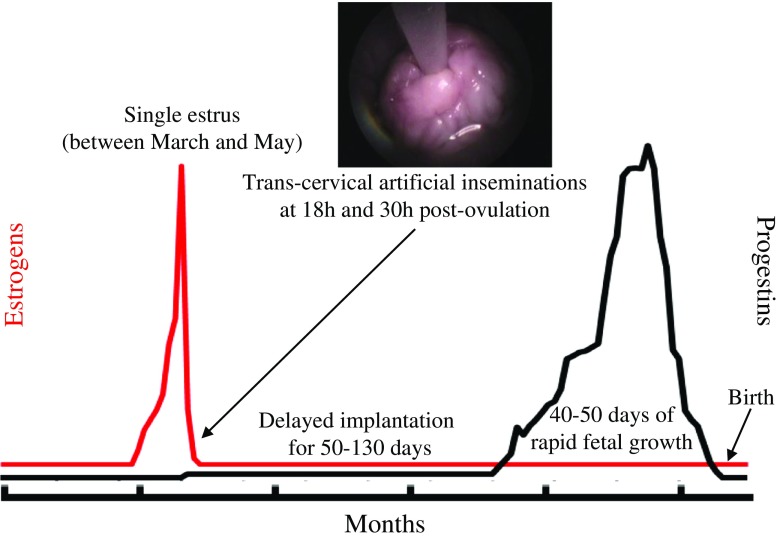
For most wildlife species, clinical examinations or blood sample collections have to be performed under anesthesia, which is highly constraining, risky, and cannot be repeated frequently. Endocrine functions now are monitored non-invasively through analyses of hormone metabolites excreted in urine or feces. Of course, major differences in metabolism and routes of excretion exist, not only between species, but also among hormone types within a species [5]. This means that “species by species” and sometimes “hormone by hormone” approaches are essential for developing effective reproductive monitoring and control strategies. Non-invasive hormone monitoring is fundamental to make decisions about treatment with exogenous hormones, insemination timing, and pregnancy monitoring [5]. For instance, the use of steroid metabolites in the urine to monitor the reproductive status in giant pandas has contributed to the vast number of cubs born from AI [37] (Fig. 1). Recent studies about non-invasive identification of fecal biomarkers for early pregnancy diagnosis in the cheetah could also be inspiring to human reproductive medicine [38]. While patients’ IVF clinics need daily blood draws, which can be constraining and uncomfortable, non-invasive monitoring techniques using urines or saliva might be more attractive.
Fig. 1.
Non-invasive monitoring of natural estrus and timing of artificial insemination using urine metabolites of estrogens and progestins in the giant panda
Improving the success of in vitro maturation and fertilization
The first baby born after conception by IVF and autologous embryo transfer was a medical breakthrough [39]. Fertilization methods have progressed from early IVF protocols mixing of sperm and eggs in a petri dish, to the advanced technologies used today, such as ICSI [40]. Many in vitro culture techniques that are successful in humans now may be vital for the preservation of endangered wildlife species. For instance, culture media developed for humans have proven to be very efficient in felids [41]. There is the potential, however, for techniques developed in endangered species to be vital for human IVF. Clinical IVF includes fertilizing in vivo matured oocytes that are retrieved from a patient that has undergone exogenous hormone injections. While there have been live and normal births from in vitro matured (IVM) oocytes, the pregnancy rate is consistently lower than those of conventional human IVF [42]. For many endangered species, the use of conventional IVF is not possible because giving exogenous hormones is not practical or even impossible. In the felid model, paracrine factors produced by cumulus-oocyte complexes (COCs) could assist in enhancing the culture capacity of denuded/lower quality oocytes [43, 44]. Interestingly, studies in mouse and bovine models also have reported the opposite (beneficial effect of denuded oocytes on the COC competence) [45, 46], thus giving another reason to study diverse animal models for potentially improving human IVF.
Grading of human spermatozoa via traditional metrics, including swimming velocity, acrosomal integrity, concentration, and general morphology, have been shown to be good markers for intrauterine insemination cycles and in IVF cycles [47]. For ICSI, however, this is not necessarily the case. Evaluating DNA fragmentation has been proposed in this case, and it has been shown that higher fragmented chromatin in a sperm sample has negative effects on implantation rate after IVF and ICSI in humans [48]. It has also been proposed that more specific features sperm morphology may be of consideration for viable spermatozoa, including the presence of nuclear vacuoles and their effect on chromatin packaging [49]. Recent studies also have shown that heat shock protein (HSPA2) is compromised in the human spermatozoa and leads to oocyte recognition defects. The characterization of these HSPA2-interacting proteins provides an important marker and insight into the complexity of the cellular pathways that may be affected in the spermatozoa of infertile individuals [50] and possibly in wild species. There are a lot of reports about sperm subpopulations in different species showing that data as mean ± SEM is open to misleading interpretations [51]. The development of new and simple ways for the identification of sperm subpopulations should enhance our ability to predict in vivo fertility in humans.
Lastly, human clinical studies have shown that a more specific and intensive oocyte scoring successfully predicted embryo quality after IVF [52]. Accurately assessing oocyte quality could save subsequent costs associated with the rest of the IVF cycle and help determine which oocytes and embryos are selected for future cycles. Additionally, as more physicians are opting for single embryo transfer, better assessment of embryo quality can assist in selecting the best embryo for the transfer. It is possible that a higher oocyte score will make the difference in determining which of two similarly scored embryos are transferred. This may also prove useful in a conservation perspective, where assessing alternative species gametes and embryos can be difficult, and gametes can be rare. For example, recent research in felids has revealed new markers of oocyte competence present in the germinal vesicle which also could shed some lights on the quality assessment of human immature oocytes [53].
Micromanipulations of gametes
The germinal vesicle (GV) is another attractive target for ART because it is the source of the maternal genome, and because its quality and competence appear to remain homogeneous even in the presence of cytoplasmic incompetence and defects, including mitochondrial DNA (mtDNA) diseases, the latter causing most developmental failures [54]. Transfer of healthy cytoplasm from a disease-free oocyte into an oocyte with disease-carrying mtDNA is inherently imperfect, as it has been shown that some of the babies are heteroplasmic [55]. Another proposed method is the transfer of the germinal vesicle (GV) from the donor oocyte, into a recipient oocyte with healthy mtDNA that has had its own GV removed. This method has been tested in humans, but has yet to be fully developed, or routinely used in clinical settings [56]. Studies in mice and rabbits indicate that there is still very little understood about the outcomes of these manipulated oocytes and subsequent embryos. Other animal models, including felids, appear to be particularly compatible for such studies due to a large-sized GV and chromatin configuration analogous to the human [57]. GV transfer into a competent, enucleated oocyte is a viable option for “rescuing” the genome from oocytes that are (1) morphologically abnormal or of poor quality that consistently fail to mature or fertilize in vitro (including aged donors) or (2) incompetent due to being derived from a preantral or early antral follicle.
Absence of spermatozoa in the ejaculate due to testicular failure, tract obstructions, or other physiological challenges can cause up to 20% of infertility [58]. Through advances in assisted reproduction, however, it is possible to circumvent this problem by extracting immature male gametes from epididymal or testicular sources, and using ICSI to produce a viable embryo. ICSI with immature testicular sperm cells has lower embryo development rates than epididymal spermatozoa suggesting post-testicular maturation [59]. Recent experiments have demonstrated that this decrease in testicular sperm cell efficiency in humans is mirrored in the cat [60]. Sperm centrosome is not functional in testicular spermatozoa and matures as it moves through the male reproductive tract, especially the epididymis. Laboratory rodents are not ideal models as the centrosome is of maternal inheritance, rather than paternal, like in other non-rodent mammals [61]. Thus, looking for alternative mammalian species may be more useful as models for human ART. In felids, the centrosome of individual testicular spermatozoa can be replaced with those from ejaculated counterparts by micromanipulation. Reconstructed cat spermatozoa (with mature centrosomes) have better functionality and, when injected into oocytes, support successful embryo development [62]. Recent results in the cat model also suggest that epididymosomes play a critical role in epididymal sperm centrosomal maturation and could be ideal vehicles to assist in the enhancement of male fertility [63].
New tools for fertility preservation and cryo-banking
There are specific components of the rapidly growing field of fertility preservation in men and women that are highly compatible with preserving valuable genomes of individuals or populations of threatened wildlife. Besides more “classical” approaches focusing on sperm and oocyte freezing, strategies associated with gonadal tissue cryopreservation and in vitro culture are especially attractive for better protecting and extending fertility for wild individuals [17, 64]. This is especially related to “oncofertility” which aims at developing reliable methods for preserving paternal and maternal germplasm from adult and prepubertal patients that may lose the capacity to produce viable gametes after therapeutic treatments [65]. For prepubertal females whose germ cells are too immature to be fertilized, the preservation of ovarian tissues for future transplantation is currently their only option for long-term fertility preservation [66]. Animal models are crucial for improving practical methods to preserve gametes and gonadal tissues with findings applicable to conserving valuable genomes and extending fertility of rare animal species [17, 60]. Approaches being explored in wild species range from enhancements in conventional cryopreservation technologies for gonadal tissue, gametes, and embryos to use of in vitro culture of immature follicles. The ovarian cortex contains thousands of follicles at different developmental stages that are recoverable from individuals at the time of ovariectomy [65]. The value of vitrification over slow cooling for preserving ovarian cortex and primordial follicles has been demonstrated for prepubertal and adult cats [67].
In association with the freezing gonadal tissues, the capacity to culture the follicles in vitro (to the point of recovering viable oocytes that can achieve nuclear maturation and then be fertilized) offers enormous opportunities for maternal genome conservation. Particularly inspiring have been studies in primates [68] where secondary follicles were able to grow, maintain architecture, and produce steroids in vitro for 15 to 30 days. However, the follicle culture strategy still needs to be improved using larger-sized animal models than the mouse. Using cat and dog tissues, encouraging results of in vitro culture methods for early-stage follicles were obtained [18, 69]. Other cutting-edge approaches also are investigated in carnivores such as the preservation of the GV at room temperature [70]. To address needs in man fertility preservation (that tends to be less developed than in women), novel approaches developed in carnivores in testicular tissue freezing [60] or sperm drying using microwaves [71] are very relevant.
In terms of bio-banking of germplasms, systematic gathering and cryo-storage of biomaterials from diverse wild species have been ongoing for > 25 years to save gene diversity and improve captive (ex situ) and wild (in situ) animal management. There are commonalities between human and wildlife bio-banking programs, including similar needs to harmonize sample and data collection, management, and most effective use as well as finding ways to be financially sustainable. There is a need to build bridges between these two “repository worlds,” sharing what we do, addressing the substantial remaining challenges and considering the advantages of a bigger, more integrated field of global bio-banking science to benefit humans and diverse species [72].
Conclusion and next steps
There is a need for more fundamental knowledge in reproductive biology for the vast majority of animal species on our planet. Development and application of assisted reproduction are not advanced enough in many species to be successfully implemented into conservation efforts and make a difference. In addition, we are facing new challenges (amphibian crisis, coral reef bleaching, mass extinctions of species) that create more demands and expectations. The value of ART in animal conservation is undeniable, but there is an urgent need for more options and faster progress. While ART in humans is much more advanced than in wild species, many infertility syndromes still have to be deciphered and lessons can be learned from non-traditional animal models. In times where animal experimentation is more and more questioned, here is a unique opportunity to take advantage of huge amount of knowledge generated in wild species to benefit human reproduction. Research and collaborations between wild species and human ART can inspire each other to address most of the issues mentioned above. Although not mentioned in this review, the use and integration of genomics and epigenomics into ART (for instance, to customize hormonal treatments or to evaluate gamete quality) will be developed more efficiently if applications in human and wild species are studied simultaneously. New precision/personalized reproductive medicine in human, innovative paradigms for genetic management of wild species, and a refined knowledge about microbiome and fertility are the expected products of this common effort. In addition, the influence of the environment on the sperm or oocyte epigenome and the possible transmission of fertility issues to the offspring is an emerging discipline that will benefit from intense collaborations with wildlife and human reproduction specialists. We look forward to advancing the exchange to foster successful multi-disciplinary approaches and address more efficiently emerging fertility issues in human and wildlife.
References
- 1.Monfort SL. “Mayday mayday mayday”, the Millennium ark is sinking! Adv Exp Med Biol. 2014;753:15–31. [cited 2017 Dec 28]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25091904. [DOI] [PubMed]
- 2.Wildt DE, Comizzoli P, Pukazhenthi B, Songsasen N. Lessons from biodiversity—the value of nontraditional species to advance reproductive science, conservation, and human health. Mol Reprod Dev. 2010;77:397–409. [DOI] [PMC free article] [PubMed]
- 3.Holt WV, Brown JL, Comizzoli P. Reproductive science as an essential component of conservation biology. Adv Exp Med Biol. 2014;753:3–14. doi: 10.1007/978-1-4939-0820-2_1. [DOI] [PubMed] [Google Scholar]
- 4.Brown JL, Comizzoli P. Female cat reproduction. In: Skinner MA, editor. Encyclopedia of reproduction, 2nd Edition. 2018. In press.
- 5.Brown JL. Comparative ovarian function and reproductive monitoring of endangered mammals. Theriogenology. 2017. 10.1016/j.theriogenology.2017.12.004. [cited 2017 Dec 28]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29249329. [DOI] [PubMed]
- 6.Comizzoli P, Mermillod P, Mauget R. Reproductive biotechnologies for endangered mammalian species. Reprod Nutr Dev. 2000;40:493–504. doi: 10.1051/rnd:2000113. [DOI] [PubMed] [Google Scholar]
- 7.Comizzoli P, Songsasen N, Hagedorn M, Wildt DE. Comparative cryobiological traits and requirements for gametes and gonadal tissues collected from wildlife species. Theriogenology. 2012;78:1666–1681. doi: 10.1016/j.theriogenology.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Comizzoli P, Wildt DE. Mammalian fertility preservation through cryobiology: value of classical comparative studies and the need for new preservation options. Reprod Fertil Dev. 2013;26:91–98. doi: 10.1071/RD13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildt DE, Schiewe MC, Schmidt PM, Goodrowe KL, Howard JG, Phillips LG, et al. Developing animal model systems for embryo technologies in rare and endangered wildlife. Theriogenology. 1986;25:33–51. doi: 10.1016/0093-691X(86)90182-2. [DOI] [Google Scholar]
- 10.Lasley BL, Loskutoff NM, Anderson GB. The limitation of conventional breeding programs and the need and promise of assisted reproduction in nondomestic species. Theriogenology. 1994;41:119–132. doi: 10.1016/S0093-691X(05)80057-3. [DOI] [Google Scholar]
- 11.Pukazhenthi B, Comizzoli P, Travis AJAJ, Wildt DE. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod Fertil Dev. 2006;18:77–90. doi: 10.1071/RD05117. [DOI] [PubMed] [Google Scholar]
- 12.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. Low N, editor. PLoS Med Public Libr Sci; 2012;9:e1001356. Available from: doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed]
- 13.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted reproductive technology surveillance—United States, 2014. MMWR. Surveill Summ. 2017;66:1–24. doi: 10.15585/mmwr.ss6606a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jose-Miller AB, Boyden JW, Frey KA. Infertility. Am Fam Physician. 2007;75:849–856. [PubMed] [Google Scholar]
- 15.Chang MC. Fertilization of rabbit ova in vitro. Nature. 1959;184(Suppl 7):466–467. doi: 10.1038/184466a0. [DOI] [PubMed] [Google Scholar]
- 16.Gould KG, Cline EM, Williams WL. Observations on the induction of ovulation and fertilization in vitro in the squirrel monkey (Saimiri sciureus) Fertil Steril. 1973;24:260–268. doi: 10.1016/S0015-0282(16)39610-8. [DOI] [PubMed] [Google Scholar]
- 17.Comizzoli P, Songsasen N, Wildt DE. Protecting and extending fertility for females of wild and endangered mammals. Cancer Treat Res. 2010;156:87–100. [DOI] [PMC free article] [PubMed]
- 18.Songsasen N, Comizzoli P, Nagashima J, Fujihara M, Wildt DE. The domestic dog and cat as models for understanding the regulation of ovarian follicle development in vitro. Reprod Domest Anim. 2012;47 Suppl 6:13–18. doi: 10.1111/rda.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farstad W. Customizing semen preservation protocols for individual dogs and individual species: sperm preservation beyond the state of the art. Reprod Domest Anim. 2012;47:269–273. doi: 10.1111/rda.12020. [DOI] [PubMed] [Google Scholar]
- 20.Brown JL. Comparative reproductive biology of elephants. Adv Exp Med Biol. 2014;753:135–69. [cited 2017 Dec 31]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25091910. [DOI] [PubMed]
- 21.Savasi V, Leone FPG, Fusè F, Parisi F, Cetin I. Assisted reproductive technologies and uterine factors influencing their success. Minerva Ginecol. 2013;65:505–524. [PubMed] [Google Scholar]
- 22.Crosier AE, Comizzoli P, Baker T, Davidson A, Munson L, Howard J, et al. Increasing age influences uterine integrity, but not ovarian function or oocyte quality, in the cheetah (Acinonyx jubatus). Biol Reprod. 2011;85:243–53. [DOI] [PubMed]
- 23.Pukazhenthi BS, Neubauer K, Jewgenow K, Howard J, Wildt DE. The impact and potential etiology of teratospermia in the domestic cat and its wild relatives. Theriogenology. 2006;66:112–121. doi: 10.1016/j.theriogenology.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 24.De Braekeleer M, Nguyen MH, Morel F, Perrin A. Genetic aspects of monomorphic teratozoospermia: a review. J Assist Reprod Genet. 2015;32:615–623. doi: 10.1007/s10815-015-0433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard JG, Wildt DE. Approaches and efficacy of artificial insemination in felids and mustelids. Theriogenology. 2009;71:130–148. doi: 10.1016/j.theriogenology.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 26.Jones OR, Scheuerlein A, Salguero-Gómez R, Camarda CG, Schaible R, Casper BB, et al. Diversity of ageing across the tree of life. Nature. 2014;505:169–173. doi: 10.1038/nature12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gleicher N, Kushnir VA, Albertini DF, Barad DH. Improvements in IVF in women of advanced age. J Endocrinol. 2016;230:F1–F6. doi: 10.1530/JOE-16-0105. [DOI] [PubMed] [Google Scholar]
- 28.Sharma R, Agarwal A, Rohra VK, Assidi M, Abu-Elmagd M, Turki RF. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod Biol Endocrinol. 2015;13:35. doi: 10.1186/s12958-015-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Place NJ, Crosier AE, Comizzoli P, Nagashima JB, Haefele H, Schmidt-Küntzel A, et al. Age-associated and deslorelin-induced declines in serum anti-Müllerian hormone concentrations in female cheetahs, Acinonyx jubatus. Gen Comp Endocrinol. 2017;250:54–57. doi: 10.1016/j.ygcen.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Mossa F, Jimenez-Krassel F, Scheetz D, Weber-Nielsen M, Evans ACO, Ireland JJ. Anti-Müllerian hormone (AMH) and fertility management in agricultural species. Reproduction. 2017;154:R1–11. doi: 10.1530/REP-17-0104. [DOI] [PubMed] [Google Scholar]
- 31.Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, et al. Fifteen years after “wingspread”—environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci. 2008;105:235–259. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Pinho JC, Aghajanova L, Herndon CN. Prepubertal gynecomastia due to indirect exposure to nonformulary bioidentical hormonal replacement therapy: a case report. J Reprod Med. 2016;61:73–7. cited 2018 Jan 5]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/26995893 [PubMed]
- 33.Myers SL, Yang CZ, Bittner GD, Witt KL, Tice RR, Baird DD. Estrogenic and anti-estrogenic activity of off-the-shelf hair and skin care products. J Expo Sci Environ Epidemiol. 2015;25:271–277. doi: 10.1038/jes.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bornman MS, Aneck-Hahn NH, de Jager C, Wagenaar GM, Bouwman H, Barnhoorn IEJ, et al. Endocrine disruptors and health effects in Africa: a call for action. Environ Health Perspect. 2017;125:085005. [cited 2017 Dec 29]; Available from: http://ehp.niehs.nih.gov/EHP1774 [DOI] [PMC free article] [PubMed]
- 35.Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. J Anim Sci. 1995;73:1509–1515. doi: 10.2527/1995.7351509x. [DOI] [PubMed] [Google Scholar]
- 36.Tubbs CW, Moley LA, Ivy JA, Metrione LC, LaClaire S, Felton RG, et al. Estrogenicity of captive southern white rhinoceros diets and their association with fertility. Gen Comp Endocrinol. 2016;238:32–38. doi: 10.1016/j.ygcen.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Kersey DC, Aitken-Palmer C, Rivera S, Willis EL, Liang LY, Snyder RJ. The birth of a giant panda: tracking the biological factors that successfully contribute to conception through to postnatal development. Theriogenology. 2016;85:671–677. doi: 10.1016/j.theriogenology.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Koester DC, Wildt DE, Maly M, Comizzoli P, Crosier AE. Non-invasive identification of protein biomarkers for early pregnancy diagnosis in the cheetah (Acinonyx jubatus). PLoS One. 2017;12:e0188575. [cited 2017 Dec 29]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29236714. [DOI] [PMC free article] [PubMed]
- 39.Johnson MH. Robert Edwards: the path to IVF. Reprod BioMed Online. 2011;23:245–262. doi: 10.1016/j.rbmo.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palermo GD, O’Neill CL, Chow S, Cheung S, Parrella A, Pereira N, et al. Intracytoplasmic sperm injection: state of the art in humans. Reproduction. 2017;154:F93–110. doi: 10.1530/REP-17-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nestle E, Graves-Herring J, Keefer C, Comizzoli P. Source of protein supplementation during in vitro culture does not affect the quality of resulting blastocysts in the domestic cat. Reprod Domest Anim. 2012;47 Suppl 6:152–155. doi: 10.1111/rda.12047. [DOI] [PubMed] [Google Scholar]
- 42.Chang EM, Song HS, Lee DR, Lee WS, Yoon TK. In vitro maturation of human oocytes: its role in infertility treatment and new possibilities. Clin Exp Reprod Med. 2014;41:41. doi: 10.5653/cerm.2014.41.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Godard NM, Pukazhenthi BS, Wildt DE, Comizzoli P. Paracrine factors from cumulus-enclosed oocytes ensure the successful maturation and fertilization in vitro of denuded oocytes in the cat model. Fertil Steril. 2009;91:2051–60. [DOI] [PMC free article] [PubMed]
- 44.Morselli MG, Luvoni GC, Comizzoli P. The nuclear and developmental competence of cumulus–oocyte complexes is enhanced by three-dimensional coculture with conspecific denuded oocytes during in vitro maturation in the domestic cat model. Reprod Domest Anim. 2017;52 Suppl 2:82–87. [DOI] [PubMed]
- 45.Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol. 2006;296:514–521. doi: 10.1016/j.ydbio.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 46.Sudiman J, Ritter LJ, Feil DK, Wang X, Chan K, Mottershead DG, et al. Effects of differing oocyte-secreted factors during mouse in vitro maturation on subsequent embryo and fetal development. J Assist Reprod Genet. 2014;31:295–306. doi: 10.1007/s10815-013-0152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berkovitz A, Eltes F, Soffer Y, Zabludovsky N, Beyth Y, Farhi J, et al. ART success and in vivo sperm cell selection depend on the ultramorphological status of spermatozoa. Andrologia. 1999;31:1–8. doi: 10.1046/j.1439-0272.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- 48.Achour-Frydman N. Amélioration des résultats et optimisation du contrôle de qualité. J Gynécologie Obs Biol la Reprod. 2008;37:S1–S3. doi: 10.1016/S0368-2315(08)73819-5. [DOI] [PubMed] [Google Scholar]
- 49.Falagario D, Brucculeri AM, Depalo R, Trerotoli P, Cittadini E. Ruvolo G. Sperm head vacuolization affects clinical outcome in ICSI cycle. A proposal of a cut-off value. J Assist Reprod Genet. 2012;29:1281–1287. doi: 10.1007/s10815-012-9858-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bromfield EG, McLaughlin EA, Aitken RJ, Nixon B. Heat shock protein member A2 forms a stable complex with angiotensin converting enzyme and protein disulfide isomerase A6 in human spermatozoa. Mol Hum Reprod. 2016;22:93–109. doi: 10.1093/molehr/gav073. [DOI] [PubMed] [Google Scholar]
- 51.Holt WV, Fazeli A. Sperm selection in the female mammalian reproductive tract. Focus on the oviduct: hypotheses, mechanisms, and new opportunities. Theriogenology. 2016;85:105–112. doi: 10.1016/j.theriogenology.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 52.Lazzaroni-Tealdi E, Barad DH, Albertini DF, Yu Y, Kushnir VA, Russell H, et al. Oocyte scoring enhances embryo-scoring in predicting pregnancy chances with IVF where it counts most. Kim S, editor. PLoS One. 2015;10:e0143632. doi: 10.1371/journal.pone.0143632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee P-C, Wildt DE, Comizzoli P. Proteomic analysis of germinal vesicles in the domestic cat model reveals candidate nuclear proteins involved in oocyte competence acquisition. Mol Hum Reprod. 2018;24:14–26. [cited 2017 Dec 31]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29126204. [DOI] [PMC free article] [PubMed]
- 54.Chappel S. The role of mitochondria from mature oocyte to viable blastocyst. Obstet Gynecol Int. 2013;2013:183024. doi: 10.1155/2013/183024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16:513–516. doi: 10.1093/humrep/16.3.513. [DOI] [PubMed] [Google Scholar]
- 56.Takeuchi T, Gong J, Veeck LL, Rosenwaks Z, Palermo GD. Preliminary findings in germinal vesicle transplantation of immature human oocytes. Hum Reprod. 2001;16:730–736. doi: 10.1093/humrep/16.4.730. [DOI] [PubMed] [Google Scholar]
- 57.Comizzoli P, Pukazhenthi BS, Wildt DE. The competence of germinal vesicle oocytes is unrelated to nuclear chromatin configuration and strictly depends on cytoplasmic quantity and quality in the cat model. Hum Reprod. 2011;26:2165–2177. doi: 10.1093/humrep/der176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarvi K, Lo K, Fischer A, Grantmyre J, Zini A, Chow V, et al. CUA guideline: the workup of azoospermic males. Can Urol Assoc J. 2010;4:163–167. doi: 10.5489/cuaj.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Wely M, Barbey N, Meissner A, Repping S, Silber SJ. Live birth rates after MESA or TESE in men with obstructive azoospermia: is there a difference? Hum Reprod. 2015;30:761–766. doi: 10.1093/humrep/dev032. [DOI] [PubMed] [Google Scholar]
- 60.Comizzoli P. Biobanking efforts and new advances in male fertility preservation for rare and endangered species. Asian J. Androl. 2015;17:640–5. [DOI] [PMC free article] [PubMed]
- 61.Howe K, FitzHarris G. Recent insights into spindle function in mammalian oocytes and early Embryos1. Biol Reprod. 2013;89:71. doi: 10.1095/biolreprod.113.112151. [DOI] [PubMed] [Google Scholar]
- 62.Comizzoli P, Wildt DE, Pukazhenthi BS. Poor centrosomal function of cat testicular spermatozoa impairs embryo development in vitro after intracytoplasmic sperm injection. Biol Reprod. 2006;75:252–260. doi: 10.1095/biolreprod.106.051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rowlison T, Ottinger MA, Comizzoli P. Key factors enhancing sperm fertilizing ability are transferred from the epididymis to the spermatozoa via epididymosomes in the domestic cat model. J Assist Reprod Genet. 2017. 10.1007/s10815-017-1083-3. [DOI] [PMC free article] [PubMed]
- 64.Comizzoli P, Wildt DE. On the horizon for fertility preservation in domestic and wild carnivores. Reprod Domest Anim. 2012;47 Suppl 6:261–265. doi: 10.1111/rda.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodruff TK. Fertility lost–fertility found: narratives from the leading edge of oncofertility. Narrat Inq Bioeth. 2017;7:147–150. doi: 10.1353/nib.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet. 2016;33:1595–1603. doi: 10.1007/s10815-016-0814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mouttham L, Comizzoli P. The preservation of vital functions in cat ovarian tissues during vitrification depends more on the temperature of the cryoprotectant exposure than on the sucrose supplementation. Cryobiology. 2016;73:187–95. [DOI] [PubMed]
- 68.Xu J, Xu M, Bernuci MP, Fisher TE, Shea LD, Woodruff TK, et al. Primate follicular development and oocyte maturation in vitro. Adv Exp Med Biol. 2013;761:43–67. [cited 2017 Dec 30]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/24097381 [DOI] [PMC free article] [PubMed]
- 69.Songsasen N, Thongkittidilok C, Yamamizu K, Wildt DE, Comizzoli P. Short-term hypertonic exposure enhances in vitro follicle growth and meiotic competence of enclosed oocytes while modestly affecting mRNA expression of aquaporin and steroidogenic genes in the domestic cat model. Theriogenology. 2017;90:228–36. [DOI] [PMC free article] [PubMed]
- 70.Elliott GD, Lee P-C, Paramore E, Van Vorst M, Comizzoli P. Resilience of oocyte germinal vesicles to microwave-assisted drying in the domestic cat model. Biopreserv Biobank. 2015;13:164–171. doi: 10.1089/bio.2014.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patrick JL, Elliott GD, Comizzoli P. Structural integrity and developmental potential of spermatozoa following microwave-assisted drying in the domestic cat model. Theriogenology. 2017;103:36–43. [DOI] [PubMed]
- 72.Comizzoli P, Wildt DE. Cryobanking biomaterials from wild animal species to conserve genes and biodiversity: relevance to human biobanking and biomedical research. In: Hainaut P, Vaught J, Zatloukal K, Pasterk M, editors. Biobanking of human biospecimens. Cham: Springer; 2017.