Abstract
Purpose
Even with 86 live births reported globally so far, the mechanism of primordial follicle loss following autotransplantation of the frozen-thawed ovarian tissue needs further evaluation. Pten, Tsc1, p27, and Amh are the inhibitor proteins that play crucial roles in suppressing the transition from the primordial follicle to primary state, maintaining the primordial follicle reserve. In this study, we aimed to evaluate whether the expression patterns of these proteins change and it may be related to the global primordial follicle loss after autotransplantation of the frozen-thawed ovarian tissue.
Methods
Four groups were established in rats: fresh-control, frozen/thawed, fresh-transplanted, and frozen/thawed and transplanted. After slow freezing and thawing process, two ovarian pieces were transplanted into the back muscle of the same rat. After 2 weeks, grafts were harvested, fixed, and embedded into the paraffin block. Normal and atretic primordial/growing follicle count was performed in all groups. Ovarian tissues were evaluated for the dynamic expressions of the Pten, Tsc1, p27, and Amh proteins using immunohistochemistry, and H-score analyses were done.
Results
Ovarian tissue cryopreservation does not change the expression patterns of inhibitory proteins that control ovarian reserve. Both in fresh and frozen/thawed autotransplanted groups, the expression of inhibitory proteins and Amh decreased significantly in primordial follicles and in growing follicles, respectively. In control group and in frozen/thawed group, primordial follicle counts were similar but decreased by almost half in both fresh-transplanted and frozen/thawed and transplanted groups.
Conclusions
One of the causes of primordial follicle loss after transplantation of ovarian graft may be decreased expression of the inhibitory proteins that guard the ovarian reserve and transplantation itself seems to be the major cause for disruption of inhibitory molecular signaling. Our findings highlight important molecular aspects for future clinical applications for fertility preservation in humans.
Keywords: Ovarian tissue cryopreservation, Primordial follicle, Pten, Tsc1, p27, AMH
Introduction
Fertility preservation techniques are vital in tackling infertility and hormonal insufficiency in premature ovarian failure (POF) caused by destructive implementations like ovarian surgeries and chemo/radiotherapy. Embryo, oocyte, and ovarian tissue (OT) cryopreservation options that are offered by the utilization of assisted reproductive techniques are crucial for preserving the ovarian reserve of female patients, and thus their fertility success [1]. However, even though embryo cryopreservation method is considered as the golden standard, it is not realistic for unmarried or pre-pubertal female patients. OT cryopreservation stands as an exceptional fertility preservation method for a group of certain group of patients [2]. Albeit the American Society for Reproductive Medicine still considers as an experimental procedure, OT cryopreservation, and retransplantation have resulted in more than 86 cases of successful live births all over the world [3].
Under normal conditions, in vivo primordial follicles (PFs) are resistant to further development, a phenomenon that prevents follicle depletion. Hormone-insensitive PFs are elected to proceed for further maturation by a delicately balanced and well-regulated expression of growth and inhibitory factors as well as precise interaction between granulosa cells and the enclosing oocyte [4]. Those well-balanced molecular activators and suppressors are needed for preserving the PF pool [5]. Situations causing a misbalance in these activators and suppressors result in a global activation and a rapid burnout in the PF pool, known as POF [6]. Coordinated and synergistic paracrine actions of signals that promote in vivo activation of PFs to primary follicles include Kit-ligand, BMP4/7/15, FGF2, KGF, LIF, and GDF9 that expressed from oocytes, granulosa cells, and stromal cells. On the other hand, PFs are maintained in a quiescence state by the interactions of various inhibitory molecules to preserve the follicle pool, such as phosphatase and tensin homolog deleted on chromosome 10 (Pten), tuberous sclerosis complex-1 (Tsc1), p27, and anti-Mullerian hormone (Amh) [5, 7].
Pten is a tumor suppressor gene that is often deleted in many human tumors [8]. Homozygous deletion of Pten in mice causes embryonic lethality [9]. In mouse ovaries lacking Pten in their oocytes, prematurely activated follicles were found to undergo atresia, and POF appeared around the third month of postnatal development [10]. Pten signaling pathway governs the follicular activation through control of the initiation of oocyte growth [11]. Pten is also expressed in the granulosa cells during folliculogenesis. An increase in Pten may lead to changes in proliferation and/or differentiation of granulosa cells during follicular growth via regulation of Akt phosphorylation [12].
Akt directly phosphorylates p27, thus phosphorylated p27 shuttles from the nucleus to cytoplasm whereby its inhibitory effect is abolished [13]. p27 is expressed in pregranulosa cells in mouse ovaries and in oocyte nuclei of primordial, primary, and secondary follicles [14]. In p27 knockout mice, activation of PFs was synergistically accelerated; thus, p27 appears to suppress follicular activation [15].
Akt also inhibits Tsc1, and phosphorylated Tsc leads to the activation of mTORC1 (raptor containing rapamycin-sensitive complex). mTORC1-mediated growth factor signaling regulates protein translation. One of the most important regulators of mTORC1 activity is Tsc that is composed of Tsc1 and Tsc2 heterodimers. Tsc1 is a tumor suppressor protein that inhibits mTORC1, and its heterozygote mutation causes benign tumors of tuberous sclerosis [16]. Tsc1 negatively regulates mTORC1 and keeps PFs quiescent. In mutant mice lacking Tsc1 gene in their oocytes, the entire pool of PFs is activated prematurely due to elevated mTORC1 activity in the oocyte, ending up with follicular depletion in early adulthood and causing POF [17].
In rat and mouse ovaries, Amh is not expressed in the pregranulosa cells of PFs. Once these follicles are activated, and start growing their granulosa cells express Amh and this expression gradually reach to peak levels in granulosa cells of multilayer primary/secondary and small antral follicles [18]. Amh has a critical role in the cyclic recruitment of ovarian follicles, and its expression from growing follicles suppresses PF reserve [19].
Since the timely and adequate expression of inhibitory proteins that regulate PF activation is critical for the preservation of ovarian reserve and considerable loss of PFs is a major concern after OT cryopreservation and transplantation, we aimed to investigate whether cryopreservation alone or following retransplantation cycles disturb the expression of the inhibitory proteins that suppress PF activation in the rat ovary. We found that the number of PFs and the expression of Pten, Tsc1, p27, and Amh proteins decreased significantly in PFs of OTs after cryopreservation and transplantation independent of freezing and thawing cycles, indicating that transplantation itself seems to be the major cause for disruption of molecular signaling that controls the ovarian reserve and PF loss.
Materials and methods
Experimental animals and study design
Five-week-old Wistar female rats (n = 28) were obtained from Ankara University Animal Research Unit, and the experimental protocol was approved by the local ethical committee (protocol #2013-14-101). Rats were housed under temperature and light-controlled conditions (22 ± 1 °C, 12 h light/12 h dark cycle) with ad libitum access to food.
Both ovaries taken from each animal were divided into eight equal pieces (almost 1 mm3 tissue) and divided into four experimental groups. By doing so, study groups and control tissues belonging to the same animal and the estrous cycle differences between the groups were eliminated.
Groups were designed as follows:
Fresh control (FC) group (n = 28): Two ovarian pieces from each rat were immediately fixed in Bouin’s solution and embedded into paraffin blocks.
Frozen/thawed (FT) group (n = 28): Two ovarian pieces from each rat were frozen in a temperature-controlled slow freezer, thawed after 2 weeks and then fixed in Bouin’s solution for paraffin sectioning.
Fresh-transplanted (T) group (n = 28): Two ovarian pieces from each rat were directly transplanted into the back muscle (m. trapezius) of the same rat (autotransplanted heterotopic). Two weeks after autotransplantation, ovarian grafts were removed and fixed in Bouin’s solution and embedded into paraffin blocks.
Frozen/thawed and transplanted (FTT) group (n = 28): After a slow-freezing and thawing process, two ovarian pieces from each rat were transplanted into the back muscle (m. trapezius) of the same rat (autotransplanted heterotopic). After 2 weeks, grafts were harvested, fixed in Bouin’s solution, and embedded into paraffin blocks.
Ovarian tissue cryopreservation: slow-freezing and thawing
Slow-freezing of OTs was performed using a temperature-controlled freezer (IceCube; SyLab, Neupurkersdorf, Austria). The procedure was carried out according to the protocol described previously [20]. Briefly, OTs were taken into cryovials containing 1 mL of cryoprotective medium composed of 1.5 M dimethyl sulfoxide (DMSO), 20% fetal bovine serum (FBS), and 0.1 M sucrose and incubated at 4 °C for 30 min prior to loading into the freezer. As the first step, OTs were cooled from 0 to − 7 °C at the rate of 2 °C/min, then soaked at − 7 °C for 10 min, and nucleation was initiated through manual seeding by touching on the side walls of the vial with a pre-cooled metal object. After manual seeding, samples were cooled down to − 40 °C at a rate of 0.3 °C/min, followed by cooling down to − 80 °C at a rate of 5 °C/min. At the final step, samples were cooled down to − 120 °C at 10 °C/min rate and then plunged into liquid nitrogen at the end of the freezing program.
After 2 weeks, frozen OTs were gently removed from the cryovials, thawed in 37 °C water bath for 1 min until melted completely. The tissue pieces were quickly transferred into 60-mm-diameter culture dishes in Leibovitz L-15 (L-15) medium that contains 1 M DMSO + 0.1 M sucrose and incubated for 5 min. OTs were subsequently kept in L-15 medium that consists of 0.5 M DMSO + 0.1 M sucrose and 0.05 M sucrose only, respectively. Finally, OTs were kept in the L-15 culture medium solely and transferred into their matching groups.
Histological analysis and follicle count
Ovarian tissues were fixed in Bouin solution (75 mL of picric acid, 25 mL of 40% formaldehyde, 5 mL of glacial acetic acid) for 2 h at room temperature for histological evaluation and follicle count. After fixation, ovaries were embedded in paraffin and sectioned at 5 μm, and morphological grading and follicle counts were carried out on hematoxylin and eosin-stained (Merck, Darmstadt, Germany) sections. Every fifth section obtained from each ovarian paraffin block was taken into evaluation to avoid a consecutive assessment of the same follicle; follicles were counted in both ovaries from each rat in each group.
Follicle assessment and follicle counting were performed according to Galdolfi [21]. Primordial follicles were defined as having an oocyte surrounded by a single layer of flat granulosa cells. All other follicles at more advanced stages of maturation were grouped and defined as growing follicles. All counted primordial, primary, secondary, and tertiary follicles were classified as normal or atretic according to the following criteria: (i) primordial, single layer of flattened pre-granulosa cells; (ii) primary, single or few layers of cuboidal granulosa cells where the antrum is absent; (iii) secondary, multiple layers of cuboidal granulosa cells where a multifocal antrum is present; (iv) tertiary, multiple layers of cuboidal granulosa cells where a single antrum is present; (v) atresia was defined as granulosa cells pulled away from the edge of follicles with intact oocyte or disruption/loss of granulosa cells and theca cells with broken or missing oocyte.
Remaining serial sections were selected for immunohistochemical analysis. For immunohistochemistry, ovary sections were initially deparaffinized in xylene and then rehydrated using decreasing ethanol concentrations.
Immunohistochemistry
Immunostaining was carried out using the avidin-biotin peroxidase complex method. After deparaffinization and rehydration, citrate buffer (pH 6.0) was used for antigen retrieval using microwave. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide in methanol for 15 min at room temperature. Then, tissues were treated with blocking buffer (Thermo Scientific TA-125-UB) at room temperature for 7 min in order to prevent nonspecific staining. After blocking, sections were incubated with the following rabbit primary antibodies [phosphates and tensin homolog (p-Pten) (Cell Signaling-catalog no. 9551 Ser380, 1:50), p27 (Santa Cruz-catalog no. #Sc-528, 1:400), tuberous sclerosis complex 1 (Tsc1) (Abcam-catalog no. ab59274 p-T1462, 1:50), and Amh (Abcam-catalog no. #ab84952, 1:50) overnight at 4 °C. After washing out the primary antibody, slides were incubated for 60 min at room temperature with the anti-rabbit secondary antibody (Vector, catalog no. ba-1000, 1:1000) followed by the incubation with avidin-biotin complex (Vectastain elite kit catalog no. pk-6100) for 30 min. Rabbit IgG control at the same amount of protein concentrations for each protein (Vector#I-1000) was used to validate nonspecific background signal caused by primary antibodies. All incubation steps were performed in a humidified chamber to avoid dehydration of the slides. Immunohistochemical reactions were visualized using diaminobenzidine chromogen (Sigma-Aldrich #D4168) under a light microscope. For nuclear counterstaining, Mayer’s hematoxylin (Merck, Darmstadt, Germany) was applied for 15 s. Pten, p27, Tsc1, and Amh expressions were evaluated and photographed under a Zeiss (Oberkochen, Germany) light microscope by an × 40 Plan-apo objective (440350-99G).
All PFs were evaluated with respect to the intensity of their cytoplasmic and/or nuclear expressions [0 (no staining), + 1 (weak staining), + 2 (moderate staining), and + 3 (intensive staining)] in oocytes and granulosa cells. H-score formula [∑Pi(i + l): “I,” score of staining density, “Pi,” rate of stained cells] was used to calculate the expression levels of the aforementioned proteins [22]. In brief, HSCORE = Pi (i + 1), where i = intensity of staining with a value of (±), (+), (++), or (+++) (minimal, mild, moderate, or strong, respectively), and Pi is the percentage of oocyte and granulosa cells stained with each intensity, varying between 0 and 100%.
Statistical analysis
Kruskal-Wallis nonparametric variance analysis was used for follicle counts and H-score comparison of nonparametric test assumptions between all groups. Tukey post hoc tests were performed for follicle count and H-score results to describe any differences between the groups. Statistical analyses were done using Sigma Stat v3.0 (Jandel Scientific Corp.), and p < 0.05 was considered as statistically significant.
Results
Primordial follicle counts and rate of primordial follicles
Normal and atretic PFs were evaluated and counted in fresh control (FC), frozen/thawed without transplantation (FT), fresh-transplanted (T), and frozen/thawed and transplanted (FTT) groups.
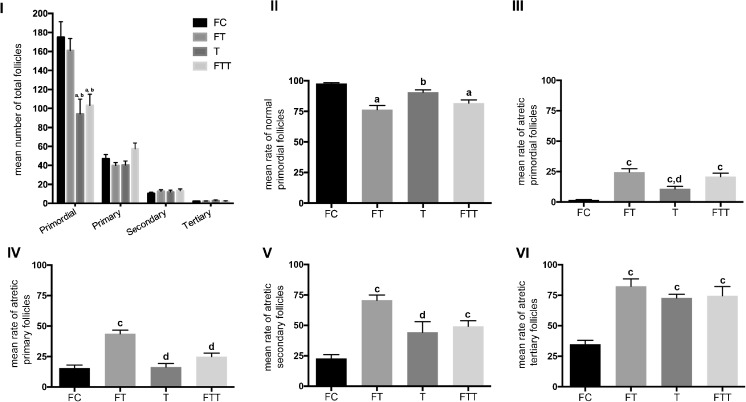
The mean number of total (normal and atretic) PFs was 174.8 ± 16.6, 161 ± 12.6, 94.1 ± 15.9, and 103.3 ± 11.8 in FC, FT, T, and FTT groups, respectively (Fig. 1 (I)). There was no significant difference between FC and FT groups, regarding the PF numbers. The mean number of PFs both in T and FTT groups was significantly lower than FC and FT groups (p < 0.001). No significant difference was noted between T and FTT groups regarding PF numbers.
Fig. 1.
Mean number of total follicles (I), mean rate of normal primordial follicles (PFs) (II), mean rate of atretic PFs (III), mean rate of atretic primary follicles (IV), mean rate of atretic secondary follicles (V), mean rate of atretic tertiary follicles (VI) in fresh control group (FC), frozen and thawed group (FT), fresh-transplanted group (T), and frozen and thawed before transplanted group (FTT). a Statistically significant from FC group (p < 0.001). b Statistically significant from FT group (p < 0.001). c Statistically significant from FC group (p < 0.05). d Statistically significant from FT group (p < 0.05)
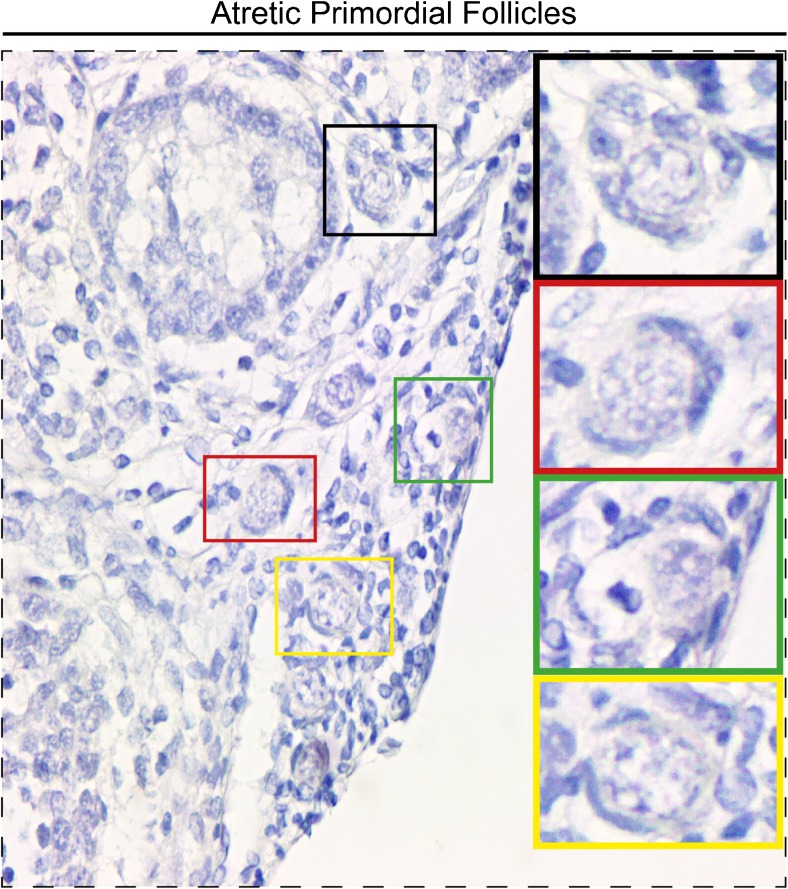
Mean rate of normal PFs (the proportion of the primordial follicles that were normal) in FC, FT, T, and FTT groups was 98 ± 0.8, 76.4 ± 3.5, 90.7 ± 1.9, and 81.7 ± 2.6%, respectively (Fig. 1 (II)). The mean rate of normal PFs in FT and FTT groups was significantly lower than the FC group (p < 0.001). Mean rate of normal PFs in T group was significantly higher than FT group (p < 0.001). The mean rate of atretic PFs (the proportion of primordial follicles that were atretic) in FC, FT, T, and FTT groups was 1.67 ± 0.4, 24.5 ± 2.8, 10.7 ± 2.1, and 20.9 ± 2.8%, respectively (Fig. 1 (III)). The mean rate of atretic PFs in FT, T, and FTT groups was significantly higher than the FC group (p < 0.05). The mean rate of atretic PFs in group T was significantly lower than in the FT group (p < 0.05) whereas their rate in FT and FTT groups were similar. Atretic PFs were demonstrated in Fig. 2.
Fig. 2.
Atretic primordial follicles. Higher magnification of designated areas with the colored squares are shown at the right side
Growing follicle counts
Normal and atretic growing follicles were evaluated and counted in all groups. The mean number of total (normal and atretic) primary follicles was 47.1 ± 4.4, 39.8 ± 3.1, 40.4 ± 4.2, and 57.4 ± 6.1, respectively (Fig. 1 (I)). No significant difference was noted between all groups regarding primary follicle mean numbers (p = 0.15). The mean numbers of total (normal and atretic) secondary follicles were 10.5 ± 1.3, 12.9 ± 1.6, 12 ± 2.1, and 13.4 ± 1.9, respectively (Fig. 1 (I)). No significant difference was noted between groups regarding secondary follicle mean numbers (p = 0.42). The mean number of total (normal and atretic) tertiary follicles was 2.3 ± 0.3, 2.2 ± 0.3, 2.9 ± 0.6, and 2.3 ± 0.4, respectively (Fig. 1 (I)). No significant difference was noted between groups regarding tertiary follicle mean numbers (p = 0.99).
The mean rate of atretic primary follicles in FC, FT, T, and FTT groups were 15.4 ± 2.6, 43.7 ± 2.9, 16.3 ± 2.9, and 24.9 ± 2.9, respectively (Fig. 1 (IV)). The mean rate of atretic primary follicles in FT group was significantly higher than the FC group (p < 0.05). Moreover, atretic primary follicle ratio in T and FTT groups were significantly lower than the FT group (p < 0.05).
The mean rate of atretic secondary follicles in FC, FT, T, and FTT groups were 22.7 ± 3.3, 70.6 ± 4.3, 44.3 ± 8.6, and 49.2 ± 4.5, respectively (Fig. 1 (V)). The mean rate of atretic secondary follicles in FT and FTT group was significantly higher than the FC group (p < 0.05). Atretic secondary follicle ratio in T group was lower than the FT group (p < 0.05).
The mean rate of atretic tertiary follicles in FC, FT, T, and FTT groups were 34.7 ± 3.3, 82.4 ± 6, 72.8 ± 11.3, and 74.5 ± 7.6, respectively (Fig. 1 (VI)). The mean rate of atretic tertiary follicles in FT, T, and FTT groups were significantly higher than the FC group (p < 0.05).
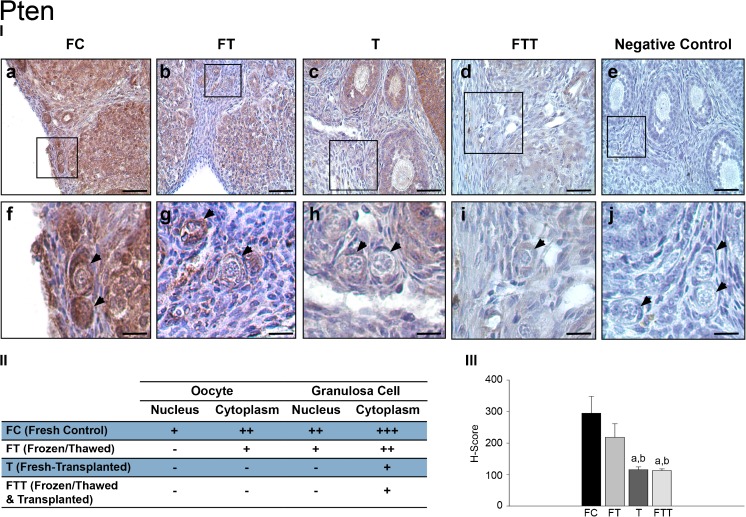
Immunohistochemistry findings—phosphatase and tensin homolog (Pten)
Expression of Pten protein in PFs was assessed in immunostained ovarian sections. In the FC group, Pten expression was distributed throughout the cytoplasm, the nuclei of PF granulosa cells and oocytes (Fig. 3 (I-a and II-f)). In the FT group, nuclear Pten expression was absent in oocytes, while its expression was retained both in the nucleus and cytoplasm of granulosa cells. However, its cytoplasmic expression was found lower when compared to those in the FC group (Fig. 3 (I-b and II-g)). In group T, a remarkable Pten expression loss was noted in both the nucleus and cytoplasm of PF oocytes and granulosa cells (Fig. 3 (I-c and II-h)). Granulosa cells in the same PF displayed a faint cytoplasmic immunopositivity for Pten. In the FTT group, the expression of Pten in oocytes and granulosa cells was similar to those in the T group (Fig. 3 (I-d and II-i)). Lack of the Pten staining was demonstrated in negative control samples (Fig. 3 (I-e and I-j)).
Fig. 3.
Expressions of Pten in primordial follicles. (I) Pten immunohistochemistry results: a, fresh control group (FC); b, frozen and thawed group (FT); c, fresh-transplanted group (T); d, frozen and thawed before transplanted group (FTT); e, negative control (IgG); f, higher magnification of designated area with the square in FC group; g, higher magnification of designated area with the square in FT group; h, higher magnification of designated area with the square in T group; i, higher magnification of designated area with the square in FTT group; j, higher magnification of negative control. (II) H-score results: a, statistically significant from FC group (p < 0.001); b, statistically significant from FT group (p < 0.001); arrowheads, primordial follicle. (III) The distribution of primordial follicles expression of Pten (scale bars = 50 μm)
Expression levels of Pten in PFs between groups were semiquantitatively evaluated with H-score. The distribution and the intensity of Pten expression in PFs were scored in Fig. 3 (II). No significant difference was present between the FC and FT groups, while a significant decrease in Pten expression was observed in the grafted groups when compared to the FC and FT groups (p < 0.001) (Fig. 3 (III)).
Tuberous sclerosis complex 1 (Tsc1)
In the FC group, Tsc1 expression was detected, both in the cytoplasm and in the nucleus of oocytes and granulosa cells (Fig. 4 (I-a and II-f)). In FT group, expression of Tsc1 was similar to those in the FC group but slightly lower in oocyte nuclei (Fig. 4 (I-b and II-g)). In group T, Tsc1 expression almost disappeared both in the oocyte nucleus and cytoplasm. Nuclear expression of Tsc1 was significantly lower in granulosa cells (Fig. 4 (I-c and II-h)). In the FTT group, the expression of Tsc1 in oocytes and granulosa cells was similar to those in the T group, and there was a notable loss in nuclear and cytoplasmic Tsc1 immunoreactivity when compared to the control group (Fig. 4 (I-d and II-i)). Negative control samples in Fig. 4 (I-e and I-j) demonstrate the specificity of the Tsc1 antibody.
Fig. 4.
Expressions of Tsc1 in primordial follicles. (I) Tsc1 immunohistochemistry results: a, fresh control group (FC); b, frozen and thawed group (FT); c, fresh-transplanted group (T); d, frozen and thawed before transplanted group (FTT); e, negative control (IgG); f, higher magnification of designated area with the square in FC group; g, higher magnification of designated area with the square in FT group; h, higher magnification of designated area with the square in T group; i, higher magnification of designated area with the square in FTT group; j, higher magnification of negative control. (II) H-score results: a, statistically significant from FC group (p < 0.001); b, statistically significant from FT group (p < 0.001); arrowheads, primordial follicle. (III) The distribution of primordial follicles expression of Tsc1 (scale bars = 50 μm)
Expression levels of Tsc1 in PFs between the groups were semiquantitatively evaluated with H-score. No significant difference was present between the FC and FT groups, while a significant decrease in Tsc1 expression was observed in the T and FTT groups when compared to FC and FT groups (p < 0.001) (Fig. 4 (III)). The distribution and intensity of Tsc1 expression in PFs were scored in Fig. 4 (II).
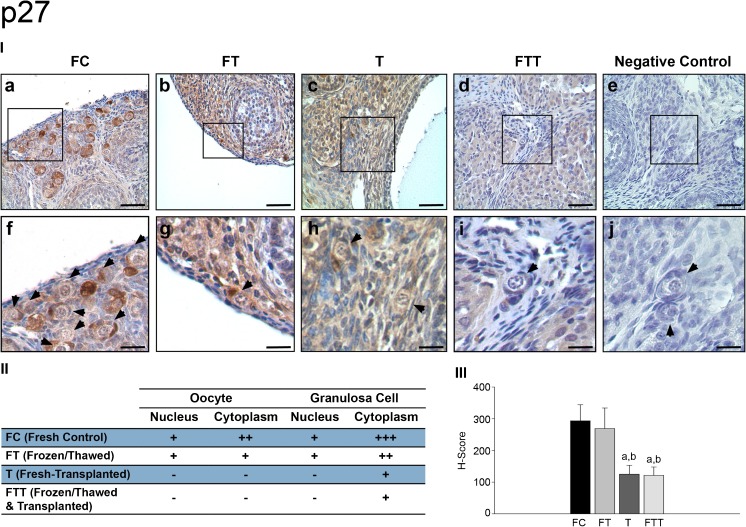
p27
In the FC group, p27 expression was detected both in the cytoplasm and in the nucleus of oocytes and granulosa cells (Fig. 5 (I-a and II-f)). In FT group, expression of p27 was similar to the FC group but slightly decreased in the oocyte cytoplasm (Fig. 5 (I-b and II-g)). In group T, expression of p27 diminished significantly both in the nucleus and cytoplasm of oocytes and granulosa cells of PFs (Fig. 5 (I-c and II-h)). In the FTT group, the expression of p27 in oocytes and granulosa cells was similar to those in group T, and there was a remarkable loss in nuclear and cytoplasmic p27 immunoreactivity when compared to the control group (Fig. 5 (I-d and I-i)). Lack of the p27 staining was demonstrated in negative control samples (Fig. 5 (I-e and I-j)).
Fig. 5.
Expressions of p27 in primordial follicles. (I) p27 immunohistochemistry results: a, fresh control group (FC); b, frozen and thawed group (FT); c, fresh-transplanted group (T); d, frozen and thawed before transplanted group (FTT); e, negative control (IgG); f, higher magnification of designated area with the square in FC group; g, higher magnification of designated area with the square in FT group; h, higher magnification of designated area with the square in T group; i, higher magnification of designated area with the square in FTT group; j, higher magnification of negative control. (II) H-score results: a, statistically significant from FC group (p < 0.001); b, statistically significant from FT group (p < 0.001); arrow heads, primordial follicle. (III) The distribution of primordial follicles expression of p27 (scale bars = 50 μm)
Expression levels of p27 in PFs between the groups were semiquantitatively evaluated with H-score. No significant difference was noted between the FC and FT groups, while a significant decrease in p27 expression was observed in T and FTT groups when compared to FC and FT groups (p < 0.001) (Fig. 5 (III)). The distribution and intensity of p27 expression in PFs were shown in Fig. 5 (II).
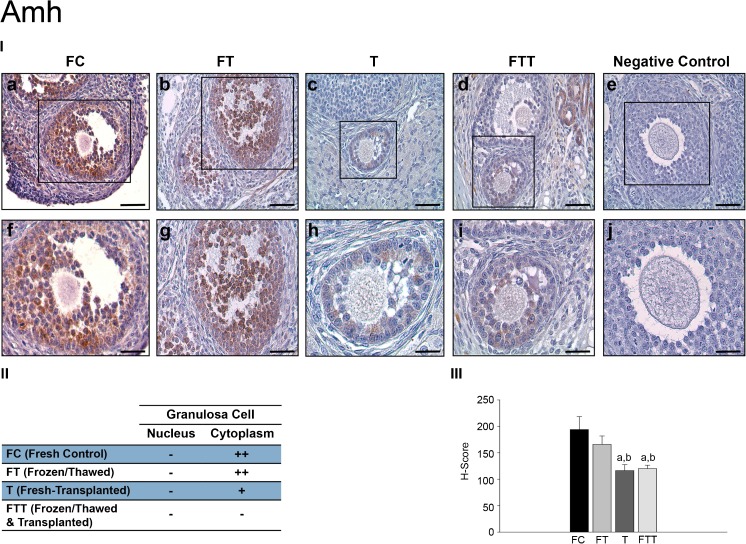
Anti-Mullerian hormone (Amh)
The expression of Amh protein in growing follicles was evaluated in immunostained ovarian sections. In the FC group, the Amh expression was present in granulosa cells (Fig. 6 (I-a and II-f)). In the FT group, the Amh expression was similar to the FC group (Fig. 6 (I-b and I-g)). In group T, the expression of Amh significantly decreased in growing follicles when compared with the FC and FT groups (Fig. 6 (I-c and II-h)). In the FTT group, the expression of Amh in granulosa cells was similar to those in group T, and there was a remarkable loss in its immunoreactivity when compared to the control group (Fig. 6 (I-d and I-i)). Negative control samples demonstrate the specificity of Amh antibody (Fig. 6 (I-e and I-j)).
Fig. 6.
Expressions of AMH in growing follicles. (I) AMH immunohistochemistry results: a, fresh control group (FC); b, frozen and thawed group (FT); c, fresh-transplanted group (T); d, frozen and thawed before transplanted group (FTT); e, negative control (IgG); f, higher magnification of designated area with the square in FC group; g, higher magnification of designated area with the square in FT group; h, higher magnification of designated area with the square in T group; i, higher magnification of designated area with the square in FTT group; j, higher magnification of negative control. (II) H-score results: a, statistically significant from FC group (p < 0.001); b, statistically significant from FT group (p < 0.001). (III) The distribution of growing follicles expression of AMH (scale bars = 50 μm)
Amh expression levels in growing follicles were semiquantitatively evaluated with H-score. No significant difference was present between the FC and FT groups, while a significant decrease in the Amh expression was observed in the T and FTT groups when compared to the FC and FT groups (p < 0.001) (Fig. 6 (III)). The distribution and intensity of the Amh expression in growing follicles are shown in Fig. 6 (II).
Discussion
The OT cryopreservation aims to increase the chance of prospective fertility in cancer patients under chemotherapy by utilizing either transplantation or in vitro culture techniques. Despite promising experimental and clinical outcomes, there is still restricted scientific evidence regarding the follicle preservation mechanisms of retransplanted frozen-thawed OT. Freezing, thawing, and retransplantation cycles are known to somehow diminish the ovarian reserve; however, it is not clear which procedure and/or mechanism(s) are substantially responsible for the PF loss. Understanding the mechanisms involved in dormancy of PFs may lead to various clinical applications like in vitro activation of PFs which has recently been announced [23].
Our present study indicated that transplantation of either fresh or frozen OT causes a significant loss of PF reserve. Increased PF atresia rate in FT, T, and FTT groups when compared to control group indicates that follicle reserve is affected by both freezing alone and also fresh or frozen transplantation processes. The number of growing follicles was not affected by cryopreservation and/or transplantation since there was no significant difference regarding the mean number of primary, secondary, and tertiary follicles between the groups. However, the rate of atretic growing follicles increased significantly after cryopreservation and transplantation. When we evaluated the possible underlying mechanism, our immunohistochemical results demonstrated that expressions of Pten, Tsc1, p27, and Amh remarkably decreased after OT cryopreservation and transplantation. Thus, autotransplantation, regardless of cryopreservation, seems to be the main reason for the loss of those protein expressions. Therefore, global follicle loss, which is a common phenomenon observed after transplanted ovaries following cryopreservation, may be a consequence of the decline of these follicle growth-inhibiting proteins.
For a successful retransplantation, a very small-sized (1–2 mm) ovarian cortical tissue must be cryopreserved [24]. Successful engraftment of tissue grafts obviously depends on the degree of neovascularization, as implants get exposed to ischemic damage during the post-transplantation period until revasculogenesis. Studies on mice show that ovarian graft perfusion begins on the third day after transplantation [25]. In rats, functional angiogenesis of ovarian xenograft is established on the seventh day after transplantation [26]. In humans, the first 5 days of the hypoxia period is present after transplantation [27]. Delay in revascularization commonly causes loss of the PF pool and limits successful transplantation of the ovarian graft. Ischemic damage also induces the depletion of follicle mass around 60–95% after the autografting process [28, 29]. Thus, neoangiogenesis should be improved after ovarian transplantation [30]. A recent study investigated the effect of revascularization and survival of cryopreserved/transplanted mouse OT in a time-dependent manner at 1st, 2nd, and 3rd weeks and found that the grafted ovary may recover to a stable status, at least, 2 weeks after transplantation with a reduction in angiogenic elevation [31]. In addition, follicles were well-preserved in the 2-week subcutaneously transplanted mouse OTs [31]. Thus, our transplantation model was appropriate to evaluate follicle survival rates and expression levels of PF growth inhibitory proteins after 2 weeks of a waiting period.
In a study that investigates the effect of graft thickness on follicle activation, it has been found that ovarian grafts with reduced thickness are more prone to activation and “burn-out” of PFs after 1-week after transplantation. Relatively high numbers of growing follicles and increased Ki67 expression in all recovered grafts indicated that follicle activation was depleting the resting follicle pool at early periods [32]. Another recent study found that extensive PF activation and loss was observed as early as 3 days posttransplantation in multispecies OT grafts, compared to frozen-thawed nongrafted controls indicating that follicle activation plays an important role in transplantation-induced follicle loss, and that it occurs within a very short time frame after grafting [33]. It has been found that the hypoxic stress that OT is subjected to during the first few days after avascular grafting could promote follicle recruitment via activation of the PTK/PI3K/Akt phosphorylation cascade, as this is one of the pathways controlling postgrafting ischemic stress in other tissue types [34, 35]. It is already known that decreased expression of inhibitory proteins leads to activation of PI3K/Akt and mTOR pathways [36]. Decreased inhibitory protein expression was evident after 2 weeks of fresh or frozen transplantation indicating that the balance of PF activation and inhibition may vary with related to the grafting period. Possible roles of activation pathway proteins need to be investigated further in this model.
The loss of inhibitory protein expression in PFs after OT transplantation could be related to hypoxia since in a study where the effect of hypoxia on lung cancer cells was assessed in a xenografted tumor model, loss of Pten expression was observed in hypoxic tumor lesions [37]. Studies on acute myeloid leukemia (AML) showed that hypoxia induces PI3K/Akt pathway and downregulation of p27 on AML cells in the S phase [38]. Activation of Akt pathway via inhibition of Pten causes expression of p27 to stop the cell cycle as a reaction to hypoxia [39]. In our study, we found that both Pten and p27 expression, which support the aforementioned cascade of cellular signaling, were downregulated. We found that p27 expression in granulosa cell nuclei in PFs is almost absent due to ovarian cryopreservation and autotransplantation. Premature follicle activation in PFs was reported in p27 knockout mice [14] and p27 plays an important role in progression control of PFs, essentially retaining them in their dormant state [40].
REDD1 is highly induced in response to hypoxia, and overexpression of REDD1 is sufficient to potently inhibit mTORC1 activity such as Tsc1 while the genetic loss of REDD1 leads to a profound failure to appropriate inhibition of mTORC1 activity in response to hypoxia [41, 42]. Also, deletion of Tsc1 gene triggers over activation of PFs on postnatal days 4 to 23 in mice [17]. These genetic studies have shown the Tsc1 protein to be essential for hypoxia regulation of mTOR activity in mammalian cells. In the present study, we showed that expression of Tsc1, an important PF activity inhibitor, significantly decreased following both fresh and cryopreserved/thawed OT transplantation. Therefore, we suggest that the remarkable loss of Tsc1 expression may cause a reduction in PF pool.
Amh, as a member of TGFβ superfamily being expressed by granulosa cells of the growing follicles, plays a crucial physiological role in adult women in the inhibition of PF activation and follicular recruitment [18]. Amh produced by developing follicles has been suggested to suppress the activation of PFs and thus plays a regulatory role in the recruitment, survival, and growth of PFs [43]. In our study, we noted that the expression of Amh in growing follicles reduced significantly after fresh transplantation or frozen/thawed transplantation which may result in PF depletion. In Amh gene deleted mice, depletion of PF pool occurs on postnatal 4th month [19]. Amh was also found to control PF recruitment by inhibiting the initiation of PF growth and by attenuating the effects of FSH on follicle development in the mouse ovary [19]. Reduction of growing follicles 40–50% was reported when in vitro cultured neonatal mice ovaries were incubated with Amh, which inhibits the PF recruitment into the growing follicles and reduces FSH responsiveness of growing follicles [44].
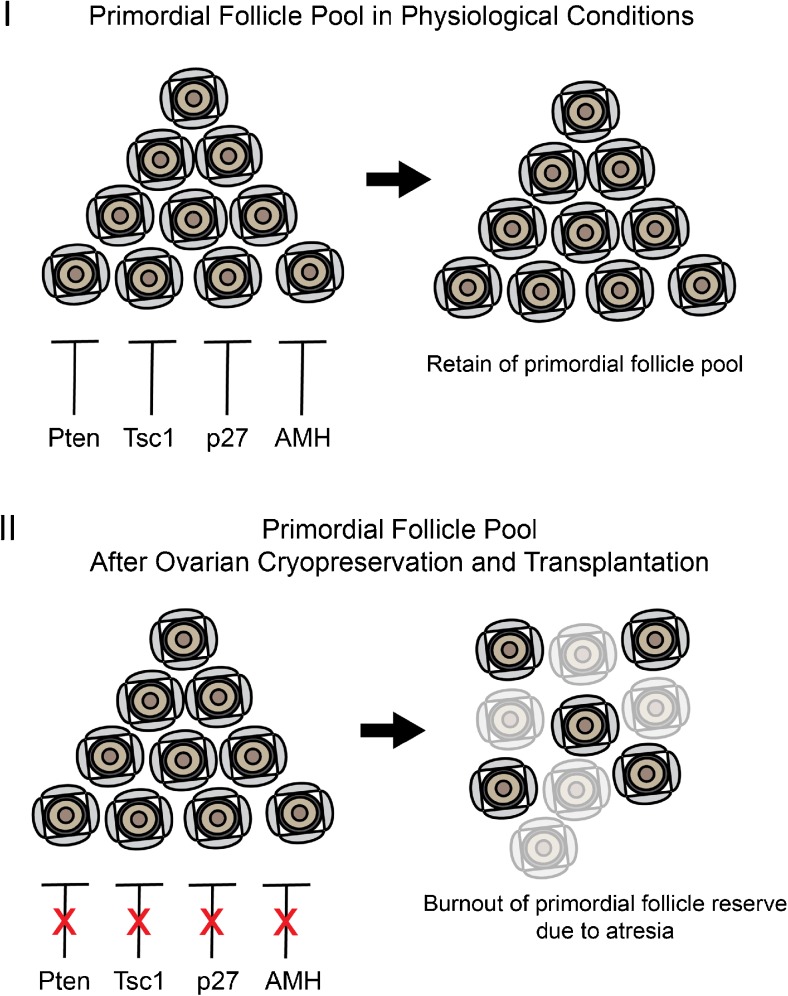
To correlate the results of the aforementioned studies to our findings, we suggest that autotransplantation of removed ovary ends with PFs loss which may be a consequence of decreased expression of inhibitory proteins that control PF growth. A hypothetical mechanism related to OT cryopreservation/transplantation and “depletion” of PFs is illustrated in Fig. 7. Under physiological circumstances, PFs remain in a dormant state by the inhibitory effects of Pten, Tsc1, p27, and Amh that are expressed in oocytes and/or granulosa cells (Fig. 7 (I)), whereas a decrease in the expression of Pten, Tsc1, p27, and Amh occurs after autotransplantation of OT pieces and a global loss of PFs due to atresia (Fig. 7 (II)).
Fig. 7.
A hypothetical mechanism regarding the possible roles of inhibitory proteins that regulate primordial follicle pool in physiological conditions (I) and after ovarian cryopreservation and transplantation (II)
In conclusion, the results of this study demonstrate that suppressor proteins that regulate the PF reserve are disturbed following ovarian cryopreservation, particularly after transplantation. Decreased expression of Pten, Tsc1, p27 in PFs, and Amh in growing follicles after transplantation, independent of freezing and thawing cycles, leads to decreased ovarian reserve and increased atresia of the follicles. Thus, autotransplantation seems to be the major cause for PF loss and disruption of inhibitory molecular signaling that controls the ovarian reserve.
References
- 1.Sonmezer M, Ozkavukcu S. Fertility preservation in females with malignant disease-1: causes, clinical needs and indications. Turk J Hematol. 2009;26. [PubMed]
- 2.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM. Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet. 2015;32(8):1167–1170. doi: 10.1007/s10815-015-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci. 2003;78(3–4):135–163. doi: 10.1016/S0378-4320(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 5.Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25(12):2944–2954. doi: 10.1093/humrep/deq275. [DOI] [PubMed] [Google Scholar]
- 6.Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr Rev. 2009;30(5):438–464. doi: 10.1210/er.2008-0048. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- 8.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco BI, et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8(21):1169–1178. doi: 10.1016/S0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 10.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319(5863):611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 11.John GB, Gallardo TD, Shirley LJ, Castrillon DH. Foxo3 is a PI3K-dependent molecular switch controlling the initiation of oocyte growth. Dev Biol. 2008;321(1):197–204. doi: 10.1016/j.ydbio.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto M, Iwase A, Ando H, Kurotsuchi S, Harata T, Kikkawa F. PTEN and Akt expression during growth of human ovarian follicles. J Assist Reprod Genet. 2007;24(11):541–546. doi: 10.1007/s10815-007-9156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin I, Rotty J, Wu FY, Arteaga CL. Phosphorylation of p27Kip1 at Thr-157 interferes with its association with importin alpha during G1 and prevents nuclear re-entry. J Biol Chem. 2005;280(7):6055–6063. doi: 10.1074/jbc.M412367200. [DOI] [PubMed] [Google Scholar]
- 14.Rajareddy S, Reddy P, Du C, Liu L, Jagarlamudi K, Tang W, et al. p27kip1 (cyclin-dependent kinase inhibitor 1B) controls ovarian development by suppressing follicle endowment and activation and promoting follicle atresia in mice. Mol Endocrinol. 2007;21(9):2189–2202. doi: 10.1210/me.2007-0172. [DOI] [PubMed] [Google Scholar]
- 15.Kaldis P. Another piece of the p27Kip1 puzzle. Cell. 2007;128(2):241–244. doi: 10.1016/j.cell.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355(13):1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 17.Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, et al. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19(3):397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, et al. Anti-mullerian hormone and anti-Mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology. 1995;136(11):4951–4962. doi: 10.1210/endo.136.11.7588229. [DOI] [PubMed] [Google Scholar]
- 19.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 20.Topal-Celikkan F, Ozkavukcu S, Balci D, Serin-Kilicoglu S, Atabenli-Erdemli E. Mouse ovarian tissue vitrification on copper electron microscope grids versus slow freezing: a comparative ultrastructural study. Reprod Fertil Dev. 2014; 10.1071/RD13262. [DOI] [PubMed]
- 21.Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini TA, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006;85(Suppl 1):1150–1156. doi: 10.1016/j.fertnstert.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 22.Inan S, Vatansever S, Celik-Ozenci C, Sanci M, Dicle N, Demir R. Immunolocalizations of VEGF, its receptors flt-1, KDR and TGF-beta’s in epithelial ovarian tumors. Histol Histopathol. 2006;21(10):1055–1064. doi: 10.14670/HH-21.1055. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura K, Kawamura N, Hsueh AJ. Activation of dormant follicles: a new treatment for premature ovarian failure? Curr Opin Obstet Gynecol. 2016;28(3):217–222. doi: 10.1097/GCO.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18(2):59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]
- 25.Nugent D, Newton H, Gallivan L, Gosden RG. Protective effect of vitamin E on ischaemia-reperfusion injury in ovarian grafts. J Reprod Fertil. 1998;114(2):341–346. doi: 10.1530/jrf.0.1140341. [DOI] [PubMed] [Google Scholar]
- 26.Israely T, Dafni H, Nevo N, Tsafriri A, Neeman M. Angiogenesis in ectopic ovarian xenotransplantation: multiparameter characterization of the neovasculature by dynamic contrast-enhanced MRI. Magn Reson Med. 2004;52(4):741–750. doi: 10.1002/mrm.20203. [DOI] [PubMed] [Google Scholar]
- 27.Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92(1):374–381. doi: 10.1016/j.fertnstert.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15(6):649–665. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93(5):1676–1685. doi: 10.1016/j.fertnstert.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 30.Kim SS. Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril. 2006;85(1):1–11. doi: 10.1016/j.fertnstert.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 31.Li SH, Hwu YM, Lu CH, Chang HH, Hsieh CE, Lee RK. VEGF and FGF2 improve revascularization, survival, and oocyte quality of cryopreserved, subcutaneously-transplanted mouse ovarian tissues. Int J Mol Sci. 2016;17(8) 10.3390/ijms17081237. [DOI] [PMC free article] [PubMed]
- 32.Gavish Z, Peer G, Roness H, Cohen Y, Meirow D. Follicle activation and ‘burn-out’ contribute to post-transplantation follicle loss in ovarian tissue grafts: the effect of graft thickness. Hum Reprod. 2014;29(5):989–996. doi: 10.1093/humrep/deu015. [DOI] [PubMed] [Google Scholar]
- 33.Gavish Z, Spector I, Peer G, Schlatt S, Wistuba J, Roness H, et al. Follicle activation is a significant and immediate cause of follicle loss after ovarian tissue transplantation. J Assist Reprod Genet. 2017; 10.1007/s10815-017-1079-z. [DOI] [PMC free article] [PubMed]
- 34.Lopez-Neblina F, Toledo-Pereyra LH. Phosphoregulation of signal transduction pathways in ischemia and reperfusion. J Surg Res. 2006;134(2):292–299. doi: 10.1016/j.jss.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 35.David A, Van Langendonckt A, Gilliaux S, Dolmans MM, Donnez J, Amorim CA. Effect of cryopreservation and transplantation on the expression of kit ligand and anti-Mullerian hormone in human ovarian tissue. Hum Reprod. 2012;27(4):1088–1095. doi: 10.1093/humrep/des013. [DOI] [PubMed] [Google Scholar]
- 36.Goldman KN, Chenette D, Arju R, Duncan FE, Keefe DL, Grifo JA, et al. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc Natl Acad Sci U S A. 2017;114(12):3186–3191. doi: 10.1073/pnas.1617233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohnoh T, Hashimoto N, Ando A, Sakamoto K, Miyazaki S, Aoyama D, et al. Hypoxia-induced modulation of PTEN activity and EMT phenotypes in lung cancers. Cancer Cell Int. 2016;16:33. doi: 10.1186/s12935-016-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drolle H, Wagner M, Vasold J, Kutt A, Deniffel C, Sotlar K, et al. Hypoxia regulates proliferation of acute myeloid leukemia and sensitivity against chemotherapy. Leuk Res. 2015;39(7):779–785. doi: 10.1016/j.leukres.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285(5436):2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 40.Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996;85(5):733–744. doi: 10.1016/S0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 41.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18(23):2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeYoung MP, Horak P, Sofer A, Sgroi D, Ellisen LW. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008;22(2):239–251. doi: 10.1101/gad.1617608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong HS, Kim SK, Lee J, Youm HW, Lee JR, Suh CS, et al. Effect of exogenous anti-Mullerian hormone treatment on cryopreserved and transplanted mouse ovaries. Reprod Sci. 2016;23(1):51–60. doi: 10.1177/1933719115594021. [DOI] [PubMed] [Google Scholar]
- 44.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124(5):601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]