Abstract
Purpose
The purpose of the study is to review all peer-reviewed published reports of women receiving ovarian tissue transplantation (OTT) with frozen/thawed tissue (OTC) with respect to age, diagnosis, transplantation site, fertility outcome, and potential side effects, including data from all women in the Danish program.
Methods
A systematic review of the literature was performed in PubMed combined with results from all patients who had received OTT in Denmark up to December 2017.
Results
OTT has been reported from 21 different countries comprising a total of 360 OTT procedures in 318 women. In nine women, malignancy was diagnosed after OTT; none were considered to be directly caused by the OTT. Despite a potential under reporting of cancer recurrence, there is currently no evidence to suggest that OTT causes reseeding of the original cancer. Renewed ovarian endocrine function was reported in 95% of the women. Half of all children born following OTT resulted from natural conception, and newborns were reported to be healthy except for one neonate with a chromosome anomaly with a family disposition. Women who conceived after OTT were significantly younger than those who failed.
Conclusion
This study found no indications of sufficient numbers of malignant cells present in the ovarian tissue to cause recurrence of cancer after OTT. Further, it is unlikely that OTC affects the well-being of children born. OTC is now an established method of fertility preservation in Denmark with public reimbursement. The current data encourage that women who require gonadotoxic treatment should be offered an individual evaluation considering fertility preservation.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1144-2) contains supplementary material, which is available to authorized users.
Keywords: Ovarian transplantation, Ovarian tissue cryopreservation, Outcome, Safety, Relapse
Introduction
Ovarian tissue cryopreservation (OTC) and transplantation (OTT) is a valid option to be considered by women, adolescents, parents of children, and their doctors for fertility preservation, supplementing the established technologies of oocyte and embryo cryopreservation. Many countries worldwide have embarked on developing clinical services to include OTC, and more than 20 countries have published manuscripts describing the birth of one or more children after OTT, although activity is still relatively modest and only performed in specialized centers [1, 2]. Furthermore, a recent report stated that in the relatively few children on whom data were available, all were healthy and had neonatal outcome similar to that of normal pregnancies [1].
One of the major concerns of more widespread use of OTT is the potential risk of grafting malignant cells harbored in the tissue at OTC, which could potentially result in reintroduction of the disease after transplantation. A large number of in vitro studies have been conducted evaluating the safety of OTT. Different studies have focused on different patient categories, who most frequently receive OTC and OTT including diagnoses like breast cancer, lymphoma cancers, sarcoma, and leukemia cancer [3–9]. The methods used to evaluate potential malignant cell re-introduction usually involved histology including immunohistochemistry, molecular biology techniques such as q-PCR, and transplantation to immunodeficient mice to determine whether a few, if any, surviving malignant cells are transplanted, to become subsequently manifest. Most of these studies concluded that in patients with non-disseminated (i.e., early stage cancer) disease, OTT appeared safe except for leukemia, where the ovarian tissue may harbor malignant cells unless patients are in full remission at the time of OTC [7]. If the tissue proves to be negative for potential molecular biomarkers, it is considered risk free and has justified transplantation in few cases where the patient previously suffered from leukemia [10, 11].
However, despite these reassuring observations, there are still uncertainties about OTT in a clinical setting since some questions remain unanswered, notably, how many malignant cells are sufficient to cause a relapse, in the context of more malignant cells being present with respect to a larger amount of tissue grafted, leading to the possibility of a higher risk of relapse. But if only a few cells can lead to relapse, it may be impossible to confidently identify or exclude their presence prior to grafting. Furthermore, in this respect, potential differences may exist between different types of cancer.
To assess the safety and efficacy of OTC and OTT, the aim of this review was to examine all peer-reviewed reports of OTT with respect to age, diagnosis, transplantation site and amount of transplanted tissue, fertility outcome, and potential side effects, combined with data from additional patients who had OTT in the Danish program, as yet unpublished.
Material and methods
A systematic review of the literature was performed in PubMed including all relevant peer-reviewed publications in the English language up to December 2017. The search terms were “ovary,” “cryopreservation,” “transplantation,” “autotransplantation,” and “ovarian tissue.” Additionally, we manually searched the reference lists of the identified articles for other references, which were not identified in the PubMed search.
We included all publications mentioning information on OTC and OTT in humans and excluded cases involving fresh ovarian tissue transplantation. In addition, data from pregnancies and live births from oocyte donations [12, 13] and patients in whom the date of conception was estimated to coincide with the date of transplantation were also excluded [14]. It was also decided to exclude OTT in patients in whom premature ovarian insufficiency (POI) had been diagnosed at the time of OTC [15–17]. A large proportion of these women did not present with ovarian follicles at the time of OTC explaining why ovarian function did not commence upon transplantation. Thus, these women represent a different group from those with known ovarian function at OTC.
Furthermore, we included 89 patients who had had OTT prior to December 2017 in the Danish cohort. Data from 41 of these women were previously published [1], but not from the remaining 48 women. In the Danish cohort, 89 women had currently undergone a total of 115 transplantation procedures, leading to 33 clinical pregnancies in 23 women resulting in 16 live births and 17 children born (one twin pregnancy).
Results
Transplantation of frozen-thawed ovarian tissue has been reported from 21 different countries comprising a total of 360 transplantation procedures in 318 women (Table 1). Thirty-nine patients had a second OTT, while three patients had three OTT procedures.
Table 1.
Number of transplantations and number of transplanted women as published in peer-reviewed papers and in the Danish cohort
| Country | No. of transplantations | No. of transplanted women | Reference |
|---|---|---|---|
| Argentina | 2 | 1 | [18, 19] |
| Australia | 23 | 21 | [20–25] |
| Belgium | 35 | 30 | [26–45] |
| Denmark | 113 | 89 | [1, 13, 31, 46–56] |
| Finland | 4 | 4 | [57] |
| France | 4 | 4 | [31, 58–60] |
| Germany, Austria, Switzerland | 76 | 75 | [12, 61–65] |
| Israel | 25 | 22 | [10, 14, 31, 45, 66–69] |
| Italy | 4 | 4 | [70, 71] |
| Norway | 2 | 2 | [1, 72] |
| Poland | 1 | 1 | [73] |
| Portugal | 1 | 1 | [74] |
| Russia | 1 | 1 | [75, 76] |
| South Korea | 1 | 1 | [77] |
| Spain | 24 | 24 | [26, 78–81] |
| Sweden | 12 | 10 | [57, 82–84] |
| Turkey | 1 | 1 | [85] |
| UK | 2 | 2 | [86, 87] |
| USA | 29 | 25 | [11, 31, 88–100] |
| Total | 360 | 318 |
Information concerning the average time from OTC to the first, second, and third transplantation procedure was available for 156, 38, and 3 patients, respectively (Table 2). The mean age at OTC was 28.9 years (range 9–47 years), the first transplantation being performed at a mean age of 33.5 years (range 13–47 years). At the time of the second OTT, the mean age was 33.9 years, (range 25–42 years), and at the third 33.7 years (range 26–39 years) (Table 2).
Table 2.
Mean age, time from freezing to transplantation, and total number of women with a first, second, or third ovarian tissue transplantation (OTT)
| No. of patients | Mean age (years) | Range (years) | Years from cryopreservation to transplantation (range) | |
|---|---|---|---|---|
| Cryopreservation | 318 | 28.9 | 9–47 | |
| First OTT | 318 | 33.5 | 13–47 | 4.4 (0.3–13.9) |
| Second OTT | 39 | 33.9 | 25–42 | 6.1 (2.0–14.5) |
| Third OTT | 3 | 33.7 | 26–39 | 10.2 (6.6—12.5) |
Safety
Recurrence of malignancy
In 318 women who had OTC, a diagnosis was available in 264, being malignant disease in 230 (87%) cases and non-malignant disease in 34 (13%) (Fig. 1). From the cohort of women with malignancy, nine experienced a recurrence after OTT, four of whom are now deceased, some details of which are summarized below.
Fig. 1.
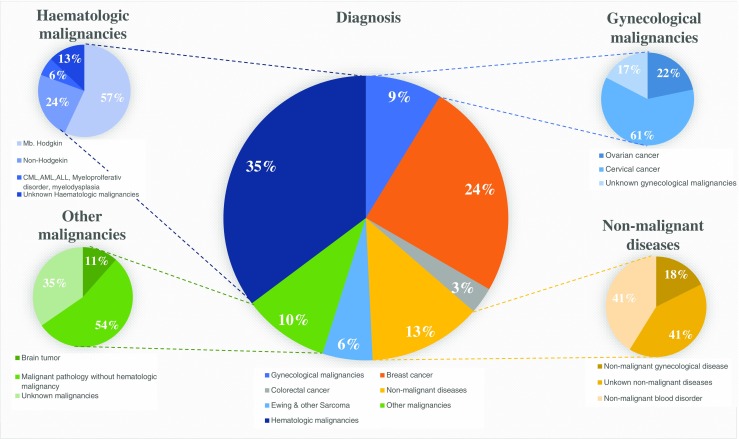
The diagnosis at OTC
Kim and colleagues [88] reported the recurrence of cervical cancer. Ovarian function returned 5 months after transplantation; however, the patient died due to recurrence of cancer soon thereafter. Local recurrence of disease in a breast cancer survivor was also reported by Dittrich and colleagues in 2015 [12]. In neither of these women was the recurrence considered to be related to the transplantation.
In 2014, Stern et al. [20] published a case report where a woman diagnosed with a granulosa cell tumor received OTT at a heterotopic graft site. She became pregnancy with twins. At delivery during cesarean section, macroscopic evidence of tumor was found involving the diaphragm and a peritoneal deposit located at the left pelvic brim. All the macroscopic tumor tissue was removed, and a granulosa cell tumor was confirmed by histology. When analyzing the previously grafted tissue, it showed no evidence of malignancy. Subsequently, the patient was treated and recovered from the tumor.
In the Danish cohort, six have experienced a relapse of whom three are deceased. Two women formerly diagnosed with breast cancer experienced local relapses. One of the two was pregnant and had an abortion in the eighth week of pregnancy just prior to chemotherapy [47], and the other patient has deceased [46]. A former Ewing’s sarcoma patient had her tissue transplanted at the age of 13 to induce puberty being 9 years at OTC. She experienced a relapse occurring in the hemithorax 4.5 years after transplantation and died subsequently [46, 48, 49]. The remaining frozen tissue was analyzed for the possible presence of malignant cells, and was found negative. Additionally, three unpublished local relapses have occurred: one in a cervical cancer patient where a relapse occurred locally next to where cancer tissue was originally removed, one in a patient with former thymic cancer, and another in a patient with brain cancer, who has deceased. All six relapses were considered to be unrelated to the transplantation of ovarian tissue.
In an article from 2016 [14], a patient formerly diagnosed with Ewing’s sarcoma was diagnosed with breast cancer shortly after transplantation, which was considered unrelated to transplantation.
Fajau-Prevot et al. reported in 2017 [58] of a woman diagnosed with an Ewing sarcoma in the neck. After OTT, she became spontaneously pregnant and delivered a health child by cesarean section. During the delivery, a 5 × 3 cm benign mucinous cystadenoma was removed from the grafted ovarian tissue. Two cortical strips were evaluated histologically prior to OTT and found negative for Ewing sarcoma cells, both histologically and using molecular markers [58]. When analyzing the tissue from the cystectomy, no histologic evidence of malignancy was found.
Diagnosis for OTC
In 264 of the cases with OTT, the diagnosis at OTC was specified. Among the diagnosis, breast cancer and hematological malignancies, including both Morbus Hodgkin and non-Hodgkin, were the main reasons for fertility preservation (Fig. 1) (Supplementary Table 1).
Transplantation site and amount of tissue transplanted
Ovarian tissue may be grafted either orthotopically and/or heterotopically. Here, we considered an orthotopic transplantation as one where natural conception was possible and a heterotopic transplantation as one that would require assisted reproductive technology (ART) for conception. Therefore, graft sites in the remaining ovary and/or in peritoneal pockets in the pelvis were considered orthotopic, whereas graft sites in the subperitoneal space of the abdomen and in the subcutaneous space of the forearm or abdomen were considered heterotopic. The location of the graft site was available in 242, 28, and 3 of the cases who had a first, second, and third OTT performed, respectively (Tables 3 and 4).
Table 3.
The graft site of ovarian tissue transplantation (OTT) for the patients who had a first, second, and third transplantation
| Transplantation site | |||
|---|---|---|---|
| First OTT | Second OTT | Third OTT | |
| Heterotopic | 17 (5%) | 6 (15%) | 0 (0%) |
| Orthotopic | 213 (67%) | 17 (44%) | 3 (100%) |
| Both heterotopic and orthotopic | 12 (4%) | 5 (13%) | 0 (0%) |
| Unavailable | 76 (24%) | 11 (28%) | 0 (0%) |
| Total | 318 (100%) | 39 (100%) | 3 (100%) |
Table 4.
Location of the graft at orthotopic transplantation
| Orthotopic graft site | |||
|---|---|---|---|
| First OTT | Second OTT | Third OTT | |
| Ovary | 62 (28%) | 11 (50%) | 0 (0%) |
| Peritoneal pocket | 59 (26%) | 6 (27%) | 2 (67%) |
| Both ovary and peritoneal pocket | 38 (17%) | 5 (23%) | 1 (33%) |
| Unavailable | 66 (29%) | 0 (0%) | 0 (0%) |
| Total | 225 (100%) | 22 (100%) | 3 (100%) |
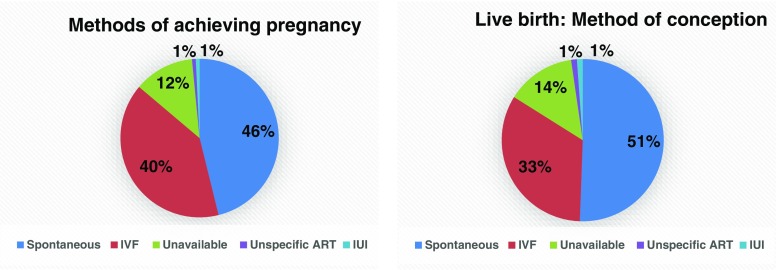
Only considering women who had a live birth, the vast majority were conceived in patients with an orthotopic or combined heterotropic/orthotopic graft, and only a single woman so far has managed to conceive through a heterotopic graft [21] (Fig. 2).
Fig. 2.
Frequency of methods used to obtain a pregnancy (N = 131) and b live birth (N = 87)
Women who wished to avoid menopause but who did not have a pregnancy wish comprised a total of 25 cases. Thirteen women had tissue transplanted orthotopically, eight heterotopically, and in four cases, the information was unavailable.
The average amount of transplanted tissue at the first OTT corresponded to 46% of the total amount of frozen tissue, with an average area of 294 mm2. During the second OTT, an average amount of 37% of the total amount of frozen tissue was transplanted comprising an average area of 263 mm2, while the third transplantation used an average amount of 38%, with an average area of 455 mm2 (Table 5).
Table 5.
Average amount of tissue transplanted at the first, second, and third ovarian tissue transplantation
| No. of cases | Average amount | Range | |
|---|---|---|---|
| First transplantation | |||
| Amount of tissue transplanted per total amount of frozen stisue | 161 | 46% | 10–100% |
| Fraction of ovary transplanted when one whole ovary was frozen | 108 | 45% | 12–100% |
| Area of transplanted ovary | 110 | 294 mm2 | 24–896 mm2 |
| Second transplantation | |||
| Amount of tissue transplanted per total amount of frozen tissue | 28 | 37% | 10–56% |
| Part of ovary transplanted when one whole ovary was frozen | 25 | 37% | 10–56% |
| Area of transplanted ovary | 33 | 263 mm2 | 24–735 mm2 |
| Third transplantation | |||
| Amount of tissue transplanted per total amount of frozen tissue | 2 | 38% | 24–51% |
| Part ovary transplanted when one whole ovary was frozen | 2 | 38% | 24–51% |
| Area of transplanted ovary | 2 | 455 mm2 | 175–735 mm2 |
Outcome after ovarian tissue transplantation
Endocrine function
Out of the 318 first OTT, the status of endocrine function was available in 237 of the cases, reporting 12 (5%) cases of non-restored function and 225 (95%) cases of restored function.
The presence of endocrine function was measured by either follicular growth or the recurrence of menstruation. The period from the first OTT to follicular function was on average 4.0 months (SD 1.5 months, range 1–8, n = 83). In cases reporting absence of endocrine restoration, so far only one case has been reported to have additional transplantations [82]. The second transplantation resulted in restored endocrine function, and the third transplantation resulted in the birth of a healthy girl, conceived by intracytoplasmic sperm injection (ICSI) [82].
Of the 39 women who underwent a second OTT and 3 women who underwent a third transplantation, endocrine function was reported in 29 cases, while information from the remaining 10 cases was missing. The period from the second OTT and third OTT to follicular function was on average 3.9 months (SD 1.4 months, range 2–6, n = 13) and 4.5 months (SD 2.1 months, range 3–6, n = 2), respectively.
Pregnancies and live births
In a total of 318 women, 170 cases wished to restore fertility—7 were excluded, 25 specifically only wished for restoration of the ovarian endocrine function, and in 123 patients, data was unavailable. In total, 131 pregnancies were obtained in 95 patients, counting both biochemical and clinical pregnancies. This resulted in 87 live births in 69 women, and a total of 93 children born (Fig. 2). Hereof, 81 singleton pregnancies and 6 twin pregnancies [14, 20, 26, 46, 78, 83] with 10 pregnancies ongoing [10, 12, 14, 22, 26–28, 57] (Danish cohort) from which 7 women will have their first child.
The age of patients who succeeded in having a live birth (LB) or ongoing pregnancy (OGP) (26.4 years (SD 6.3), range 9–38 years) were significantly younger at OTC (P value = 0.0019) compared to patients who failed to conceive but had a pregnancy wish (29.6 years (SD 5.4), range 14–39 years).
In total, three women managed to deliver three healthy babies during three consecutive pregnancies; all were menopausal before OTT. A woman in Belgium had her ovarian tissue cryopreserved at the age of 17, due to a neuroectodermal tumor, and has delivered three times after one orthotopic OTT in the ovary [26]. In Israel, a patient with an OTC at age 24 conceived four times after an orthotopic transplantation; hereof, two in vitro fertilization (IVF) pregnancies resulted in one live birth and one early abortion, and two successful spontaneous pregnancies [14]. In Denmark, a woman had her tissue frozen at the age of 27, due to an Ewing’s sarcoma. After OTT, she has had three healthy children, delivering the third baby almost 6 years after having six pieces of ovarian tissue transplanted [50].
Three legal abortions following OTT have been published. One occurred due to a genetic diagnosis showing that the embryo carried the same BRCA1 mutation as the mother [29]. A second abortion took place because the woman was separating from her partner [51], and a third because the woman experienced a relapse of her breast cancer [47]. One woman who had radiation to the pelvis experienced miscarriage twice, both times in gestational week 19, due to premature preterm rupture of membranes (PPROM) [46].
Method of conception
Out of the 131 pregnancies and the 87 live births, 46% (Fig. 2a) and 51%, respectively (Fig. 2b), were achieved spontaneously. The couples who have achieved spontaneous conceptions that have led to live births, 84% of the women were menopausal before OTT. The conceptions achieved by IVF, 75% of the women were menopausal before OTT. Three of the total 87 live births were from patients who had previously undergone bilateral oophorectomy. Two women conceived by IVF from an orthotopic peritoneal pocket [30, 79], and one conceived by IVF from a heterotopic abdominal pocket [23].
Children born
The health and perinatal outcome of children born from OTC and OTT has recently been reported [1]. From the 40 children in whom data were available showed similar birth weight and similar gestational age as children born from normal pregnancies. From the total 93 children born, only one has been reported with a chromosome anomaly. In Meirow’s group [14], they reported one patient who delivered a child with fetal arthrogryposis. The patient had a family history of other limb malformations.
Discussion
The aim of this review was to account for the worldwide activity of OTC and OTT, as published in peer-reviewed papers in combination with updated results from the Danish cohort. This study identified a total of 360 transplantations performed in a total of 318 patients. However, a high number of unreported cases have also been performed, and the total transplantation activity is obviously well above 360 cases. This was recently highlighted in a review presenting a total of 130 children born from OTC and OTT taking also abstracts and congress proceedings into account [2]. Nevertheless, data from peer-reviewed publications offer more reliable data and as these procedures are not yet considered established by many professional organizations and authorities, it is important to present the collective experience with the best-quality data, especially in connection with evaluating the safety of the procedure. The above notwithstanding, the collective data presented in this review offer reassurance on the safety of the procedure. Transplantation to women with a known former malignant diagnosis has been performed 230 times, and there have been no reports on recurrence of the original cancer in connection with OTT (except for the granulosa cell tumor that most likely developed as a new cancer after grafting). Although reported relapses are likely to be under-represented in published cases, it does not seem to be a major problem with current policies employed for grafting and the results from Denmark alone are encouraging. The transplanted women represent a group of women where relapse may occur, and in this context, it is interesting that the frequency of women who actually experience relapse in Denmark has remained constant on 7% from our first account representing 41 women [46] until today where 89 women have been grafted. This suggests that the relapse rate does not increase over time within the Danish cohort representing women who have now had tissue transplanted for less than a year and up to 13 years.
When appropriate safety tests have been applied to secure transplantation as practically possible outside a clinical setting, real cases of transplantation provide the ultimate answer to the safety issue of OTT. Often cortical tissue from one third to half of one entire ovary is transplanted, if not more, which obviously makes it impossible to check all pieces on beforehand as the tissue is not suitable for transplantation after the evaluation process. Therefore, close long-term follow-up of these patients is of utmost importance. With a total of 230 women having tissue transplanted with a former malignant diagnosis without reported relapses relating to the ovarian tissue, it appears appropriate to conclude that metastasis to the ovary rarely occur to an extend that causes relapse and that currently the method is safe. In this context, it is also noticeable that 31 women have had two or three transplantations, some of whom have had all the retrieved tissue transplanted, without affecting the relapse rate. Breast cancer is the most frequent diagnosis for.
OTT accounting for 24% (N = 65) and Morbus Hodgkins disease 20% (N = 53) of all cases showing that these two diagnoses have now been tested for safety in good numbers. However, it is obviously of outmost importance that relapses following OTT are reported in the literature, especially if the ovarian tissue transplanted is suspected to be involved as a causative factor.
The different diagnosis used for OTT more or less reflects the frequency by which most of the common cancers occur in girls and young women. However, cancers prevalent in the young years such as cancer to the brain, sarcomas, leukemia, and genetic diseases are probably under represented since these girls have not yet reached childbearing age, and OTT will likely increase in this category in the years to come. It is noticeable that the vast majority of transplantations occur to the remaining ovary or to an orthotopic site probably reflecting that most women undergo OTT in order to become pregnant and only a minority wishes only to avoid menopausal symptoms where a heterothopic site will allow for follicular development and sex steroid production. The use of the remaining ovary present in the women as a site for transplantation is further supported by the fact that half of all children conceived occur by natural conception and indeed highlight the ovary as the preferred transplantation site for regaining fertility. Furthermore, a few studies have indicated that ovarian tissue transplanted outside of the ovaries may support follicular development and production of oocytes capable of supporting fertilization less frequently than when transplanted to the ovaries [101, 102].
Currently, it is difficult to predict the lifespan of grafted tissue. It is dependent on a number of conditions including age of the woman, the follicular density, the amount of tissue grafted, and how fast revascularization occur to the transplanted tissue among others. Indeed, this study demonstrated that women giving birth to child after OTC are significantly younger compared to those who fail to conceive despite a pregnancy wish. This suggests that the number of follicles transplanted affect the chances of conceiving. However, the amount of tissue transplanted is not a very precise term, since two women of the same age may have very different-sized ovaries, e.g., 4 and 12 ml in volume. These two women will most likely produce a different number of cortical pieces for freezing, and assuming they have equal number of follicles, the same number of pieces transplanted will represent different number of follicles, therefore most likely providing fertility with different efficacy. We have attempted to provide figures on the amount of tissue transplanted as a fraction of one ovary or as a measure of the surface area, when data were available. Perhaps these figures will prove to improve prognosis for the longevity of the transplanted tissue, but future studies will need to prove that. It is also noticeable how variable the duration of the grafts is and how well they provide fertility. Some women may only experience ovarian function for a limited period of time around 1 year, while others have ovarian function for up to 10 years [46] and may achieve three children from three independent pregnancies. This most likely reflect differences in the number of follicles available at transplantation, but probably also reflects that the transplantation procedures have not yet been optimized and may provide a variable number of follicles available for the woman.
In conclusion, based on OTT as published in peer-reviewed publications combined with data from the Danish cohort, in total more than 300 cases of OTT, this study has found no evidence of reseeding malignant cells present in the frozen ovarian tissue in sufficient numbers to cause recurrence of the cancer. Neither does it seem likely that OTC and OTT affect the well-being of children born from this procedure. Together with the reassuring results of 93 children born OTC and OTT is considered an established method of fertility preservation in Denmark. Worldwide, we support that if patient needs to undergo gonadotoxic treatment, an individual evaluation considering fertility preservation should always be conducted.
Electronic supplementary material
(DOCX 18 kb)
Acknowledgements
All personnel including the clinical activities in fertility preservation are thanked for their passionate work.
Funding information
The University Hospital of Copenhagen, the EU interregional project ReproUnion, and Vera and Carl Johan Michaelsens Legat are gratefully acknowledged for financial support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1144-2) contains supplementary material, which is available to authorized users.
References
- 1.Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY. 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet. 2017;34(3):325–336. doi: 10.1007/s10815-016-0843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/NEJMra1614676. [DOI] [PubMed] [Google Scholar]
- 3.Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30(1):11–24. doi: 10.1007/s10815-012-9912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SS, Donnez J, Barri P, Pellicer A, Patrizio P, Rosenwaks Z, et al. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J Assist Reprod Genet. 2012;29(6):465–468. doi: 10.1007/s10815-012-9786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt KT, Andersen CY. Recommendations for fertility preservation in patients with lymphomas. J Assist Reprod Genet. 2012;29(6):473–477. doi: 10.1007/s10815-012-9787-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greve T, Wielenga VT, Grauslund M, Sorensen N, Christiansen DB, Rosendahl M, et al. Ovarian tissue cryopreserved for fertility preservation from patients with Ewing or other sarcomas appear to have no tumour cell contamination. Eur J Cancer (Oxf, Engl: 1990) 2013;49(8):1932–1938. doi: 10.1016/j.ejca.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Greve T, Clasen-Linde E, Andersen MT, Andersen MK, Sorensen SD, Rosendahl M, et al. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood. 2012;120(22):4311–4316. doi: 10.1182/blood-2012-01-403022. [DOI] [PubMed] [Google Scholar]
- 8.Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99(6):1514–1522. doi: 10.1016/j.fertnstert.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Soares M, Saussoy P, Sahrari K, Amorim CA, Donnez J, Dolmans MM. Is transplantation of a few leukemic cells inside an artificial ovary able to induce leukemia in an experimental model? J Assist Reprod Genet. 2015;32(4):597–606. doi: 10.1007/s10815-015-0438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapira M, Raanani H, Barshack I, Amariglio N, Derech-Haim S, Marciano MN, et al. First delivery in a leukemia survivor after transplantation of cryopreserved ovarian tissue, evaluated for leukemia cells contamination. Fertil Steril. 2017; [DOI] [PubMed]
- 11.Silber S. How ovarian transplantation works and how resting follicle recruitment occurs: a review of results reported from one center. Women's Health (Lond Engl) 2016;12(2):217–227. doi: 10.2217/whe.15.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittrich R, Hackl J, Lotz L, Hoffmann I, Beckmann MW. Pregnancies and live births after 20 transplantations of cryopreserved ovarian tissue in a single center. Fertil Steril. 2015;103(2):462–468. doi: 10.1016/j.fertnstert.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt KT, Rosendahl M, Ernst E, Loft A, Andersen AN, Dueholm M, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril. 2011;95(2):695–701. doi: 10.1016/j.fertnstert.2010.07.1080. [DOI] [PubMed] [Google Scholar]
- 14.Meirow D, Ra’anani H, Shapira M, Brenghausen M, Derech Chaim S, Aviel-Ronen S, et al. Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril. 2016;106(2):467–474. doi: 10.1016/j.fertnstert.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 15.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110(43):17474–17479. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod (Oxf, Engl) 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 17.Zhai J, Yao G, Dong F, Bu Z, Cheng Y, Sato Y, et al. In vitro activation of follicles and fresh tissue auto-transplantation in primary ovarian insufficiency patients. J Clin Endocrinol Metab. 2016;101(11):4405–4412. doi: 10.1210/jc.2016-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzo FVM, Buzzi PJ, Marconi G, Tiberon M, Young ET. Live birth with intrauterine insemination (IUH) after orthotopic transplantation of cryopreserved ovarian tissue in a young patient previously treated with chemotherapy for a skin’s disease. Argentina e160 ASRM Abstracts. Fertil Steril. 2014;102(3 Suppl):64. [Google Scholar]
- 19.Lorenzo FVM, Viola J, Tiveron M, Young E. Second children born after autotransplantation of cryopreserved ovarian tissue in a young patient previously treated with chemotherapy for Askin’s disease. The successful of fertility preservation program. Fertil Steril. 2016;106(suppl 3):e29. doi: 10.1016/j.fertnstert.2016.07.096. [DOI] [Google Scholar]
- 20.Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. Delivery of twins following heterotopic grafting of frozen-thawed ovarian tissue. Hum Reprod (Oxf Engl) 2014;29(8):1828. doi: 10.1093/humrep/deu119. [DOI] [PubMed] [Google Scholar]
- 21.Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Hum Reprod (Oxf Engl) 2013;28(11):2996–2999. doi: 10.1093/humrep/det360. [DOI] [PubMed] [Google Scholar]
- 22.Burmeister L, Kovacs GT, Osianlis T. First Australian pregnancy after ovarian tissue cryopreservation and subsequent autotransplantation. Med J Aust. 2013;198(3):158–159. doi: 10.5694/mja12.11768. [DOI] [PubMed] [Google Scholar]
- 23.Stern CJ, Toledo MG, Hale LG, Gook DA, Edgar DH. The first Australian experience of heterotopic grafting of cryopreserved ovarian tissue: evidence of establishment of normal ovarian function. Aust N Z J Obstet Gynaecol. 2011;51(3):268–275. doi: 10.1111/j.1479-828X.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 24.Rozen G, Agresta F, Gook D, Braat D, Stern CJ. Success and challenges in fertility preservation after ovarian tissue grafting. Lancet (Lond, Engl) 2015;385(9981):1947. doi: 10.1016/S0140-6736(15)60959-X. [DOI] [PubMed] [Google Scholar]
- 25.Stern K RG, Gook D, Agresta F, Braat D, Hale L. Are there factors which predict the success of ovarian tissue grafting in oncofertility patients? Abstract presented at: 32nd Annual Meeting of the European Society of Human Reproduction and Embryology.3–6 July, 2016; Helsinki, Finland.
- 26.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Imbert R, Moffa F, Tsepelidis S, Simon P, Delbaere A, Devreker F, et al. Safety and usefulness of cryopreservation of ovarian tissue to preserve fertility: a 12-year retrospective analysis. Hum Reprod (Oxf Engl) 2014;29(9):1931–1940. doi: 10.1093/humrep/deu158. [DOI] [PubMed] [Google Scholar]
- 28.Donnez J, Dolmans MM, Diaz C, Pellicer A. Ovarian cortex transplantation: time to move on from experimental studies to open clinical application. Fertil Steril. 2015;104(5):1097–1098. doi: 10.1016/j.fertnstert.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Lambertini M, Goldrat O, Ferreira AR, Dechene J, Azim HA, Jr, Desir J, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients. Ann Oncol: Off J Eur Soc Med Oncol. 2017;29(1):237–243. doi: 10.1093/annonc/mdx639. [DOI] [PubMed] [Google Scholar]
- 30.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98(3):720–725. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43(6):437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 32.Demeestere I, Simon P, Dedeken L, Moffa F, Tsepelidis S, Brachet C, et al. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod (Oxf, Engl) 2015;30(9):2107–2109. doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 33.Demeestere I, Simon P, Buxant F, Robin V, Fernandez SA, Centner J, et al. Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod (Oxf Engl) 2006;21(8):2010–2014. doi: 10.1093/humrep/del092. [DOI] [PubMed] [Google Scholar]
- 34.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin’s disease. Oncologist. 2007;12(12):1437–1442. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 35.Demeestere I, Simon P, Moffa F, Delbaere A, Englert Y. Birth of a second healthy girl more than 3 years after cryopreserved ovarian graft. Hum Reprod (Oxf, Engl) 2010;25(6):1590–1591. doi: 10.1093/humrep/deq096. [DOI] [PubMed] [Google Scholar]
- 36.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet (Lond Engl) 2004;364(9443):1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 37.Jadoul P, Guilmain A, Squifflet J, Luyckx M, Votino R, Wyns C, et al. Efficacy of ovarian tissue cryopreservation for fertility preservation: lessons learned from 545 cases. Hum Reprod (Oxf Engl) 2017;32(5):1046–1054. doi: 10.1093/humrep/dex040. [DOI] [PubMed] [Google Scholar]
- 38.Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Van Langendonckt A, et al. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril. 2011;95(5):1787.e1–1787.e4. doi: 10.1016/j.fertnstert.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 39.Donnez J, Martinez-Madrid B, Jadoul P, Van Langendonckt A, Demylle D, Dolmans MM. Ovarian tissue cryopreservation and transplantation: a review. Hum Reprod Update. 2006;12(5):519–535. doi: 10.1093/humupd/dml032. [DOI] [PubMed] [Google Scholar]
- 40.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Restoration of ovarian function after orthotopic (intraovarian and periovarian) transplantation of cryopreserved ovarian tissue in a woman treated by bone marrow transplantation for sickle cell anaemia: case report. Hum Reprod (Oxf Engl) 2006;21(1):183–188. doi: 10.1093/humrep/dei268. [DOI] [PubMed] [Google Scholar]
- 41.Donnez J, Squifflet J, Van Eyck AS, Demylle D, Jadoul P, Van Langendonckt A, et al. Restoration of ovarian function in orthotopically transplanted cryopreserved ovarian tissue: a pilot experience. Reprod BioMed Online. 2008;16(5):694–704. doi: 10.1016/S1472-6483(10)60484-1. [DOI] [PubMed] [Google Scholar]
- 42.Janse F, Donnez J, Anckaert E, de Jong FH, Fauser BC, Dolmans MM. Limited value of ovarian function markers following orthotopic transplantation of ovarian tissue after gonadotoxic treatment. J Clin Endocrinol Metab. 2011;96(4):1136–1144. doi: 10.1210/jc.2010-2188. [DOI] [PubMed] [Google Scholar]
- 43.Dolmans MM, Donnez J, Camboni A, Demylle D, Amorim C, Van Langendonckt A, et al. IVF outcome in patients with orthotopically transplanted ovarian tissue. Hum Reprod (Oxf Engl) 2009;24(11):2778–2787. doi: 10.1093/humrep/dep289. [DOI] [PubMed] [Google Scholar]
- 44.Hulsbosch S, Koskas M, Tomassetti C, De Sutter P, Wildiers H, Neven P, et al. A real-life analysis of reproductive outcome after fertility preservation in female cancer patients. Gynecol Obstet Investig. 2017. [DOI] [PubMed]
- 45.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Ernst E, Macklon KT, et al. Fertility preservation for age-related fertility decline. Lancet (Lond Engl) 2015;385(9967):506–507. doi: 10.1016/S0140-6736(15)60198-2. [DOI] [PubMed] [Google Scholar]
- 46.Jensen AK, Kristensen SG, Macklon KT, Jeppesen JV, Fedder J, Ernst E, et al. Outcomes of transplantations of cryopreserved ovarian tissue to 41 women in Denmark. Hum ReProduct (Oxf Engl) 2015;30(12):2838–2845. doi: 10.1093/humrep/dev230. [DOI] [PubMed] [Google Scholar]
- 47.Ernst EH, Offersen BV, Andersen CY, Ernst E. Legal termination of a pregnancy resulting from transplanted cryopreserved ovarian tissue due to cancer recurrence. J Assist Reprod Genet. 2013;30(7):975–978. doi: 10.1007/s10815-013-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ernst E, Kjaersgaard M, Birkebaek NH, Clausen N, Andersen CY. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer (Oxf Engl: 1990) 2013;49(4):911–914. doi: 10.1016/j.ejca.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 49.Yding Andersen C, Ernst E, Baerentzen S, Birkebaek NH, Clausen N. No malignancy detected in surplus ovarian tissue from a former Ewing sarcoma patient who experienced relapse four years after being grafted with frozen/thawed ovarian tissue. J Assist Reprod Genet. 2014;31(11):1567–1568. doi: 10.1007/s10815-014-0357-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen CY, Silber SJ, Bergholdt SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod BioMed Online. 2012;25(2):128–132. doi: 10.1016/j.rbmo.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 51.Greve T, Ernst E, Markholt S, Schmidt KT, Andersen CY. Legal termination of a pregnancy resulting from transplanted cryopreserved ovarian tissue. Acta Obstet Gynecol Scand. 2010;89(12):1589–1591. doi: 10.3109/00016349.2010.512074. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt KL, Andersen CY, Loft A, Byskov AG, Ernst E, Andersen AN. Follow-up of ovarian function post-chemotherapy following ovarian cryopreservation and transplantation. Hum Reprod (Oxf Engl) 2005;20(12):3539–3546. doi: 10.1093/humrep/dei250. [DOI] [PubMed] [Google Scholar]
- 53.Rosendahl M, Loft A, Byskov AG, Ziebe S, Schmidt KT, Andersen AN, et al. Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report. Hum Reprod (Oxf Engl) 2006;21(8):2006–2009. doi: 10.1093/humrep/del140. [DOI] [PubMed] [Google Scholar]
- 54.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod (Oxf Engl) 2008;23(10):2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 55.Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. J Assist Reprod Genet. 2014;31(11):1557–1564. doi: 10.1007/s10815-014-0331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ernst E, Bergholdt S, Jorgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reproduct (Oxf Engl) 2010;25(5):1280–1281. doi: 10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez-Wallberg KA, Tanbo T, Tinkanen H, Thurin-Kjellberg A, Nedstrand E, Kitlinski ML, et al. Ovarian tissue cryopreservation and transplantation among alternatives for fertility preservation in the Nordic countries—compilation of 20 years of multicenter experience. Acta Obstet Gynecol Scand. 2016;95(9):1015–1026. doi: 10.1111/aogs.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fajau-Prevot C, Le Gac YT, Chevreau C, Cohade C, Gatimel N, Parinaud J, et al. Ovarian mucinous cystadenoma after ovarian graft. Obstet Gynecol. 2017;129(6):1035–1036. doi: 10.1097/AOG.0000000000001990. [DOI] [PubMed] [Google Scholar]
- 59.Poirot C, Abirached F, Prades M, Coussieu C, Bernaudin F, Piver P. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet (Lond Engl) 2012;379(9815):588. doi: 10.1016/S0140-6736(11)61781-9. [DOI] [PubMed] [Google Scholar]
- 60.Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93(7):2413.e15–2413.e19. doi: 10.1016/j.fertnstert.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 61.Dittrich R, Mueller A, Maltaris T, Hoffmann I, Magener A, Oppelt PG, et al. Hormonal and histologic findings in human cryopreserved ovarian autografts. Fertil Steril. 2009;91(4 Suppl):1503–1506. doi: 10.1016/j.fertnstert.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 62.Dittrich R, Mueller A, Binder H, Oppelt PG, Renner SP, Goecke T, et al. First retransplantation of cryopreserved ovarian tissue following cancer therapy in Germany. Deutsch Arztebl Int. 2008;105(15):274–278. doi: 10.3238/arztebl.2008.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller A, Keller K, Wacker J, Dittrich R, Keck G, Montag M, et al. Retransplantation of cryopreserved ovarian tissue: the first live birth in Germany. Deutsch Arztebl Int. 2012;109(1–2):8–13. doi: 10.3238/arztebl.2012.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van der Ven H, Liebenthron J, Beckmann M, Toth B, Korell M, Krussel J, et al. Ninety-five orthotopic transplantations in 74 women of ovarian tissue after cytotoxic treatment in a fertility preservation network: tissue activity, pregnancy and delivery rates. Hum Reprod (Oxf Engl) 2016;31(9):2031–2041. doi: 10.1093/humrep/dew165. [DOI] [PubMed] [Google Scholar]
- 65.Isachenko V, Orth I, Isachenko E, Mallmann P, Peters D, Schmidt T, et al. Viability of human ovarian tissue confirmed 5 years after freezing with spontaneous ice-formation by autografting and chorio-allantoic membrane culture. Cryobiology. 2013;66(3):233–238. doi: 10.1016/j.cryobiol.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Yemini Z, et al. Monitoring the ovaries after autotransplantation of cryopreserved ovarian tissue: endocrine studies, in vitro fertilization cycles, and live birth. Fertil Steril. 2007;87(2):418.e7–418e15. doi: 10.1016/j.fertnstert.2006.05.086. [DOI] [PubMed] [Google Scholar]
- 67.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353(3):318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 68.Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod (Oxf Engl) 2011;26(5):1097–1103. doi: 10.1093/humrep/der063. [DOI] [PubMed] [Google Scholar]
- 69.Azem FAA, Vagman I, Mey Raz N, Ben-Yosef D, Lessing J. Successful pregnancy and birth following avascular micro ovarian tissue orthotopic transplantation in a patient with Hodgkin disease. Scientific Congress Supplement: oral and poster abstracts. Fertil Steril. 2012;98(3):S95–S96. doi: 10.1016/j.fertnstert.2012.07.349. [DOI] [Google Scholar]
- 70.Fabbri R, Pasquinelli G, Magnani V, Macciocca M, Vicenti R, Parazza I, et al. Autotransplantation of cryopreserved ovarian tissue in oncological patients: recovery of ovarian function. Future Oncol (Lond Engl) 2014;10(4):549–561. doi: 10.2217/fon.13.234. [DOI] [PubMed] [Google Scholar]
- 71.Revelli A, Marchino G, Dolfin E, Molinari E, Delle Piane L, Salvagno F, et al. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertil Steril. 2013;99(1):227–230. doi: 10.1016/j.fertnstert.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 72.Tanbo T, Greggains G, Storeng R, Busund B, Langebrekke A, Fedorcsak P. Autotransplantation of cryopreserved ovarian tissue after treatment for malignant disease—the first Norwegian results. Acta Obstet Gynecol Scand. 2015;94(9):937–941. doi: 10.1111/aogs.12700. [DOI] [PubMed] [Google Scholar]
- 73.Radwan P, Abramik A, Wilczynski J, Radwan M. Successful autotransplantation of cryopreserved ovarian tissue with recovery of the ovarian function. Ginekol Pol. 2016;87(3):235–240. doi: 10.17772/gp/61981. [DOI] [PubMed] [Google Scholar]
- 74.Povoa A, Xavier P, Calejo L, Soares S, Sousa M, Silva J, et al. First transplantation of cryopreserved ovarian tissue in Portugal, stored for 10 years: an unexpected indication. Reprod BioMed Online. 2016;32(3):334–336. doi: 10.1016/j.rbmo.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Kiseleva M, Malinova I, Komarova E, Shvedova T, Chudakov K. The Russian experience of autotransplantation of vitrified ovarian tissue to a cancer patient. Gynecological Endocrinol. 2014;30(Suppl 1):30–31. doi: 10.3109/09513590.2014.945781. [DOI] [PubMed] [Google Scholar]
- 76.Filatov MA, Khramova YV, Kiseleva MV, Malinova IV, Komarova EV, Semenova ML. Female fertility preservation strategies: cryopreservation and ovarian tissue in vitro culture, current state of the art and future perspectives. Zygote (Cambridge, England) 2016;24(5):635–653. doi: 10.1017/S096719941600006X. [DOI] [PubMed] [Google Scholar]
- 77.Kim SS, Hwang IT, Lee HC. Heterotopic autotransplantation of cryobanked human ovarian tissue as a strategy to restore ovarian function. Fertil Steril. 2004;82(4):930–932. doi: 10.1016/j.fertnstert.2004.02.137. [DOI] [PubMed] [Google Scholar]
- 78.Sanchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escriba MJ, Simon C, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93(1):268.e11–268.e13. doi: 10.1016/j.fertnstert.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 79.Callejo J, Salvador C, Gonzalez-Nunez S, Almeida L, Rodriguez L, Marques L, et al. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res. 2013;6(1):33. doi: 10.1186/1757-2215-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sanchez M, Novella-Maestre E, Teruel J, Ortiz E, Pellicer A. The Valencia Programme for fertility preservation. Clin Transl Oncol. 2008;10(7):433–438. doi: 10.1007/s12094-008-0227-4. [DOI] [PubMed] [Google Scholar]
- 81.Callejo J, Salvador C, Miralles A, Vilaseca S, Lailla JM, Balasch J. Long-term ovarian function evaluation after autografting by implantation with fresh and frozen-thawed human ovarian tissue. J Clin Endocrinol Metab. 2001;86(9):4489–4494. doi: 10.1210/jcem.86.9.7871. [DOI] [PubMed] [Google Scholar]
- 82.Rodriguez-Wallberg KA, Karlstrom PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, et al. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet Gynecol Scand. 2015;94(3):324–328. doi: 10.1111/aogs.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milenkovic M, Brannstrom M, Diaz-Garcia C, Lundin K, Selleskog U, Soderlund B, et al. Spontaneous twin pregnancy with live births after cryopreservation and re-implantation of ovarian tissue. Gynecol Surg. 2017;14(1):9. doi: 10.1186/s10397-017-1012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolner-Hanssen P, Hagglund L, Ploman F, Ramirez A, Manthorpe R, Thuring A. Autotransplantation of cryopreserved ovarian tissue to the right forearm 4(1/2) years after autologous stem cell transplantation. Acta Obstet Gynecol Scand. 2005;84(7):695–698. doi: 10.1111/j.0001-6349.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 85.Akar ME, Carrillo AJ, Jennell JL, Yalcinkaya TM. Robotic-assisted laparoscopic ovarian tissue transplantation. Fertil Steril. 2011;95(3):1120.e5–1120.e8. doi: 10.1016/j.fertnstert.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 86.Dunlop CE, Brady BM, McLaughlin M, Telfer EE, White J, Cowie F, et al. Re-implantation of cryopreserved ovarian cortex resulting in restoration of ovarian function, natural conception and successful pregnancy after haematopoietic stem cell transplantation for Wilms tumour. J Assist Reprod Genet. 2016;33(12):1615–1620. doi: 10.1007/s10815-016-0805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Radford JA, Lieberman BA, Brison DR, Smith AR, Critchlow JD, Russell SA, et al. Orthotopic reimplantation of cryopreserved ovarian cortical strips after high-dose chemotherapy for Hodgkin’s lymphoma. Lancet (Lond Engl) 2001;357(9263):1172–1175. doi: 10.1016/S0140-6736(00)04335-X. [DOI] [PubMed] [Google Scholar]
- 88.Kim SS. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J Assist Reprod Genet. 2012;29(6):489–493. doi: 10.1007/s10815-012-9757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med. 2000;342(25):1919. doi: 10.1056/NEJM200006223422516. [DOI] [PubMed] [Google Scholar]
- 90.Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience. Fertil Steril. 2010;93(3):762–768. doi: 10.1016/j.fertnstert.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 91.Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis. Reprod Sci (Thousand Oaks, Calif) 2017;24(8):1111–1120. doi: 10.1177/1933719117702251. [DOI] [PubMed] [Google Scholar]
- 92.Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet (Lond Engl) 2004;363(9412):837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 93.Oktay K. Spontaneous conceptions and live birth after heterotopic ovarian transplantation: is there a germline stem cell connection? Hum Reprod (Oxf Engl) 2006;21(6):1345–1348. doi: 10.1093/humrep/del007. [DOI] [PubMed] [Google Scholar]
- 94.Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94.e1–94.e9. doi: 10.1016/j.ajog.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oktay K, Taylan E, Sugishita Y, Goldberg GM. Robot-assisted laparoscopic transplantation of frozen-thawed ovarian tissue. J Minim Invasive Gynecol. 2017;24(6):897–898. doi: 10.1016/j.jmig.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 96.Silber S, Pineda J, Lenahan K, DeRosa M, Melnick J. Fresh and cryopreserved ovary transplantation and resting follicle recruitment. Reprod BioMed Online. 2015;30(6):643–650. doi: 10.1016/j.rbmo.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 97.Silber S. Chapter 13 human ovarian tissue vitrification. Methods Mol Biol (Clifton, NJ) 2017;1568:177–194. doi: 10.1007/978-1-4939-6828-2_13. [DOI] [PubMed] [Google Scholar]
- 98.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94(6):2191–2196. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 99.Silber S. Ovarian tissue cryopreservation and transplantation: scientific implications. J Assist Reprod Genet. 2016;33(12):1595–1603. doi: 10.1007/s10815-016-0814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim SS, Lee WS, Chung MK, Lee HC, Lee HH, Hill D. Long-term ovarian function and fertility after heterotopic autotransplantation of cryobanked human ovarian tissue: 8-year experience in cancer patients. Fertil Steril. 2009;91(6):2349–2354. doi: 10.1016/j.fertnstert.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 101.Greve T, Schmidt KT, Kristensen SG, Ernst E, Andersen CY. Evaluation of the ovarian reserve in women transplanted with frozen and thawed ovarian cortical tissue. Fertil Steril. 2012;97(6):1394–8.e1. doi: 10.1016/j.fertnstert.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 102.Dolmans MM, Marotta ML, Pirard C, Donnez J, Donnez O. Ovarian tissue cryopreservation followed by controlled ovarian stimulation and pick-up of mature oocytes does not impair the number or quality of retrieved oocytes. J Ovarian Res. 2014;7:80. doi: 10.1186/s13048-014-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 18 kb)