Abstract
Gastric cancer (GC) is regarded as the fifth most common cancer and the third cause of cancer-related deaths worldwide. Mechanism of GC pathogenesis is still unclear and relies on multiple factors, including environmental and genetic characteristics. One of the most important environmental factors of GC occurrence is infection with Helicobacter pylori that is classified as class one carcinogens. Dysregulation of several genes and pathways play an essential role during gastric carcinogenesis. Dysregulation of developmental pathways such as Wnt/β-catenin signaling, Hedgehog signaling, Hippo pathway, Notch signaling, nuclear factor-kB, and epidermal growth factor receptor have been found in GC. Epithelial-mesenchymal transition, as an important process during embryogenesis and tumorigenesis, is supposed to play a role in initiation, invasion, metastasis, and progression of GC. Although surgery is the main therapeutic modality of the disease, the understanding of biological processes of cell signaling pathways may help to develop new therapeutic targets for GC.
Keywords: Beta Catenin, Epithelial-mesenchymal transition, Hedgehogs, Helicobacter pylori, NF-kappa B
INTRODUCTION
Gastric cancer (GC) is one of the most common and lethal cancers worldwide. More than 950,000 new cases are diagnosed annually[1]. The incidence of GC is higher in Eastern Asia, Eastern Europe, and Southern America than Northern America and Northern Africa[2]. In Iran, GC is prevalent in northern and northwestern regions, and men are twice as likely to be affected than women[3]. GC is the fourth most common cancer (after lung, prostate, and colorectal cancers) in men and the fifth most common cancer (after breast, cervical, colorectal, and lung cancers) in women globally[4]. Despite the declining rate of GC incidence and advances in diagnosis, GC causes more than 700,000 death annually, and a five-year survival rate is nearly 20%[5].
Gastric adenocarcinoma has recently been classified genetically to four molecular subtypes, including chromosomal instability, microsatellite instability, genome stable, and Epstein-Barr virus-positive[6]. There are two main histological types of GC consisting of intestinal and diffuse types. Development of the intestinal type includes the transformation of normal mucosa to the similar mucosa of the intestinal epithelium. These series of mucosal alterations are triggered by chronic inflammation (gastritis), which eventually leads to metaplasia, dysplasia, and cancer. The diffuse type appears as single-cell that changes in the mucous neck area of gastric glands[7]. Thirty to 50% of the diffuse types are caused by either point or small frameshift mutations in CDH1 gene, which encodes E-cadherin and plays an essential role in cell adhesion[8].
Some of the main risk factors of GC are summarized in Table 1, including Helicobacter pylori infection and atrophic gastritis, tobacco smoking, dietary salt and food preservation, pernicious anemia, and abnormalities in E-cadherin gene[9]. The aim of this review is to summarize several important signaling pathways in GC, which helps to have a better understanding of GC biology.
Table 1.
GC risk factors
| GC risk factor | Explanations | Reference |
|---|---|---|
| H. pylori infection | Most important risk factor, long-term infection, leads to chronic atrophic gastritis and pre-cancerous alterations. The international agency for research on cancer (IARC) classified H. Pylori as the first class carcinogen. People with GC have a higher rate of H. pylori infection. | [105] |
| Smoking | Smoking increased the risk of GC. Studies have reported that smokers have higher hazard ratio in GC in cardia (2.86–4.10) compared with the distal region of stomach (1.52–1.94). | [106] |
| E-cadherin gene | Hereditary diffuse GC caused by the mutation in CDH1 gene encodes E-cadherin. | [107] |
| Pernicious anemia | People with Pernicious anemia have increased the risk of GC. More studies are needed to confirm this condition. | [9] |
| Diet | Diet play important role in prevention and development of GC. Salt and salt-preserved foods increased the risk of GC. Intake twice or more of fruits and vegetables in a day decreased the risk of GC. | [7] |
| Epstein-Barr virus (EBV) | 5% to 10% of GCs are associated with EBV. Its mechanism is DNA methylation (gene silencing). | [108] |
Molecular pathways of GC
There are several cell signaling pathways playing a role in gastric carcinogenesis. Here, we review different cell signaling pathways that are involved in GC tumorigenesis, highlighting either the expression pattern or contributed mutations in related genes.
Hedgehog (Hh) signaling pathway
The Hh signaling pathway is important in embryonic development, differentiation, proliferation, and maintenance of some adult tissues. Ligands of this pathway in mammals include Sonic, Indian, and Desert. In the absence of these ligands, the transmembrane receptor ptch inhibits another transmembrane protein (smoothened [SMO]), resulting in deactivation of Hh pathway. By binding ligands to the ptch receptor, the inhibitory effect of patch is eliminated from SMO, and SMO activates the downstream transcription factors, including GLI (GLI1, GLI2, and GLI3) proteins. Then GLI translocates to the nucleus and activates Hh-related target genes[10] (Fig. 1).
Fig. 1.
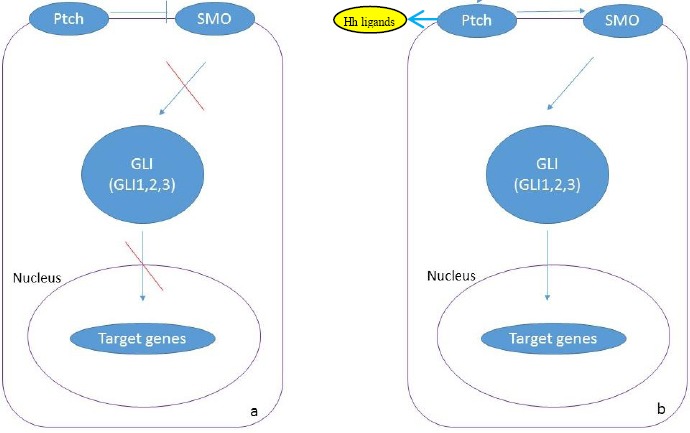
Hh pathway in Hh signaling. In the absence of ligands Ptch inhibits SMO and then inactivates the signaling pathway (a). (b) In the presence of ligands, ligands bind to the Ptch, and the activation of SMO and signaling pathway occurs (b).
In the gastrointestinal tract, where epithelial cells are continuously replenished from progenitor cell populations, Hh signaling appears to be essential for restoration. During GC processing, chronic H. pylori infection causes mucosal damage. Furthermore, the overexpression of sonic Hh has been detected in progenitor cells (in gastric mucosa), which restore the damaged gastric mucosa[11]. In addition, overexpression of GLI1 was correlated to the lymph node metastasis in esophageal squamous cell carcinoma (ESCC) patients[12]. The expression changes of this pathway in GC are summarized in Table 2.
Table 2.
Hh pathway
| Up-regulated genes | Explanations | Reference |
|---|---|---|
| SHH, PTCH, and GLI1 | Up-regulation of these genes is observed during H. pylori infection in GC cells. CagA-positive H. pylori was correlated with the higher expression of SHH. | [11] |
| PTCH1, SMO, and GLI Shh and Ihh | Overexpression of these genes is documented in diffuse types of GC. Expression of Shh and Ihh is detected in the intestinal type of GC. | [109] |
| GLI1 | The up-regulation of Gli1 and down-regulation of SuFu have been reported in GC tissue. Gli1 overexpression is correlated with aggressive phenotype. | [110] |
| SHH | SHH overexpression is related with age, tumor differentiation state, T staging, and N stage in GC. In another study, SHH expression is correlated with lymphatic metastasis and poor prognosis. Furthermore, in xenograft of human GC, the up-regulation of SHH significantly enhances the incidence of lung metastasis. | [111] |
| SHH, PTCH, and Gli3 | The expression of these genes increases in CD44+ and CD24 + subpopulation, which is comparable with the CD44−CD24−subpopulation. | [112] |
Wnt/β-catenin pathway
Wnt proteins are cysteine-rich glycoproteins that bind to the extracellular domain of frizzled receptor and lipoprotein receptor-related protein 5/6. Wnt signaling regulates different cellular processes, including cell fate, movement, polarity, and organogenesis. There are three types of Wnt pathways. The first is canonical or β-catenin-dependent pathway that involves in the stabilization of the proto-oncogene β-catenin. The second is planar cell polarity pathway that involves in cell ciliogenesis. The last is Wnt/Ca2+-dependent pathway that stimulates the intracellular release of calcium and activates Ca2+-dependent mediators controlling cell movement and behavior. The planar cell polarity and Wnt/Ca2+ pathways are collectively called either non-canonical or β-catenin-independent pathway[13].
In the absence of Wnt, GSK3 in APC complex (including APC, AXIN, CK1, and GSK3) phosphorylates β-catenin, which in turn leads to the degradation of β-catenin in proteasome complex. Binding of Wnt ligand to the frizzled receptor inhibits GSK3 activity through dishevelled, resulting in dephosphorylation and stabilization of the β-catenin. Therefore, β-catenin accumulates in the nucleus, and its interaction with the T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factor family stimulates the transcription of Wnt target genes[14]. Two signaling pathways, including nuclear factor (NF)-kB and Wnt/β-catenin are dysregulated in 70% of the GC patients[15]. Wnt pathway is a key element in cell proliferation during both normal and cancerous gut development. SALL4, as an embryonic stem cell marker, has a direct interaction with Wnt signaling. Its overexpression is correlated with lymph node metastasis in GC[16]. Furthermore, overexpression of SALL4 and SOX2, members of the sex-determining region Y-related high-mobility group (HMG), are observed in ESCC, and the expression levels of these two genes are correlated with each other[17]. The overexpression of SALL4 has also been detected in patients with colorectal cancer, and its overexpression is associated with the grade of tumor cell differentiation and tumor cell metastasis to the lymph node[18]. Some of the genetic alterations of this pathway are summarized in Table 3.
Table 3.
Genetic alteration of Wnt pathway
| Gene | Genetics alteration | Explanation | Reference |
|---|---|---|---|
| Wnt-1 | Up-regulated | [113] | |
| Wnt-2 | Up-regulated | The overexpression of WNT2 is correlated with cytoplasmic/nuclear β-catenin accumulation in both intestinal- and diffuse-type ofr GC in Chinese people. Moreover, the expression of WNT2 positively is correlated with lymph node metastasis. | [114] |
| Wnt-5 | Up-regulated | Its expression is correlated with poor prognosis. | [115] |
| Fzd-3 | Up-regulated | Its overexpression is correlated with the activation of Wnt signaling in GC. | [116] |
| CTNNB1 | Mutation | Mutation in the gene (CTNNB1) is found in diffuse and intestinal type of GC. | [117] |
| TCF7L2 | Somatic frame shift mutation | Somatic frame shift mutation is detected in GC with microsatellite instability. | [118] |
| APC | Mutation | Mutation and deletion | [119] |
| Sox10 | Down-regulated | Sox10 is a transcription factor that regulates Wnt signaling. | [120] |
| WNT10A | Up-regulated | H. Pylori infection induces this overexpression. | [121] |
H. pylori infection dysregulates Wnt signaling pathway. CagA, the most important virulence factor of H. pylori, causes the activation of the β-catenin through an independent phosphorylation manner. CagA interacts with E-cadherin, leading to β-catenin accumulation in cytoplasm and nucleus. Moreover, CagA transactivates CDX1 and P21 genes that are involved in the intestinal differentiation of gastric epithelial cells[19]. VacA, another H. pylori virulence factor, induces Wnt/β-catenin signaling through the activation of PI3K/Akt pathway, resulting in phosphorylation of GSK3β and translocation of the β-catenin to the nucleus to activate CCND1 gene[20]. Moreover, H. pylori infection increases the expression levels of Oct4 and Nanog, two cancer stem cell (CSC) markers, through Wnt signaling that promotes CSC-properties in GC cells[21].
Transglutaminase (TGM) family plays an essential factor in drug resistance and progression of cancers. The expression level of TGM1, a member of TGM family, is elevated in GC that indicates TGM1 participation in the development of this disease. Moreover, the reduced levels of TGM1 in GC cells result in the suppression of Wnt signaling activities. This result suggests that the TGM1 may function in GC by affecting Wnt signaling pathway[22].
Cell cycle
Dysregulation of the cell cycle components is a defining factor in gastric tumorigenesis. Activation of the cyclin-dependent kinase (CDK) results in cell cycle progression. Cyclin D1 and cyclin D2 are up-regulated in GC[23]. Furthermore, cyclin D1 is up-regulated in co-cultured GC cells with H. pylori infection[24].
Tp53, the guardian of human genome, is a tumor suppressor gene that is commonly mutated in all types of human cancer. TP53 gene mutation is observed in GC[25]. Moreover, P21Waf1/Cip1, as a target for p53, binds to cyclin A-CDK2 and cyclin D1-CDK4 complexes and inhibits their function. Loss of P21Waf1/Cip1 expression has been reported in the 60% of GC tissues. Moreover, the underexpression of P21Waf1/Cip1 is correlated with tumor invasiveness and metastasis, as well as poor prognosis in GC[26]. Besides, down-regulation of p27Kip1, a CDK inhibitor, has been observed in GC, and its down-regulation is correlated with advanced stages and invasiveness of the tumor[27].
P16 is a regulator of cell cycle that causes G1 phase arrest by the inhibition of CDK4 and CDK6. The expression of P16 is observed in tissues and serum of GC patients, while its expression is not detected in normal tissues and sera. P16 DNA methylation can be used as a serum biomarker for early detection of GC[28].
Notch signaling
Notch signaling is an important pathway in tumorigenesis through the regulation of cell proliferation, apoptosis, and differentiation. Jagged1 is a ligand of Notch signaling. After binding Jagged1 to the Notch receptor, Notch1 receptor intracellular domain is cleaved by matrix metalloproteinase (MMP) and Y-secretase and consequently translocates into the nucleus to activate transcription machinery[29].
H. pylori infection can induce Notch signaling. Moreover, jagged1 expression is associated with aggressiveness of GC. Notch signaling induces expression of the cyclooxygenase-2 (COX-2) through the binding of the Notch1 receptor intracellular domain to the Cox-2 promoter, which results in GC progression[30]. The expression of Notch1 is detected in human GC, especially in well-differentiated intestinal type[31]. Furthermore, up-regulation of Notch1, Notch3, Jagged1, and Jagged2 are significantly correlated with the intestinal type of GC[32]. In addition, inhibition of Notch signaling pathway in GC leads to the activation of PTEN, which consequently induces G2/M cell cycle arrest[33]. Overexpression of Notch signaling target genes, such as HEY1 and HEY2, has been reported in ESCC with significant correlation to the different indices of poor prognosis, including stage of tumor progression and lymph node metastasis[34].
Hippo signaling
The Hippo signaling pathway is a key element in cell growth and organ size, as well as in the homeostasis of the gastrointestinal tissues. Moreover, dysregulation of Hippo pathway is associated with initiation, development, and distant metastasis of GC[35]. The main components of this pathway are MST1/2, LATS1/2, Mob1, YAP1, and TAZ1. MST1/2 phosphorylates and activates LATS1/2 and Mob1. Then LATS1/2 phosphorylates YAP1 and TAZ and increases 14-3-3 binding to phosphorylated YAP1/TAZ, leading to the oncogenic accumulation of the YAP1/TAZ in the cytoplasm. The unphosphorylated YAP1/TAZ translocates to the nucleus and binds to the TEAD1-4 transcription factors to induce transcriptional activity for cell growth and differentiation[36] (Fig. 2).
Fig. 2.
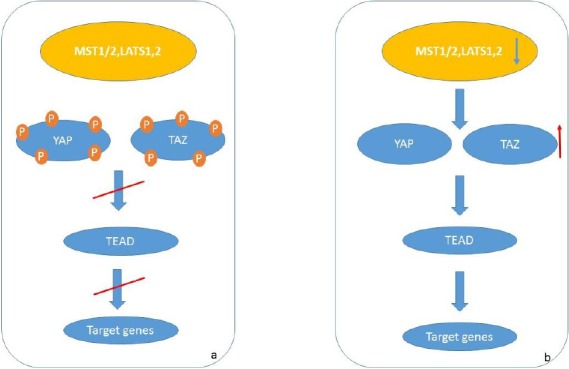
Hippo pathway. (a) During signaling pathway; the upstream components (MST1/1, LATS1/2) phosphorylate the downstream components and result in inactivation of pathway. (b) During GC; the expression of MST1/2 and LATS1/2 decreased and failed to phosphorylate YAP/TAZ. YAP/TAZ translocates to the nucleus and binds to the TEAD, resulting in transcription of target genes.
While the down-regulation of upstream components of Hippo pathway, such as MST1/2 and LATS1/2, is detected in GC, up-regulation of YAP1 that is the main downstream component is observed in high-grade dysplasia and metastatic GC[37]. Moreover, YAP1 is negatively regulated by tumor suppressor microRNAs, including miR-15a, miR-16-1, and miR-506 in GC[38].
The gain of function mutation in RhoA, an activator of YAP1, has been detected in diffuse type of GC[39]. TEAD4 gene, as the main transcription factor of this pathway, is significantly hypo-methylated, and its overexpression is observed in GC[40]. Furthermore, the expression of TAZ, another key effector of the Hippo pathway, is associated with the overexpression of β-catenin and poor prognosis in GC[41].
Epithelial-mesenchymal transition (EMT)
EMT is a cellular process that normally occurs during heart morphogenesis, mesoderm and neural crest formation, embryogenesis, wound healing, as well as fibrotic disease and cancer[42]. There are three types of EMT process. The type one of EMT is involved in generating mesenchymal cells; these cells can undergo a MET process to produce secondary epithelial cells. Actually, this type of EMT plays a role during embryogenesis and organ development. The type two of EMT involves in wound healing and tissue reconstruction and organ fibrosis. Moreover, type two is an essential factor during inflammation. The type three of EMT has a key role in neoplastic cells; these cells have enormous genetic and epigenetic changes, especially in oncogenes and tumor suppressor genes. Those neoplastic cells that undergo the type three of EMT may invade and metastasize, thereby leading to cancer progression[43]. Through the EMT process, cell phenotype changes from epithelial to mesenchymal. Indeed, epithelial cells lose their cell-cell adhesion, alter their polarity, rearrange their cytoskeleton and become isolated[44].
During EMT process, the down-regulation of E-cadherin, which is essential for the cell adhesion and is expressed at the surface of the epithelial cells, occurs. Moreover, the overexpression of N-cadherin, which is expressed in the mesanchymal cells, is another important event in EMT[45]. Other proteins such as FSP1, β-catenin, α-SMA, extracellular matrix (ECM), and cytoskeleton proteins are also determinant in EMT progress[46]. Besides, WNT5A induces EMT-related genes in GC and probably regulats EMT process[47]. In addition, paired-related homeobox 1 is up-regulated in GC. Additionally, PRPX1 induces EMT through the activation of the Wnt/B-catenin pathway[48]. Furthermore, the overexpression of the Twist1, a regulatory protein of EMT, and Vimentin as well as PDCD4 and E-cadherin downregulation have been detected in GC samples. Moreover, CagA transfection into GC cells can activate TWIST1 and Vimentin. Besides, CagA can decrease the expression levels of the E-cadherin through the down-regulation of the PDCD4[49]. Down-regulation of the Twist1 is associated with the up-regulation of the E-cadherin, suggesting that Twist1 induces EMT in GC[50]. Furthermore, the expression of the erythropoietin-producing hepatocellular A2 is positively associated with the EMT markers in GC[51]. Moreover, Fas signaling induces EMT and increases metastasis in GC. During the progression of GC, the overexpression of the FasL, phospho-GSK-3β, Snail, and B-catenin is observed[52].
The overexpression of the transforming growth factor beta (TGF-β1), Twist1, Snail, Slug, and Vimentin, as well as CD44, which is a CSC marker, is found in patients with dysplasia or early GC. Moreover, the expression levels of E-cadherin, an epithelial marker, decreased. Furthermore, eradication of the H. pylori infection decreased the levels of the TGF-β1, Twist, Snail, Slug, and Vimentin, while the levels of the E-cadherin increased. These data suggests that H. pylori may induce EMT through TGF-β1[53].
EMT is a key factor in gastric tumorigenesis. GC stem cells are significantly correlated with the expression of the EMT activating transcription factors. Moreover, CD44 expression is significantly associated with the expression of the Snail-1, ZEB-1, and E-cadherin in GC[54]. Overexpression of MAML1 and TWIST1 is significantly correlated with lymph node metastasis in ESCC patient[55]. Furthermore, the expression levels of TWIST1 and SNAIL genes are significantly correlated with invasion in ESCC cell line KYSE-30 where ectopic expression of TWIST1 results in the significant down-regulation of SNAIL[56]. Some of the important factors of EMT have been summarized in Table 4.
Table 4.
EMT factors
| Gene | Function | Cancer | Reference |
|---|---|---|---|
| E-Cadherin | Cell adhesion Expressed in epithelial cell | During EMT, the loss of E-cadherin expression occurs. | [122] |
| N-Cadherin | Expressed in mesenchymal cells | Gain of N-Cadherin expression during EMT occurs. | [122] |
| TWIST1 | A transcription factor induces EMT and increases metastasis | Overexpression in GC and EMT happens. | [123] |
| SNAIL | Transcription factor that controls EMT during embryogenesis and tumorigenesis | Its expression is associated with tumorigenesis in GC during EMT. | [124] |
| ZEB-1 | A transcription factor that induces EMT and metastasis | It overexpressed in GC. | [125] |
| Vimentin | Mesenchymal marker in EMT | Its overexpression is observed in GC during EMT. | [126] |
| Slug | Regulator of EMT | It overexpressed in GC. | [127] |
Matrix metalloproteinase
MMPs) break down the components of the ECM. MMPs and their tissue inhibitors act in tumor invasion and metastasis. The levels of the MMPs and tissue inhibitors increased in GC[57]. Besides, the overexpression of MMP9 in GC is associated with tumor invasion, and its serum level has a relation with the lymph node metastasis. Therefore, this data suggests that MMP9 is a novel biomarker for diagnosis and prognosis of GC[58]. The overexpression of MMP2, MMP7, and MMP9 has also been observed in GC[59]. Interestingly, the expression of MMP1 is associated with the metastasis of GC cells[60]. Expression of the integrin αvβ6, which is an epithelial-specific receptor for fibronectin (an ECM protein), is associated with MMP9 in GC[61].
TGF-β signaling pathway
TGF-β signaling pathway involves in many cellular processes such as cell growth, cell differentiation, and apoptosis. This pathway has many ligands, including TGF-β, activin, inhibin, bone morphogenetic proteins, Nodal, and others[62]. Furthermore, this pathway has two receptors: type I and type II, which are serine/threonine kinase receptors. During signaling, the ligands bind to the type II receptor where it catalyzes and phosphorylates the type I receptor. Then type I receptor phosphorylates SMADs proteins such as SMAD2/3; these proteins heterodimerize with SMAD4 and translocate into the nucleus to activate the transcription of target genes[63]. Dysregulation of the components of this pathway occurs in GC. The overexpression of TGF-β1 is detected in GC[64]. Besides, its expression is associated with lymph node metastasis[65]. Moreover, the polymorphism -509C>T in the promoter region of TGF-β1 has a connection with worse prognosis in GC[64]. RUNX3 is one of the target proteins in TGF signaling that is a defining factor in induction of apoptosis in GC cells and its inactivation has been found in GC[66]. Furthermore, H. pylori infection leads to the methylation of RUNX3 and inhibits its expression in GC[67]. Moreover, inactivation of SMAD4 has been reported in GC[68]. Additionally, mutations in TGFβRII occur in GC tissues, which are likely the result of microsatellites’ instability. TGFβRII gene has 10 poly-A repeats that make them as hotspot regions for mutation[69]. Besides, mutations in TGFβRI are less frequent in GC, andits downregulation is associated with poor prognosis[70].
Cyclooxygenase-2 and lipoxygenase (LOX) pathways
COX-2/ Prostaglandin E2 is one of the important pathways during gastric carcinogenesis. The COX enzymes, COX-1 and COX-2, are key effectors in prostaglandin synthesis. COX-1 has a function in the maintenance of the gastric mucosa integrity, while COX-2 is an inducible enzyme and can produce the prostaglandins. Prostaglandins are necessary for the reactions during the inflammatory processes. The normal mucosa of gastric produces COX-1, but the expression level of COX-2 is too low or undetectable. Moreover, COX-2 takes part in inflammation and carcinogenesis[71]. Many studies have reported the overexpression of COX-2 in GC[72-74]. Besides, the H. pylori infection may induce the expression of COX-2 in GC. H. Pylori infection induces the COX2 expression through p38 mitogen-activated protein kinase/activating transcription factor-2 signaling pathway in MKN45 GC cells[75]. Therefore, this pathway could be a novel therapeutic target for patients who have H. pylori-associated GC. Furthermore, H. pylori leads to the overexpression of vascular endothelial growth factor (VEGF) in MKN45 cells, which may be mediated by COX-2[76]. Moreover, the correlation between COX-2 expression and VEGF expression has been reported in GC, suggesting the important role of prostaglandins in gastric carcinogenesis[77]. Additionally, COX-2 regulates the expression of Snail through Notch signaling pathway. The COX-2 expression has an inverse correlation with the Notch1 expression in GC cells[78].
LOX pathway is an important pathway in producing leukotrienes and hydroxyeicosatetraenoic acids from arachidonic acid[79]. This pathway is also dysregulated during gastric carcinogenesis. In addition, 12-LOX is important during tumorigenesis. Its expression is found in GC cells, including AGS and MKN-28. Furthermore, 12-LOX regulates the apoptosis and cell proliferation in GC cells, and blocking the activity of 12-LOX leads to the inhibition of cell growth and activation of apoptosis[80,81]. Furthermore, the overexpression of LOX-5 has been reported in GC where its expression is associated with lymph node metastasis and TNM staging of the tumor[82]. Moreover, during H. Pylori infection, the activity of 5-LOX and the amount of 5-hydroxyeicosatetraenoic acid, which is the product of the function of 5-LOX on arachidonic acid, increased in GC cells[83]. Besides, the inhibition of 5-LOX led to the activation of apoptosis in GC cells[84].
Epidermal growth factor receptor (EGFR), Human epidermal growth factor receptor 2 (HER2) signaling pathway
EGFR, a member of Erb-B family receptors, has a role in gastric mucosa proliferation and development of GC, and its overexpression is associated with poor prognosis in GC[85]. Furthermore, the overexpression and amplification of HER2, another member of ErbB family, has been detected in GC[86].
One of the downstream components of HER2 and EGFR pathways is Ras, an oncogenic GTPase that has three isoforms, including K-Ras, H-Ras, and N-Ras. Mutation in K-RAS gene has been detected in intestinal type of GC[87]. Moreover, mutations in K-RAS gene in H. pylori-associated chronic gastritis is more frequent in GC patients than those who did not have cancer. This finding suggests that K-RAS gene mutation is involved in the early stages of gastric carcinogenesis of the intestinal type[88]. Besides, fluorescent in situ hybridization study on gastric tumors, cell lines, and patients-derived xenografts shows the amplification of RTK/Ras components, including FGFR (fibroblast growth factor receptor) 2, HER2, and K-Ras[89].
Nuclear factor-kB
NF-kB is a family of bipartite transcription factors that include NFKB1, NFKB2, c-Rel, RelA, and RelB. The common form of NF-kB in mammalian is RelA/NFkB1 dimer. Activation of this pathway occurs during inflammation. NF-kB normally binds to its inhibitor, inhibitory proteins of kB family (IkB), which leads to NF-kB being restricted in the cytoplasm. During inflammation, IkB kinase complex phosphorylates IkB, and then the degradation of IkB and activation of NF-kB occur[90]. H. pylori infection induces NF-kB activation in GC. Besides, H. pylori infection induces the expression of the pro-inflammatory cytokine IL-8 through the activation of the NF-kB[91]. HuR, a RNA-binding factor, is a direct transcript target of NF-kB and its activation in GC cell lines depends on phosphatidylinositol 3-kinase/ AKT signaling. HuR activation has proliferative and anti-apoptotic effects on GC[92]. Fructose-1,6-bisphosphatase-1 is an antagonist of the glycolysis process. The NF-kB is involved in glycolsis process through downregulation of FBP1 expression in GC[93]. Furthermore, the aberrant expression of NF-kB has anti-apoptotic effects and leads to drug resistant in GC[94,95].
Treatment of gastric cancer
Surgery is the only curative treatment of GC, whereas perioperative and adjuvant chemotherapy, in addition to chemoradiation can improve the outcome of resectable GC with extended lymph node dissection.
According to the National Comprehensive Cancer Network (NCCN), the treatment of the early stages of GC guidelines includes endoscopic resection or complete surgical resection for long-term survival. Furthermore, in advanced stages of GC, the treatment includes preoperative chemotherapy, or chemoradio-therapy after surgery. The patients who have extended lymph node resection (D2) are recommended to have postoperative chemoradiation or chemotherapy. The recommendation for patients who have unresectable tumors is treating with fluoropyrimidine- or taxane-based chemoradiotherapy[96].
There are several genes with altered expression pattern in GC that can be a target for cancer-therapy (Table 5). Trastuzumab, a humanized anti-HER2 monoclonal antibody, is used against HER2-positive GCs[97]. Cetuximab, an anti-EGFR monoclonal antibody, cannot induce any response in GC when used alone. It is shown that VEGF and their receptors are overexpressed in GC[98]. In this regard, Ramucirumab, a fully human IgG1 antibody against VEGFR2, is now approved by FDA for the treatment GC[99].
Table 5.
Genetic alteration targets for treatment of GC
| Gene | Function | Expression in GC | Treatment | Reference |
|---|---|---|---|---|
| HER2 | Regulation of cell growth and differentiation, | Over, Amp | Trastuzumab | [97] |
| EGFR | Cell growth, cell profilation, and cellular survival | Over, Amp | Cetuximab Nimotuzumab | [103,104] |
| MET | Embryogenesis, cellular survival, and cellular migration | Over, Amp | Onartuzumab | [102] |
| HGF | Regulation of cell motility and cell growth, morphogenesis of numerous cells and tissues, and angiogenesis | Over | Rilotumumab | [101] |
| VEGF | Angiogenesis, bone formation, hematopoiesis, wound healing, and development | Over | Bevacizumab | [128] |
| VEGFR2 | Tyrosine kinase receptor, angiogenesis, embryonic hemopoiesis, regulation of cell profilation, and organization of ECM | Over | Ramucirumab | [99] |
| FGFR2 | Cell division, cell growth, formation of blood vessels, wound healing, and embryonic development | Over, Amp | AZD4547 | [100] |
| IGFR-IR | Cell growth | Over | Figitumumab | [129] |
| NF-κB | Immune response to infection | Over | Bortezomib | [130] |
| mTOR | Cell growth, cell proliferation, and cell cycle | Over | Everolimus | [131] |
| MMPs | Degradation and destruction of ECM | Over | Marimastat | [132] |
Over, overexpression; Amp, amplification
AZD4547, as a selective ATP-competitive receptor tyrosine kinase inhibitor of FGFR, is effective against patients who have amplification of FGFR2[100]. Furthermore, hepatocyte growth factor (rilotumumab, a fully human IgG2 monoclonal antibody against HGF), hepatocyte growth factor receptor (onartuzumab, humanized monoclonal antibody directed against HGFR), and EGFR (cetuximab, an anti-EGFR monoclonal antibody and a nimotuzumab that is a humanized monoclonal IgG1 antibody to EGFR), are also the targets of treatment in GC[101-104].
Here, we summarized multiple pathways involving in GC carcinogenesis. A better understanding of molecular mechanisms of GC progression and development, as well as crosstalk between signaling pathways can help to identify new targets for anticancer drugs. Although many studies have been done on GC, the mechanism of GC carcinogenesis is still unclear. Understanding the molecular processes of GC could help to design more efficient genetic studies. With the novel technology advances, it will be easier to find new and useful targets in signaling pathways; these targets will be a potential marker for the early diagnosis and treatment of GC. Therefore, the management and the efficiency of treatment in patients with GC will be improved in future.
ACKNOWLEDGMENTS
This work was supported by a grant from the Vice Chancellor for Research at Mashhad University of Medical Sciences (Mashhad, Iran) and is a part of a M.Sc. student’s dissertation, No. 940720.
Footnotes
CONFLICT OF INTEREST. None declared.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA:a cancer journal for clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Tan P, Yeoh KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149(5):1153–1162. doi: 10.1053/j.gastro.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 3.Bowles MJ, Benjamin IS. Cancer of the stomach and pancreas. The BMJ. 2001;323(7326):1413–1416. doi: 10.1136/bmj.323.7326.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA:a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Nagini S. Carcinoma of the stomach:A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World journal of gastrointestinal oncology. 2012;4(7):156–169. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalano V, Labianca R, Beretta GD, Gatta G, De Braud F, Van Cutsem E. Gastric cancer. Critical reviews in oncology/hematology. 2009;71(2):127–164. doi: 10.1016/j.critrevonc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira C, Senz J, Kaurah P, Pinheiro H, Sanges R, Haegert A, Corso G, Schouten J, Fitzgerald R, Vogelsang H, Keller G, Dwerryhouse S, Grimmer D, Chin SF, Yang HK, Jackson CE, Seruca R, Roviello F, Stupka E, Caldas C, Huntsman D. Germline CDH1 deletions in hereditary diffuse gastric cancer families. Human molecular genetics. 2009;18(9):1545–1555. doi: 10.1093/hmg/ddp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thrumurthy SG, Chaudry MA, Hochhauser D, Mughal M. The diagnosis and management of gastric cancer. The BMJ. 2013;347:f6367. doi: 10.1136/bmj.f6367. [DOI] [PubMed] [Google Scholar]
- 10.Pasca di Magliano MP, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nature reviews cancer. 2003;3(12):903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Choi YJ, Lee SH, Shin HS, Lee IO, Kim YJ, Kim H, Yang WI, Kim H, Lee YC. Effect ofHelicobacter pyloriinfection on the sonic hedgehog signaling pathway in gastric cancer cells. Oncology reports. 2010;23(6):1523–1528. doi: 10.3892/or_00000791. [DOI] [PubMed] [Google Scholar]
- 12.Najafi M, Abbaszadegan MR, Rad A, Dastpak M, Boroumand-Noughabi S, Forghanifard MM. Crosstalk between SHH and stemness state signaling pathways in esophageal squamous cell carcinoma. Journal of cell communication and signaling. 2017;11(2):147–153. doi: 10.1007/s12079-016-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling:components, mechanisms, and diseases. Developmental cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nojima M, Suzuki H, Toyota M, Watanabe Y, Maruyama R, Sasaki S, Sasaki Y, Mita H, Nishikawa N, Yamaguchi K, Hirata K, Itoh F, Tokino T, Mori M, Imai K, Shinomura Y. Frequent epigenetic inactivation of SFRP genes and constitutive activation of Wnt signaling in gastric cancer. Oncogene. 2007;26(32):4699–4713. doi: 10.1038/sj.onc.1210259. [DOI] [PubMed] [Google Scholar]
- 15.Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, Ward L, Koo JH, Gopalakrishnan V, Zhu Y, Cheng LL, Lee J, Rha SY, Chung HC, Ganesan K, So J, Soo KC, Lim D, Chan WH, Wong WK, Bowtell D, Yeoh KG, Grabsch H, Boussioutas A, Tan P. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5(10):e1000676. doi: 10.1371/journal.pgen.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Xu Z, Xu X, Zhang B, Wu H, Wang M, Zhang X, Yang T, Cai J, Yan Y, Mao F, Zhu W, Shao Q, Qian H, Xu W. SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene. 2014;33(48):5491–5500. doi: 10.1038/onc.2013.495. [DOI] [PubMed] [Google Scholar]
- 17.Forghanifard MM, Khales SA, Javdani-Mallak A, Rad A, Farshchian M, Abbaszadegan MR. Stemness state regulators SALL4 and SOX2 are involved in progression and invasiveness of esophageal squamous cell carcinoma. Medical oncology. 2014;31(4):1–8. doi: 10.1007/s12032-014-0922-7. [DOI] [PubMed] [Google Scholar]
- 18.Forghanifard MM, Moghbeli M, Raeisossadati R, Tavassoli A, Mallak AJ, Boroumand-Noughabi S, Abbaszadegan MR. Role of SALL4 in the progression and metastasis of colorectal cancer. Journal of biomedical science. 2013;20(1):6. doi: 10.1186/1423-0127-20-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM, Jr, Azuma T, Hatakeyama M. Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26(32):4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 20.Nakayama M, Hisatsune J, Yamasaki E, Isomoto H, Kurazono H, Hatakeyama M, Azuma T, Yamaoka Y, Yahiro K, Moss J, Hirayama T. Helicobacter pyloriVacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. Journal of biological chemistry. 2009;284(3):1612–1619. doi: 10.1074/jbc.M806981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, Hu C-J, Dong H, Yang SM. Helicobacter pyloriupregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer letters. 2016;374(2):292–303. doi: 10.1016/j.canlet.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, Chen Z, Ni X. Tissue transglutaminase-1 promotes stemness and chemoresistance in gastric cancer cells by regulating Wnt/β-catenin signaling. Experimental biology and medicine (Maywood) 2017;242(2):194–202. doi: 10.1177/1535370216670541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arici D, Tuncer E, Ozer H, Simek G, Koyuncu A. Expression of retinoblastoma and cyclin D1 in gastric carcinoma. Neoplasma. 2008;56(1):63–67. doi: 10.4149/neo_2009_01_63. [DOI] [PubMed] [Google Scholar]
- 24.Hirata Y, Maeda S, Mitsuno Y, Akanuma M, Yamaji Y, Ogura K, Yoshida H, Shiratori Y, Omata M. Helicobacter pyloriactivates the cyclin D1 gene through mitogen-activated protein kinase pathway in gastric cancer cells. Infection and immunity. 2001;69(6):3965–3971. doi: 10.1128/IAI.69.6.3965-3971.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenoglio-Preiser CM, Wang J, Stemmermann G, Noffsinger A. TP53 and gastric carcinoma:a review. Human mutation. 2003;21(3):258–270. doi: 10.1002/humu.10180. [DOI] [PubMed] [Google Scholar]
- 26.Gamboa-Dominguez A, Seidl S, Reyes-Gutierrez E, Hermannstädter C, Quintanilla-Martinez L, Busch R, Höfler H, Fend F, Luber B. Prognostic significance of p21WAF1/CIP1, p27Kip1, p53 and E-cadherin expression in gastric cancer. Journal of clinical pathology. 2007;60(7):756–761. doi: 10.1136/jcp.2006.038976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nitti D, Belluco C, Mammano E, Marchet A, Ambrosi A, Mencarelli R, Segato P, Lise M. Low level of p27 (Kip1) protein expression in gastric adenocarcinoma is associated with disease progression and poor outcome. Journal of surgical oncology. 2002;81(4):167–175. doi: 10.1002/jso.10172. [DOI] [PubMed] [Google Scholar]
- 28.Abbaszadegan MR, Moaven O, Sima HR, Ghafarzadegan K, A'rabi A, Forghani MN, Raziee HR, Mashhadinejad A, Jafarzadeh M, Esmaili-Shandiz E, Dadkhah E. p16 promoter hypermethylation:a useful serum marker for early detection of gastric cancer. World journal of gastroenterology. 2008;14(13):2055–2060. doi: 10.3748/wjg.14.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopan R, Ilagan MXG. The canonical Notch signaling pathway:unfolding the activation mechanism. Cell. 2009;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC, Li AF, Wang AM, Kuo ML, Chi CW. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer research. 2009;69(12):5039–5048. doi: 10.1158/0008-5472.CAN-08-4021. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Gao X, Liu J, Kong QY, Wang XW, Chen XY, Wang Q, Cheng YF, Qu XX, Li H. Differential Notch1 and Notch2 expression and frequent activation of Notch signaling in gastric cancers. Archives of pathology and laboratory medicine. 2011;135(4):451–458. doi: 10.5858/2009-0665-OA.1. [DOI] [PubMed] [Google Scholar]
- 32.Kang H, An HJ, Song JY, Kim TH, Heo JH, Ahn DH, Kim G. Notch3 and Jagged2 contribute to gastric cancer development and to glandular differentiation associated with MUC2 and MUC5AC expression. Histopathology. 2012;61(4):576–586. doi: 10.1111/j.1365-2559.2012.04274.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim S, Lee H, Baek J, Cho Y, Kang H, Jeong J, Song J, Park H, Chun K. Activation of nuclear PTEN by inhibition of Notch signaling induces G2/M cell cycle arrest in gastric cancer. Oncogene. 2016;35(2):251–260. doi: 10.1038/onc.2015.80. [DOI] [PubMed] [Google Scholar]
- 34.Forghanifard MM, Taleb S, Abbaszadegan MR. Notch signaling target genes are directly correlated to esophageal squamous cell carcinoma tumorigenesis. Pathology and oncology research. 2015;21(2):463–467. doi: 10.1007/s12253-014-9849-8. [DOI] [PubMed] [Google Scholar]
- 35.Zhou GX, Li XY, Zhang Q, Zhao K, Zhang CP, Xue CH, Yang K, Tian ZB. Effects of the hippo signaling pathway in human gastric cancer. Asian Pacific journal of cancer prevention. 2013;14(9):5199–5205. doi: 10.7314/apjcp.2013.14.9.5199. [DOI] [PubMed] [Google Scholar]
- 36.Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Molecular and cellular biology. 2008;28(7):2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam-Himlin DM, Daniels JA, Gayyed MF, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. The hippo pathway in human upper gastrointestinal dysplasia and carcinoma:a novel oncogenic pathway. Journal of gastrointestinal cancer. 2006;37(4):103–109. doi: 10.1007/s12029-007-0010-8. [DOI] [PubMed] [Google Scholar]
- 38.Deng J, Lei W, Xiang X, Zhang L, Yu F, Chen J, Feng M, Xiong J. MicroRNA-506 inhibits gastric cancer proliferation and invasion by directly targeting Yap1. Tumor biology. 2015;36(9):6823–6831. doi: 10.1007/s13277-015-3364-8. [DOI] [PubMed] [Google Scholar]
- 39.Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, Yamamoto S, Tatsuno K, Katoh H, Watanabe Y, Ichimura T, Ushiku T, Funahashi S, Tateishi K, Wada I, Shimizu N, Nomura S, Koike K, Seto Y, Fukayama M, Aburatani H, Ishikawa S. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nature genetics. 2014;46(6):583–587. doi: 10.1038/ng.2984. [DOI] [PubMed] [Google Scholar]
- 40.Lim B, Park JL, Kim HJ, Park YK, Kim JH, Sohn HA, Noh SM, Song KS, Kim WH, Kim YS, Kim SY. Integrative genomics analysis reveals the multilevel dysregulation and oncogenic characteristics of TEAD4 in gastric cancer. Carcinogenesis. 2014;35(5):1020–1027. doi: 10.1093/carcin/bgt409. [DOI] [PubMed] [Google Scholar]
- 41.Sun L, Chen F, Shi W, Qi L, Zhao Z, Zhang J. Prognostic impact of TAZ and β-catenin expression in adenocarcinoma of the esophagogastric junction. Diagnostic pathology. 2014;9:125. doi: 10.1186/1746-1596-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nature reviews molecular cell biology. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 43.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valdés F, Álvarez AM, Locascio A, Vega S, Herrera B, Fernández M, Benito M, Nieto MA, Fabregat I. The epithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor βin fetal rat hepatocytes. Molecular cancer research. 2002;1(1):68–78. [PubMed] [Google Scholar]
- 45.Qiao Y, Jiang X, Lee ST, Karuturi RM, Hooi SC, Yu Q. FOXQ1 regulates epithelial-mesenchymal transition in human cancers. Cancer research. 2011;71(8):3076–3086. doi: 10.1158/0008-5472.CAN-10-2787. [DOI] [PubMed] [Google Scholar]
- 46.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. The journal of clinical investigation. 2009;119(6):1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanzawa M, Semba S, Hara S, Itoh T, Yokozaki H. WNT5A is a key regulator of the epithelial-mesenchymal transition and cancer stem cell properties in human gastric carcinoma cells. Pathobiology. 2013;80(5):235–244. doi: 10.1159/000346843. [DOI] [PubMed] [Google Scholar]
- 48.Guo J, Fu Z, Wei J, Lu W, Feng J, Zhang S. PRRX1 promotes epithelial–mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer. Medical oncology. 2015;32(1):393. doi: 10.1007/s12032-014-0393-x. [DOI] [PubMed] [Google Scholar]
- 49.Yu H, Zeng J, Liang X, Wang W, Zhou Y, Sun Y, Liu S, Li W, Chen C, Jia J. Helicobacter pyloripromotes epithelial–mesenchymal transition in gastric cancer by downregulating programmed cell death protein 4 (PDCD4) PloS one. 2014;9(8):e105306. doi: 10.1371/journal.pone.0105306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu AN, Zhu ZH, Chang SJ, Hang XS. Twist expression associated with the epithelial-mesenchymal transition in gastric cancer. Molecular and cellular biochemistry. 2012;367(1-2):195–203. doi: 10.1007/s11010-012-1333-8. [DOI] [PubMed] [Google Scholar]
- 51.Huang J, Xiao D, Li G, Ma J, Chen P, Yuan W, Hou F, Ge J, Zhong M, Tang Y, Xia X, Chen Z. EphA2 promotes epithelial-mesenchymal transition through the Wnt/β-catenin pathway in gastric cancer cells. Oncogene. 2014;33(21):2737–2747. doi: 10.1038/onc.2013.238. [DOI] [PubMed] [Google Scholar]
- 52.Zheng H, Li W, Wang Y, Liu Z, Cai Y, Xie T, Shi M, Wang Z, Jiang B. Glycogen synthase kinase-3 beta regulates Snail and β-catenin expression during Fas-induced epithelial–mesenchymal transition in gastrointestinal cancer. European journal of cancer. 2013;49(12):2734–2746. doi: 10.1016/j.ejca.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Choi YJ, Kim N, Chang H, Lee HS, Park SM, Park JH, Shin CM, Kim JM, Kim JS, Lee DH. Helicobacter pylori-induced epithelial-mesenchymal transition, a potential role of gastric cancer initiation and an emergence of stem cells. Carcinogenesis. 2015;36(5):553–563. doi: 10.1093/carcin/bgv022. [DOI] [PubMed] [Google Scholar]
- 54.Ryu HS, Park DJ, Kim HH, Kim WH, Lee HS. Combination of epithelial-mesenchymal transition and cancer stem cell–like phenotypes has independent prognostic value in gastric cancer. Human pathology. 2012;43(4):520–528. doi: 10.1016/j.humpath.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Forghanifard MM, Moaven O, Farshchian M, Montazer M, Raeisossadati R, Abdollahi A, Moghbeli M, Nejadsattari T, Parivar K, Abbaszadegan MR. Expression analysis elucidates the roles of MAML1 and Twist1 in esophageal squamous cell carcinoma aggressiveness and metastasis. Annals of surgical oncology. 2012;19(3):743–749. doi: 10.1245/s10434-011-2074-8. [DOI] [PubMed] [Google Scholar]
- 56.Forghanifard MM, Khales SA, Farshchian M, Rad A, Homayouni-Tabrizi M, Abbaszadegan MR. Negative regulatory role of TWIST1 on SNAIL gene expression. Pathology and oncology research. 2017;23(1):85–90. doi: 10.1007/s12253-016-0093-2. [DOI] [PubMed] [Google Scholar]
- 57.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarker s and potential therapeutic targets in human cancer. Journal of clinical oncology. 2009;27(31):5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen SZ, Yao HQ, Zhu SZ, Li QY, Guo GH, Yu J. Expression levels of matrix metalloproteinase-9 in human gastric carcinoma. Oncology letters. 2015;9(2):915–919. doi: 10.3892/ol.2014.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sier C, Kubben FJ, Ganesh S, Heerding MM, Griffioen G, Hanemaaijer R, Van Krieken JH, Lamers CB, Verspaget HW. Tissue levels of matrix metalloproteinases MMP-2 and MMP-9 are related to the overall survival of patients with gastric carcinoma. British journal of cancer. 1996;74(3):413–417. doi: 10.1038/bjc.1996.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inoue T, Yashiro M, Nishimura S, Maeda K, Sawada T, Ogawa Y, Sowa M, Chung K. Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. International journal of molecular medicine. 1999;4(1):73–77. doi: 10.3892/ijmm.4.1.73. [DOI] [PubMed] [Google Scholar]
- 61.Lian PL, Liu Z, Yang GY, Zhao R, Zhang ZY, Chen YG, Zhuang ZN, Xu KS. Integrin αvβ6 and matrix metalloproteinase 9 correlate with survival in gastric cancer. World journal of gastroenterology. 2016;22(14):3852–3859. doi: 10.3748/wjg.v22.i14.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katz LH, Li Y, Chen JS, Muñoz NM, Majumdar A, Chen J, Mishra L. Targeting TGF-βsignaling in cancer. Expert opinion on therapeutic targets. 2013;17(7):743–760. doi: 10.1517/14728222.2013.782287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyazono K, Suzuki H, Imamura T. Regulation of TGF-βsignaling and its roles in progression of tumors. Cancer science. 2003;94(3):230–234. doi: 10.1111/j.1349-7006.2003.tb01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ananiev J, Manolova I, Aleksandrova E, Gulubova M. Impact of TGF-β1 expression and-509C>T polymorphism in the TGF-β1 gene on the progression and survival of gastric cancer. Polish journal of pathology. 68(3):234–240. doi: 10.5114/pjp.2017.71530. [DOI] [PubMed] [Google Scholar]
- 65.Hu WQ, Wang LW, Yuan JP, Yan SG, Li JD, Zhao HL, Peng CW, Yang GF, Li Y. High expression of transform growth factor beta 1 in gastric cancer confers worse outcome:results of a cohort study on 184 patients. Hepato-gastroenterology. 2014;61(129):245–250. [PubMed] [Google Scholar]
- 66.Ito K, Liu Q, Salto-Tellez M, Yano T, Tada K, Ida H, Huang C, Shah N, Inoue M, Rajnakova A, Peh BK, Han HC, Ito T, Teh M, Yeoh KG, Ito Y. RUNX3, a novel tumor suppressor, is frequently inactivated in gastric cancer by protein mislocalization. Cancer research. 2005;65(17):7743–7750. doi: 10.1158/0008-5472.CAN-05-0743. [DOI] [PubMed] [Google Scholar]
- 67.Katayama Y, Takahashi M, Kuwayama H. Helicobacter pyloricauses runx3 gene methylation and its loss of expression in gastric epithelial cells, which is mediated by nitric oxide produced by macrophages. Biochemical and biophysical research communications. 2009;388(3):496–500. doi: 10.1016/j.bbrc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Powell SM, Harper JC, Hamilton SR, Robinson CR, Cummings OW. Inactivation of Smad4 in gastric carcinomas. Cancer research. 1997;57(19):4221–4224. [PubMed] [Google Scholar]
- 69.Kim JJ, Baek MJ, Kim L, Kim NG, Lee YC, Song SY, Noh SH, Kim H. Accumulated frameshift mutations at coding nucleotide repeats during the progression of gastric carcinoma with microsatellite instability. Laboratory investigation. 1999;79(9):1113–1120. [PubMed] [Google Scholar]
- 70.Kang SH, Bang YJ, Im YH, Yang HK, Lee DA, Lee HY, Lee HS, Kim NK, Kim SJ. Transcriptional repression of the transforming growth factor-βtype I receptor gene by DNA methylation results in the development of TGF-βresistance in human gastric cancer. Oncogene. 1999;18(51):7280–7286. doi: 10.1038/sj.onc.1203146. [DOI] [PubMed] [Google Scholar]
- 71.Kargman S, Charleson S, Cartwright M, Frank J, Riendeau D, Mancini J, Evans J, O'Neill G. Characterization of prostaglandin G/H synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology. 1996;111(2):445–454. doi: 10.1053/gast.1996.v111.pm8690211. [DOI] [PubMed] [Google Scholar]
- 72.Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer research. 1997;57(7):1276–1280. [PubMed] [Google Scholar]
- 73.Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. Journal of surgical oncology. 2001;76(1):26–30. doi: 10.1002/1096-9098(200101)76:1<26::aid-jso1005>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 74.Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91(10):1876–1881. [PubMed] [Google Scholar]
- 75.Li Q, Liu N, Shen B, Zhou L, Wang Y, Wang Y, Sun J, Fan Z, Liu RH. Helicobacter pylorienhances cyclooxygenase 2 expression via p38MAPK/ATF-2 signaling pathway in MKN45 cells. Cancer letters. 2009;278(1):97–103. doi: 10.1016/j.canlet.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 76.Caputo R, Tuccillo C, Manzo BA, Zarrilli R, Tortora G, Blanco CDV, Ricci V, Ciardiello F, Romano M. Helicobacter pyloriVacA toxin up-regulates vascular endothelial growth factor expression in MKN 28 gastric cells through an epidermal growth factor receptor-, cyclooxygenase-2-dependent mechanism. Clinical cancer research. 2003;9(6):2015–2021. [PubMed] [Google Scholar]
- 77.Huang S-P, Wu M-S, Shun C-T, Wang H-P, Hsieh C-Y, Kuo M-L, Lin J-T. Cyclooxygenase-2 increases hypoxia-inducible factor-1 and vascular endothelial growth factor to promote angiogenesis in gastric carcinoma. Journal of biomedical science. 2005;12(1):229–241. doi: 10.1007/s11373-004-8177-5. [DOI] [PubMed] [Google Scholar]
- 78.Ye Y, Liu M, Yuan H, Ning S, Wang Y, Chen Z, Ji R, Guo Q, Li Q, Zhou Y. COX-2 regulates Snail expression in gastric cancer via the Notch1 signaling pathway. International journal of molecular medicine. 2017;40(2):512–522. doi: 10.3892/ijmm.2017.3011. [DOI] [PubMed] [Google Scholar]
- 79.Henderson WR. The role of leukotrienes in inflammation. Annals of internal medicine. 1994;121(9):684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 80.Chen F-L, Wang X-Z, Li J-Y, Yu J-P, Huang C-Y, Chen Z-X. 12-lipoxygenase induces apoptosis of human gastric cancer AGS cells via the ERK1/2 signal pathway. Digestive diseases and sciences. 2008;53(1):181–187. doi: 10.1007/s10620-007-9841-1. [DOI] [PubMed] [Google Scholar]
- 81.Wong BCY, Wang WP, Cho CH, Fan XM, Lin MCM, Kung HF, Lam SK. 12-Lipoxygenase inhibition induced apoptosis in human gastric cancer cells. Carcinogenesis. 2001;22(9):1349–1354. doi: 10.1093/carcin/22.9.1349. [DOI] [PubMed] [Google Scholar]
- 82.Zou L-Y, Li J-Y, Chen F-L, Chen Z-X, Wang X-Z. Tumor 5-lipoxygenase expression correlates with gastric cancer metastasis and its selective inhibitor induces cancer cell apoptosis. Journal of Cancer Molecules. 2006;2(6):227–233. [Google Scholar]
- 83.Park S, Yeo M, Jin J-H, Lee K-M, Kim SS, Choi SY, Hahm K-B. Inhibitory activities and attenuated expressions of 5-LOX with red ginseng inHelicobacter pylori-infected gastric epithelial cells. Digestive diseases and sciences. 2007;52(4):973–982. doi: 10.1007/s10620-006-9440-6. [DOI] [PubMed] [Google Scholar]
- 84.Fan XM, Tu SP, Lam SK, Wang WP, Wu J, Wong WM, Yuen MF, Lin MCM, Kung HF, WONG BCY. Five-lipoxygenase-activating protein inhibitor MK-886 induces apoptosis in gastric cancer through upregulation of p27kip1 and bax. Journal of gastroenterology and hepatology. 2004;19(1):31–37. doi: 10.1111/j.1440-1746.2004.03194.x. [DOI] [PubMed] [Google Scholar]
- 85.Matsubara J, Yamada Y, Hirashima Y, Takahari D, Okita NT, Kato K, Hamaguchi T, Shirao K, Shimada Y, Shimoda T. Impact of insulin-like growth factor type 1 receptor, epidermal growth factor receptor, and HER2 expressions on outcomes of patients with gastric cancer. Clinical cancer research. 2008;14(10):3022–3029. doi: 10.1158/1078-0432.CCR-07-1898. [DOI] [PubMed] [Google Scholar]
- 86.Gravalos C, Jimeno A. HER2 in gastric cancer:a new prognostic factor and a novel therapeutic target. Annals of oncology. 2008;19(9):1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 87.Miki H, Ohmori M, Perantoni A, Enomoto T. K-ras activation in gastric epithelial tumors in Japanese. Cancer letters. 1991;58(1):107–113. doi: 10.1016/0304-3835(91)90031-c. [DOI] [PubMed] [Google Scholar]
- 88.Gong C, Mera R, Bravo JC, Ruiz B, Diaz-Escamilla R, Fontham ET, Correa P, Hunt JD. KRAS mutations predict progression of preneoplastic gastric lesions. Cancer epidemiology biomarkers and prevention. 1999;8(2):167–171. [PubMed] [Google Scholar]
- 89.Das K, Gunasegaran B, Tan IB, Deng N, Lim KH, Tan P. Mutually exclusive FGFR2, HER2, and KRAS gene amplifications in gastric cancer revealed by multicolour FISH. Cancer letters. 2014;353(2):167–175. doi: 10.1016/j.canlet.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 90.Spehlmann ME, Eckmann L. Nuclear factor-kappa B in intestinal protection and destruction. Current opinion in gastroenterology. 2009;25(2):92–99. doi: 10.1097/MOG.0b013e328324f857. [DOI] [PubMed] [Google Scholar]
- 91.Sharma SA, Tummuru MKR, Blaser MJ, Kerr LD. Activation of IL-8 gene expression byHelicobacter pyloriis regulated by transcription factor nuclear factor-κB in gastric epithelial cells. The journal of immunology. 1998;160(5):2401–2407. [PubMed] [Google Scholar]
- 92.Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha TK, Byun DS, Chae KS, Lee BH, Chun HS, Lee KY, Kim HJ, Chi SG. NF-κB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology. 2008;135(6):2030–2042.e2033. doi: 10.1053/j.gastro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 93.Liu X, Wang X, Zhang J, Lam E, Shin VY, Cheng AS, Yu J, Chan FK, Sung JJ, Jin HC. Warburg effect revisited:an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene. 2010;29(3):442–450. doi: 10.1038/onc.2009.332. [DOI] [PubMed] [Google Scholar]
- 94.Liu CA, Wang MJ, Chi CW, Wu Cw, Chen JY. Rho/Rhotekin-mediated NF-kappaB activation confers resistance to apoptosis. Oncogene. 2004;23(54):8731–8742. doi: 10.1038/sj.onc.1208106. [DOI] [PubMed] [Google Scholar]
- 95.Cho SJ, Park JW, Kang JS, Kim WH, Juhnn YS, Lee JS, Kim YH, Ko YS, Nam SY, Lee BL. Nuclear factor-κB dependency of doxorubicin sensitivity in gastric cancer cells is determined by manganese superoxide dismutase expression. Cancer science. 2008;99(6):1117–1124. doi: 10.1111/j.1349-7006.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hofstetter WL, Ilson DH, Keswani RN, Kleinberg LR, Korn WM, Lockhart AC, Meredith K, Mulcahy MF, Orringer MB, Posey JA, Sasson AR, Scott WJ, Strong VE, Varghese TK Jr, Warren G, Washington MK, Willett C, Wright CD, McMillian NR, Sundar H National Comprehensive Cancer Network. Gastric cancer, version 2.2013:featured updates to the NCCN guidelines. Journal of the national comprehensive cancer network. 2013;11(5):531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 97.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA):a phase 3, open-label, randomised controlled trial. The Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 98.Hirashima Y, Yamada Y, Matsubara J, Takahari D, Okita N, Takashima A, Kato K, Hamaguchi T, Shirao K, Shimada Y, Taniguchi H, Shimoda T. Impact of vascular endothelial growth factor receptor 1, 2, and 3 expression on the outcome of patients with gastric cancer. Cancer science. 2009;100(2):310–315. doi: 10.1111/j.1349-7006.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fuchs CS, Tabernero J, Tomasek J, Chau I, Melichar B, Safran H, Tehfe MA, Dumitru F, Topuzov E, Schlittler L, Anghel Adrian Udrea, William Campbell, Stephen Brincat, Michael Emig, Symantha A. Melemed, Rebecca R. Hozak, David Ferry, William Caldwell, Jaffer A. Ajani. Candidate biomarker analyses in gastric or gastro-esophageal junction carcinoma:REGARD trial of single-agent ramucirumab (RAM) vs. placebo (PL) Journal of clinical oncology. 2015 May 20;15:4029. [Google Scholar]
- 100.Xie L, Su X, Zhang L, Yin X, Tang L, Zhang X, Xu Y, Gao Z, Liu K, Zhou M, Gao B, Shen D, Zhang L, Ji J, Gavine PR, Zhang J, Kilgour E, Zhang X, Ji Q. FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clinical cancer research. 2013;19(9):2572–2583. doi: 10.1158/1078-0432.CCR-12-3898. [DOI] [PubMed] [Google Scholar]
- 101.Iveson T, Donehower RC, Davidenko I, Tjulandin S, Deptala A, Harrison M, Nirni S, Lakshmaiah K, Thomas A, Jiang Y, Zhu M, Tang R, Anderson A, Dubey S, Onliner KS, Loh E. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma:an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. The lancet oncology. 2014;15(9):1007–1018. doi: 10.1016/S1470-2045(14)70023-3. [DOI] [PubMed] [Google Scholar]
- 102.Shah MA, Bang YJ, Lordick F, Tabernero J, Chen M, Hack SP, Phan SC, Shames DS, Cunningham D. METGastric:A phase III study of onartuzumab plus mFOLFOX6 in patients with metastatic HER2-negative (HER2-) and MET-positive (MET+) adenocarcinoma of the stomach or gastroesophageal junction (GEC) Journal of clinical oncology. 2015 May 20;15:4012. [Google Scholar]
- 103.Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Götte H, Melezínková H, Moehler M Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND):a randomised, open-label phase 3 trial. The lancet oncology. 2013;14(6):490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 104.Satoh T, Lee KH, Rha SY, Sasaki Y, Park SH, Komatsu Y, Yasui H, Kim TY, Yamaguchi K, Fuse N, Yasuhide Yamada, Takashi Ura, Si-Young Kim, Masaki Munakata, Soh Saitoh, Kazuto Nishio, Satoshi Morita, Eriko Yamamoto, Qingwei Zhang, Jung-mi Kim, Yeul Hong Kim, Yuh Sakata. Randomized phase II trial of nimotuzumab plus irinotecan versus irinotecan alone as second-line therapy for patients with advanced gastric cancer. Gastric cancer. 2015;18(4):824–832. doi: 10.1007/s10120-014-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nardone G, Compare D. Epigenetic alterations due to diet andHelicobacter pyloriinfection in gastric carcinogenesis. Expert review of gastroenterology and hepatology. 2008;2(2):243–248. doi: 10.1586/17474124.2.2.243. [DOI] [PubMed] [Google Scholar]
- 106.Nomura AM, Wilkens LR, Henderson BE, Epplein M, Kolonel LN. The association of cigarette smoking with gastric cancer:the multiethnic cohort study. Cancer causes and control. 2012;23(1):51–58. doi: 10.1007/s10552-011-9854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, Chung DC, Norton J, Ragunath K, Van Krieken JH, Dwerryhouse S, Caldas C International Gastric Cancer Linkage Consortium. Hereditary diffuse gastric cancer:updated consensus guidelines for clinical management and directions for future research. Journal of medical genetics. 2010;47(7):436–444. doi: 10.1136/jmg.2009.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boysen T, Mohammadi M, Melbye M, Hamilton-Dutoit S, Vainer B, Hansen A, Wohlfahrt J, Friborg J. EBV-associated gastric carcinoma in high-and low-incidence areas for nasopharyngeal carcinoma. British journal of cancer. 2009;101(3):530–533. doi: 10.1038/sj.bjc.6605168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fukaya M, Isohata N, Ohta H, Aoyagi K, Ochiya T, Saeki N, Yanagihara K, Nakanishi Y, Taniguchi H, Sakamoto H, Shimoda T, Nimura Y, Yoshida T, Sasaki H. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology. 2006;131(1):14–29. doi: 10.1053/j.gastro.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 110.Yan R, Peng X, Yuan X, Huang D, Chen J, Lu Q, Lv N, Luo S. Suppression of growth and migration by blocking the Hedgehog signaling pathway in gastric cancer cells. Cellular oncology (Dordr) 2013;36(5):421–435. doi: 10.1007/s13402-013-0149-1. [DOI] [PubMed] [Google Scholar]
- 111.Niu Y, Li F, Tang B, Shi Y, Hao Y, Yu P. Clinicopathological correlation and prognostic significance of sonic hedgehog protein overexpression in human gastric cancer. International journal of clinical and experimental pathology. 2014;7(8):5144–5153. [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang C, Li C, He F, Cai Y, Yang H. Identification of CD44+CD24+gastric cancer stem cells. Journal of cancer research and clinical oncology. 2011;137(11):1679–1686. doi: 10.1007/s00432-011-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, Wang L, Song B, Li L. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell death and disease. 2014;5(1):e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng XX, Wang ZC, Chen XY, Sun Y, Kong QY, Liu J, Li H. Correlation of Wnt-2 expression and β-catenin intracellular accumulation in Chinese gastric cancers:relevance with tumour dissemination. Cancer letters. 2005;223(2):339–347. doi: 10.1016/j.canlet.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 115.Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer research. 2006;66(21):10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 116.To KF, Chan MW, Leung WK, Yu J, Tong JH, Lee TL, Chan FK, Sung JJ. Alterations of frizzled (FzE3) and secreted frizzled related protein (hsFRP) expression in gastric cancer. Life sciences. 2001;70(4):483–489. doi: 10.1016/s0024-3205(01)01422-9. [DOI] [PubMed] [Google Scholar]
- 117.Pan KF, Liu WG, Zhang L, You WC, Lu YY. Mutations in components of the Wnt signaling pathway in gastric cancer. World journal of gastroenterology. 2008;14(10):1570–1574. doi: 10.3748/wjg.14.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim MS, Kim SS, Ahn CH, Yoo NJ, Lee SH. Frameshift mutations of Wnt pathway genes AXIN2 and TCF7L2 in gastric carcinomas with high microsatellite instability. Human pathology. 2009;40(1):58–64. doi: 10.1016/j.humpath.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 119.Rhyu MG, Park WS, Jung YJ, Choi SW, Meltzer SJ. Allelic deletions of MCC/APC and p53 are frequent late events in human gastric carcinogenesis. Gastroentology. 1994;106(6):1584–1584. doi: 10.1016/0016-5085(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 120.Tong X, Li L, Li X, Heng L, Zhong L, Su X, Rong R, Hu S, Liu W, Jia B, Xing Liu, Geng Kou, Jun Han, Shangjing Guo, Yi Hu, Cheng Li, Qian Tao, Yajun Guo. SOX10, a novel HMG-box-containing tumor suppressor, inhibits growth and metastasis of digestive cancers by suppressing the Wnt/β-catenin pathway. Oncotarget. 2014;5(21):10571–10583. doi: 10.18632/oncotarget.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kirikoshi H, Sekihara H, Katoh M. Up-regulation of WNT10A by tumor necrosis factor αandHelicobacter pyloriin gastric cancer. International journal of oncology. 2001;19(3):533–536. [PubMed] [Google Scholar]
- 122.Murai T, Yamada S, Fuchs BC, Fujii T, Nakayama G, Sugimoto H, Koike M, Fujiwara M, Tanabe KK, Kodera Y. Epithelial-to-mesenchymal transition predicts prognosis in clinical gastric cancer. Journal of surgical oncology. 2014;109(7):684–689. doi: 10.1002/jso.23564. [DOI] [PubMed] [Google Scholar]
- 123.Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Höfler H, Becker KF. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. The American journal of pathology. 2002;161(5):1881–1891. doi: 10.1016/S0002-9440(10)64464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shin NR, Jeong EH, Choi CI, Moon HJ, Kwon CH, Chu IS, Kim GH, Jeon TY, Kim DH, Lee JH, Park DY. Overexpression of Snail is associated with lymph node metastasis and poor prognosis in patients with gastric cancer. BMC cancer. 2012;12:521. doi: 10.1186/1471-2407-12-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jia B, Liu H, Kong Q, Li B. Overexpression of ZEB1 associated with metastasis and invasion in patients with gastric carcinoma. Molecular and cellular biochemistry. 2012;366(1-2):223–229. doi: 10.1007/s11010-012-1299-6. [DOI] [PubMed] [Google Scholar]
- 126.Otsuki S, Inokuchi M, Enjoji M, Ishikawa T, Takagi Y, Kato K, Yamada H, Kojima K, Sugihara K. Vimentin expression is associated with decreased survival in gastric cancer. Oncology reports. 2011;25(5):1235–1242. doi: 10.3892/or.2011.1185. [DOI] [PubMed] [Google Scholar]
- 127.Alves CC, Rosivatz E, Schott C, Hollweck R, Becker I, Sarbia M, Carneiro F, Becker KF. Slug is overexpressed in gastric carcinomas and may act synergistically with SIP1 and Snail in the down-regulation of E-cadherin. The Journal of pathology. 2007;211(5):507–515. doi: 10.1002/path.2138. [DOI] [PubMed] [Google Scholar]
- 128.Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Xu JM, Yong WP, Langer B, Delmar P, Scherer SJ, Shah MA. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer:a biomarker evaluation from the AVAGAST randomized phase III trial. Journal of clinical oncology. 2012;30(17):2119–2127. doi: 10.1200/JCO.2011.39.9824. [DOI] [PubMed] [Google Scholar]
- 129.Molife L, Fong P, Paccagnella L, Reid A, Shaw HM, Vidal L, Arkenau HT, Karavasilis V, Yap TA, Olmos D, Spicer J, Postel-Vinay S, Yin D, Lipton A, Demers L, Leitzel K, Gualberto A, de Bono JS. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours:results of a phase Ib dose-escalation, open-label study. British journal of cancer. 2010;103(3):332–339. doi: 10.1038/sj.bjc.6605767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakata W, Hayakawa Y, Nakagawa H, Sakamoto K, Kinoshita H, Takahashi R, Hirata Y, Maeda S, Koike K. Anti-tumor activity of the proteasome inhibitor bortezomib in gastric cancer. International journal of oncology. 2011;39(6):1529–1536. doi: 10.3892/ijo.2011.1141. [DOI] [PubMed] [Google Scholar]
- 131.Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, Muro K, Kim YH, Ferry D, Tebbutt NC, Al-Batran SE, Smith H, Costantini C, Rizvi S, Lebwohl D, Van Cutsem E. Everolimus for previously treated advanced gastric cancer:results of the randomized, double-blind, phase III GRANITE-1 study. Journal of Clinical Oncology. 2013;31(31):3935–3943. doi: 10.1200/JCO.2012.48.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bramhall S, Hallissey M, Whiting J, Scholefield J, Tierney G, Stuart RC, Hawkins RE, McCulloch P, Maughan T, Brown PD, Baillet M, Fielding JWL. Marimastat as maintenance therapy for patients with advanced gastric cancer:a randomised trial. British journal of cancer. 2002;86(12):1864–1870. doi: 10.1038/sj.bjc.6600310. [DOI] [PMC free article] [PubMed] [Google Scholar]


