Abstract
We investigated the characteristics of ethylene biosynthesis associated with ripening in banana (Musa sp. [AAA group, Cavendish subgroup] cv Grand Nain) fruit. MA-ACS1 encoding 1-aminocyclopropane-1-carboxylic acid (ACC) synthase in banana fruit was the gene related to the ripening process and was inducible by exogenous ethylene. At the onset of the climacteric period in naturally ripened fruit, ethylene production increased greatly, with a sharp peak concomitant with an increase in the accumulation of MA-ACS1 mRNA, and then decreased rapidly. At the onset of ripening, the in vivo ACC oxidase activity was enhanced greatly, followed by an immediate and rapid decrease. Expression of the MA-ACO1 gene encoding banana ACC oxidase was detectable at the preclimacteric stage, increased when ripening commenced, and then remained high throughout the later ripening stage despite of a rapid reduction in the ACC oxidase activity. This discrepancy between enzyme activity and gene expression of ACC oxidase could be, at least in part, due to reduced contents of ascorbate and iron, cofactors for the enzyme, during ripening. Addition of these cofactors to the incubation medium greatly stimulated the in vivo ACC oxidase activity during late ripening stages. The results suggest that ethylene production in banana fruit is regulated by transcription of MA-ACS1 until climacteric rise and by reduction of ACC oxidase activity possibly through limited in situ availability of its cofactors once ripening has commenced, which in turn characterizes the sharp peak of ethylene production.
Ethylene has profound effects on many developmental events and environmental responses of plants (Yang and Hoffman, 1984). Endogenous production of ethylene increases during certain stages of growth and development, such as seed germination, fruit ripening, and leaf and flower senescence and abscission, and in response to drought, flooding, physical wounding, chilling injury, pathogen infection, and chemical inducers (Yang and Hoffman, 1984; Theologis, 1992). In higher plants, ethylene is biosynthesized from Met by a well-defined pathway in which 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase catalyze the reactions from S-adenosylmethionine to ACC and ACC to ethylene, respectively (Yang, 1987). With advancement in molecular biology techniques, cDNA and genomic clones for both enzymes have been isolated from various plant species, and both enzymes appear to be encoded by multigene families. Using these cDNA clones, expression of individual members has been characterized in different tissues and in response to specific stimuli known to induce ethylene biosynthesis (Kende, 1993; Zarembinski and Theologis, 1994; Fluhr and Mattoo, 1996).
Fruits have been classified as climacteric and nonclimacteric on the basis of their patterns of respiration and ethylene production during maturation and ripening (Biale and Young, 1981). In climacteric fruits, it has been accepted that ethylene plays an important role in ripening in that a massive production of ethylene commences at the onset of the respiratory climacteric period, and exogenously applied ethylene induces ripening and endogenous ethylene production. In ripening climacteric fruits, both ACC synthase and ACC oxidase are induced and contribute to the regulation of ethylene biosynthesis (Yang and Hoffman, 1984). Expression of ACC synthase genes has been investigated in many fruits, including apple (Dong et al., 1991), tomato (Olson et al., 1991; Rottmann et al., 1991; Lincoln et al., 1993; Nakatsuka et al., 1998), melon (Yamamoto et al., 1995), pear (Lelièvre et al., 1997b), passion fruit (Mita et al., 1998), and cucumber (Shiomi et al., 1998). Expression of ACC oxidase genes has also been investigated in fruits such as tomato (Barry et al., 1996; Nakatsuka et al., 1998), apple (Ross et al., 1992), melon (Balaguè et al., 1993; Yamamoto et al., 1995; Lasserre et al., 1996), kiwi (Whittaker et al., 1997), pear (Lelièvre et al., 1997b), cucumber (Shiomi et al., 1998), passion fruit (Mita et al., 1998), and banana (Huang et al., 1997; López-Gómez et al., 1997).
Banana (Musa spp.), a typical climacteric fruit, is commercially ripened by treatment with exogenous ethylene (Inaba and Nakamura, 1986; Golding et al., 1998). In most climacteric fruits, ethylene production begins to increase at the onset of the climacteric period and thereafter increases and decreases in parallel with the changes in respiratory climacteric toward the full-ripe stage. In the reports cited above, it was demonstrated at the molecular level that this pattern of change in the rate of ethylene production is well correlated with the pattern of changes in the levels of ACC synthase and ACC oxidase gene transcripts. However, unlike most of the climacteric fruits mentioned above, banana fruit exhibits a sharp rise and fall in the rate of ethylene production during the early climacteric rise of respiration (Burg and Burg, 1962, 1965; Karikari et al., 1979). A similar trend has also been recognized in avocado fruit (Hoffman and Yang, 1980). For this reason, it is considered that the regulatory mechanism(s) of ethylene biosynthesis in banana fruit may be different from that of other climacteric fruits. Therefore, it is important to investigate at both the biochemical and molecular levels the possible mechanism(s) involved in the sharp peak of ethylene production at the early climacteric stage in banana fruit. An ACC oxidase gene has been cloned from banana fruit and its expression pattern during fruit ripening has been characterized (Clendennen et al., 1997; Huang et al., 1997; López-Gómez et al., 1997). However, to our knowledge, there has been no report related to the expression of ACC synthase genes, despite the fact that six sequences for banana ACC synthase have already been registered in the database.
In the present study, we isolated three different cDNAs for ACC synthase and one for ACC oxidase from banana fruit, and analyzed the expression characteristics of these genes during ripening. We demonstrate a possible regulatory mechanism of the sharp peak in ethylene production at the early climacteric rise in banana fruit, showing a possible involvement of a sharp decrease in ACC oxidase activity through limited availability of its cofactors.
MATERIALS AND METHODS
Plant Material and Treatment
Preclimacteric banana (Musa sp. [AAA group, Cavendish subgroup] cv Grand Nain) fruit imported from the Philippines were supplied by a local importer. Each banana hand was separated into individual fingers and ripened at 22°C naturally or after treatment with 100 μL L−1 ethylene for 18 h. In each experiment, fingers from the same hand were used as a sample group to avoid variation in ripening behaviors of fingers among different hands (Inaba and Nakamura, 1986). During ripening, ethylene production and in vivo ACC oxidase activity were measured on a daily basis. Based on the rate of ethylene production, flesh tissue was frozen in liquid nitrogen at the appropriate time points and stored at −80°C for the extraction of total RNA, ACC, ACC oxidase, ascorbate, and soluble iron. For wounding, flesh tissue was cut into cubes of about 5 mm and incubated at 25°C for 6 h under humidified conditions. The cubes were frozen in liquid nitrogen and stored at −80°C until used. All experiments except RNA extraction were repeated at least three times. However, the rate of ethylene biosynthesis associated with ripening varied slightly in each hand, and since there is limitation in the number of fingers in one hand, only representative data are shown.
Ethylene Production
Ethylene production was measured by enclosing fruit samples or flesh cubes in an airtight container for 1 h at 25°C, withdrawing 1 mL of the headspace gas, and injecting it into a gas chromatograph fitted with a flame ionization detector and an activated alumina column.
Contents of ACC, Ascorbate, and Iron
ACC was measured by the method of Lizada and Yang (1979), with 80% (v/v) ethanol extract from frozen flesh tissue. Reduced ascorbate content was determined according to the method described by the Association of Official Analytical Chemists (1980) with only slight modifications. Five grams of frozen flesh tissue was homogenized with 10 mL of 5% (w/v) metaphosphate solution and centrifuged at 30,000g for 15 min. Five milliliters of 2,6-dichlorophenolindophenol solution, whose factor had previously been determined using authentic ascorbate, was titrated with the extracted solution. Soluble iron was extracted with water. Ten grams of frozen flesh tissue was homogenized with 20 mL of distilled water and 250 mg of insoluble polyvinylpyrrolidone, and then centrifuged at 30,000g for 20 min. The supernatant was dried overnight at 70°C and heated at 350°C for 5 h, followed by overnight ashing at 550°C. The ash was dissolved in small amount of 0.3 m HCl and diluted with deionized water. Soluble iron was measured according to the o-phenanthroline method (Sandell, 1959).
ACC Oxidase Activity
For the measurement of in vivo ACC oxidase activity, flesh slices of 1 mm thickness (approximately 1 g) were put into 40-mL Erlenmeyer flasks containing 2 mL of incubation buffer consisting of 1 mm ACC, 0.4 m mannitol, and 0.1 m Tricine (pH 7.5). In vivo ACC oxidase activity was determined both in the absence and in the presence of 30 mm sodium ascorbate, 0.1 mm FeSO4, and 20 mm NaHCO3 according to the method described by Moya-Leòn and John (1994). The flasks were incubated at 30°C for 1 h and the ethylene formed was determined as described above. The activity was expressed as ethylene (in nanomoles) produced per gram fresh weight per hour.
In vitro ACC oxidase activity was measured according to the method by Moya-Leòn and John (1994). Ten grams of frozen flesh was ground to a fine powder in liquid nitrogen in the presence of 5% (w/w) polyvinylpyrrolidone. The powder was transferred to a 50-mL centrifuge tube containing 20 mL of an extraction buffer consisting of 0.1 m Tris-HCl (pH 7.5), 10% (v/v) glycerol, 2 mm dithiothreitol, and 30 mm sodium ascorbate. After the slurry had thawed completely, the tube was centrifuged at 30,000g for 20 min. The supernatant was passed through a membrane filter (Cellulose Nitrate, 0.45 μm, Toyo Roshi, Tokyo) and desalted by passage through Sephadex G-25 columns previously equilibrated with the extraction buffer. All steps were carried out at 4°C. In vitro ACC oxidase activity was assayed by incubating 1 mL of the enzyme preparation with 1 mL of a reaction mixture consisting of 0.1 m Tricine (pH 7.5), 10% (v/v) glycerol, 1 mm ACC, 30 mm sodium ascorbate, 0.1 mm FeSO4, and 20 mm NaHCO3 at 30°C for 1 h, and the ethylene produced was determined.
RNA Isolation, Cloning, and Sequencing
Total RNA was extracted by the hot borate method (Wan and Wilkins, 1994). Poly(A+) RNA was isolated using Oligotex-dT30 (TaKaRa, Kyoto) according to the manufacturer's protocol. The first-strand cDNAs synthesized by reverse transcription from 2 μg of poly(A+) RNA isolated from ripe, ethylene-treated, and wounded banana flesh were used as templates for the reverse transcriptase (RT)-PCR using degenerate oligonucleotide primers for ACC synthase, ACC oxidase, and actin. The primers for ACC synthase and ACC oxidase were synthesized with reference to the conserved amino acid sequences reported for other plant organs (Kende, 1993) with restriction site sequences of BamHI or PstI, as we previously described (Nakatsuka et al., 1998). Degenerate oligonucleotide primers for actin cDNA were synthesized based on the conserved domain in actin amino acid sequences registered in the database from various plant sources: 5′-CGCGGATCCGARAARATGACNCARATHATGTT-3′ as the upstream primer and 5′-AAACTGCAGATRTCNACR- TCRCAYTTCAT-3′ as the downstream primer, where the underlined sequences were restriction sites of BamHI and PstI, respectively.
Reactions for the RT-PCR were subjected to 30 cycles of 94°C for 1 min, 55°C for 2 min, and 72°C for 3 min. The amplified cDNAs were digested with the restriction enzymes and ligated into pUC118 plasmid. The nucleotide sequences of the cDNA inserts were determined using a DNA sequencer (model DSQ-1000, Shimadzu, Kyoto) using either the −21M13 or M13 sequencing primers according to the manufacturer's instructions (Amersham, Uppsala). The sequences obtained were analyzed with the GenomeNet database to determine whether the cloned cDNAs were the fragments for ACC synthase or ACC oxidase genes. To determine the full-length nucleotide sequences for MA-ACS1, RACE-PCR was performed using a cDNA amplification kit (Marathon, CLONTECH, Palo Alto, CA) according to the manufacturer's protocol.
Northern-Blot Analysis
The mRNA isolated from ripening, ethylene-treated, and wounded flesh tissues was separated by electrophoresis on 1% (w/v) agarose gels containing 0.66 m formaldehyde, blotted onto nylon membranes (Hybond N, Amersham), and fixed by UV cross-linker (Amersham). The membranes were hybridized with 32P-labeled cDNA probes obtained from the RT-PCR products mentioned above and hybridized as previously described (Nakatsuka et al., 1998). Following hybridization, the membranes were washed at 60°C in 2× SSPE (1× SSPE = 0.15 m NaCl, 10 mm NaH2PO4, and 1 mm EDTA [pH 7.4]) and 0.1% (w/v) SDS for 30 min, in 0.5× SSPE and 0.1% (w/v) SDS for 30 min, and in 0.2× SSPE and 0.1% (w/v) SDS for 30 min. The cDNA probes were labeled using the random-primed DNA labeling kit (Boehringer Mannheim, Basel) with [32P]dCTP. The membranes were subsequently exposed to an imaging plate (Fuji Photo Film, Tokyo) at room temperature. Equal reactivity and amount of RNA in all samples were verified by hybridization with 32P-labeled MA-Actin.
Southern Analysis
Genomic DNA was isolated from the unfurling leaves immediately after emergence from the banana corm or rhizome by the method of Murray and Thompson (1980). Five-microgram samples of DNA were digested with restriction enzymes, EcoRI, HindIII, KpnI, and BamHI, separated on 0.8% (w/v) agarose gels, and blotted onto nylon membranes as described above. Membranes were hybridized with 32P-labeled cDNA probes obtained from RT-PCR clones for ACC synthase, washed once at 55°C in 5× SSPE and 0.1% (w/v) SDS for 30 min, twice at 55°C in 0.2× SSPE and 0.1% (w/v) SDS, and then exposed to an imaging plate as described above.
RESULTS
Isolation and Identification of cDNA Clones
Using degenerate oligonucleotide primers, we cloned five fragments including three different cDNAs for ACC synthase (MA-ACS1, MA-ACS2, and MA-ACS3), one for ACC oxidase (MA-ACO1), and one for actin (MA-Actin) based on their amino acid sequences. When the sequence of each fragment for ACC synthase was compared with those already registered in the database, MA-ACS1 had high sequence similarity to Y15739 with 97.3% and 95.9% at the nucleotide and amino acid levels, respectively (Table I). However, the other two cDNAs for ACC synthase cloned in this study had low sequence similarity compared with those already registered (less than 80%). Among the cloned ACC synthase cDNAs, MA-ACS1 was the gene expressed during fruit ripening as described below; therefore, we determined the full-length sequence of its cDNA using RACE-PCR and registered it in the database (accession no. AB021906). Full-length cDNA of MA-ACS1 contained an open reading frame of 1623 bp encoding a sequence of 541 amino acids.
Table I.
Percentage sequence identity between ACC synthases encoded by multigene family in banana
| Deduced Amino Acid Sequence | Nucleotide Sequence
|
|||||
|---|---|---|---|---|---|---|
| MA-ACS1 | MA-ACS2 | MA-ACS3 | Y15739 | X96946 | AJ223186 | |
| MA-ACS1 | – | 61.3 | 64.4 | 97.3 | 64.3 | 61.7 |
| MA-ACS2 | 52.5 | – | 83.5 | 60.5 | 77.6 | 61.0 |
| MA-ACS3 | 56.6 | 79.8 | – | 64.1 | 80.2 | 62.7 |
| Y15739 | 95.7 | 51.4 | 54.9 | – | 63.2 | 61.3 |
| X96946 | 57.4 | 74.2 | 78.0 | 56.4 | – | 63.4 |
| AJ223186 | 61.0 | 55.2 | 58.0 | 59.2 | 59.1 | – |
The nucleotide sequence of the MA-ACO1 fragment cloned in the present study had homology of more than 99.5% compared with those of banana ACC oxidase cDNAs registered in the databases: MA-ACO1 (accession no. X91076); MA-ACO (accession no. Z93121); and MA-ACO (accession no. U86045). The mismatch of sequences between our fragment and the registered cDNAs could be due to PCR errors or to differences in banana strains. The deduced amino acid sequence of the MA-Actin fragment was identical by more than 88% to that for actin in potato (accession no. U60486), rice (accession no. X15864), tobacco (accession no. U60491), maize (accession no. J01238), and soybean (accession no. U60497).
Differential Expression and Genome Structures of ACC Synthase Genes
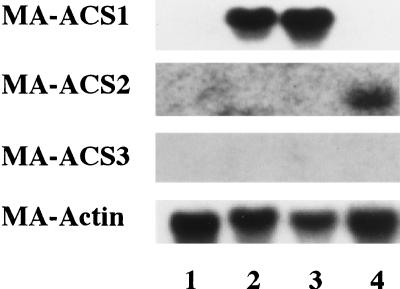
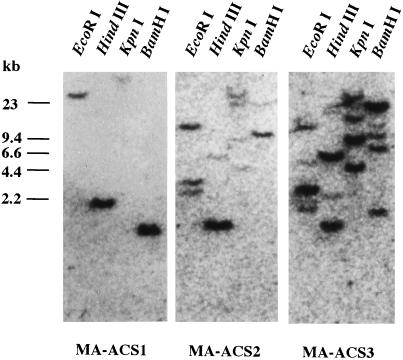
To determine the most significant ACC synthase gene(s) related to ripening in banana fruit, northern analysis was performed with mRNAs from fruit ripened naturally or by treatment with exogenous ethylene or subjected to wounding. As shown in Figure 1, only signals for the MA-ACS1 gene were detected in the ripening flesh and MA-ACS2 mRNA accumulated only in the wounded flesh. Signals for the MA-ACS3 gene were not detected in any of the treatments. Thus, among the three members of the MA-ACS gene family cloned, MA-ACS1 was the only gene that seemed to be expressed during fruit ripening. To verify the organization of these three genes in the banana genome, Southern analysis was performed using cDNA clones obtained in this study as probes. The blot probed with MA-ACS1 yielded a single band on each lane (Fig. 2). However, one strong band and several weak bands were observed in each restriction enzyme digest on the blots probed with MA-ACS2 or MA-ACS3. The strong band on the blot of MA-ACS2 corresponded to one of weak bands observed in the MA-ACS3-probed blot, suggesting cross-hybridization due to high homology in the nucleotide sequence, whereas the band in MA-ACS1 did not correspond to any of the bands in MA-ACS2 or MA-ACS3. Southern analysis showed that MA-ACS1 exists as a single copy and an additional two or more genes homologous to MA-ACS2 and MA-ACS3 exist in the banana genome. One of the additional genes could be X96946 registered in the database due to its high similarity in nucleotide sequence with MA-ACS2 and MA-ACS3 (Table I).
Figure 1.
RNA blot showing the differential expression of three banana ACC synthase genes in the flesh tissue of preclimacteric, ripening, and wounded fruit. The lanes are: 1, preclimacteric fruit; 2, fruit ripened naturally; 3, fruit ripened by application of exogenous ethylene; and 4, fruit subjected to wounding. Each lane contains 5 μg of mRNA. MA-Actin was used as an internal control to normalize the amount of mRNA loaded.
Figure 2.
Genomic Southern-blot analysis of banana ACC synthase genes. DNA purified from sprouting leaves was digested with EcoRI, HindIII, KpnI, or BamHI, fractionated on a 0.8% (w/v) agarose gel, and blotted to a nylon membrane. The membrane was hybridized to the 32P-labeled MA-ACS1, MA-ACS2, and MA-ACS3 probes. Blots were washed with high-stringency buffer as described in “Materials and Methods,” and subsequently subjected to autoradiography.
Ethylene Biosynthesis and Expression of ACC Synthase and ACC Oxidase Genes during Ripening
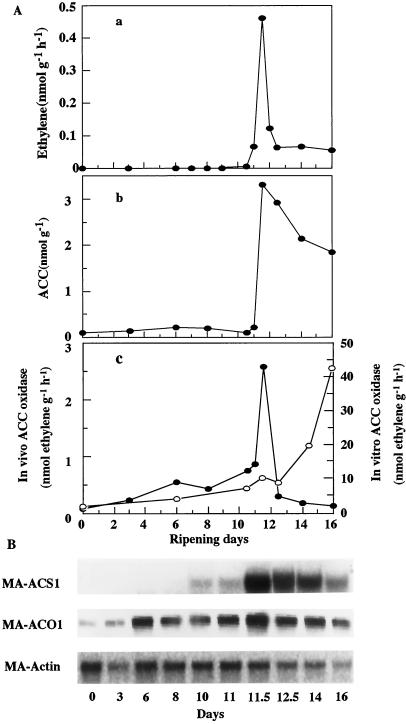
Figure 3 shows the changes in the rate of ethylene production, ACC content, and in vivo ACC oxidase activity and northern analysis in banana fruit ripened naturally at 22°C. Ethylene production commenced on d 11, and immediately thereafter increased greatly with a sharp peak for one-half day and then decreased rapidly. ACC content remained at a low level until commencement of ethylene production and then increased abruptly in parallel with the ethylene peak followed by a gradual decline. In vivo ACC oxidase activity increased gradually during the preclimacteric period and then showed a sharp rise and fall in parallel with that of ethylene production. On the contrary, in vitro ACC oxidase activity increased steadily from the initiation of ripening to the full-ripe stage. MA-ACS1 mRNA was undetectable in the fruit at the preclimacteric stage, but increased from the onset of the climacteric and reached the highest level on d 11 followed by a slight decline. Thus, the slight accumulation of MA-ACS1 mRNA preceded the increase in ACC content and the highest signal of the gene was observed on d 11 when an abrupt increase in ACC content and ethylene production occurred. The MA-ACO1 gene was already expressed in the fruit at the beginning of the experiment. However, the abundance of its mRNA increased slightly toward commencement of ripening and thereafter decreased slightly.
Figure 3.
A, Changes in ethylene biosynthesis in banana fruit ripened naturally. a, Ethylene production rate; b, ACC content; c, in vivo (●) and in vitro (○) ACC oxidase activities. B, Expression of ACC synthase and ACC oxidase genes. Individual fruit was separated from one hand and ripened naturally at 22°C. On each sampling day, ethylene production was determined in one fruit, and then flesh from the same fruit was used for the determination of ACC content, ACC oxidase activity assay, and RNA extraction for northern analysis. Each lane (B) contains 10 μg of mRNA for MA-ACS1 and MA-Actin and 1.5 μg for MA-ACO1. MA-Actin was used as an internal control to normalize the amount of mRNA loaded.
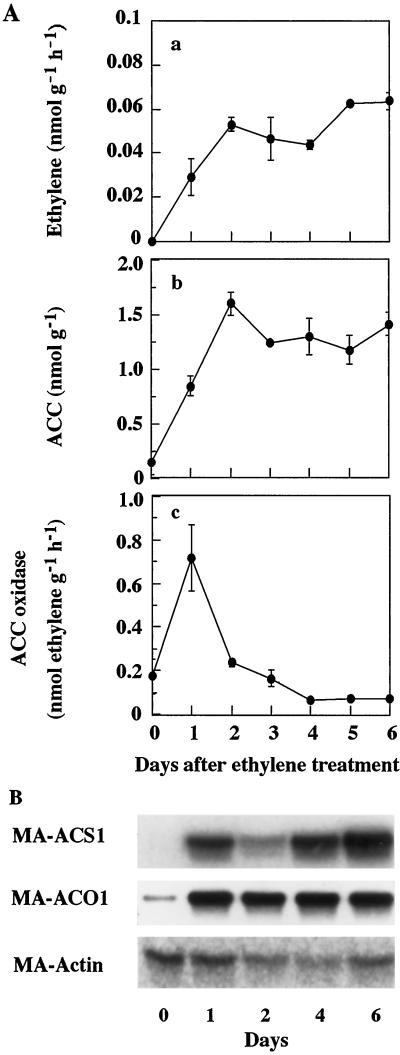
In the fruit ripened by exogenous ethylene treatment, ethylene biosynthesis was activated immediately after the treatment (Fig. 4). Ethylene production by the fruit increased gradually throughout ripening, but no sharp peak was observed like the one observed in fruit ripened naturally. ACC content increased for 2 to 4 d and remained high toward the full-ripe stage. In vivo ACC oxidase activity in the flesh was enhanced immediately after treatment with exogenous ethylene, but declined rapidly thereafter to the preclimacteric level. From these fruit, mRNA was extracted and northern analysis was performed as shown in Figure 4. MA-ACS1 mRNA was not detectable in the preclimacteric fruit but increased greatly by treatment with exogenous ethylene. Although the MA-ACO1 gene was expressed in preclimacteric fruit, the abundance of its mRNA increased further upon commencement of ripening initiated by exposure to exogenous ethylene, and remained at a high level until the full-ripe stage.
Figure 4.
A, Changes in ethylene biosynthesis in banana fruit ripened by exogenous ethylene. a, Ethylene production rate; b, ACC content; c, in vivo ACC oxidase activity. B, Expression of ACC synthase and ACC oxidase genes. Individual fruit was separated from one hand, treated with 100 μL L−1 ethylene for 18 h, and then ripened at 22°C. On each sampling day, ethylene production was determined in one fruit and then the flesh was used for determination of in vivo ACC oxidase activity, ACC content, and gene expression. Vertical bars represent means ± se (n = 3). Each lane (B) contains 10 μg of mRNA for MA-ACS1 and MA-Actin and 1.5 μg for MA-ACO1. MA-Actin was used as an internal control to normalize the amount of mRNA loaded.
Characteristics of ACC Oxidase Activity
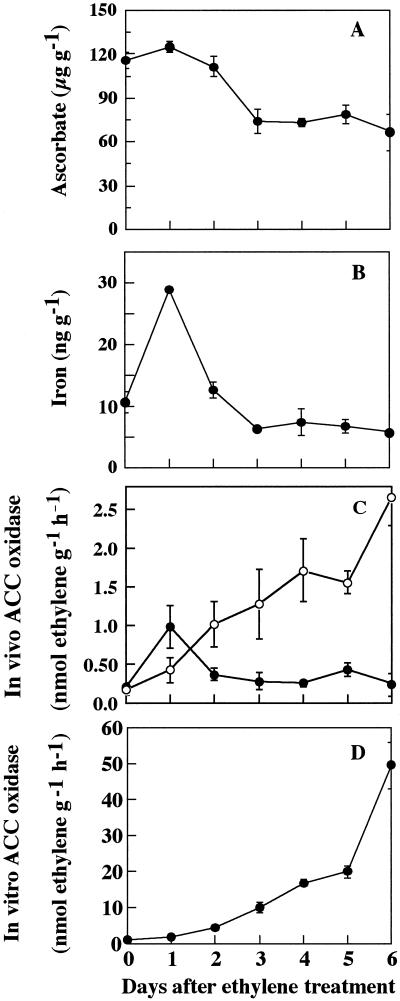
In the results described above, the level of MA-ACO1 mRNA in the flesh was inconsistent with ACC oxidase activity in the flesh slices determined in the absence of ascorbate and iron. Since ACC oxidase has been shown to require ascorbate and iron as cofactors (Ververidis and John, 1991), we analyzed free ascorbate and soluble iron contents in the flesh of the fruit ripened by treatment with exogenous ethylene (Fig. 5). Both ascorbate and iron contents increased at the beginning of ripening and then decreased toward the full-ripe stage. The rate of decrease of the iron content was higher than that of ascorbate. When these cofactors were added to the incubation mixture, in vivo ACC oxidase activity was greatly activated especially during the later stages of ripening (Fig. 5). The pattern of the in vivo ACC oxidase activity in the presence of cofactors closely resembled that of the in vitro activity throughout ripening.
Figure 5.
Changes in free ascorbate (A), soluble iron content (B), in vivo ACC oxidase activity measured in the presence (○) and absence (●) of ascorbate and iron (C), and in vitro ACC oxidase activity (D) during ripening in banana fruit treated with ethylene. Individual fruit was separated from one hand, treated with 100 μL L−1 ethylene for 18 h, and then ripened at 22°C. Vertical bars represents means ± se (n = 3).
DISCUSSION
Isolation of cDNAs Encoding ACC Synthase and ACC Oxidase from Banana Fruit
To understand ethylene biosynthesis in banana fruit at the molecular level, we cloned three cDNA fragments for ACC synthase and one for ACC oxidase. Among the cDNAs cloned, MA-ACS1 had a high sequence similarity (more than 97%) to one banana ACC synthase gene registered in the database (accession no. Y15739) at the nucleotide level (Table I). However, the other two cDNAs for ACC synthase cloned in this study, MA-ACS2 and MA-ACS3, showed low sequence similarity (less than 80%) to any registered cDNAs, indicating that these two cDNA fragments are novel (Table I). Genomic Southern analysis showed that in addition to MA-ACS1 being a single-copy gene, four or five genes homologous to MA-ACS2 or MA-ACS3 belonging to the same subfamily exist in banana (Fig. 2). Since the nucleotide sequence of AJ223186 has relatively low homology (about 60%) with any sequence obtained in our study or others (Table I), at least one more gene must exist in the ACC synthase gene family in the banana genome. Therefore, six or more members of ACC synthase gene family may exist in banana. MA-ACO1 showed a high sequence similarity (more than 98%) to the one registered as the ACC oxidase gene related to banana ripening (Huang et al., 1997; López-Gómez et al., 1997).
To our knowledge, there has been no report on the expression of ACC synthase genes in banana fruit, despite there being six sequences already registered in the database. Therefore, we determined the most significant ACC synthase gene related to ripening in banana fruit based on northern analysis (Fig. 1). Among the three members of the MA-ACS gene family we cloned, only the MA-ACS1 mRNA was detected in ripening fruit. This gene was also inducible by exogenous ethylene treatment. MA-ACS2 was the gene inducible by wounding, whereas the transcript of MA-ACS3 was not detected by northern analysis, suggesting its extremely low level of expression in banana fruit. Therefore, we concluded that among the ACS genes cloned in this study MA-ACS1 is a significant member of the ACC synthase gene family related to ripening in banana fruit.
Induction of Ripening Ethylene in Banana Fruit
In the present study, ethylene production by intact banana fruit ripened naturally was undetectable during the preclimacteric period but increased abruptly and reached a value of about 0.5 nmol g−1 h−1 followed by a rapid decline to a level of 0.06 nmol g−1 h−1 (Fig. 3). A sharp peak of ethylene production at the onset of climacteric has been recognized as a characteristic ripening feature of banana fruit (Burg and Burg, 1962, 1965; Karikari et al., 1979). At the onset of the climacteric, accumulation of MA-ACS1 mRNA and ACC in the banana flesh increased dramatically coincidentally with the increase in ethylene production (Fig. 3). In vivo and in vitro activities of ACC oxidase and the abundance of MA-ACO1 mRNA gradually increased during the preclimacteric period. In vivo activity of the enzyme increased abruptly at the ethylene burst, whereas in vitro activity showed only a slight increase and the level of MA-ACO1 mRNA did not show any remarkable change at this time.
The sudden increase in ACC content concomitant with a burst of ethylene production (Hoffman and Yang, 1980) due to newly synthesized mRNA for ACC synthase at the onset of ripening is a well-known phenomenon in climacteric fruits (Lelièvre et al., 1997a). At the onset of ripening, a burst in ethylene production and ACC oxidase activity occurs in many climacteric fruits (Lelièvre et al., 1997a), as was observed for banana in this study. The accumulation of MA-ACS1 mRNA correlated well with the induction of ethylene production, while relatively large accumulation of MA-ACO1 mRNA was observed before the event. This was also true in the fruit ripened by external ethylene treatment (Fig. 4). These results suggest that the induction of ripening ethylene in banana fruit is primarily regulated by MA-ACS1 gene expression.
Possible Mechanism of the Rapid Decline in Ethylene Production at the Early Climacteric Phase
In most climacteric fruits, ethylene production during ripening has been widely recognized to have a climacteric pattern in parallel with the respiration rate; however, ethylene production in banana and avocado declines in the early climacteric phase, resulting in a sharp and short peak, as was observed in naturally ripened fruit in this study. Thus, the pertinent question is what mechanism is involved in the rapid decline of ethylene production that characterizes the unique ethylene biosynthesis in ripening banana fruit? Since the level of the MA-ACS1 transcript and ACC content remained high even after the reduction of ethylene production (Fig. 3), ACC synthase limitation was excluded from the mechanism to explain the rapid fall of ethylene production during the early ripening stage. Although ACC synthase in general is the rate-limiting enzyme in ethylene biosynthesis, evidence has accumulated to suggest that ACC oxidase plays an important role in the regulation of ethylene production in fruits such as tomato (Barry et al., 1996; Blume and Grierson, 1997), melon (Lasserre et al., 1996), banana (Dominiguez and Vendrell, 1994), apple (Lelièvre, 1995), and pear (Lelièvre et al., 1997b).
Surprisingly, in vivo ACC oxidase activity in the flesh showed a rapid decline similar to that observed for ethylene production at the early ripening stage, despite the fact that the abundance of MA-ACO1 mRNA remained high and in vitro activity of the enzyme increased consistently until the full-ripe stage (Fig. 3). High message levels of the MA-ACO1 gene in ripening banana fruit have previously been demonstrated by López-Gómez et al. (1997) and Huang et al. (1997). The in vivo and in vitro activities of ACC oxidase are reflected in the accumulated level of ACC oxidase mRNAs in various climacteric fruits such as apple (Dong et al., 1992), tomato (Nakatsuka et al., 1997), peach (Tonutti et al., 1997), melon (Bouquin et al., 1997), and pear (Lelièvre et al., 1997b). However, in banana, changes in ACC oxidase activity determined in vivo were inconsistent with the abundance of MA-ACO1 mRNA in the ripening fruit. These observations suggest that a mechanism(s) other than gene transcription of MA-ACO1 may be responsible for the rapid decline in ethylene production at the early ripening stages.
Since the work by Ververidis and John (1992), it has been established that ACC oxidase has an absolute requirement for ascorbate and iron as cofactors for its activity. That study demonstrated that ACC oxidase activity determined in vivo was also stimulated by these cofactors at the later ripening stages in melon fruit. This finding led us to consider an involvement of the limitation of these cofactors during the rapid decline of in vivo ACC oxidase activity, which in turn could have limited ethylene production at the late stages of banana ripening. To clarify this hypothesis, we used banana fruit ripened by application of ethylene, because the ripening behavior of the fruit ripened naturally is identical to that of the fruit ripened by exogenous ethylene treatment (Inaba and Nakamura, 1986). Exogenous ethylene induced ripening with elevation in the ethylene production rate, ACC content, and in vivo ACC oxidase activity, but no sharp peak was observed in these ripening parameters (Fig. 4). The peak may have occurred in the period during ethylene treatment, because ethylene production, ACC content, and in vivo ACC oxidase activity in the fruit ripened by exogenous ethylene were almost the same as those in the naturally ripened fruit at the stage after the ethylene peak (Fig. 4). In addition, we previously observed that endogenous ethylene production during exposure to exogenous ethylene was induced in a similar pattern as in the naturally ripened banana fruit (Inaba et al., 1989).
We measured the contents of reduced ascorbate and soluble iron in banana fruit treated with exogenous ethylene (Fig. 5). Both ascorbate and iron contents increased temporarily at the onset of ripening and then decreased rapidly coinciding with the rapid decrease in the in vivo ACC oxidase activity. A similar trend was also observed in the naturally ripened fruit (data not shown). These results suggest that the decreased contents of ascorbate and iron, especially the latter, might limit ACC oxidase activity in banana fruit. Indeed, the addition of these cofactors stimulated the in vivo ACC oxidase activity in fruit at late ripening stages but not at the preclimacteric or early ripening stages. Although the reasons for higher in vitro activity than in vivo activity are not known, similar observations have been reported in ripening banana pulp (Moya-Leòn and John, 1994). Thus, by addition of cofactors, in vivo ACC oxidase activity showed a similar pattern to that of the in vitro activity with progress of ripening.
When we determined the dependence of the in vivo ACC oxidase activity on the concentrations of the cofactors, we found that it was saturated at concentrations of about 100 and 1 mm for ascorbate and iron, respectively (data not shown). The apparent Km value of these cofactors under in vivo assay conditions is difficult to determine accurately because of uncertainties regarding the penetration rates of the added cofactors into the cells and/or the compartmentation of both ACC oxidase and cofactors. However, it can be assumed that the in vivo ACC oxidase activity determined in the absence of exogenous ascorbate and iron reflects the in situ activity acting within intact fruit. Therefore, our results suggest that in banana fruit the in vivo ACC oxidase activity could be limited neither by the accumulated level of MA-ACO1 mRNA nor the activity of its protein but by availability of its cofactors. However, there is a possibility that other mechanisms such as inhibitors of enzyme activity might be responsible for the decreased ACO activity at the late ripening stage. Further study is needed to understand the regulatory mechanism of ethylene biosynthesis in ripening banana fruit.
Based on our results, ethylene production in ripening banana fruit can be characterized as follows: (a) increase in the abundance of MA-ACS1 mRNA in the flesh is the first step of ethylene induction at the onset of the climacteric; (b) the increased content of ACC in the flesh induces a rapid increase of climacteric ethylene whereby ACC oxidase is activated with the enhanced accumulation of MA-ACO1 mRNA once ripening commences; (c) ACC oxidase activity decreases rapidly through limitation of its cofactors, that is, decline of ascorbate and iron contents or through other unknown factors; (d) which in turn causes a rapid rise and fall of ethylene production at the early phase of ripening.
ACKNOWLEDGMENTS
We thank Dr. Hideo Shimizu (Atagawa Tropical and Alligator Garden, Shizuoka, Japan) for his kind gift of banana sprouts for the extraction of genomic DNA. We also thank Dr. Francis M. Mathooko (Jomo Kenyatta University of Agriculture and Technology, Kenya) for his careful reading of the manuscript. The nucleotide sequence data for the full-length and fragment cDNAs reported in this article appear in the nucleotide sequence databases (accession nos. AB021906 for MA-ACS1, AB021907 for MA-ACS2, AB021908 for MA-ACS3, and AB022041 for MA-ACTIN).
Footnotes
This work was supported in part by a Grant-in-Aid for Scientific Research to A.I. (no. 08456020) from The Ministry of Education, Science, Sports and Culture of Japan, and by a grant for the specific research “The Study of the Development of Organisms Effective to Environmental Conservation for Human Life” at Okayama University in 1998–1999.
LITERATURE CITED
- Association of Official Analytical Chemists. Official Methods of Analysis. Ed 13. Washington, DC: Association of Official Analytical Chemists; 1980. [Google Scholar]
- Balaguè C, Watson CF, Turner AJ, Rouge P, Picton S, Pech JC, Grierson D. Isolation of a ripening and wound-induced cDNA from Cucumis melo L. encoding a protein with homology to the ethylene-forming enzyme. Eur J Biochem. 1993;212:27–34. doi: 10.1111/j.1432-1033.1993.tb17628.x. [DOI] [PubMed] [Google Scholar]
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, Grierson D. Differential expression of the 1-aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996;9:525–535. doi: 10.1046/j.1365-313x.1996.09040525.x. [DOI] [PubMed] [Google Scholar]
- Biale JB, Young RE. Respiration and ripening in fruits: retrospect and prospect. In: Friend J, Rhodes MJC, editors. Recent Advances in the Biochemistry of Fruits and Vegetables. London: Academic Press; 1981. pp. 1–39. [Google Scholar]
- Blume B, Grierson D. Expression of ACC oxidase promoter-GUS fusions in tomato and Nicotiana plumbaginifolia regulated by developmental and environmental stimuli. Plant J. 1997;12:731–746. doi: 10.1046/j.1365-313x.1997.12040731.x. [DOI] [PubMed] [Google Scholar]
- Bouquin T, Lasserre E, Paradier J, Pech JC, Balagué C. Wound and ethylene induction of the ACC oxidase melon gene CM-ACO1 occurs via two direct and independent transduction pathways. Plant Mol Biol. 1997;35:1029–1035. doi: 10.1023/a:1005902226054. [DOI] [PubMed] [Google Scholar]
- Burg SP, Burg EA. Role of ethylene in fruit ripening. Plant Physiol. 1962;37:179–189. doi: 10.1104/pp.37.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP, Burg EA. Relationship between ethylene production and ripening in bananas. Bot Gaz. 1965;126:200–204. [Google Scholar]
- Clendennen SK, Kipp PB, May GD. The role of ethylene in banana fruit ripening. In: Kanellis AK, Chang C, Kende H, Grierson D, editors. Biology and Biotechnology of the Plant Hormone Ethylene. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 141–148. [Google Scholar]
- Domìniguez M, Vendrell M. Effect of ethylene treatment on ethylene production, EFE activity and ACC levels in peel and pulp of banana fruit. Postharvest Biol Technol. 1994;4:167–177. [Google Scholar]
- Dong JG, Fernández-Maculet JC, Yang SF. Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc Natl Acad Sci USA. 1991;89:9789–9793. doi: 10.1073/pnas.89.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JG, Kim WT, Yip WK, Thompson GA, Li L, Bennett AB, Yang SF. Cloning of a cDNA encoding 1-aminocyclopropane-1-carboxylate synthase and expression of its mRNA in ripening apple fruit. Planta. 1991;185:38–45. doi: 10.1007/BF00194512. [DOI] [PubMed] [Google Scholar]
- Fluhr R, Mattoo AK. Ethylene: biosynthesis and perception. CRC Crit Rev Plant Sci. 1996;15:479–523. [Google Scholar]
- Golding JB, Shearer D, Wyllie SG, McGlasson WB. Application of 1-MCP and propylene to identify ethylene-dependent ripening processes in mature banana fruit. Postharvest Biol Technol. 1998;14:87–98. [Google Scholar]
- Hoffman NE, Yang SF. Changes of 1-aminocyclopropane-1-carboxylic acid content in ripening fruits in relation to their ethylene production rates. J Am Soc Hortic Sci. 1980;105:492–495. [Google Scholar]
- Huang PL, Do YY, Huang FC, Thay TS, Chang TW. Characterization and expression analysis of a banana gene encoding 1-aminocyclopropane-1-carboxylate oxidase. Biochem Mol Biol Int. 1997;41:941–950. doi: 10.1080/15216549700202001. [DOI] [PubMed] [Google Scholar]
- Inaba A, Kubo Y, Nakamura R. Automated microcomputer system for measurement of O2 uptake, CO2 output, and C2H4 evolution by fruit and vegetables. J Jpn Soc Hortic Sci. 1989;58:443–448. [Google Scholar]
- Inaba A, Nakamura R. Effect of exogenous ethylene concentration and fruit temperature on the minimum treatment time necessary to induce ripening in banana fruit. J Jpn Soc Hortic Sci. 1986;55:348–354. [Google Scholar]
- Karikari SK, Marriott J, Hutchins P. Changes during the respiratory climacteric in ripening plantain fruits. Sci Hortic. 1979;10:369–376. [Google Scholar]
- Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- Lasserre E, Bouquin T, Hernandez JA, Bull J, Pech JC, Balagué C. Structure and expression of three genes encoding ACC oxidase homologs from melon (Cucumis melo L.) Mol Gen Genet. 1996;251:81–90. doi: 10.1007/BF02174348. [DOI] [PubMed] [Google Scholar]
- Leliévre JM, Latché A, Jones B, Bouzayen M, Pech JC. Ethylene and fruit ripening. Physiol Plant. 1997a;101:727–739. [Google Scholar]
- Leliévre JM, Tichit L, Dao P, Fillion L, Nam YW, Pech JC, Latché A. Effects of chilling on the expression of ethylene biosynthetic genes in Passe-Crassane pear (Pyrus communis L.) fruits. Plant Mol Biol. 1997b;33:847–855. doi: 10.1023/a:1005750324531. [DOI] [PubMed] [Google Scholar]
- Leliévre JM, Tichit L, Larrigaudiére C, Vendrell M, Pech JC. Cold-induced accumulation of 1-aminocyclopropane-1-carboxylate oxidase protein in Granny Smith apples. Postharvest Biol Technol. 1995;5:11–17. [Google Scholar]
- Lincoln JE, Campbell AD, Oetiker J, Rottmann WH, Oeller PW, Shen NF, Theologis A. LE-ACS4, a fruit ripening and wound-induced 1-aminocyclopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum) J Biol Chem. 1993;268:19422–19430. [PubMed] [Google Scholar]
- Lizada MCC, Yang SF. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979;100:140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Lòpez-Gòmez R, Campbell A, Dong JG, Yang SF, Gòmez-Lim MA. Ethylene biosynthesis in banana fruit: isolation of a genomic clone to ACC oxidase and expression studies. Plant Sci. 1997;123:123–131. [Google Scholar]
- Mita S, Kawamura S, Yamawaki K, Nakamura K, Hyodo H. Differential expression of genes involved in the biosynthesis and perception of ethylene during ripening of passion fruit (Passiflora edulis Sims) Plant Cell Physiol. 1998;39:1209–1217. doi: 10.1093/oxfordjournals.pcp.a029322. [DOI] [PubMed] [Google Scholar]
- Moya-León MA, John P. Activity of 1-aminocyclopropane-1-carboxylate (ACC) oxidase (ethylene-forming enzyme) in the pulp and peel of ripening bananas. J Hortic Sci. 1994;69:243–250. [Google Scholar]
- Murray MG, Thompson WF. Rapid isolation of high molecular weight DNA. Nucleic Acids Res. 1980;8:4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998;118:1295–1305. doi: 10.1104/pp.118.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka A, Shiomi S, Kubo Y, Inaba A. Expression and internal feedback regulation of ACC synthase and ACC oxidase genes in ripening tomato fruit. Plant Cell Physiol. 1997;38:1103–1110. doi: 10.1093/oxfordjournals.pcp.a029094. [DOI] [PubMed] [Google Scholar]
- Olson DC, White JA, Edelman L, Harkins RN, Kende H. Differential expression of two genes for 1-aminocyclopropane-1-carboxylate synthase in tomato fruits. Proc Natl Acad Sci USA. 1991;88:5340–5344. doi: 10.1073/pnas.88.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G, Knighton ML, Lay-Yee M. An ethylene-related cDNA from ripening apples. Plant Mol Biol. 1992;19:231–238. doi: 10.1007/BF00027344. [DOI] [PubMed] [Google Scholar]
- Rottmann WH, Peter GF, Oeller PW, Keller JA, Shen NF, Nagy BP, Taylor LP, Campbell AD, Theologis A. 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senescence. J Mol Biol. 1991;222:937–961. doi: 10.1016/0022-2836(91)90587-v. [DOI] [PubMed] [Google Scholar]
- Sandell EB. Colorimetric Determination of Traces of Metals. Ed 3. New York: Interscience Publishers; 1959. pp. 522–544. [Google Scholar]
- Shiomi S, Yamamoto M, Ono T, Kakiuchi K, Nakamoto J, Nakatsuka A, Kubo Y, Nakamura R, Inaba A, Imaseki H. cDNA cloning of ACC synthase and ACC oxidase in cucumber fruit and their differential expression by wounding and auxin. J Jpn Soc Hortic Sci. 1998;67:685–692. [Google Scholar]
- Theologis A. One rotten apple spoils the whole bushel: the role of ethylene in fruit ripening. Cell. 1992;70:181–184. doi: 10.1016/0092-8674(92)90093-r. [DOI] [PubMed] [Google Scholar]
- Tonutti P, Bonghi C, Ruperti B, Tornielli GB, Ramina A. Ethylene evolution and 1-aminocyclopropane-1-carboxylate oxidase gene expression during early development and ripening of peach fruit. J Am Soc Hortic Sci. 1997;122:642–647. [Google Scholar]
- Ververidis P, John P. Complete recovery in vitro of ethylene-forming enzyme activity. Phytochemistry. 1991;30:725–727. [Google Scholar]
- Wan CY, Wilkins TA. A modified hot borate method significantly enhance the yield of high-quality RNA from cotton (Gossypium hirsutum L.) Anal Biochem. 1994;223:7–12. doi: 10.1006/abio.1994.1538. [DOI] [PubMed] [Google Scholar]
- Whittaker DJ, Smith GS, Gardner RC. Expression of ethylene biosynthetic genes in Actinidia chinensis fruit. Plant Mol Biol. 1997;34:45–55. doi: 10.1023/a:1005789220668. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Miki T, Ishiki Y, Fujinami K, Yanagisawa Y, Nakagawa H, Ogura N, Hirabayashi T, Sato T. The synthesis of ethylene in melon fruit during the early stage of ripening. Plant Cell Physiol. 1995;36:591–596. [Google Scholar]
- Yang SF. The role of ethylene and ethylene synthesis in fruit ripening. In: Thompson WW, Nothnagel EA, Huffaker RC, editors. Plant Senescence: Its Biochemistry and Physiology. Rockville, MD: American Society of Plant Physiologists; 1987. pp. 156–166. [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Zarembinski TI, Theologis A. Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol. 1994;26:1579–1597. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]