Abstract
Background
Reward seeking and avoidance of punishment are key motivational processes. Brain-imaging studies often use the Monetary Incentive Delay Task (MIDT) to evaluate motivational processes involved in maladaptive behavior. Although the bulk of research has been done on the MIDT reward events, little is known about the neural basis of avoidance of punishment. Therefore, we conducted a meta-analysis of brain activations during anticipation and receipt of monetary losses in healthy controls.
Methods
All functional neuro-imaging studies using the MIDT in healthy controls were retrieved using PubMed, Google Scholar & EMBASE databases. Functional neuro-imaging data was analyzed using the Seed-based d Mapping Software.
Results
Thirty-five studies met the inclusion criteria, comprising 699 healthy adults. In both anticipation and loss outcome phases, participants showed large and robust activations in the bilateral striatum, (anterior) insula, and anterior cingulate gyrus relatively to Loss > Neutral contrast. Although relatively similar activation patterns were observed during the two event types, they differed in the pattern of prefrontal activations: ventro-lateral prefrontal activations were observed during loss anticipation, while medial prefrontal activations were observed during loss receipt.
Discussion
Considering that previous meta-analyses highlighted activations in the medial prefrontal cortex/anterior cingulate cortex, the anterior insula and the ventral striatum, the current meta-analysis highlighted the potential specificity of the ventro-lateral prefrontal regions, the median cingulate cortex and the amygdala in the loss events. Future studies can rely on these latter results to examine the neural correlates of loss processing in psychiatric populations characterized by harm avoidance or insensitivity to punishment.
Keywords: Monetary Incentive Delay Task, Meta-analysis, fMRI, Punishment, Loss avoidance
Introduction
Reward seeking and avoidance of punishment both play a key role in human motivation (Navratilova & Porreca, 2014). Both components of motivation, when expressed in excess or insufficiency, can be associated with maladaptive behavior. Indeed, several studies have shown that individuals with major depressive disorder and schizophrenia both lack motivation for rewards (Takamura et al., 2017; Whitton, Treadway & Pizzagalli, 2015), whereas individuals with substance use disorders have uncontrolled motivation for substance seeking but decreased motivation for alternative natural rewards (Baker et al., 2017). There is also evidence individuals with anxiety disorders are characterized by harm avoidance (Ottenbreit, Dobson & Quigley, 2014; Wright, Lebell & Carleton, 2016), whereas individuals with antisocial behavior tend to be insensitive to punishment (Byrd, Loeber & Pardini, 2014; Loney et al., 2003).
In view of the importance of reward seeking and avoidance of punishment to human behavior and maladaptive behavior, diverse cognitive tasks have been developed to study both processes in humans; the most employed being the Monetary Incentive Delay Task (MIDT) (Knutson & Greer, 2008). Although several versions of the task exist, they similarly subdivide events of reward anticipation and receipt, with relatively fewer versions also comprising events of loss anticipation and outcome (Balodis & Potenza, 2015; Patel et al., 2013). The MIDT and its several variants have been very useful in studying the neurobiological mechanisms in reward processing. Knutson & Greer (2008) performed a neuro-imaging meta-analysis which showed that healthy participants recruit the bilateral nucleus accumbens (NAC), thalamus, the right (anterior) insula and the medial frontal gyrus during reward anticipation (n = 20 studies), while they activate the bilateral NAC, the right caudate nucleus, the left amygdala and the right sub-callosal gyrus during reward receipt (n = 12 studies). Since then, a much larger meta-analysis has been performed by Liu et al. (2011), which did not restrict the inclusion of studies to those using the MIDT specifically. In this meta-analysis, comprising of a total of 142 neuro-imaging studies, it was shown that the reward anticipation is associated, in healthy volunteers, with activations in the bilateral NAC, bilateral (anterior) insula, bilateral (dorsal) anterior cingulate cortex (ACC) and the left medial orbito-frontal cortex (OFC), while reward receipt is associated with similar activations in the bilateral NAC, insula, medial OFC, the right amygdala and thalamus. Taken together, the results of these meta-analytic studies highlight activations during reward processing in dopamine-rich brain regions (e.g., NAC, insula, ACC and medial OFC), a finding consistent with the vast pre-clinical literature showing that meso-cortico-limbic dopaminergic neurons are critically involved in the processing of both drug and natural rewards (Lammel et al., 2011; Pignatelli & Bonci, 2015; Pitchers et al., 2010).
The growing understanding of the neurobiological bases of reward processing has fueled research on motivational alterations in psychiatric disorders. Thus far, several studies and meta-analyses have highlighted reduced activations in the ventral striatum (VS) during reward anticipation and receipt in schizophrenia (Radua et al., 2015); reduced VS activations during reward anticipation and increased VS activations during reward receipt in addiction (Luijten et al., 2017); as well as decreased sub-cortical and limbic regions and increased cortical responses during reward processing in major depressive disorder (Zhang et al., 2013). Likewise, blunted VS responses have been observed in large-scale studies of adolescents at risk of addictive behaviors (Büchel et al., 2017; Jia et al., 2016). Comparatively, it is striking to observe that little attention has been paid to the study of loss anticipation and receipt events in both healthy and psychiatric populations. This means that at the present, the neural map of activations associated with loss events is unknown, although one of the lead theories of antisocial behavior proposes that individuals will not readily obey to the law if they are insensitive to punishment (Byrd, Loeber & Pardini, 2014; Crowley et al., 2010). In the meta-analysis of Liu et al. (2011) involving healthy participants, no specific analysis was performed on loss events (anticipation or receipt). In the meta-analysis of Knutson & Greer (2008), also involving healthy participants, a sub-analysis on 12 studies revealed activations of the right caudate nucleus, the left putamen, the left thalamus and the left (dorsal) insula during loss anticipation, while activations of the superior temporal gyrus was observed during loss receipt. The reliability of the latter result is especially uncertain, given that it was based on the pooling of only six studies. Moreover, about a third of the studies included in this meta-analysis were studies using predefined regions-of-interest (ROIs) rather than performing whole-brain analyses, and this methodological choice may have biased results. In this context, some authors have noticed that the regions and pathways that are differently activated in healthy participants during reward versus loss events remain unknown (Knutson & Greer, 2008; Lutz & Widmer, 2014). In theory, it has been proposed that rewarding events may elicit stronger activations in the medial prefrontal cortex (medial OFC and ventral ACC) and VS (Dillon et al., 2008; Schlagenhauf et al., 2009), given that these regions are well known core regions of the brain reward system (Lammel et al., 2011; Pignatelli & Bonci, 2015; Pitchers et al., 2010). Conversely, some authors have proposed that stronger activations may occur during loss events in the amygdala (Hahn et al., 2010; Lutz & Widmer, 2014), a region critically involved in threat detection (LeDoux, 2014), as well as the hippocampus (Hahn et al., 2010), which plays an important role in memory retrieval of negative emotions (Fossati, 2012). On the other hand, some authors have argued that certain brain regions may be involved in the processing of both rewarding and loss events. For instance, Hahn et al. (2010) have postulated that the dorsal ACC could be activated during both reward and loss anticipation (regardless of valence), since the anticipatory phase is characterized by heightened arousal and increased attention. Finally, Wu et al. (2014) noticed that the (anterior) insula is likely to play a role in the processing of both rewarding and loss events, since this brain region responds to affective stimuli of positive and negative valence (Fusar-Poli et al., 2009; Liu et al., 2011; Palermo et al., 2015). These hypotheses need to be further investigated.
In view of our poor understanding of the neurobiological bases of punishment processing, we sought to perform a functional neuro-imaging meta-analysis of loss events (anticipation and receipt) in healthy participants. Analyses were restricted to the studies using MIDT in order to reduce task heterogeneity.
Method
Selection procedures
Search strategies
A systematic search strategy was employed to identify relevant studies for the present meta-analysis. The literature search was performed by two researchers (JD, SP), independently, with the use of PubMed, Google Scholar and EMBASE search engines, up to September 2017. The following search terms were used: “MID” (monetary incentive delay) AND “loss” or “loss-avoidance” or “punishment” AND “fMRI” (functional magnetic resonance imaging). Also, a cross-referencing method was used by manually examining reference lists of the articles included in the meta-analysis.
Selection criteria
Studies were included if they met the following criteria: (1) were reported in an original paper from a peer-reviewed journal, (2) had involved healthy subjects (i.e., no psychiatric or organic disorders reported) as a primary or control group, (3) had employed the Monetary Incentive Delay Task (MIDT) (Knutson et al., 2000) or a modified version of the MIDT (Bjork et al., 2004), (4) had included punishment cues (loss or receipt) in the task and reported brain map activations for this component. Studies were reviewed by two researchers (JD, SP) and inclusion criteria were evaluated by consensus. To achieve a high reporting standard, we followed the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines (for more information, see Table S1) (Moher et al., 2009).
Recorded variables
The variables included in the present meta-analysis, for each article, were: sample size, mean age of participants, magnet intensity and repetition time (TR) of functional volumes. Also, recent research has suggested that the use of full width at half maximum (FWHM) of the smoothing kernel (Sacchet & Knutson, 2013) are leading to heterogeneous results in neuro-imaging studies. Therefore, these variables were also recorded in the meta-analysis (see Table 1 for complete data reports).
Table 1. Description of the studies included in the meta-analysis (n = 35).
| First author (year) | N | Type? | Loss outcome | Mean age | Software | Tesla | FWHM | TR | Incentive magnitude | Percentage of winning |
|---|---|---|---|---|---|---|---|---|---|---|
| Balodis et al. (2012) | 14 | ROI | X | 37.1 | spm5 | 3 | 6 | 1500 | 1$ & 5$ | 66.6% |
| Bayer, Bandurski & Sommer (2013) | 23 | ROI | – | 26 | spm8 | 3 | 8 | 2630 | €0.20, €3 | 50.0% |
| Beck et al. (2009) | 19 | WB + ROI | X | 41.68 | spm5 | 1.5 | 8 | 1870 | €0.10, €0.60, €3 | 66.6% |
| Bjork et al. (2004) | 12 | ROI | X | 23.8 | AFNI | 3 | 2 | 2000 | 0.20$, 1$, 5$ | 66.6% |
| Bjork, Smith & Hommer (2008) | 23 | ROI | – | 32 | AFNI | 3 | 8 | 2000 | 0.50$, 5$ | 66.6% |
| Bjork et al. (2010) | 24 | ROI | X | 29.3 | AFNI | 3 | 8 | 1000 | 0.50$, 5$ | 67.0% |
| Bustamante et al. (2014) | 18 | WB | – | 37.44 | spm8 | 1.5 | 8 | 2000 | €0.20, €3 | 75.0% |
| Cho et al. (2013) | 30 | ROI | – | 28.8 | spm8 | 3 | 8 | 2500 | 0.20$, 1$, 5$ | 66.6% |
| Cooper et al. (2009) | 12 | WB | X | NA | spm2 | 1.5 | 4 | 2000 | 0.05$, 5$ | 66.6% |
| Dillon et al. (2008) | 8 | ROI | X | 28.13 | AFNI | 1.5 | 6 | 2500 | Range (1.81 to 2.19$) | 50.0% |
| Enzi et al. (2012) | 15 | ROI | – | 34.7 | spm5 | 1.5 | 8 | 1900 | €0.10, €0.60, €3 | 66.6% |
| Hahn et al. (2010) | 45 | WB | – | 29.1 | spm5 | 1.5 | NA | 2000 | €0.05, €1 | 67.0% |
| Herbort et al. (2016) | 23 | WB | – | 25.78 | spm8 | 3 | 8 | 2000 | €0.50, €10 | 66.6% |
| Juckel et al. (2006) | 10 | WB + ROI | – | 31.7 | spm2 | 1.5 | 8 | 1900 | €0.10, €0.60, €3 | 66.6% |
| Juckel et al. (2012) | 13 | WB | – | 25.69 | spm5 | 1.5 | 8 | 1987 | NA | NA |
| Kaufmann et al. (2013) | 19 | WB | – | 34.9 | spm8 | 1.5 | 8 | 1870 | €0.10, €0.60, €3 | 66.6% |
| Kirk, Brown & Downar (2014) | 44 | ROI | X | 36.5 | spm8 | 3 | 8 | 2000 | 1$, 5$ | 55.0% |
| Knutson et al. (2001) | 8 | ROI | – | 31 | AFNI | 1.5 | 4 | 2000 | 0.20$, 5$ | 66.6% |
| Knutson et al. (2003) | 12 | ROI | – | 31 | AFNI | 1.5 | 4 | 2000 | 0.20$, 1$, 5$ | 66.6% |
| Knutson & Greer (2008) | 12 | WB | X | 28.67 | AFNI | 1.5 | 4 | 2000 | 0.20$, 1$, 5$ | 66.6% |
| Kocsel et al. (2017) | 37 | WB | X | 25.92 | spm12 | 3 | 8 | 2500 | Range (1.76 to €2.12) | NA |
| Pfabigan et al. (2014) | 25 | ROI | – | 23.8 | spm8 | 3 | 8 | 1800 | €2.00 | 50.0% |
| Romanczuk-Seiferth et al. (2015) | 17 | WB | X | 37.41 | spm8 | 3 | 8 | 2500 | €1.00 | 67.0% |
| Samanez-Larkin et al. (2007) | 12 | WB | X | 23.75 | AFNI | 1.5 | 4 | 2000 | 0.50$, 5$ | 66.6% |
| Samanez-Larkin et al. (2007) | 12 | WB | X | 72.92 | AFNI | 1.5 | 4 | 2000 | 0.50$, 5$ | 66.6% |
| Schlagenhauf et al. (2008) | 10 | WB | – | 31.8 | spm2 | 1.5 | 4 | 1900 | €0.10, €0.60, €3 | 66.6% |
| Schlagenhauf et al. (2009) | 15 | WB + ROI | X | 30.1 | spm5 | 1.5 | 8 | 1987 | €0.10, €0.60, €3 | 66.6% |
| Stoy et al. (2011) | 12 | WB + ROI | – | 28.08 | spm5 | 1.5 | 8 | 1900 | €0.10, €0.60, €3 | 66.6% |
| Stoy et al. (2012) | 15 | WB | – | 39.5 | spm5 | 1.5 | 8 | 1900 | €0.10, €0.60, €3 | 66.6% |
| Treadway, Buckholtz & Zald (2013) | 38 | WB | X | 22 | spm5 | 3 | 6 | 2000 | 0.20$, 1$, 5$ | 66.6% |
| Ubl et al. (2015) | 28 | WB + ROI | X | 43.96 | spm5 | 3 | 6 | 2700 | €0.20, €2 | 65.0% |
| Van Duin et al. (2016) | 12 | WB | – | 29 | spm8 | 3 | 8 | 2000 | 0.20$, 1$, 5$ | NA |
| Wrase et al. (2007a) | 14 | WB | – | 39.9 | spm2 | 1.5 | 6 | 1870 | €0.10, €0.60, €3 | 67.0% |
| Wrase et al. (2007b) | 16 | ROI | – | 39.94 | spm2 | 1.5 | 4 | 1800 | €0.10, €0.60, €3 | 67.0% |
| Wu et al. (2014) | 52 | WB | X | 50 | AFNI | 1.5 | 4 | 2000 | 0.50$, 5$ | 66.6% |
Note:
- WB
- Whole-Brain
- ROI
- Region Of Interest
- AFNI
- Analysis of Functional NeuroImages
- SPM
- Statistical Parametric Mapping
- Tesla
- Scanner Magnet Intensity
- FWHM
- Full Width at Half Maximum Smoothing Kernel level
- TR
- Repetition Time
Meta-analysis
The meta-analysis was performed by using the Effect-size Seed-based d Mapping (formerly Signed Differential Mapping) (ES-SDM) (Radua et al., 2012a; Radua et al., 2012b). This method is based on the use of peak coordinates to recreate, for each study, an effect-size map of contrast results. A standard random-effects variance weighted meta-analysis for each voxel is then executed. Default ES-SDM kernel size and thresholds were used (FWHM = 20 mm, voxel P = 0.005, peak height Z = 1, cluster extent = 10 voxels) (Radua et al., 2012a; Radua et al., 2012b).
Also, robustness of the significant results was assessed by means of exploration of the residual heterogeneity, jack-knife and subgroup analyses. Furthermore, we investigated if the findings had been driven by a small subset of studies or studies including small samples. Publication bias was assessed by examining Egger’s tests (Egger et al., 1997) for asymmetry of the funnel plots (Sterne et al., 2011). Jack-knife sensitivity analyses consisted of repeating the meta-analysis iteratively by removing one study at a time to assess the replicability of the results (Radua et al., 2012a; Radua et al., 2012b). Subgroup analyses were conducted on magnet intensity (1.5 tesla versus 3 tesla) and the smoothing kernel used (FWHM = 4 versus FWHM = 8). Finally, a meta-regression was performed on mean age of participants and TR across studies. Following previous meta-analyses, we decreased the probability threshold to minimize the detection of spurious results (please refer to Radua et al., 2012a; Radua et al., 2012b; Radua & Mataix-Cols, 2009 for further details on robustness analyses).
Results
Number of studies found
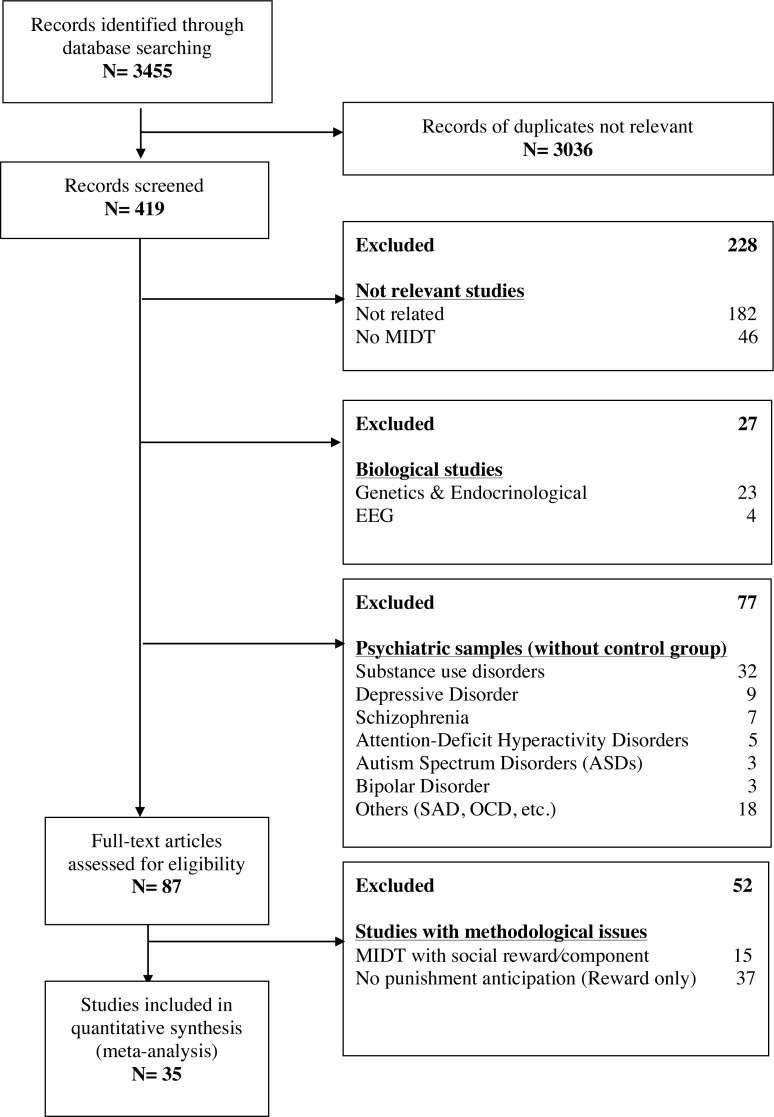
Thirty-five studies met inclusion criteria for this loss anticipation meta-analysis (see Fig. 1 for the flow chart). More specifically, we included 699 healthy individuals (mean TR 2014.69 ms, range 1,000–2,700 ms; mean age 33.28, range 22–72.92). Twenty-three studies used a whole-brain analysis and twelve used predefined regions-of-interest in their statistical analyses. Also, a large majority of studies used a MID task with ≥65% chance of successful trial (n = 28, 80%). All studies reported similar monetary incentive per stimulus (largest inventive = 5$ or 3 euros, except for one study reporting 10$). Of these 35 studies, 16 also reported loss outcome brain activations in their results comprising 356 healthy subjects (mean TR 2034.81ms, range 1,000–2,700 ms; mean age 35.42, range 22–72.92). Ten studies examined whole brain activations and six used predefined region-of-interest. For more details of the included studies, please see Table 1.
Figure 1. Flow chart of the studies included in the meta-analysis.
Brain responses during loss anticipation
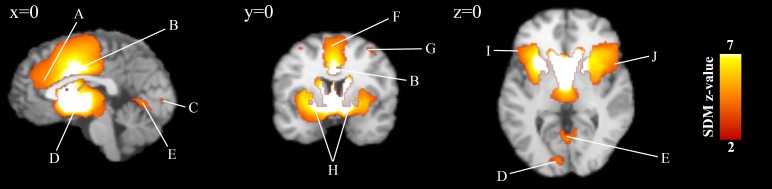
Subjects showed a large and robust bilateral striato-insular activation cluster (Z = 6.86, Cluster size = 10,623, p < 0.001) that includes the bilateral anterior insula, the putamen, the thalamus, the caudate nucleus and the amygdala, as well as the bilateral ventro-lateral sub-regions. Significant increased activations were also observed in the bilateral median cingulate gyri (Z = 5.69, Cluster size = 4,845, p < 0.001), the left precentral gyrus (Z = 3.95, Cluster size = 846, p < 0.001), the bilateral cerebellum hemispheric lobule VI (Left: Z = 3.50, Cluster size = 551, p < 0.001, Right: Z = 3.13, Cluster size = 144, p = 0.002), the bilateral lingual gyrus (Left: Z = 3.42, Cluster size = 129, p < 0.001; Right: Z = 3.70, Cluster size = 182, p < 0.001) as well as the right middle frontal gyrus (Z = 3.17, Cluster size = 130, p = 0.002) (Table 2; Fig. 2). The analyses of robustness showed that regions were highly replicable with the exception of the right cerebellum hemispheric lobules VI, the left lingual gyrus and the right middle frontal gyrus, which were found in only 68.6%, 74.3% and 68.6% of the 35 studies (see Table S3).
Table 2. Increased activations during anticipation of monetary loss (all studies; n = 35).
| MNI Coordinates | SDM z-valuea | P Valueb | No. of voxelsc | Breakdown (No. of voxels)d | |
|---|---|---|---|---|---|
| Left lenticular nucleus | −12,4,0 | 6.863 | ∼0 | 10,623 | Bilateral insula (1,635) |
| Bilateral anterior thalamic projections (1,253) | |||||
| Bilateral striatum (1,243) | |||||
| Bilateral putamen (755) | |||||
| Bilateral caudate nucleus (581) | |||||
| Bilateral thalamus (563) | |||||
| Bilateral BA 47 (374) | |||||
| Bilateral BA 25 (331) | |||||
| Bilateral BA 45 (239) | |||||
| Corpus callosum (230) | |||||
| Bilateral BA 11 (217) | |||||
| Bilateral BA 34 (130) | |||||
| Bilateral amygdala (129) | |||||
| Anterior commissure (112) | |||||
| Left median cingulate gyri | 0,0,32 | 5.694 | ∼0 | 4,845 | Bilateral median cingulate gyri (1,917) |
| Bilateral supplementary motor area (1548) | |||||
| Bilateral Anterior cingulate gyri (655) | |||||
| Corpus callosum (327) | |||||
| Bilateral superior frontal gyrus, medial (255) | |||||
| Left precentral gyrus | −34,−24,58 | 3.945 | 0.000026 | 846 | L precentral gyrus (473) |
| L postcentral gyrus (319) | |||||
| Corpus callosum (33) | |||||
| Left cerebellum, lobule VI | −20, −70, −14 | 3.503 | 0.00037 | 551 | L hemispheric lobule VI (366) |
| L crus I (116) | |||||
| Right Lingual Gyrus | 0, −66,0 | 3.704 | 0.00012 | 182 | R Lingual Gyrus (60) |
| Cerebellum, vermic lobule IV/V (30) | |||||
| Right cerebellum, lobule VI | 16, −62, −16 | 3.134 | 0.0024 | 144 | R hemispheric lobule VI (108) |
| R lingual gyrus (13) | |||||
| Left lingual gyrus | −8, −86,2 | 3.416 | 0.00059 | 129 | L BA 17 (49) |
| Corpus callosum (31) | |||||
| Right middle frontal gyrus | 36,0,54 | 3.168 | 0.002 | 130 | R BA 6 (124) |
Notes.
- BA
- Brodmann Area
- SDM
- Seed-based d Mapping
Voxel probability threshold: p = 0.005.
Peak height threshold: z = 1.
Cluster extent threshold: 100 voxels.
Regions with less than 10 voxels are not reported in the cluster breakdown.
Figure 2. Overlay of brain areas activated in loss anticipation events (x = 0, y = 0, z = 0).
These blobs were generated using the SDM p-value threshold of p = 0.005 derived from the original analysis. SDM = Seed-Based d Mapping. (A) Anterior Cingulate Cortex. (B) Median Cingulate Cortex. (C) Lingual Gyrus. (D) Striatum. (E) Vermis. (F) Precentral Gyrus. (G) Medial Frontal Gyrus. (H) Lentiform Nucleus. (I) Inferior Frontal Gyrus. (J) Insula.
Furthermore, funnel plots revealed that only the peak activation of the striato-insular cluster may have been driven by small or noisy studies. In fact, significant publication bias was observed in the peak of the striato-insular cluster (x = 12, y = − 4, z = 0), as shown by the Egger’s test result (Bias: 3.24, t: 3.93, df: 33, p < 0.001). However, every study included in the meta-analysis reported activations in the striato-insular cluster. Considering the large size of this cluster and its very large effect size, the publication bias found in the main peak is unlikely to reduce the robustness and validity of the results. In fact, results within this cluster comprised an outlier study (e.g., Kaufmann et al., 2013). When removing the peak activations results within the striato-insular cluster from this outlier study, we still observed highly similar results (Peak at x = − 2, y = 4, z = − 2; Z = 7.11, Cluster size = 10,030, p < 0.001) but no publication bias (Bias: 1.03, t: 1.34, df: 33, p = 0.191) (see Table S2 and Fig. S1 for Funnel Plot). No publications bias was observed for the bilateral median cingulate gyri (Bias: 0.76, t: 1.24, df: 33, p: 0.225), the left precentral gyrus (Bias: −0.46, t: − 0.68, df: 33, p:0.503), the bilateral cerebellum hemispheric lobule VI (Left: Bias: 0.26, t: 0.39, df: 33, p: 0.700; Right: Bias: 1.13, t: 1.29, df: 33, p: 0.207), the bilateral lingual gyrus (Left: Bias: 1.24, t: 1.85, df: 33, p: 0.074; Right: Bias: −0.53, t: −0.76, df: 33, p: 0.450) as well as the right middle frontal gyrus (Bias: 0.14, t: 0.21, df: 33, p: 0.836).
We also found significant residual heterogeneity between studies in activations during loss anticipation (τ = 0.06, Q = 66.1, df = 34, p = 0.0008). To better understand this heterogeneity, we performed subgroup analyses. First, no significant difference was observed between whole-brain studies versus region-of-interest studies. Second, magnet intensity subgroup analysis yielded significant results. In fact, studies using a 3 Tesla magnet reported more frequently increased activations in the left cerebellum (hemispheric lobule VI) (Z = 2.86, Cluster size = 1,295, p < 0.001) and the left thalamus (Z = 3.58, Cluster size = 350, p < 0.001) while studies using a 1.5 Tesla magnet reported more frequently increased activations in the bilateral left inferior frontal gyrus (Left: Z = 2.07, Cluster size = 297, p < 0.001; Right: Z = 2.24, Cluster size = 1,062, p < 0.001) (see Table S4). Third, comparisons between the kernel density employed in smoothing parameterizations showed that studies using a 8 mm FWHM reported more increased activations in the left lingual gyrus ( Z = 2.25, Cluster size = 512, p < 0.001) and the left thalamus (Z = 2.14, Cluster size = 120, p < 0.001) (see Table S4) while 4 mm FWHM yielded in increased activations in the right insula (Z = 3.82, Cluster size = 186, p < 0.001). Finally, the meta-regression revealed significant age and TR effects. In fact, studies with older participants reported more increased activations in the left inferior frontal gyrus (opercular part) (Z = 2.62, Cluster size=190, p = 0.0011) as well as the left median/posterior cingulate gyrus (Z = 2.83, Cluster size = 182, p < 0.001) while studies with younger participants reported more increased activations in the left anterior cingulate gyrus (Z = 2.25, Cluster size = 941, p < 0.001), right olfactory cortex (Z = 2.75, Cluster size=611, p < 0.001), left thalamus (Z = 2.59, Cluster size=550, p < 0.001) as well as the right lingual gyrus (Z = 2.09, Cluster size = 182, p < 0.001) (see Table S6). Finally, regarding the functional TR, shortest TR was significantly associated with increased activations in the lingual gyrus (Z = 2.99, Cluster size = 461, p < 0.001) as well as the right cerebellum (vermic lobule VI) (Z = 2.60, Cluster size = 297, p < 0.001) (see Table S7).
Brain responses during loss receipt/outcome
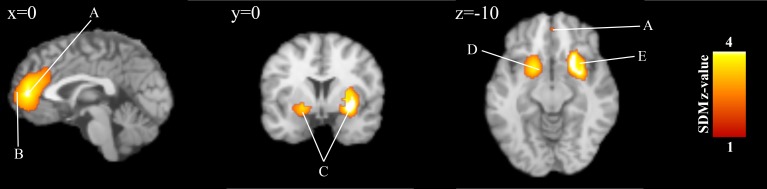
Subjects also showed increased activations in a bilateral striato-insular cluster (Left: Z = 2.92, Cluster size = 567, p < 0.001; Right: Z = 3.39, Cluster size = 1,475, p < 0.001) that includes the putamen, the anterior insula and the amygdala (Table 3; Fig. 3). We also observed significant increased activations in the bilateral anterior cingulate/paracingulate gyri (encompassing the medial PFC) (Z = 3.14, Cluster size = 1,625, p < 0.001) during the loss outcome. These results were highly replicable as shown by the jack-knife analysis (See Table S8). No publication bias was observed for the bilateral striato-insular cluster (Left: Bias: 0.94, t: 1.11, df: 14, p: 0.285; Right: Bias: −0.06, t: −0.07, df: 14, p: 0.946), the bilateral anterior cingulate/paracingulate gyri (Bias: 0.49, t: 0.61, df: 14, p: 0.550) suggesting that these regions were not driven by few small or noisy studies. Finally, no significant residual heterogeneity was observed between studies (τ = 0.04, Q = 22.4, df = 15, p = 0.098).
Table 3. Increased activations during monetary loss (outcome) (n = 16).
| MNI Coordinates | SDM z-valuea | P Valueb | No. of voxelsc | Breakdown (No. of voxels)d | |
|---|---|---|---|---|---|
| Right striatum (putamen) | 22,16,−8 | 3.389 | 0.000005 | 1,475 | R BA 48 (775) |
| R striatum (301) | |||||
| R Insula (94) | |||||
| R inferior network (79) | |||||
| R BA 47 (45) | |||||
| R putamen (27) | |||||
| R amygdala (20) | |||||
| Left anterior cingulate gyri | −2,48,8 | 3.138 | 0.000015 | 1,625 | Bilateral anterior cingulate gyri (1,060) |
| Bilateral superior frontal gyrus (510) | |||||
| Left striatum (putamen) | −18,6,−8 | 2.924 | 0.000026 | 567 | L striatum (208) |
| L putamen (118) | |||||
| L BA 48 (89) | |||||
| L BA 25 (32) | |||||
| L amygdala (28) | |||||
| L BA 11 (11) |
Notes.
- BA
- Brodmann Area
- SDM
- Seed-based d Mapping
Voxel probability threshold: p = 0.005.
Peak height threshold: z = 1.
Cluster extent threshold: 100 voxels.
Regions with less than 10 voxels are not reported in the cluster breakdown.
Figure 3. Overlay of brain areas activated in reception of loss events (outcome)(x = 0, y = 0, z = − 10).
These blobs were generated using the SDM p-value threshold of p = 0.005 derived from the original analysis. SDM = Seed-Based d Mapping. (A) Anterior Cingulate Cortex. (B) mFG = Medial Frontal Gyrus. (C) Lentiform Nucleus. (D) Globus Pallidus. (E) Claustrum.
No subgroup analyses were performed in order to avoid abnormal cluster activations resulting from the small number of studies having reported the loss outcome results (n = 16).
Discussion
Compared to the vast neuro-imaging literature on the neural mechanisms involved in reward processing (anticipation and outcome), little attention has been paid to how humans process punishments at the brain level. Here, we performed a neuro-imaging meta-analysis of loss events during the MIDT in healthy volunteers, using the seed-based d mapping approach. The meta-analysis showed that during loss anticipation, participants activated the bilateral insula, bilateral caudate nucleus and putamen, bilateral amygdala, bilateral ventro-lateral prefrontal areas, as well as the median and anterior cingulate gyri, the left pre- and post-central gyri, and the left cerebellum. Activations were also observed, during loss anticipation, in the bilateral lingual gyrus, the right cerebellum and middle frontal gyrus, but these results were less robust, as revealed by the jacknife sensitivity analysis. Results were found to be influenced by the mean age of participants, the TR, the scanner magnet intensity and the smoothing kernel level. Relative to loss anticipation, loss outcome was associated with activations in similar brain regions, though the cluster size was smaller in the case of loss outcome. That is, the loss outcome event was associated, in healthy participants, with activations in the bilateral striatum (putamen), bilateral amygdala, right ventro-lateral prefrontal cortex, and ACC (encompassing the medial PFC). Most of these regions are related to the emotional salience network that has been identified using rest- and task-based functional connectivity analyses (Menon & Uddin, 2010). Importantly, the pattern of activations between loss anticipation and loss outcome differed in that the former was associated with activations of ventro-lateral prefrontal regions, whereas the latter was associated with activations of the medial PFC.
The finding that the loss events recruit activations in the ACC, anterior insula and striatum is consistent with a large literature showing that these brain regions are critically involved in the affective responding to a whole range of stimuli having a negative valence, such as faces expressing fear or anger, images depicting social conflicts, sad music or nociceptive stimuli (Fusar-Poli et al., 2009; Groenewold et al., 2013; Koelsch, 2010; Palermo et al., 2015). It must be noticed that in previous meta-analyses on reward anticipation and receipt, activations in the (ventral) striatum, anterior insula and ACC were also observed, meaning that the regions are commonly activated by both types of reinforcers, regardless of their valence, and do not differentiate between them. As such, this result is unsurprising, as the challenge of establishing the pattern of activity preferentially associated with reward and punishment has been noticed by several authors (Byrd, Loeber & Pardini, 2014; Liu et al., 2011; Lutz & Widmer, 2014). Before concluding that the ACC, anterior insula and striatum lack specificity for the valence of reinforcers, it is important to point out, however, that the similarities in activations between the processing of reward and punishment may stem from a bias in the selection of ROIs. Indeed, most studies included in the meta-analysis which performed ROI analyses used regions previously found (by them or others) to be activated during reward outcomes. Obviously, this selection of ROIs may have introduced a bias towards finding activations in reward-related brain regions during punishment. Because we were conscious of this potential bias at the beginning, we performed a sub-analysis comparing ROI to whole-brain analyses. Importantly, we found no significant differences in activations between studies that perform whole-brain analyses versus those who used a priori defined ROIs. This strongly suggests that the similarities between our results and results of previous meta-analyses on reward processing are unlikely to be explained by a biased selection of ROIs.
Perhaps more interestingly, the meta-analysis produced results suggesting that there might be subtle differences between the neural processing of reward and punishment. First, whereas the previous meta-analysis of Liu et al. (2011) showed clear activations in the medial orbito-frontal cortex during reward anticipation and receipt, the current meta-analysis shows that loss anticipation recruits instead the activity of ventro-lateral prefrontal regions (note: the medial PFC was actived during loss outcome, however). Compared to the medial OFC, which is involved in the subjective valuation of reinforcers (Noonan et al., 2012), the ventro-lateral prefrontal regions have been shown to play a significant role in emotion regulation and cognitive control (Frank et al., 2014; Levy & Wagner, 2011). This differential pattern of activity means that reward anticipation may have greater subjective value than the anticipation of loss. (note: comparatively, both reward receipt and loss receipt seem to activate medial prefrontal regions). Another difference between both types of events is that both the median cingulate gyrus is activated during loss anticipation, but not during reward anticipation and receipt (Knutson & Greer, 2008; Liu et al., 2011). Although the fMRI literature has paid far less attention to the median cingulate gyrus compared to the ACC, there is growing evidence showing that this region is a hub linking incoming affective information with brain regions involved in goal-directed behavior, and that it uses information about punishment (e.g., painful stimuli) to control action motivated by aversive events (for a review and meta-analysis, see Shackman et al., 2011). In that regard, this particular result is clearly consistent with a novel interpretation of the key roles of the median cingulate gyrus. Another noteworthy difference that we observed in the current meta-analysis is that loss events (anticipation and receipt) were associated with activations in the bilateral amygdala, which was not significantly activated in the previous meta-analyses on reward processing (Knutson & Greer, 2008; Liu et al., 2011). As such, this result lends support to the notion that the amygdala would play a significant role in the processing of negative outcomes due to its well-established role as a threat detector (LeDoux, 2014; Lutz & Widmer, 2014).
The similarity of findings observed in the current meta-analysis with the results of previous meta-analyses on the neural processing of aversive emotional stimuli raises the question of the specificity of the findings reported here. As in the current meta-analysis, previous meta-analyses of neuroimaging studies on negative emotions have shown that the ACC, (anterior) insula and amygdala are consistently activated across studies, regardless of the type of emotional stimuli (Frank et al., 2014; Fusar-Poli et al., 2009; Groenewold et al., 2013). As such, these observations suggest that the loss events and aversive emotional stimuli are processed (at least in part) via common neurobiological mechanisms. Striatal activations have also been observed in previous meta-analyses of neuroimaging studies on aversive emotional stimuli (Frank et al., 2014; Groenewold et al., 2013); however, it is important to note that striatal activations are not observed in the case of every type of negative emotion (Fusar-Poli et al., 2009). This tentatively suggests that the striatum (putamen and caudate nucleus) may play a more important role in the processing of loss events than the processing of aversive emotional stimuli. Based on the current state of knowledge, the clearest difference between loss events and aversive emotional stimuli is that the parahippocampal gyrus has been consistently found to be activated in previous meta-analyses on aversive emotional stimuli (Frank et al., 2014; Fusar-Poli et al., 2009; Groenewold et al., 2013), but not in the current meta-analysis. As such, this latter result suggests that the neurobiological mechanisms involved in the processing of loss events and aversive emotional stimuli may not be fully overlapping. Future studies will need to formally test these assumptions in head-to-head comparison of both types of stimuli.
The current meta-analysis has some limitations that need to be acknowledged. The first limitation has to do with the number of studies included in the meta-analysis. In the current meta-analysis, we were able to retrieve a significantly larger sample of studies than in the previous one focusing on the MIDT (35 vs 12 studies) (Knutson & Greer, 2008). Still, this sample of studies is not comparable to the number of studies included in the meta-analyses on reward events, and as such, our results should not be considered reliable. Due to this clear imbalance between the number of studies on reward and punishment, we did not perform a direct comparison between both types of events. In the same vein, the finding of similar though smaller brain regions activated during loss receipt relative to loss anticipation may simply be explained by the fact that the analysis on loss receipt was based on a smaller sample of studies. As in several other fMRI meta-analyses (Bartra, McGuire & Kable, 2013; Costafreda et al., 2008), heterogeneity is another limitation of the current meta-analysis. However, in an effort to explain this heterogeneity, we performed sub-analyses on age, scanner magnet intensity, smoothing kernel level and TR. We found that studies including younger participants reported stronger activations in the (dorsal) ACC and median cingulate gyrus but decreased activations in the inferior frontal gyrus, which may reflect differences in self-regulation of affective responses to punishment. Also, older participants had increased activations in the posterior cingulate cortex, a core region of the default mode network (Lin et al., 2016), which may mean that older individuals are better able to anticipate the personal implications of loss. Studies performed on a 1.5 Tesla scanner produced activations in the left inferior frontal gyrus, a region playing a key role in emotion regulation (Frank et al., 2014); this was not the case of studies using 3 Tesla scanners. Since the majority of studies included in the meta-analysis were performed on 1.5 Tesla scanners, known to have lower signal-to-noise ratio (Parra-Robles, Cross & Santyr, 2005), it could explain why activations in the left inferior frontal gyrus were not observed in our global analysis. Also, the relationship between TR and occipital and cerebellar activations during loss anticipation suggests that studies with long TR parameters may lack statistical power to detect activations in these regions. Finally, greater activations were observed in the right striatum in studies using a smoothing level of 4 FWHM, whereas studies a smoothing level of 8 FWHM found greater activations in the thalamus and the lingual gyrus, which is consistent with the notion that smaller smoothing levels increase the chance of finding activations in small brain regions and bias the spatial localization of striatal activity (Sacchet & Knutson, 2013).
In the largest neuro-imaging meta-analysis on loss anticipation and receipt, we found that healthy participants recruit activations in brain regions, such as the ACC, anterior insula and striatum, that are involved in affective responding. Although these regions have been shown to be activated also during reward anticipation and receipt (Knutson & Greer, 2008; Liu et al., 2011), punishment seems to recruit to a greater extent ventro-lateral prefrontal regions (loss anticipation) and the amygdala (loss anticipation and receipt), which are involved in emotion regulation and threat detection, respectively. In the future, more neuro-imaging research is needed on the head-to-head comparison of reward and punishment events within the same sample of participants. In order to improve the ability to detect differences between both types of events, it will be relevant to perform uni- and multi-variate analyses. In recent years, several fMRI studies have shown that multivariate analyses help overcome the multiple comparisons problem inherent to mass-univariate approaches and to improve analytic accuracy (Valente et al., 2014). Importantly, it has been shown that multi-variate approaches can also be used in the case of rapid event-related designs (Mumford et al., 2012), which are typically employed in the case of the MIDT. Given that several of the regions examined in the current meta-analysis tap into neural networks identified in large datasets of resting-state functional connectivity data (Gu et al., 2010; Seeley et al., 2007), such as the mesolimbic and salience networks, future studies will need to not only examine the activity of the brain regions involved in punishment processing, but also the functional and effective connectivity between them. Future studies will also need to pay greater attention to the differences in neural activity between loss anticipation and loss receipt, which seem to mostly differ in terms of prefrontal activations (ventro-lateral versus medial, respectively). Future studies will also need to study the neurobiological bases of the altered responses to punishment seen in some psychiatric disorders, starting with populations having high levels of harm avoidance (e.g., anxiety disorders) and those displaying, on the contrary, a relative insensitivity to punishment (e.g., psychopathy/callous-unemotional traits).
Finally, it is noteworthy to mention that the most variants of the MIDT are designed in such fashion that participants win money over the entire task. This means the most variants of the task have slightly more power to detect consistent activations during reward than loss outcomes. In the present meta-analysis, a large majority of studies used the MID task with a probability of successful trial of ≥65% (n = 28, 80%). As pointed out by Dillon et al. (2008) and Ubl et al. (2015), this type of design could have led to possible under-estimations of the loss-related effects (i.e., significantly more successful trial than losses). In fact, striatal and medial frontal regions were found to be maximally responsive when rewards were unpredictable (i.e., probability of successful trial at 50%) (Berns et al., 2001). Therefore, using a more unpredictable variant of the original MIDT could also be a good alternative to increase the sensitivity of mapping loss processing. Even if the results of the current meta-analysis were relatively robust, it would be of interest to use other variants of the MIDT, in the future, that are more optimized for studying loss events in terms of power and instructions. For instance, Hahn et al. (2010) used a modified version of the MIDT in which the participants started with 10 euros and were instructed to lose as little as possible. This modified version of the task could represent a good alternative to recruit more negative salience activations during loss events.
Conclusion
To our knowledge, this is the first sufficiently powered meta-analysis to be performed on the neural mechanisms involved in both loss anticipation and receipt. The results of the meta-analysis provide insights on the regions that are commonly activated by reward and punishment events, as well as the regions that are potentially specific to each event type. The meta-analysis also provide a map of the brain regions that are activated during loss events that can be used a regions-of-interest for future neuro-imaging investigations on the neurobiology of psychiatric disorders characterized by harm avoidance or by an insensitivity to punishment.
Supplemental Information
Funding Statement
This study was funded by a grant from the Natural Sciences and Engineering Research Council to Stéphane Potvin. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Stéphane Potvin and Alexandre Dumais are holders of grants from Otsuka Pharmaceuticals and HLS Therapeutics, unrelated to the current study.
Author Contributions
Jules R. Dugré performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Alexandre Dumais conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Nathalie Bitar performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Stéphane Potvin conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This research was a meta-analysis, therefore this article did not generate any data or code. Any data used were collected from the literature, and are provided in the Supplemental Files.
References
- Baker et al. (2017).Baker TE, Lesperance P, Tucholka A, Potvin S, Larcher K, Zhang Y, Jutras-Aswad D, Conrod P. Reversing the atypical valuation of drug and nondrug rewards in smokers using multimodal neuroimaging. Biological Psychiatry. 2017;82(11):819–827. doi: 10.1016/j.biopsych.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Balodis et al. (2012).Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Potenza MN. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biological Psychiatry. 2012;71(8):749–757. doi: 10.1016/j.biopsych.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis & Potenza (2015).Balodis IM, Potenza MN. Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biological Psychiatry. 2015;77(5):434–444. doi: 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra, McGuire & Kable (2013).Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer, Bandurski & Sommer (2013).Bayer J, Bandurski P, Sommer T. Differential modulation of activity related to the anticipation of monetary gains and losses across the menstrual cycle. European Journal of Neuroscience. 2013;38(10):3519–3526. doi: 10.1111/ejn.12347. [DOI] [PubMed] [Google Scholar]
- Beck et al. (2009).Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hägele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry. 2009;66(8):734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berns et al. (2001).Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. Journal of Neuroscience. 2001;21(8):2793–2798. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork et al. (2004).Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork et al. (2010).Bjork JM, Smith AR, Chen G, Hommer DW. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLOS ONE. 2010;5(7):e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork, Smith & Hommer (2008).Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. NeuroImage. 2008;42(4):1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel et al. (2017).Büchel C, Peters J, Banaschewski T, Bokde AL, Bromberg U, Conrod PJ, Flor H, Papadopoulos D, Garavan H, Gowland P, Heinz A, Walter H, Ittermann B, Mann K, Martinot JL, Paillére-Martinot ML, Nees F, Paus T, Pausova Z, Poustka L, Rietschel M, Robbins TW, Smolka MN, Gallinat J, Schumann G, Knutson B, IMAGEN consortium Blunted ventral striatal responses to anticipated rewards foreshadow problematic drug use in novelty-seeking adolescents. Nature Communications. 2017;8:14140. doi: 10.1038/ncomms14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante et al. (2014).Bustamante JC, Barrós-Loscertales A, Costumero V, Fuentes-Claramonte P, Rosell-Negre P, Ventura-Campos N, Llopis JJ, Ávila C. Abstinence duration modulates striatal functioning during monetary reward processing in cocaine patients. Addiction Biology. 2014;19(5):885–894. doi: 10.1111/adb.12041. [DOI] [PubMed] [Google Scholar]
- Byrd, Loeber & Pardini (2014).Byrd AL, Loeber R, Pardini DA. Antisocial behavior, psychopathic features and abnormalities in reward and punishment processing in youth. Clinical Child and Family Psychology Review. 2014;17(2):125–156. doi: 10.1007/s10567-013-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho et al. (2013).Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. NeuroImage. 2013;66:508–521. doi: 10.1016/j.neuroimage.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper et al. (2009).Cooper JC, Hollon NG, Wimmer GE, Knutson B. Available alternative incentives modulate anticipatory nucleus accumbens activation. Social Cognitive and Affective Neuroscience. 2009;4(4):409–416. doi: 10.1093/scan/nsp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda et al. (2008).Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Crowley et al. (2010).Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, Banich MT. Risky decisions and their consequences: neural processing by boys with antisocial substance disorder. PLOS ONE. 2010;5(9):e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon et al. (2008).Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA. Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology. 2008;45(1):36–49. doi: 10.1111/j.1469-8986.2007.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger et al. (1997).Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzi et al. (2012).Enzi B, Edel M-A, Lissek S, Peters S, Hoffmann R, Nicolas V, Tegenthoff M, Juckel G, Saft C. Altered ventral striatal activation during reward and punishment processing in premanifest Huntington’s disease: a functional magnetic resonance study. Experimental Neurology. 2012;235(1):256–264. doi: 10.1016/j.expneurol.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Fossati (2012).Fossati P. Neural correlates of emotion processing: from emotional to social brain. European Neuropsychopharmacology. 2012;22:S487–S491. doi: 10.1016/j.euroneuro.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Frank et al. (2014).Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neuroscience & Biobehavioral Reviews. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli et al. (2009).Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Groenewold et al. (2013).Groenewold NA, Opmeer EM, De Jonge P, Aleman A, Costafreda SG. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews. 2013;37(2):152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Gu et al. (2010).Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage. 2010;53(2):593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn et al. (2010).Hahn T, Dresler T, Plichta MM, Ehlis A-C, Ernst LH, Markulin F, Polak T, Blaimer M, Deckert J, Lesch K-P, Jakob PM, Fallgatter AJ. Functional amygdala-hippocampus connectivity during anticipation of aversive events is associated with Gray’s trait “sensitivity to punishment”. Biological Psychiatry. 2010;68(5):459–464. doi: 10.1016/j.biopsych.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Herbort et al. (2016).Herbort MC, Soch J, Wüstenberg T, Krauel K, Pujara M, Koenigs M, Gallinat J, Walter H, Roepke S, Schott BH. A negative relationship between ventral striatal loss anticipation response and impulsivity in borderline personality disorder. NeuroImage: Clinical. 2016;12:724–736. doi: 10.1016/j.nicl.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia et al. (2016).Jia T, Macare C, Desrivières S, Gonzalez DA, Tao C, Ji X, Ruggeri B, Nees F, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Büchel C, Conrod PJ, Dove R, Frouin V, Gallinat J, Garavan H, Gowland PA, Heinz A, Ittermann B, Lathrop M, Lemaitre H, Martinot JL, Paus T, Pausova Z, Poline JB, Rietschel M, Robbins T, Smolka MN, Müller CP, Feng J, Rothenfluh A, Flor H, Schumann G, IMAGEN Consortium Neural basis of reward anticipation and its genetic determinants. Proceedings of the National Academy of Sciences. 2016;113(14):3879–3884. doi: 10.1073/pnas.1503252113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel et al. (2012).Juckel G, Friedel E, Koslowski M, Witthaus H, Özgürdal S, Gudlowski Y, Knutson B, Wrase J, Brüne M, Heinz A, Schlagenhauf F. Ventral striatal activation during reward processing in subjects with ultra-high risk for schizophrenia. Neuropsychobiology. 2012;66(1):50–56. doi: 10.1159/000337130. [DOI] [PubMed] [Google Scholar]
- Juckel et al. (2006).Juckel G, Schlagenhauf F, Koslowski M, Wüstenberg T, Villringer A, Knutson B, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kaufmann et al. (2013).Kaufmann C, Beucke J, Preuße F, Endrass T, Schlagenhauf F, Heinz A, Juckel G, Kathmann N. Medial prefrontal brain activation to anticipated reward and loss in obsessive–compulsive disorder. NeuroImage: Clinical. 2013;2:212–220. doi: 10.1016/j.nicl.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk, Brown & Downar (2014).Kirk U, Brown KW, Downar J. Adaptive neural reward processing during anticipation and receipt of monetary rewards in mindfulness meditators. Social Cognitive and Affective Neuroscience. 2014;10(5):752–759. doi: 10.1093/scan/nsu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson et al. (2001).Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21(16):RC159–RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson et al. (2003).Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18(2):263–272. doi: 10.1016/S1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson & Greer (2008).Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363(1511):3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson et al. (2000).Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Kocsel et al. (2017).Kocsel N, Szabó E, Galambos A, Édes A, Pap D, Elliott R, Kozák LR, Bagdy G, Juhász G, Kökönyei G. Trait rumination influences neural correlates of the anticipation but not the consumption phase of reward processing. Frontiers in Behavioral Neuroscience. 2017;11:85. doi: 10.3389/fnbeh.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch (2010).Koelsch S. Towards a neural basis of music-evoked emotions. Trends in Cognitive Sciences. 2010;14(3):131–137. doi: 10.1016/j.tics.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Lammel et al. (2011).Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70(5):855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux (2014).LeDoux JE. Coming to terms with fear. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(8):2871–2878. doi: 10.1073/pnas.1400335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy & Wagner (2011).Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences. 2011;1224(1):40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2016).Lin P, Yang Y, Jovicich J, De Pisapia N, Wang X, Zuo CS, Levitt JJ. Static and dynamic posterior cingulate cortex nodal topology of default mode network predicts attention task performance. Brain Imaging and Behavior. 2016;10(1):212–225. doi: 10.1007/s11682-015-9384-6. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2011).Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2011;35(5):1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney et al. (2003).Loney BR, Frick PJ, Clements CB, Ellis ML, Kerlin K. Callous-unemotional traits, impulsivity, and emotional processing in adolescents with antisocial behavior problems. Journal of Clinical Child and Adolescent Psychology. 2003;32(1):66–80. doi: 10.1207/S15374424JCCP3201_07. [DOI] [PubMed] [Google Scholar]
- Luijten et al. (2017).Luijten M, Schellekens AF, Kühn S, Machielse MW, Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74(4):387–398. doi: 10.1001/jamapsychiatry.2016.3084. [DOI] [PubMed] [Google Scholar]
- Lutz & Widmer (2014).Lutz K, Widmer M. What can the monetary incentive delay task tell us about the neural processing of reward and punishment. Neuroscience and Neuroeconomics. 2014;3:33–35. [Google Scholar]
- Menon & Uddin (2010).Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher et al. (2009).Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford et al. (2012).Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. NeuroImage. 2012;59(3):2636–2643. doi: 10.1016/j.neuroimage.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova & Porreca (2014).Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nature Neuroscience. 2014;17(10):1304–1312. doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan et al. (2012).Noonan M, Kolling N, Walton M, Rushworth M. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. European Journal of Neuroscience. 2012;35(7):997–1010. doi: 10.1111/j.1460-9568.2012.08023.x. [DOI] [PubMed] [Google Scholar]
- Ottenbreit, Dobson & Quigley (2014).Ottenbreit ND, Dobson KS, Quigley L. An examination of avoidance in major depression in comparison to social anxiety disorder. Behaviour Research and Therapy. 2014;56:82–90. doi: 10.1016/j.brat.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Palermo et al. (2015).Palermo S, Benedetti F, Costa T, Amanzio M. Pain anticipation: an activation likelihood estimation meta-analysis of brain imaging studies. Human Brain Mapping. 2015;36(5):1648–1661. doi: 10.1002/hbm.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Robles, Cross & Santyr (2005).Parra-Robles J, Cross AR, Santyr GE. Theoretical signal-to-noise ratio and spatial resolution dependence on the magnetic field strength for hyperpolarized noble gas magnetic resonance imaging of human lungs. Medical Physics. 2005;32(1):221–229. doi: 10.1118/1.1833593. [DOI] [PubMed] [Google Scholar]
- Patel et al. (2013).Patel KT, Stevens MC, Meda SA, Muska C, Thomas AD, Potenza MN, Pearlson GD. Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biological Psychiatry. 2013;74(7):529–537. doi: 10.1016/j.biopsych.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfabigan et al. (2014).Pfabigan DM, Seidel E-M, Sladky R, Hahn A, Paul K, Grahl A, Küblböck M, Kraus C, Hummer A, Kranz GS, Windischberger C, Lanzenberger R, Lamm C. P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: an EEG and fMRI experiment. NeuroImage. 2014;96:12–21. doi: 10.1016/j.neuroimage.2014.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli & Bonci (2015).Pignatelli M, Bonci A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron. 2015;86(5):1145–1157. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Pitchers et al. (2010).Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biological Psychiatry. 2010;67(9):872–879. doi: 10.1016/j.biopsych.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua et al. (2012a).Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire P, Fusar-Poli P. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neuroscience & Biobehavioral Reviews. 2012a;36(10):2325–2333. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Radua & Mataix-Cols (2009).Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. British Journal of Psychiatry. 2009;195(5):393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Radua et al. (2012b).Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus D, Cardoner N, Surguladze S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry. 2012b;27(8):605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Radua et al. (2015).Radua J, Schmidt A, Borgwardt S, Heinz A, Schlagenhauf F, McGuire P, Fusar-Poli P. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72(12):1243–1251. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- Romanczuk-Seiferth et al. (2015).Romanczuk-Seiferth N, Koehler S, Dreesen C, Wüstenberg T, Heinz A. Pathological gambling and alcohol dependence: neural disturbances in reward and loss avoidance processing. Addiction Biology. 2015;20(3):557–569. doi: 10.1111/adb.12144. [DOI] [PubMed] [Google Scholar]
- Sacchet & Knutson (2013).Sacchet MD, Knutson B. Spatial smoothing systematically biases the localization of reward-related brain activity. NeuroImage. 2013;66:270–277. doi: 10.1016/j.neuroimage.2012.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin et al. (2007).Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10(6):787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf et al. (2008).Schlagenhauf F, Juckel G, Koslowski M, Kahnt T, Knutson B, Dembler T, Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology. 2008;196(4):673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf et al. (2009).Schlagenhauf F, Sterzer P, Schmack K, Ballmaier M, Rapp M, Wrase J, Juckel G, Gallinat J, Heinz A. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biological Psychiatry. 2009;65(12):1032–1039. doi: 10.1016/j.biopsych.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Seeley et al. (2007).Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman et al. (2011).Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne et al. (2011).Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- Stoy et al. (2011).Stoy M, Schlagenhauf F, Schlochtermeier L, Wrase J, Knutson B, Lehmkuhl U, Huss M, Heinz A, Ströhle A. Reward processing in male adults with childhood ADHD—a comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology. 2011;215(3):467–481. doi: 10.1007/s00213-011-2166-y. [DOI] [PubMed] [Google Scholar]
- Stoy et al. (2012).Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hägele C, Suchotzki K, Schmack K, Wrase J, Ricken R, Knutson B, Adli M, Bauer M, Heinz A, Ströhle A. Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. Journal of Psychopharmacology. 2012;26(5):677–688. doi: 10.1177/0269881111416686. [DOI] [PubMed] [Google Scholar]
- Takamura et al. (2017).Takamura M, Okamoto Y, Okada G, Toki S, Yamamoto T, Ichikawa N, Mori A, Minagawa H, Takaishi Y, Fujii Y, Kaichi Y, Akiyama Y, Awai K, Yamawaki S. Patients with major depressive disorder exhibit reduced reward size coding in the striatum. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2017;79:317–323. doi: 10.1016/j.pnpbp.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Treadway, Buckholtz & Zald (2013).Treadway MT, Buckholtz JW, Zald D. Perceived stress predicts altered reward and loss feedback processing in medial prefrontal cortex. Frontiers in Human Neuroscience. 2013;7:180. doi: 10.3389/fnhum.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubl et al. (2015).Ubl B, Kuehner C, Kirsch P, Ruttorf M, Diener C, Flor H. Altered neural reward and loss processing and prediction error signalling in depression. Social Cognitive and Affective Neuroscience. 2015;10(8):1102–1112. doi: 10.1093/scan/nsu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente et al. (2014).Valente G, Castellanos AL, Vanacore G, Formisano E. Multivariate linear regression of high-dimensional fMRI data with multiple target variables. Human Brain Mapping. 2014;35(5):2163–2177. doi: 10.1002/hbm.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Duin et al. (2016).Van Duin ED, Goossens L, Hernaus D, Da Silva Alves F, Schmitz N, Schruers K, Van Amelsvoort T. Neural correlates of reward processing in adults with 22q11 deletion syndrome. Journal of Neurodevelopmental Disorders. 2016;8(1):25. doi: 10.1186/s11689-016-9158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton, Treadway & Pizzagalli (2015).Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Current Opinion in Psychiatry. 2015;28(1):7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase et al. (2007a).Wrase J, Kahnt T, Schlagenhauf F, Beck A, Cohen MX, Knutson B, Heinz A. Different neural systems adjust motor behavior in response to reward and punishment. NeuroImage. 2007a;36(4):1253–1262. doi: 10.1016/j.neuroimage.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wrase et al. (2007b).Wrase J, Schlagenhauf F, Kienast T, Wüstenberg T, Bermpohl F, Kahnt T, Beck A, Ströhle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007b;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Wright, Lebell & Carleton (2016).Wright KD, Lebell MAA, Carleton RN. Intolerance of uncertainty, anxiety sensitivity, health anxiety, and anxiety disorder symptoms in youth. Journal of Anxiety Disorders. 2016;41:35–42. doi: 10.1016/j.janxdis.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2014).Wu CC, Samanez-Larkin GR, Katovich K, Knutson B. Affective traits link to reliable neural markers of incentive anticipation. NeuroImage. 2014;84:279–289. doi: 10.1016/j.neuroimage.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2013).Zhang W-N, Chang S-H, Guo L-Y, Zhang K-L, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. Journal of Affective Disorders. 2013;151(2):531–539. doi: 10.1016/j.jad.2013.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
This research was a meta-analysis, therefore this article did not generate any data or code. Any data used were collected from the literature, and are provided in the Supplemental Files.