Abstract
Clinical trials to test the safety and efficacy of analgesics across all pediatric age cohorts are needed to avoid inappropriate extrapolation of adult data to children. However, the selection of acute pain models and trial design attributes to maximize assay sensitivity, by pediatric age cohort, remains problematic. Acute pain models used for drug treatment trials in adults are not directly applicable to the pediatric age cohorts – neonates, infants, toddlers, children, and adolescents. Developmental maturation of metabolic enzymes in infants and children must be taken into consideration when designing trials to test analgesic treatments for acute pain. Assessment tools based on levels of cognitive maturation and behavioral repertoire must be selected as outcome measures. Models and designs of clinical trials of analgesic medications used in the treatment of acute pain in neonates, infants, toddlers, children, and adolescents were reviewed and discussed at an Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) Pediatric Pain Research Consortium consensus meeting. Based on extensive reviews and continuing discussions, the authors recommend a number of acute pain clinical trial models and design attributes that have the potential to improve the study of analgesic medications in pediatric populations. Recommendations are also provided regarding additional research needed to support the use of other acute pain models across pediatric age cohorts.
Keywords: ACTTION, pediatrics, acute pain models, neonates, infants, toddlers, children, adolescents, clinical trial
Introduction
The overwhelming majority (approximately 80 percent in the United States and over 50 percent in Europe) of drugs given to children are prescribed “off label” because they have not received United States Food and Drug Administration (FDA) or European Medicines Agency (EMA) approval for use in younger age groups [78]. Clinicians face a difficult dilemma: either to withhold potentially beneficial medications from young patients because they are not labeled for that age group or to give these drugs based on extrapolation from adult trials (with dosage modifications commonly adjusted to body weight). Indeed, justification offered for failing to treat pain in children is inadequate knowledge of the safety or efficacy analgesic medications; an issue that has persisted for decades [86].
Children are not merely “little adults” and early development plays a major role in disease processes, as well as drug pharmacokinetics (PK) and pharmacodynamics (PD). Many medications are metabolized and/or excreted by the liver, kidney, or other organs, which continue to mature in function until approximately 1 year of life [36]. Extrapolation is limited and thus for younger children, in particular, safety and efficacy of many medications remain to be demonstrated [10].
Historically there are a number of ethical and pragmatic elements that make the study of analgesic medications in young children very challenging. Simple placebo controlled trials, the gold standard for acute pain trials in adults, are unethical in those too young to provide informed consent [7], and many trial elements, such as sampling of blood or urine, are more difficult, intrusive, and carry greater risk in infants and young children.
The paucity of approved analgesics with pediatric labeling reflects both challenges in trial design and the lack of data to guide critical aspects of the drug development effort. Although clinical trials in children generally focus on the same drug indications as for adults, for many conditions (e.g., breakthrough cancer pain, osteoarthritis, or diabetic neuropathy), parallel pediatric indications are significantly different or absent [45]. Similarly, many chronic pain conditions in adults reflect physiological anatomical changes associated with aging and are not found in the pediatric population (e.g., degenerative arthritis is rare in 5-year-olds) [87]. In addition, even within the pediatric population, similar symptoms may have different anatomic or physiological etiologies as a function of development. These considerations make trial design a challenge and patient recruitment almost impossible for some age cohorts and some clinical conditions. Moreover, industry investment in pediatric research is questionable given the relatively small return on investment to pharmaceutical companies, as well as potential excess legal liability. As a result, pharmaceutical companies in the United States often request waivers from the FDA to avoid conducting pediatric trials; waivers which may be justified, but also contribute to the ongoing paucity of pediatric analgesic data.
Recognizing these challenges, in 2009, the FDA convened a consensus panel to address critical issues in pediatric analgesic clinical trials. The panel focused on ethical concerns, pragmatic obstacles, and limits of extrapolation from adult trials. A paper summarizing that work outlined some general issues, but offered little in terms of specific methodologies for clinical trials [10].
Consensus Process
The Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION; www.acttion.org) Pediatric Pain Research Consortium (PPRC) convened a consensus meeting in 2013 that included participants from universities, government agencies, and industry with demonstrated expertise relevant to the treatment of children with acute pain and/or conducting clinical trials focused on acute pain. All participants were from North America and did not include patients, caregivers, or their representatives. The meeting intended to provide consensus recommendations on specific trial designs for acute pain, embracing guidance from the US FDA and the European Medicines Agency (EMA) on analgesic trials for children and the definition of pain models and methodologies within clinically relevant age cohorts (i.e., neonates, infants, toddlers, children, and adolescents). Optimal criteria were presented for clinical trials of analgesic medications for acute pain, including an overview of why methods for acute pain trials in adults may not be applicable to these age cohorts. Prior work on methods for clinical trials in neonates and infants were reviewed, as well as key issues in measuring outcomes in neonatal and pediatric clinical trials. Finally, different pain models, methods of pain assessment, and trial methodologies were presented for each age cohort. This article presents the authors’ recommendations based on the background presentations, literature reviews, and discussions at and following the consensus meeting.
Review of Key Issues
Regulatory Perspective, Role, and Issues
The FDA has implemented a series of innovative programs over the past two decades to promote pediatric labeling of safe and effective medications for children [80]. In 1994, the FDA issued a final rule for the requirements of the Pediatric Use subsection of product labeling, including surveys to establish the sufficiency of existing data for pediatric use and labeling. In 1997, the FDA Modernization Act (FDAMA) provided an additional 6 months of patent exclusivity to companies on drugs for which pediatric clinical trials were conducted. In 1998, the FDA issued “Regulations Requiring Manufacturers to Assess Safety and Effectiveness of New Drugs and Biological Products in Pediatric Patients” (the Pediatric Rule), but the FDA’s legal authority to require pediatric trials was challenged in 2000 and enjoined by the Court in 2002. Also in 2002, the Best Pharmaceuticals for Children Act (BPCA) not only continued FDAMA, but also provided funding for research on the use of various medications in children through the National Institutes of Health (NIH). The Pediatric Research Equity Act (PREA), which parallels the pediatric rule, was passed by Congress in 2003. In 2007, the FDA Amendments Act (FDAAA) reauthorized BPCA and PREA. The FDA Safety and Innovation Act (FDASIA) of 2012 made these acts permanent and required FDA-approved pediatric study plans to be submitted earlier in the drug development process (i.e., before or at the time of filing a New Drug Application [NDA]).
Thus, at present the FDA has a “carrot” (BPCA) and a “stick” (PREA) to facilitate pediatric trials. The FDA has interpreted BPCA to provide exclusivity not only to the drug studied in the pediatric population, but to any of the drug’s formulations, dosage forms, and indications that contain the same moiety and have existing exclusivity. Although PREA enables the FDA to require pediatric trials as part of an NDA, such trials do not delay the availability of the drug for adults. A similar approach has been instituted in the European Union. In 1998, the International Conference of Harmonisation (ICH) defined regulatory requirements across the EU, Japan, and the US. The Directive on Good Clinical Practice for Clinical Trials (2001, 2004) presented criteria for protection of children in clinical trials. In 2002, the European Commission published Better Medicines for Children – Proposed Regulatory Actions in Paediatric Medicinal Products and in 2004, the European Commission released a first proposal for regulation of medicinal products for pediatric use, which was agreed upon by the European Parliament in 2006 and went into force in 2007 as Regulation (EC) No 1901/2006 couple with amended Regulation (EC) no 1902/2006.
According to the FDA Pediatric Labeling Information Database [79], through April 30, 2017, there have been 687 pediatric labeling changes, 622 with and 65 without new pediatric trials. Of these, 175 were related to BPCA, 366 related to PREA, 96 related to BPCA and PREA, and 49 resulted from the Pediatric Rule. However, despite over 20 years of FDA regulations, analgesic drugs remain underrepresented among the pediatric labeling changes. For example, for children under 6 months of age, there are no drugs with a pediatric label as an analgesic; for children 6 to 24 months, only ibuprofen has a pediatric label; for children who are older than 24 months, additionally only acetaminophen, a combination of hydrocodone and acetaminophen, and transdermal fentanyl have pediatric labeling; and for older children and adolescents, additionally naproxen sodium, buprenorphine injection, fentanyl citrate injection, and extended-release oxycodone have been approved and granted pediatric labeling [79]. There are other medications, often combination products, now deemed obsolete that have approval for children and adolescents of various ages (e.g., meperidine, codeine with acetaminophen). Aspirin may be used in children over 3 years, but the FDA provides warnings about doing so due to concerns for Reye’s syndrome.
Methods for Acute Pain Clinical Trials in Adults
Acute pain models and methodologies used in adults have yielded some reproducible, sensitive, and generalizable trial results upon which evidence-based therapeutic and regulatory decisions have been established. These models have followed the design of early clinical trials [9,46] and incorporate many scientific principles that apply to pediatric clinical trials as well. Prospective, comparative clinical trials that utilize negative and positive controls provide the ideal trial design for assay sensitivity (i.e., the trial designs and methods utilized are able to discriminate between less effective and more effective analgesic interventions).
A pain model is a systematic, reproducible set of methods, processes, and procedures used to design, conduct, and analyze randomized, controlled clinical trials of putative analgesic interventions. Investigators have learned that studying relatively homogeneous patient populations, using reproducible and controlled trial methods, leads to replicable trial results (high internal validity). However, the utilization of pain models has been criticized for lack of generalizability to broader healthcare settings and diverse patients (low external validity). Nonetheless, most investigators, pharmaceutical companies, and the FDA have endorsed the benefits of relatively rapid enrolling, sensitive, reliable, and internally valid trial methods [76].
Starting in 2002, the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) has sought to develop consensus reviews and recommendations for improving the design, execution, analysis, and interpretation of clinical trials for pain therapies. A 2007 IMMPACT meeting focused on research design considerations for single-dose analgesic clinical trials in acute pain and identified dental impaction and bunionectomy as the most useful adult models (Table 1) [20], which was subsequently supported by a systematic review [63]. Among the useful surgical and medical acute pain models studied in adults, few apply to pediatric age cohorts largely due to the relative prevalence of conditions as a function of age (e.g., bunions are not present in infants and most children).
Table 1.
Acute Pain Models
| Adult Acute Pain Models14 | Pediatric Acute Pain Models | ||||
|---|---|---|---|---|---|
| Neonates (0-<1month) | Infants (1month-<6months) | Toddlers (6months-<2yrs) | Children (2yrs-<10yrs) | Adolescents (≥10yrs) | |
| Surgical | |||||
| Bunionectomy* | No | No | No | No | Yes |
| Dental impaction* | No | No | No | No | Yes |
| General surgery | Yes | Yes | Yes | Yes | Yes |
| Herniorrhaphy | No | No | Yes | Yes | Yes |
| Gynecologic | No | No | No | No | Yes |
| OA knee | No | No | No | No | No |
| TJR hip/knee | No | No | No | No | No |
| Tonsillectomy | No | No | Yes | Yes | Yes |
| Medical | |||||
| Acute low back pain | No | No | No | No | Yes |
| DOMS | No | No | No | Yes | Yes |
| Dysmenorrhea | No | No | No | No | Yes |
| Sore throat | No | No | No | Yes | Yes |
| Sprains/strains | No | No | No | Yes | Yes |
| Sickle cell disease (vaso-occlusion and sequelae) | No | No | Yes1 | Yes | Yes |
DOMS = delayed onset muscle soreness; OA = osteoarthritis; TJR = total joint replacement
Recommended acute pain models19
Sickle cell crises begin to manifest in infants ≥6mon due to sufficient titers of adult hemoglobin.
In order to assess the utility of any pain model for use in pediatric clinical trials, the following questions need to be considered: (1) What types of pain problems are encountered in each pediatric age cohort? (2) Are there some pain conditions that are so common that a model may be developed and do these models have external validity? (3) Are there sufficient numbers of potential participants available who may be recruited into clinical trials? (4) What are the validated, sensitive, and reproducible outcome measures that can be used in each pediatric age group? (5) What is the performance of prototypic analgesic interventions in these pediatric models or conditions? (6) Are these results sufficiently rigorous to serve as a prototype against which new treatments may be benchmarked? To date, there is a paucity of empirical data to support recommendations of specific index acute pain models for each of the pediatric age cohorts.
The most sensitive and reproducible pain models include a consistent set of selection criteria. It became clear in adult trials that mixed populations recovering from different surgical procedures after various types of intra-operative anesthesia yielded variable results, while homogenous surgical procedures performed on patients with common diagnostic and demographic characteristics, and under comparable anesthetic regimens, yielded much more predictable results. This observation holds true for clinical trials with children.
Secondly, hypotheses, sample sizes, and the primary outcomes need to be established in advance to assure that the study will provide sufficient power to detect significant differences between the study drug and the comparator. Moreover, statistical tests must be specified before each trial is conducted. Limited access to some pediatric patient populations reduces power; as a result, multicenter trials are needed or one faces the complexities of pooling data from a number of smaller trials.
Thirdly, basic trial design principles to control for selection bias are essential. The best practices for these trial designs incorporate pre-specification of the primary endpoint, randomized allocation of trial treatments, double-blinding of the treatments, and rigorous adherence to collecting pain outcomes at predetermined intervals.
Before different models may be compared in terms of their efficiency and ability to discriminate between different treatments, there must be agreement on outcome domains and measures. For most postoperative patients who are conscious, pain intensity and pain relief may be assessed using simple categorical scales. Derived measures for total effect, Sum of Pain Intensity Differences (SPID), Total Pain Relief (TOTPAR), and peak effect can be generated. Based upon these patient responses and need for a rescue analgesic, duration of time-to-effect can be calculated (e.g., time-to-first rescue medication) [12]. Participants’ global evaluation of the effectiveness of or satisfaction with a study drug or device using a simple categorical scale is captured in virtually all well designed adult trials.
There are two elements that have contributed to the success of acute pain clinical trials in adults that may not be applied to such trials in children. First, participants are relatively homogenous with respect to the pain model, the related nature of the pain experience, and the manner in which drugs behave in their systems. In children, different pain problems are more prevalent at different ages, pain systems themselves are maturing, and renal and hepatic mechanisms related to drug excretion and metabolism (clearance) develop and become more predictable with increasing age [10].
Secondly, adults may consent to participate in a clinical trial based on their own informed assessment of risk and benefits. Young children are not capable of such analyses and thus many key organizations have defined it as unethical for a child to participate in a clinical trial unless doing so provides direct benefit to that child [90,54,13,77]. Thus, any clinical trial in which a child is at increased risk for exposure to pain or suffering as a function of research participation will not receive ethics approval. Consequently, the standard placebo-controlled clinical trial for analgesics is deemed unethical to pursue in infants and children unless there is true clinical equipoise, which is rare. Moreover, repeating trials when safety or efficacy parameters is burdensome to participants and of questionable value [27].
Tables 1 and 2 present the 2007 IMMPACT recommendations on trial design and methodologies for research on the effects of analgesics on acute pain [20], along with potential applications to pediatric age cohorts. The utility of each recommendation pertaining to adult clinical trials of acute pain varies depending upon the age cohort to be studied. For many of the IMMPACT recommendations, there is definitive applicability to the pediatric population (e.g., use of an active comparator, randomization, and reporting of observed adverse events) [65]. For some of the recommendations, there is poor applicability in the pediatric population that may be due to age-related formulation restrictions (e.g., dosage; administration of a solid, oral form to a neonate), developmental appropriateness of various pain intensity assessment tools, or different case management practices for a similar surgical procedure. In addition, there are several recommendations that may or may not be applicable to the pediatric population, depending upon the specific clinical trial. For example, a placebo may be used in children if immediate rescue medication is available as part of the trial protocol.
Table 2.
Recommendations for Acute Pain Clinical Trials
| Adult Clinical Trials – IMMPACT Recommended Attributes19 | Applicability to Pediatric Clinical Trials | ||||
|---|---|---|---|---|---|
| Neonates (0-<1month) | Infants (1month-<6months) | Toddlers (6months-<2yrs) | Children (2yrs-<10yrs) | Adolescents (≥10yrs) | |
| Placebo treatment group | Maybe* | Maybe* | Maybe* | Maybe* | Maybe* |
| Active comparator (1 or 2 dose levels) treatment group | Yes | Yes | Yes | Yes | Yes |
| Active comparator with similar mechanism of action, route of administration, and adverse event profile to study drug | No! | No! | No! | Yes | Yes |
| Randomized, parallel design | Yes | Yes | Yes | Yes | Yes |
| Baseline Pain Intensity ≥5 using VAS or NRS | Yes | Yes | Yes | Yes | Yes |
| NRS (or VAS or VRS) pain intensity or pain relief assessments | No | No | No | No | Yes |
| Multiple early observation times (PD) over the first 60-120 minutes | Yes | Yes | Yes | Yes | Yes |
| All reported and observed AEs must be collected and reported2 | Yes | Yes | Yes | Yes | Yes |
| All subjects - treat with identical surgical, anesthetic, and perioperative care regimens | Maybe | Maybe | Maybe | Maybe | Yes |
| Standardized scripts - for questioning subjects/parents (legal guardians) and answering trial methodology questions | Maybe | Maybe | Maybe | Yes | Yes |
| Same CTC per subject for entire evaluation period | Yes | Yes | Yes | Yes | Yes |
| Only trained trial personnel | Yes | Yes | Yes | Yes | Yes |
AE(s) = adverse event(s); CSC = Clinical Trial Coordinator; NRS = numerical rating scale; PD = pharmacodynamic; VAS = visual analogue scale; VRS = verbal rating scale
When true clinical equipoise exists
If solid, oral dosage formulation
Specific AE details to be reported can be found in Smith S, et al.62
US NIH/FDA Workshop on Pain Methods for Trials in Newborns
The Newborn Drug Development Initiative (NDDI), convened in March of 2004, identified an array of important perspectives on clinical trial designs, ethical constraints, gaps in knowledge, and future research needs for analgesia in neonates and infants. Highlights relevant to trials of analgesic drugs for acute pain are included here.
The fetus and the newborn respond to noxious stimuli [6,40] by activating the same brain areas as those in adults. Despite clinical and preclinical data showing that newborns are more sensitive to pain than older infants, children, and adults [3], analgesics are used inconsistently or not at all during moderately-to-severely painful procedures in newborns [33,61,52,67,14]. This undertreatment of iatrogenic pain in neonates may be due, in part, to the lack of analgesics with an approved analgesic indication for infants under 6 months. Opioid analgesics, local and general anesthetics, sedatives, and nonsteroidal anti-inflammatory drugs have all been studied for the treatment of pain in neonates to various degrees, but specific clinical trials have not been submitted to the FDA for review, approval, and labeling. NDDI discussants concluded that questions of safety and efficacy for analgesia in neonates remain unanswered.
Research issues included lack of information on the efficacy and safety of repeated doses, a paucity of adequate dose-ranging trials, a dearth of pharmacokinetic and pharmacodynamics (PK/PD) trials of single or repeated analgesic dosing to determine optimal doses and dosing intervals, lack of uniform treatment goals and reporting of outcomes, and limitations associated with extrapolation of trial results among different patient populations and across different procedures [4]. The discussants proposed heel lance as a priority pain model for the study of novel analgesic treatments of acute pain because of its frequency [4]. Other acute pain models focused on elective surgeries or major abdominal, thoracic, cardiac, pelvic, or urologic surgeries.
Trial design attributes included blinding and placebo control, with immediate rescue analgesia being available. The latter was thought to be acceptable because opioid dose sparing would decrease opioid-related adverse events, balancing the use of placebo. Moreover, use of rescue medication would ensure that placebo control groups only endured very brief periods of insufficient pain control. For postoperative analgesia, to mitigate confounding effects on clinical trial results, standardizing intraoperative management was recommended as much as possible, including analgesics, anesthetics, muscle relaxants, fluids, glucose infusion rate, body temperature, and degree of surgical stress [4].
Pain assessment tools developed for neonates lack the same scaling properties as those used in adults [16], only assess the dimension of pain intensity, and are dependent on the subjective interpretation of an observer. Thus, it is challenging to use them to evaluate outcomes [5,62,51]. Their use for clinical management is also limited because a recent survey of 243 European NICUs showed that only 10% of newborns received assessment of ongoing pain at least once a day. It is also not clear what constitutes a minimal clinically important reduction in pain in this cohort [16]; preliminary data suggest a range of 15 to 20 percent, but this may differ by the populations, types of pain assessed, and clinical context [60].
Clinical trials with neonates must include consideration of the maturity of renal clearance and drug metabolizing enzymes[10], dosing parameters (based on body weight, allometric scaling, or surface area), restrictions on blood sampling from newborns (e.g., <2–3% total blood volume for clinical research), accuracy of ultra-low blood volume sampling methods (e.g., dry blood spot, micro volume assays, scavenged blood), use of clinically indicated indwelling catheters or access lines, use of capnography or oxygen saturation, assessment of sleep-wake cycles, level of sedation or paralysis, severity of illness, developmental age, and behavioral repertoires at each stage of development. While progress has been made, gaps remain in knowledge pertaining to acute pain assessment, which informs trial design, in the neonatal population. Areas for additional research include, but are not limited to [4]:
Development and psychometric validation of new or existing pain indicators (e.g. behavioral, physiological, cortical) for neonates of varying gestational age, especially preterm or very low birth weight, and neonates at high risk for neurologic impairment, that go beyond procedural pain
Determination of a gold standard for pain measurement
Determination of the optimal PK trial design, including age cohorts, blood sampling strategies, and microassay analysis techniques
Outcome Measures in Acute Pain Clinical Trials in Pediatric Populations
Approaches to measuring pain in children include self-report, observation of behavior, physiologic responses and most recently, biomarkers and cortical electroencephalograph responses. The ideal measurement approach likely combines multiple modalities [17] to capture the multidimensional nature of pain; however, self-reported pain intensity is the most commonly used approach in pediatric clinical trials.
Self-report measures can be used with children who are developmentally mature enough to understand and use these scales, who are not overly distressed, and who do not have impaired cognitive or communicative abilities. In cases where self-report may not be feasible or the best choice for measuring pain [59], other approaches, such as behavioral observations, are invoked [85]. These approaches involve assigning quantitative values to observed behaviors indicative of pain, such as body movements, facial expression, and vocalizations [18]. For infants and preverbal children, validated multidimensional composite measures including items from multiple domains (e.g., behavioral, physiologic) assessed within a specific context (e.g., age, severity of illness, behavioral state) should be utilized [39]. These indicators have not been applied for assessing ongoing or persistent pain.
While multiple pain measures exist across all pediatric age groups, they have been used inconsistently, making it difficult to compare or pool results across trials. To address this problem, Pediatric IMMPACT (Ped-IMMPACT) recommended core outcome domains that should be considered when designing clinical trials for acute as well as recurrent and chronic pain for children 3 years of age and older. The six core outcome domains for acute pain are: (1) pain, (2) global judgment of satisfaction with treatment, (3) symptoms and adverse events, (4) physical recovery, (5) emotional recovery, and (6) economic factors [43].
Pain assessment is particularly complex and challenging in infants and preverbal children. Despite the development of over four dozen neonatal pain measures, lack of a gold standard hinders our understanding of infant pain processing and effectiveness of pain treatment [28]. Existing measures tend to incorporate a finite set of behavioral and physiologic responses (e.g., facial actions, cry, body movements, changes in heart rate, and oxygen saturation). However, given the fundamental role of cortical activation in adult pain processing [75] and with the emergence of technological advances, there are new measurement possibilities using biomarkers of stress, such as cortisol and heart rate variability. Interest is mounting in cortical indicators for determining how infants manifest neurocognitive responses to pain, including electroencephalogram, positron emission tomography, functional magnetic resonance, and near-infrared spectroscopy [64,83]. Although these indicators hold significant promise as more objective “pain” indicators for clinical trials in infants and young children, their complexity and problematic real-time measurement makes them difficult to decode and compare to more traditional behavioral indicators. Cost may also limit widespread application.
Further research should be directed towards the validation of objective multidimensional measures for assessing pain in infants and young children. There is a proportion of infants (20–25%) who do not respond with visible behavioral or physiological responses [34]. Lack of response does not necessarily indicate absence of pain, but rather that the indicator may not be sensitive enough to reflect a response triggered by a painful stimulus; investigation of cortical indicators may be prudent in these situations. There are populations of vulnerable infants and young children (e.g., extremely sick and low birth weight infants) for whom there are no validated measures.
Tools such as the Pieces of Hurt Tool and the Multiple Size Poker Chip Tool [72] might assist preschool children to comprehend and more accurately rate their pain; further psychometric testing is required. Given the high failure rates and tendency to use extremes of scales by young children, more research is needed to establish screening methods to determine which children may provide meaningful self-reports. The effects of standardized instructions and other methods that ensure children can meaningfully use self-report measures need to be established.
There are currently more than 35 self-report pain intensity measures designed for children and adolescents; however, only 10 have well-established evidence of reliability and validity [31,69,70,71] with varying degrees of responsiveness and modest evidence of interpretability. No single scale was reliable and valid across all age groups or pain types, with the majority lacking reliability and validity in preschool children. Moreover, there are important differences in failure rates and children’s preferences across measures. The recommendations for the use of self-report measures in clinical trials are summarized in Table 3 [43,28]. Although age is frequently used as a proxy for developmental level, it should not be taken literally. As the psychometric testing of measurement tools is a dynamic and ongoing process, the body of evidence will grow and these recommendations will undoubtedly change. New digital pain assessment tools are also showing promise for use in clinical trials [68].
Table 3.
Summary of recommendations of pain intensity measures for clinical trials in infants and children66
|
|
|
|
|
|
Psychometrically sound measures that are clinically sensitive are required across all age groups [25]. Minimal clinically significant differences (MCSD) need to be established at different ages, using different scales, with different types of pain. Only one trial has been conducted in children to determine the MCSD in acute pain using a visual analogue scale (VAS) [53]. Voepel-Lewis and colleagues evaluated MCSD using a 0–10 numerical rating scale in children aged 7–16 years with acute postsurgical pain, and found that a difference of ± 1 point was perceived as ‘a little better’ or ‘a little worse’ [84]. Further research needs to be done on the best way to operationalize a clinically meaningful improvement in pain, as a difference of 10 percent of the scale (e.g., 10 mm on a 100 mm VAS) is arbitrary. For clinical trials, the most cautious approach may be to define clinically ‘‘meaningful improvement’’ as being both a rating of ‘‘much improved’’ and a 30% reduction in the rating of pain intensity [22,57]. Given the recognized functional impact of postoperative pain evoked by movement and the observation that this is often of substantially greater intensity than pain experienced at rest [37,66], future research, development, and validation of methods to evaluate movement-related pain across all pediatric age cohorts are needed.
Pain Models for Developmental Stages
Figure 1 presents general model for approaches to studying treatments for acute pain in children, while specific recommendations for acute pain models and clinical trial attributes to improve assay sensitivity are summarized in Table 4. To recommend a pain model for use in acute pain trials, regardless of age, the condition needs to be prevalent (i.e., adequate patient population), specific (i.e., homogenous patient population), manifest minimal comorbidities, exhibit a predictable level of pain and response to analgesics, and show reasonable evidence that the agent has probability of efficacy. For each age group, a review of epidemiological data from the Healthcare Cost and Utilization Project (H-CUP), a project within the Agency for Healthcare Research and Quality [30], was conducted to facilitate discussion at the meeting.
Figure 1.
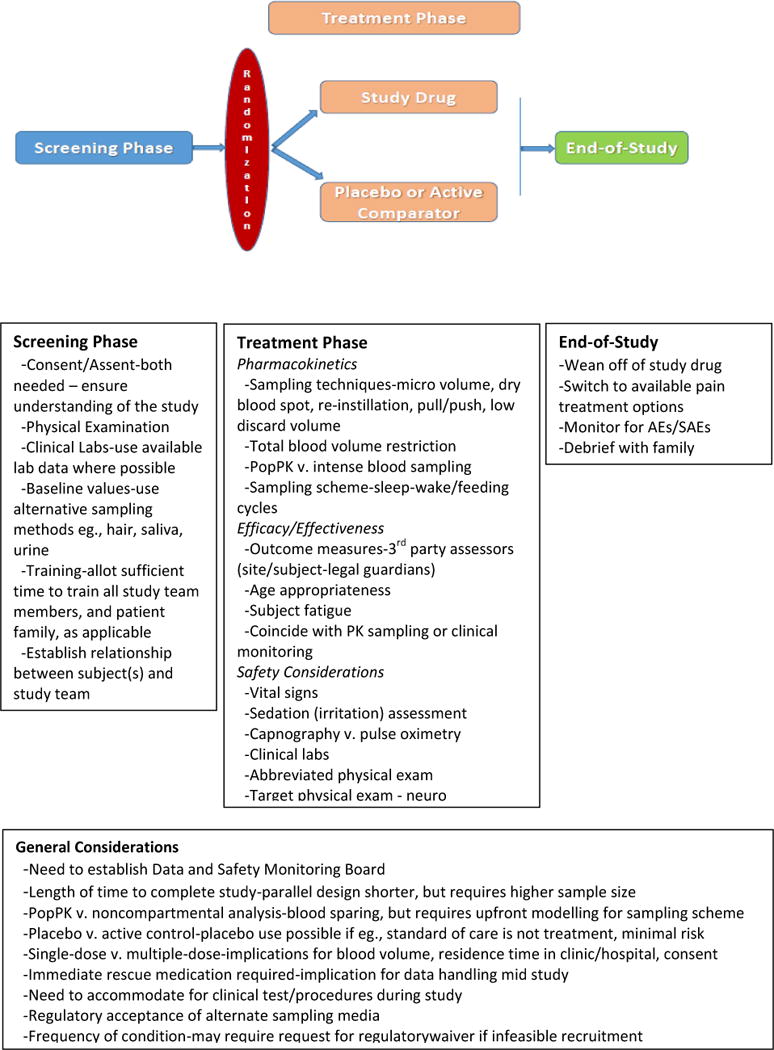
Acute Pain Analgesic Trial Design Considerations: ACTTION Recommendations
Table 4.
Recommended Acute Pain Models and Clinical Trial Attributes
| Pediatric Population | |||||
|---|---|---|---|---|---|
| Neonates (0-<1month) | Infants (1month-<6months) | Toddlers (6months-<2yrs) | Children (2yrs-<10yrs) | Adolescents (≥10yrs) | |
| Acute Pain Model | Heel lance Circumcision | Heel lance Circumcision Inguinal or femoral hernia Colorectal resection | Appendectomy T & A Spinal fusion Circumcision Inguinal or femoral hernia Cleft palate Hypospadias | Appendectomy T & A Spinal fusion Foot osteotomy Ureteral reimplant Pharyngitis | Appendectomy T & A Spinal fusion Foot osteotomy Pharyngitis |
| Clinical Trial Design | Double-blind, randomized, placebo-controlled with immediate RM, cross-over- or parallel-group; N-of-1; randomized withdrawal; flexible randomization; play-the-winner | Double-blind, randomized, placebo-controlled with immediate RM, cross-over- or parallel-group; N-of-1; randomized withdrawal; flexible randomization; play-the-winner | Double-blind, randomized, placebo-controlled with immediate RM, cross-over- or parallel-group | Double-blind, randomized, placebo-controlled with immediate RM, cross-over- or parallel-group | Double-blind, randomized, placebo-controlled with immediate RM, cross-over- or parallel-group |
| Drug Formulation | Liquid | Liquid | Liquid; microspheres/pellets sprinkled onto soft food | Liquid; solid (monolithic); microspheres/pellets sprinkled onto soft food; dissolving thin film | Liquid; solid (monolithic); microspheres/pellets sprinkled onto soft food; dissolving thin film |
| Route of Administration | IN, IV, rectal, enteral tube | IN, IV, PO, rectal, enteral tube | IN, IV, PO, rectal, enteral tube | IN, IV, PO, enteral tube; topical, buccal | IN, IV, PO, enteral tube; topical, transdermal, buccal |
| Use of PBO | Yes, with immediate RM | Yes, with immediate RM | Yes, with immediate RM | Yes, with immediate RM | Yes, with immediate RM |
| Safety Measures | Clin labs – chemistry/hematology, LFTs, urinalysis, capnography, ECGs, EEGs, sedation, WAT-1, P/E, AEs | Clin labs – chemistry/hematology, LFTs, urinalysis, capnography, ECGs, EEGs, sedation, WAT-1, P/E, AEs | Clin labs – chemistry/hematology, LFTs, urinalysis, capnography, ECGs, EEGs, sedation, WAT-1, P/E, AEs | Clin labs – chemistry/hematology, LFTs, urinalysis, capnography, ECGs, EEGs, sedation, WAT-1, P/E, AEs | Clin labs – chemistry/hematology, LFTs, urinalysis, O2 saturation and RR, ECGs, EEGs, sedation, WAT-1, P/E, AEs |
| TBV Limits | 2-3% | 2-3% | 2-3% | 2-3% | 2-3% |
| Blood Sampling Technique | Dry blood spot; Micro volume; Re-instillation; Push-pull; Discard volume <0.5mL/sample | Dry blood spot; Micro volume; Re-instillation; Push-pull; Discard volume <0.5mL/sample | Dry blood spot; Micro volume; Discard volume <0.5mL/sample | Dry blood spot; Micro volume; Discard volume <0.5mL/sample | Dry blood spot Micro or small volume; Discard volume <0.5mL/sample |
| Pharmacokinetics | Sparse sampling Population PK | Sparse sampling Population PK | Sparse sampling Population PK | Sparse sampling Population PK; traditional rich sampling PK NCA | Sparse sampling Population PK, traditional rich sampling PK NCA |
| Assay | Microvolume | Microvolume | Microvolume | Microvolume | Microvolume |
| Developmental Metabolism | CYP450 variable birth to 9months | CYP450 variable birth to 9months | Generally adult values | Generally adult values | Generally adult values |
| Use of EMLA/Synera/LMX | EMLA1 | EMLA (>3months) Synera | EMLA Synera | EMLA Synera | EMLA Synera LMX2 |
| Venous Access | Clinical line access, Catheter access | Clinical line access, Catheter access | Clinical line access, Catheter access | Clinical line access, Catheter access | Clinical line access, Catheter access |
| Sampling Media | Blood, hair, saliva, meconium | Blood, hair, urine, saliva | Blood, saliva, urine | Blood, saliva, urine | Blood, saliva, urine |
| Consent/Assent | Consent | Consent | Consent | Consent Assent (≥7yrs) | Consent Assent |
Use with caution in children under 3months due to potential for methemoglobinemia
Use with caution in children under 12yrs
AEs = adverse events; CYP = cytochrome; ECGs = electrocardiograms; EEGs = electroencephalographs; EMLA = eutectic mixed of local anesthetics; IN = intranasal (inhaled); IV = intravenous; LFTs = liver function tests; NCA = noncompartmental analysis; O2 = oxygen; PI = pain intensity; PBO = placebo; P/E = physical exam; PK = pharmacokinetic; PO = per os; RR = respiratory rate; T&A = tonsillectomy & adenoidectomy; TBV = total blood volume; WAT-1 = Withdrawal Assessment Tool-1; yrs = years
Models for Analgesic Clinical Trials for Acute Pain in Neonates and Infants
Epidemiological data illustrate the number of invasive procedures performed on preterm and term neonates and infants, as well as acute pain from surgery, inflammatory conditions, and other forms of nociceptive stimuli such as visceral pain. Carbajal et al. [14] studied 430 neonates in intensive care units over a 6-week period where each neonate experienced a median of 75 (range, 3–364) painful procedures and 10 (range, 0–51) per day. Of the 42,413 painful procedures, 79.2% were performed without specific analgesia and 34.2% were performed while the neonate was receiving analgesia for other reasons, but presumably not addressing the procedural pain. Simons et al. showed similar findings for neonates in the Netherlands [61].
Unfortunately, H-CUP data do not include a neonatal age group [23]. Available data indicate that the most common procedures in this age group are heel lance and circumcision, both of which are prevalent and recommended acute pain models (Table 4). The Center for Disease Control and Prevention estimated that in 2010, approximately 56% of all newborn males in the United States were circumcised within 28 days after birth [15]. Pain associated with heel lance and circumcision tends to be of lesser severity and duration when compared to postoperative pain, and, of course, the circumcision model is restricted to one sex. Thus, models such as hernia repair, major abdominal, cardiac, and thoracic surgeries (e.g., intestinal atresia, anorectal malformation, and lung malformations), Achilles tenotomy, or urologic procedures (e.g., pyeloplasty) may be considered. However, the variable severity of illness and co-morbidities of these patients must be considered, particularly because these factors may vary across settings.
Other trial design considerations for acute pain in neonates and infants are presented in Table 5. Operationally, clinical trials in newborns are also constrained by the stringent timing of feeds, requirement for frequent naps, and physiological restrictions that may limit routes and modes of analgesic administration.
Table 5.
Trial Design Considerations for Acute Pain in Neonates
|
|
|
|
|
|
|
NICU = neonatal intensive care unit
Although a double-blind, randomized, placebo-controlled (immediate rescue), parallel-group or crossover, multicenter trial design is ideal to study acute pain and analgesia in neonates, Table 4 provides additional, alternative trial designs for consideration. A flexible randomization design avoids isolating patients who have reservations about being in the placebo group; patients are asked about preference with respect to treatment group and those with a preference are so assigned, while others are randomized to one of the trial treatment groups. A concern with this design is uneven sample groups and potential for loss of randomization. The randomized play-the-winner adaptive design (trial based on an urn model in which a higher proportion of patients will be assigned to the more successful treatment) is suited to clinical trials with a binary outcome (success or failure) and two treatments [89,56,74]. To further isolate treatment effects, an active comparator can be added, but this requires more patients, thereby decreasing the likelihood of completing the trial within a reasonable timeframe.
Safety variables included in neonatal acute pain clinical trials should include appropriate monitoring of vital signs, sedation, agitation, capnography, clinical lab tests (clinical chemistry, hematology, urinalysis, liver function), drug withdrawal, monitoring for specific adverse events, and physical examination. Other safety variables included in a specific acute pain clinical trial will depend upon the disease model, the type of pain treated, and the study drug used (e.g., electrocardiograms, electroencephalograms). Other design considerations are summarized in Table 4.
Although factors for each of the age groups from infancy through adolescence pose unique challenges to the design and conduct of clinical trials, additional issues arise in studying neonates. Due to maturation of drug metabolism and organ system function, a “neonate” may represent six individual populations, each with unique attributes that need to be considered when designing a clinical trial:
micropreterm (23–26 weeks gestation)
early preterm (27–32 weeks gestation)
late preterm (33–37 weeks gestation)
ex-preterm (not yet 40 weeks gestation)
term neonates (38–42 weeks gestation, between birth to <1week age)
older neonates (1 week to <2 weeks, 2 weeks to <3 weeks, and 3 to <4 weeks age).
Factors that increase variability in PK and PD include gestational age, postnatal age, asphyxia at birth, patent ductus arteriosus, prenatal drug exposure, maternal smoking, neonatal surgery, and pharmacogenetic/epigenetic polymorphisms [2]. Moreover, neonates, infants, and toddlers are all undergoing rapid developmental maturation of the central nervous system, heart, lungs, kidneys, liver, blood, skin, and muscle [36]. A detailed review by Kearns et al. should be preliminary reading for any investigator designing a clinical trial with PK/PD endpoints studying neonates, infants, and toddlers [36].
These age groups show substantial differences in serum binding protein and albumin levels, intra- and extracellular water compartment volumes, fat and muscle body content, permeability of the blood brain barrier to molecules, glomerular filtration rate, and enzyme maturation (e.g., key CYP450 enzymes involved in analgesic metabolism – CYP2D6, CYP3A4, UDP-glucuronyl transferase) [36]. Metabolizer status needs to be considered in the clinical trial of a new drug to avoid adverse events in ultra-rapid or slow metabolizers. These challenges can be addressed by understanding the patient population, the type of pain being studied, the study drug, and its metabolic pathways. Each of these facets needs to be clearly understood before a successful clinical trial can be designed and conducted.
Models for Analgesic Clinical Trials for Acute Pain in Toddlers (6 to 24 months)
Many of the challenges cited above for the younger age child are overcome due to maturation of many of the physiologic systems by about 6 months of age. In addition, there are several relatively standardized surgical procedures completed in this age group that could lend themselves to study in acute pain analgesic trials. Trials of procedural pain, and needle pain associated with immunization and venipuncture, in particular, have been completed in this age cohort [26,32,19,55,82]. Ideally, acute pain trials in this age group would be studied in a homogeneous population, with a goal of studying healthy cohorts, who lack complicated, confounding co-morbidities.
Although surgical procedures are common in this age group (Table 4), approximately 1.4 million procedures a year, there is surprisingly minimal overlap in the types of surgical procedures in adjacent age cohorts [29]. Candidate surgical procedures with adequate frequency are tonsillectomy and adenoidectomy, cleft palate repair, and inguinal herniorrhaphy. These procedures are candidates for investigation in both inpatient and ambulatory settings. The potential advantage of the first candidate procedures is the more intense nociception expected, while hypospadias repair and inguinal herniorrhaphy are associated with mild to moderate postoperative pain.
Berde and colleagues published a schema for recommended trials of analgesics for pediatric patients that may be applied to this age cohort [10]. The immediate rescue trial design would support valid and clinically sensitive primary outcome measures for efficacy of the proposed study analgesic while addressing the ethical concern of a child participant being exposed to untreated pain. Parent- or nurse- controlled analgesia rescue with a standardized opioid has been used extensively in this age cohort [47,21] and may serve as the primary design in analgesic trials in this age cohort.
Pharmacokinetic trials are challenging in this age group. In situ intravenous lines are commonly of small caliber and unlikely to yield adequate sampling volumes. If a blood sampling methodology involves breaking the skin, it is essential to minimize discomfort through the use of topical anesthetic preparations, which is highly recommended for neonates; however, the recommendation applies to all other age cohorts as well.
Candidate analgesics that warrant consideration in this age cohort include morphine, hydromorphone, fentanyl, oxycodone, buprenorphine, tapentadol, diclofenac, ketorolac, and naproxen. Opioid sparing medications, such as ketamine, gabapentin, and pregabalin have potential for further study.
Models for Analgesic Clinical Trials for Acute Pain in Children 24 Months and Older
Karling et al. [35] conducted a survey (N = 6,344) in Sweden that showed 73% of children who had undergone surgery were estimated to have some pain, and 23% of those with pain (17% of the total) had moderate-to-severe pain. Moreover, of the 766 children with non-postoperative acute pain, 31% of children still had moderate-to-severe pain despite treatment. Linhares et al. [42] and Murphy et al. [48] both showed similar findings indicating the undertreatment of pain in children.
Of the three populations considered in the present article, children older than 24 months demonstrate the greatest variability in types and severity of pain. Superimposed on these variables are the effects on pain measurement of the heterogeneity of developmental psychology, cognition, and learned behaviors, which may be vast as one considers age cohorts through adolescence. In addition, generally children over the age of 7 years appropriately are asked to give assent to their participation in clinical trials, adding another potential source of bias and enrollment challenge.
The top five operative procedures performed in this age group are appendectomy (greater than fourfold in frequency compared to the others), partial excision of bone, treatment of fractures or dislocation of the hip or femur, tonsillectomy and/or adenoidectomy, and spine fusion to correct idiopathic scoliosis [30]. Because trauma often involves confounding factors, appendectomy, tonsillectomy and adenoidectomy, spine fusion, ureteral reimplantation, and foot osteotomy in children >2 years (Table 4) are the recommended acute pain models for clinical trials. A model of less severe pain, sore throat due to pharyngitis with or without tonsillitis, has been evaluated in randomized, double-blind, placebo-controlled trials demonstrating the analgesic efficacy and safety of ibuprofen and acetaminophen for children 2–12 years of age [11,58].
Because there are fewer developmental limitations in this age cohort than in younger age groups, a double-blind, randomized, placebo-controlled (immediate rescue), parallel-group or crossover, multicenter trial design can be used. Adaptive trial designs and the sequential parallel comparison design that have been used with adults aim to conserve patient numbers or yield more robust results [24] but have not been deployed with children and adolescents. An active comparator can be added, but this will require a larger sample size, and therefore more time to complete the trial. Routes of administration include intranasal, buccal, intravenous, and oral; available modes of administration include solid, monolithic tablets and capsules as well as pellet or microsphere formulations that can be sprinkled onto soft foods or administered via enteral tube. Although less common, transdermal and topical routes may be invoked.
Pharmacokinetic trials have many of the restrictions common with those applicable to the younger age cohorts, but also have greater flexibility; for example, noncompartmental analysis requiring rich blood sampling is feasible in children >10 years, albeit justification for the sampling scheme must still be evidence-based. In addition, blood sampling technique (e.g., use of a catheter with topical anesthetic pretreatment of the insertion site) and analysis assay (e.g., small- or micro-volume assay) still require minimization of patient burden and discomfort and conservation of blood volume drawn for research purposes.
Safety variables for this age group should include monitoring of vital signs, sedation, agitation, oxygen saturation, clinical labs (chemistry, hematology, urinalysis, liver function), withdrawal, adverse events, physical examination, and, when indicated, electrocardiograms, electroencephalograms; more frequent assessments should be included after dosing, but selection of assessment timing must be justified and presented in the trial protocol. Other design considerations are summarized in Table 4.
Practical Limitations and Solutions for Conducting Clinical Trials – Additional Considerations
The conduct of pediatric clinical trials presents challenges at the clinical site level, the regulatory agency level, and the pharmaceutical industry level, and at the study drug formulation level. At any clinical site, pediatric clinical trials are more likely to succeed if there is good communication among members of the research team, comprehension of the trial by parents and child assenters, and an institutional culture that embraces research and supports participation in clinical research, minimizes inconvenience for participants, and defines trial recruitment and retention plans. At the regulatory agency and pharmaceutical industry levels, limitations to the success of a pediatric clinical trial include aggressive company timelines for conduct of the trial, inadequate pediatric scientific review, and the need for a specific pediatric formulation of the study drug.
To address some of these practical concerns and limitations, a number of different potential clinical trial designs can be employed for studying new analgesics. The parallel-group, randomized, single-/multiple-dose, active-/placebo-controlled, reverse age sequence (i.e., studying older children before younger children) trial can be used across stratified age cohorts if the investigator incorporates age-specific modifications to address trial feasibility. A crossover design is less suitable for acute pain trials because these trials take longer to complete. N-of-1 trials may be considered in pediatric patient populations with rare or life-limiting conditions [41, 10]. An active run-in, responder-randomized trial design has the advantage of limiting exposure to a study drug for children who are non-responders. The Zelen design, in which randomization precedes consent and only those patients who are allocated to active treatment are then approached for consent, while others receive standard of care has been used in pediatric clinical research [49,73]. The parallel trial design with immediately available parent-, nurse-, or patient-controlled analgesia affords the inclusion of a placebo group without ethical concerns for risk of additional burden [38].
Blood-sparing techniques must be incorporated in PK trials. This may include a discard volume of <0.5 ml/sample, re-instillation sampling, pull/push sampling, or use of dry blood spots [1,8,44,50], as well as alternate sampling media (e.g., hair, saliva, sweat, urine, and meconium). Population PK analysis may be preferred over intensive blood sampling for conventional, non-compartmental PK analysis [88]. Each blood sparing technique has its own challenges and limitations that must be considered prior to use.
Summary
This article reviews the existing literature and expert consensus on ways to facilitate pediatric trials, recognizing and embracing the ethical and pragmatic challenges. Only with additional research can these trial methods be tested and refined. These recommendations focused exclusively on acute pain trials and do not pertain to recurrent or chronic pain.
To date, minimal attention and funding have been directed toward studying neonatal and pediatric analgesia. As the National Pain Strategy and the Federal Pain Research Strategy are advanced, these elements should be included for public attention and funding opportunities. Collaborative efforts among research sites are essential to move the field forward. Optimally, a stable network of institutions with collaborative agreements can move this agenda forward. A recent combined advisory meeting sponsored by the FDA (September 15–16, 2016) has again highlighted this need and will hopefully provide the impetus for accomplishing this important objective [81]. The Pediatric Research Network for Pain (PRN-Pain), a collaborative research group established thus far among pediatric organizations in the United States and Canada, may play a pivotal role in this regard.
Acknowledgments
No official endorsement by the US Food and Drug Administration or the pharmaceutical companies that provided unrestricted grants to the University of Rochester, Office of Continuing Professional Education should be inferred. The authors thank Andrea Speckin and Valorie Thompson for their invaluable assistance in the organization of the ACTTION Pediatric Pain Research Consortium meeting. The authors also thank the following participants from the FDA: Ellen Fields, MD, Sharon Hertz, MD, Allison Lin, PhD, PharmD, Diane Murphy, MD, Robert Nelson, MD, Rigoberto Roca, MD, Amy Taylor, MD, MHS, and Lynne Yao, MD.
Footnotes
The views expressed in this article are those of the authors, none of whom have financial conflicts of interest related to the specific issues discussed in this article. ACTTION has received research contracts, grants, or other revenue from the FDA, multiple pharmaceutical and device companies, philanthropy, and other sources. At the time of the consensus meeting on which this article is based, 2 of the authors were employed by companies that provided unrestricted grants to the University of Rochester Office of Continuing Professional Education to support the activities of ACTTION, including the consensus meeting; these companies were Collegium Pharmaceutical, Inc. and Purdue Pharma, LP.
References
- 1.Adlard K. Examining the push-pull method of blood sampling from central venous access devices. J Pediatr Oncol Nurs. 2008;25:200–207. doi: 10.1177/1043454208320975. [DOI] [PubMed] [Google Scholar]
- 2.Allegaert K, Vanhaesebrouck S, Verbesselt R, van den Anker JN. In vivo glucuronidation activity of drugs in neonates: Extensive interindividual variability despite their young age. Ther Drug Monit. 2009;31:411–415. doi: 10.1097/FTD.0b013e3181a8cc0a. [DOI] [PubMed] [Google Scholar]
- 3.Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- 4.Anand KJ, Aranda JV, Berde CB, Buckman S, Capparelli EV, Carlo W, Hummel P, Johnston CC, Lantos J, Tutag-Lehr V, Lynn AM, Maxwell LG, Oberlander TF, Raju TN, Soriano SG, Taddio A, Walco GA. Summary proceedings from the Neonatal Pain-Control Group. Pediatrics. 2006;117(Suppl 1):S9–S22. doi: 10.1542/peds.2005-0620C. [DOI] [PubMed] [Google Scholar]
- 5.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA, NEOPAIN Trial Investigators Group Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–1682. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 6.Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317:1321–1329. doi: 10.1056/NEJM198711193172105. [DOI] [PubMed] [Google Scholar]
- 7.Baer GR, Nelson RM, Ethics Group of the Newborn Drug Development Initiative Ethical challenges in neonatal research: Summary report of the ethics group of the newborn drug development initiative. Clin Ther. 2006;28:1399–1407. doi: 10.1016/j.clinthera.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Barton SJ, Chase T, Latham B, Rayens MK. Comparing two methods to obtain blood specimens from pediatric central venous catheters. J Pediatr Oncol Nurs. 2004;21:320–326. doi: 10.1177/1043454204269604. [DOI] [PubMed] [Google Scholar]
- 9.Beecher HK, Keats AS, Mosteller F, Lasagna L. The effectiveness of oral analgesics (morphine, codeine, acetylsalicylic acid) and the problem of placebo “reactors” and “non-reactors”. J Pharmacol Exp Ther. 1953;109:393–400. [PubMed] [Google Scholar]
- 10.Berde CB, Walco GA, Krane EJ, Anand KJ, Aranda JV, Craig KD, Dampier CD, Finkel JC, Grabois M, Johnston C, Lantos J, Lebel A, Maxwell LG, McGrath P, Oberlander TF, Schanberg LE, Stevens B, Taddio A, von Baeyer CL, Yaster M, Zempsky WT. Pediatric analgesic clinical trial designs, measures, and extrapolation: report of an FDA scientific workshop. Pediatrics. 2012;129:354–364. doi: 10.1542/peds.2010-3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertin L, Pons G, d’Athis P, Lasfarguess G, Maudelonde C, Duhamel JF, Olive G. Randomized, double-blind, multicenter, controlled trial of ibuprofen versus acetaminophen (paracetamol) and placebo for treatment of symptoms of tonsillitis and pharyngitis in children. J Pediatr. 1991;119:811–814. doi: 10.1016/s0022-3476(05)80308-7. [DOI] [PubMed] [Google Scholar]
- 12.Black P, Max MB, Desjardins PJ, Norwood T, Ardia A, Palotta T. A randomized, double-blind, placebo controlled comparison of the analgesic efficacy, onset of action, and tolerability of ibuprofen argininate and ibuprofen in postoperative dental pain. Clin Ther. 2002;24:1072–1089. doi: 10.1016/s0149-2918(02)80020-0. [DOI] [PubMed] [Google Scholar]
- 13.Canadian Tri-Council Policy Statement. Ethical conduct for research involving humans. 2010 Available at: http://www.pre.ethics.gc.ca/mwg-internal/de5fs23hu73ds/progress?id=lLGiHc2eya6Fxe61UqDZjwRxFt_jKWF8RH-fH5P_6W8 Last accessed: 2 MAY 2016.
- 14.Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, Saizou C, Lapillonne A, Granier M, Durand P, Lenclen R, Coursol A, Hubert P, de Saint Blanquat L, Boëlle PY, Annequin D, Cimerman P, Anand KJ, Bréart G. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Trends in in-hospital newborn male circumcision - United States, 1999—2010. Morbidity and Mortality Weekly Report (MMWR) 2011;60:1167–1168. [PubMed] [Google Scholar]
- 16.Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–157. doi: 10.1016/s0304-3959(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 17.Champion GD, Goodenough B, von Baeyer CL, Thomas W. Measurement of pain by self-report. In: Finley GA, McGrath PJ, editors. Measurement of Pain in Infants and Children. Seattle: IASP Press; 1998. pp. 123–160. [Google Scholar]
- 18.Chorney J, McMurtry CM. Behavioural measures of pain. In: McGrath PJ, Stevens BJ, Walker SM, Zempsky WT, editors. Oxford Textbook of Paediatric Pain. Oxford University Press; 2014. pp. 379–390. [Google Scholar]
- 19.Cohen LL, MacLaren JE, Fortson BL, Friedman A, DeMore M, Lim CS, Gangaram B. Randomized clinical trial of distraction for infant immunization pain. Pain. 2006;25:165–171. doi: 10.1016/j.pain.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Cooper SA, Desjardins PJ, Turk DC, Dworkin RH, Katz NP, Kehlet H, Ballantyne JC, Blum DE, Burke LB, Carragee E, Cowan P, Croll S, Dionne RA, Farrar JT, Gilron I, Gordon DB, Iyengar S, Jay GW, Kalso EA, Kerns RD, McDermott MP, Raja SN, Rappaport BA, Rauschkolb C, Royal MA, Segerdahl M, Stauffer JW, Todd KH, Vanhove GF, Wallace MS, West C, White RE, Wu C. Research design considerations for single-dose analgesic clinical trials in acute pain: IMMPACT recommendations. Pain. 2016;157:288–301. doi: 10.1097/j.pain.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 21.Czarnecki ML, Salamon KS, Mano KEJ, Ferrise AS, Sharp M, Weisman SJ. A preliminary report of parent/nurse-controlled analgesia (PNCA) in infants and preschoolers. Clin J Pain. 2011;27:102–107. doi: 10.1097/AJP.0b013e3181f0972c. [DOI] [PubMed] [Google Scholar]
- 22.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 23.Fingar KR (Truven Health Analytics), Stock C(AHRQ), Weiss AJ (Truven Health Analytics), Steiner CA(AHRQ) HCUP Statistical Brief #186. Agency for Healthcare Research and Quality; Rockville, MD: Dec, 2014. Most frequent operating room procedures performed in US hospitals, 2003–2012. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb186-Operating-Room-Procedures-United-States-2012.pdf. [PubMed] [Google Scholar]
- 24.Fova M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72:115–127. doi: 10.1159/000069738. Erratum in Psychother Psychosom 2004;73:123. [DOI] [PubMed] [Google Scholar]
- 25.Franck LS, Ridout D, Howard R, Peters J, Honour JW. A comparison of pain measures in newborn infants after cardiac surgery. Pain. 2011;152:1758–1765. doi: 10.1016/j.pain.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Halperin SA, McGrath P, Smith B, Houston T. Lidocaine-prilocaine patch decreases the pain associated with the subcutaneous administration of measles-mumps-rubella vaccine but does not adversely affect the antibody response. J Pediatr. 2000;136:789–794. [PubMed] [Google Scholar]
- 27.Harrison D, Bueno M, Yamada J, Adams-Webber T, Stevens B. Analgesic effects of sweet tasting solutions for infants: Current state of equipoise. Pediatrics. 2010;126:894–902. doi: 10.1542/peds.2010-1593. [DOI] [PubMed] [Google Scholar]
- 28.Hartley C, Slater R. Neurophysiological measures of nociceptive brain activity in the newborn infant. Acta Pediatr. 2014;103:238–242. doi: 10.1111/apa.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2012. HCUP Clinical Classifications Software for Services and Procedures. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/ccs_svcsproc/ccssvcproc.jsp. Last accessed: 20 AUG 2016. [Google Scholar]
- 30.Healthcare Cost and Utilization Project (H-CUP) State Ambulatory Surgery and Services Database (SASD) Available at: https://www.hcup-us.ahrq.gov/db/state/sasddbdocumentation.jsp Last accessed: 24 MAY 2017.
- 31.Huguet A, Stinson J, McGrath PJ. Measurement of self-reported pain intensity in children and adolescents. J Psychosom Res. 2010;68:329–336. doi: 10.1016/j.jpsychores.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Ipp M, Cohen E, Goldbach M, Macarthur C. Effect of choice of measles-mumps-rubella vaccine on immediate vaccination pain in infants. Arch Pediatr Adolesc Med. 2004;158:323–326. doi: 10.1001/archpedi.158.4.323. [DOI] [PubMed] [Google Scholar]
- 33.Johnston CC, Collinge JM, Henderson SJ, Anand KJS. A cross-sectional survey of pain and pharmacological analgesia in Canadian neonatal intensive care units. Clin J Pain. 1997;13:308–312. doi: 10.1097/00002508-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Johnston CC, Stevens BJ, Franck LS, Jack A, Stremler R, Platt R. Factors explaining lack of response to heel stick in preterm newborns. J Obstet Gynecol Neonatal Nurs. 1999;28:587–594. doi: 10.1111/j.1552-6909.1999.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 35.Karling M, Renström M, Ljungman G. Acute and postoperative pain in children: a Swedish nationwide survey. Acta Paediatr. 2002;91:660–666. doi: 10.1080/080352502760069070. [DOI] [PubMed] [Google Scholar]
- 36.Kearns GL, Abdel-Rohman SM, Alander SW, Blowey DL, Leeder JS, Kaufman RE. Developmental pharmacology – drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 37.Kehlet H, Dahl JB. Assessment of postoperative pain-need for action! Pain. 2011;152:1699–1700. doi: 10.1016/j.pain.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Kossowsky J, Donado C, Berde CB. Immediate rescue design in pediatric analgesic trials: A systematic review and meta analysis. Anesthesiol. 2015;122:150–171. doi: 10.1097/ALN.0000000000000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee G, Yamada J, Kyololo OB, Shorkey A, Stevens B. Pediatric clinical practice guidelines for acute procedural pain: a systematic review. Pediatrics. 2014;133:500–515. doi: 10.1542/peds.2013-2744. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Ralston HJP, Drey EA, Partridge JC, Rosen MA. Fetal pain: A systematic multidisciplinary review of the evidence. JAMA. 2005;294:947–954. doi: 10.1001/jama.294.8.947. [DOI] [PubMed] [Google Scholar]
- 41.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: The ultimate strategy for individualizing medicine. Per Med. 2012;8:161–173. doi: 10.2217/pme.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linhares MBM, Linhares MB, Doca FN, Martinez FE, Carlotti AP, Cassiano RG, Pfeifer LI, Funayama CA, Rossi LR, Finley GA. Pediatric pain: prevalence, assessment, and management in a teaching hospital. Braz J Med Biol Res. 2012;45:1287–1294. doi: 10.1590/S0100-879X2012007500147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Mendez SJ. Evidence-based practice for obtaining blood specimens from a central venous access device. Oncol Nurs Forum. 2012;39:247–251. doi: 10.1188/12.ONF.247-251. [DOI] [PubMed] [Google Scholar]
- 45.Mercadante S, Giarratano A. Pharmacological management of cancer pain in children. Crit Rev Oncol Hematol. 2014;91:93–97. doi: 10.1016/j.critrevonc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Modell W, Houde RW. Factors influencing clinical evaluation of drugs. JAMA. 1958;167:2190–2198. doi: 10.1001/jama.1958.72990350005006. [DOI] [PubMed] [Google Scholar]
- 47.Monitto CL, Greenberg RS, Kost-Byerly S, Wetzel R, Billett C, Lebet RM, Yaster M. The safety and efficacy of parent/nurse-controlled analgesia in patients less than six years of age. Anesth Analg. 2000;91:573–579. doi: 10.1097/00000539-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 48.Murphy A, McCoy S, O’Reilly K, Fogarty E, Dietz J, Crispino G, Wakai A, O’Sullivan R. A prevalence and management study of acute pain in children attending emergency departments by Ambulance. Prehosp Emerg Care. 2016;20:52–58. doi: 10.3109/10903127.2015.1037478. [DOI] [PubMed] [Google Scholar]
- 49.O’Rourke PP, Crone RK, Vacanti JP, Ware JH, Lillehei CW, Parad RB, Epstein MF. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989;84:957–963. [PubMed] [Google Scholar]
- 50.Patel P, Mulla H, Tanna S, Pandya H. Facilitating pharmacokinetic studies in children: a new use of dried blood spots. Arch Dis Child. 2010;95:484–487. doi: 10.1136/adc.2009.177592. [DOI] [PubMed] [Google Scholar]
- 51.Pillai Riddell R, Racine NR, Turcotte K, Uman L, Horton R, Ahola Kohut S, Hillgrove Stuart J, Stevens BJ, Lisi DA. Non-pharmacological management of infant and young child procedural pain. Cochrane Database of Syst Rev. 2015;12:CD006275. doi: 10.1002/14651858.CD006275.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porter FL, Anand KJS. Epidemiology of pain in neonates. Res Clin Forums. 1998;20:9–16. [Google Scholar]
- 53.Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Annals Emerg Med. 2001;37:28–31. doi: 10.1067/mem.2001.111517. [DOI] [PubMed] [Google Scholar]
- 54.Regulation of the European Parliament and of the council on clinical trials on medicinal products for human use. 2012 Available at: http://ec.europa.eu/health/human-use/clinical-trials/index_en.htm Last accessed: 2 MAY 2016.
- 55.Robieux I, Kumar R, Radhakrishnan S, Koren G. Assessing pain and analgesia with a lidocaine-prilocaine emulsion in infants and toddlers during venipuncture. J Pediatr. 1991;118(6):971–973. doi: 10.1016/s0022-3476(05)82220-6. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberger WF. Randomized play-the-winner clinical trials: Review and recommendations. Control Clin Trials. 1999;20:328–342. doi: 10.1016/s0197-2456(99)00013-6. [DOI] [PubMed] [Google Scholar]
- 57.Rowbotham MC. What is a “clinically meaningful” reduction in pain? Pain. 2001;94:131–132. doi: 10.1016/S0304-3959(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 58.Schachtel BP, Thoden WR. A placebo-controlled model for assaying systemic analgesics in children. Clin Pharmacol Ther. 1993;53:593–601. doi: 10.1038/clpt.1993.75. [DOI] [PubMed] [Google Scholar]
- 59.Schiavenato M, Craig KD. Pain assessment as a social transaction: beyond the “gold standard”. Clin J Pain. 2010;26:667–676. doi: 10.1097/AJP.0b013e3181e72507. [DOI] [PubMed] [Google Scholar]
- 60.Shah V, Ipp M, Sam J, Einarson TR, Ohlsson A, Taddio A. Eliciting the minimal clinically important difference in the pain response from parents of newborn infants and nurses [abstract] Pediatr Res. 2004;55:519. [Google Scholar]
- 61.Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003;157:1058–1064. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- 62.Simons SH, van Dijk M, van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, Bunkers C, Smink E, Anand KJ, van den Anker JN, Tibboel D. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003;290:2419–2427. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 63.Singla N, Desjardins PJ, Chang PD. A comparison of the clinical and experimental characteristics of four acute surgical pain models: Dental extraction, bunionectomy, joint replacement, and soft tissue surgery. Pain. 2014;155:441–456. doi: 10.1016/j.pain.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. Neuroimage. 2010;52:583–589. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- 65.Smith SM, Wang AT, Katz NP, McDermott MP, Burke LP, Coplon P, Gilron I, Hertz SH, Lin AH, Rapport BA, Rowbotham MC, Sampaio C, Sweeney M, Turk DC, Dworkin RH. Adverse event assessment, analysis, and reporting in recent published analgesic clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154:997–1008. doi: 10.1016/j.pain.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Srikandarajah S, Gilron I. Systematic review of movement-evoked pain versus pain at rest in postsurgical clinical trials and meta-analyses: a fundamental distinction requiring standardized measurement. Pain. 2011;152:1734–1739. doi: 10.1016/j.pain.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 67.Stevens B, McGrath P, Gibbins S, Beyene J, Breau L, Camfield C, Finley A, Franck L, Howlett A, McKeever P, O’Brien K, Ohlsson A, Yamada J. Procedural pain in newborns at risk for neurologic impairment. Pain. 2003;105:27–35. doi: 10.1016/s0304-3959(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 68.Stinson J, Jibb L, Nguyen C, Nathan P, Maloney AM, Dupuis L, Gerstle T, Alman B, Hopyan S, Strahlendorf C, Portwine C, Johnston D. Development and Testing of a Multidimensional iPhone Pain Assessment Application for Adolescents with Cancer. J Med Internet Res. 2013;15:e51. doi: 10.2196/jmir.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stinson J, Napoleone M, Nguygen C, Yohannes L, Kazazian V, Chan B, Emanuele C, Luca S, Foster J. A 2014 update of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents: A systematic literature review. Canadian Pain Society; May 22, 2015. Poster. [Google Scholar]
- 70.Stinson J, Yamada J, Kavanagh T, Gill N, Stevens B. Systematic review of the psychometric properties and feasibility of self-report pain measures for use in clinical trials in children and adolescents. Pain. 2006;125:143–157. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Stinson JN, Jibb LA, Nguyen C, Nathan PC, Maloney AM, Dupuis LL, Gerstle JT, Hopyan S, Alman BA, Strahlendorf C, Portwine C, Johnston DL. Construct validity and reliability of a real-time multidimensional smartphone app to assess pain in children and adolescents with cancer. Pain. 2015;156:2607–2615. doi: 10.1097/j.pain.0000000000000385. [DOI] [PubMed] [Google Scholar]
- 72.St-Laurent-Gagnon T, Bernard-Bonnin AC, Villeneuve E. Pain evaluation in preschool children and by their parents. Acta Paediatr. 1999;88:422–427. doi: 10.1080/08035259950169819. [DOI] [PubMed] [Google Scholar]
- 73.Torgerson D. The use of Zelen’s design in randomised clinical trials. BJOG. 2004;111:2. doi: 10.1111/j.1471-0528.2004.00033.x. [DOI] [PubMed] [Google Scholar]
- 74.Tolusso D, Wang X. Some properties of the randomized play the winner rule. J Stat Theory Applicat. 2012;11:1–8. [Google Scholar]
- 75.Tracey I, Mantyh P. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 76.United States Department of Health and Human Services, FDA, CDER. Draft Guidance for Industry – Analgesic Indications: Developing Drug and Biological Products. 2014 Feb; Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm384691.pdf Last accessed: 8 FEB 2016.
- 77.United States Food and Drug Administration. Clinical trials and human subject protection. 2015 Available at: http://www.fda.gov/ScienceResearch/SpecialTopics/RunningClinicalTrials/ Last accessed: 2 MAY 2016.
- 78.United States Food and Drug Administration. Drug Research and Children. Available at: https://www.fda.gov/drugs/resourcesforyou/consumers/ucm143565.htm.
- 79.United States Food and Drug Administration. New Pediatric Labeling Information Database. Available at: https://www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingdatabase. Last accessed: 21 JUN 2017.
- 80.United States Food and Drug Administration. Pediatric Regulations. Available at: https://www.accessdata.fda.gov/scripts/cderworld/index.cfm?action=newdrugs:main&unit=4&lesson=1&topic=1. Last accessed: 27 JUL 2017.
- 81.United States Food and Drug Administration Center for Drug Evaluation and Research. Anesthetic and Analgesic Drug Products Advisory Committee, the Drug Safety and Risk Management Advisory Committee, and the Pediatric Advisory Committee Joint Meeting 15–16 SEP 2016 Available at: https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndAnalgesicDrugProductsAdvisoryCommittee/UCM524875.pdf Last accessed: 25 MAY 2017.
- 82.Van Cleve L, Johnson L, Pothier P. Pain responses of hospitalized infants and children to venipuncture and intravenous cannulation. J Pediatr Nurs. 1996;11(3):161–168. doi: 10.1016/S0882-5963(96)80049-2. [DOI] [PubMed] [Google Scholar]
- 83.Verriotis M, Fabrizi L, Lee A, Ledwidge S, Meek J, Fitzgerald M. Cortical activity evoked by inoculation needle prick in infants up to one-year. Pain. 2015;56:222–230. doi: 10.1097/01.j.pain.0000460302.56325.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Voepel-Lewis T, Burke CN, Jeffreys N, Malviya S, Tait AR. Do 0-10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg. 2011;112:415–421. doi: 10.1213/ANE.0b013e318203f495. [DOI] [PubMed] [Google Scholar]
- 85.von Baeyer CL, Spagrud LJ. Systematic review of observational (behavioral) measures of pain for children and adolescents aged 3 to 18 years. Pain. 2007;127:140–150. doi: 10.1016/j.pain.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 86.Walco GA, Cassidy RC, Schechter NL. Pain, hurt, and harm. The ethics of pain control in infants and children. N Engl J Med. 1994;331:541–544. doi: 10.1056/NEJM199408253310812. [DOI] [PubMed] [Google Scholar]
- 87.Walco GA, Krane EJ, Schmader KE, Weiner DK. Applying a life-span developmental perspective to chronic pain: pediatrics to geriatrics. J Pain. 2016;17(9 Suppl):T108–117. doi: 10.1016/j.jpain.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, Jadhav PR, Lala M, Gobburu JV. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J Clin Pharmacol. 2012;52:1601–1606. doi: 10.1177/0091270011422812. [DOI] [PubMed] [Google Scholar]
- 89.Wie LJ, Durham S. The randomized play-the-winner rule in medical trials. J Am Stat Asso. 1978;73:840–843. [Google Scholar]
- 90.World Medical Association. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. 2008 Available at: http://www.wma.net/en/30publications/10policies/b3/17c.pdf Last accessed: 1 MAY 2016.


