Abstract
The Hedgehog (Hh) pathway plays a central role in the development of multicellular organisms, guiding cell differentiation, proliferation and survival. While many components of the vertebrate pathway were discovered two decades ago, the mechanism by which the Hh signal is transmitted across the plasma membrane remains mysterious. This fundamental task in signalling is carried out by Smoothened (SMO), a human oncoprotein and validated cancer drug target that is a member of the G-protein coupled receptor protein family. Recent structural and functional studies have advanced our mechanistic understanding of SMO activation, revealing its unique regulation by two separable but allosterically-linked ligand-binding sites. Unexpectedly, these studies have nominated cellular cholesterol as having an instructive role in SMO signalling.
Introduction
The Hedgehog (Hh) signalling pathway is essential for embryogenesis and adult stem cell homeostasis in all bilaterians [1–3]. Its dysregulation is linked to developmental abnormalities and various types of cancers, including basal cell carcinoma (BCC) and medulloblastoma. Many tissues in the early embryo are patterned by gradients of morphogens, exemplified by ligands such as Hh in Drosophila and Sonic Hedgehog (SHH) in vertebrates. Local concentrations of such morphogens are interpreted by target cells to drive cell fate decisions, ultimately forming the basis for a body plan.
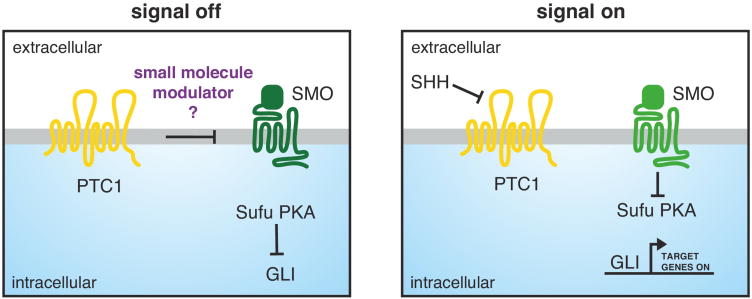
In Hh-producing cells, a precursor form of a secreted Hh ligand (e.g. SHH) is expressed and auto-catalytically cleaved into a doubly lipidated N-terminal domain to produce the mature morphogen [1–3]. Hh ligands are received on target cells by the twelve-pass transmem-brane (TM) protein Patched 1 (PTC1), in cooperation with other co-receptors that can modify ligand reception (for details see [4–6]). A unique feature of the Hh signalling cascade is that ligand reception and signal transduc-tion across the plasma membrane have been assigned to two different membrane proteins, PTC1 and Smooth-ened (SMO). SHH binding inactivatesPTC1, thereby relieving its constitutive inhibition of the G-protein coupled receptor (GPCR) SMO (Figure 1). SMO activation is the critical step that transmits the Hh signal across the membrane to the cytoplasm, ultimately resulting in the activation of the glioma-associated oncogene family members (GLI) transcription factors [1,2]. This separation of function requires that the Hh signal must be relayed from PTC1 to SMO, a mysterious step in signalling that is thought to be mediated by a small molecule second messenger [7–9] (Figure 1).
Figure 1.
PTC1 regulates SMO via an unknown mechanism. In the absence of SHH, PTC1 inhibits SMO, which allows Sufu and PKA to inhibit the GLI transcription factors (left panel). In the presence of SHH, PTC1 releases its inhibition of SMO, which in turn is able to signal downstream, ultimately resulting in the transcription of target genes by GLI (right panel).
SMO has been the focus of intense study because it is required for transmembrane signalling in all animals and because it has become a validated drug target for Hh-driven human cancers [3,10]. This review will discuss recent advances and enduring puzzles related to the mechanisms of SMO signal transduction, with a focus on the recent structural characterization of SMO and its regulation by various small molecules.
Architecture of Smoothened
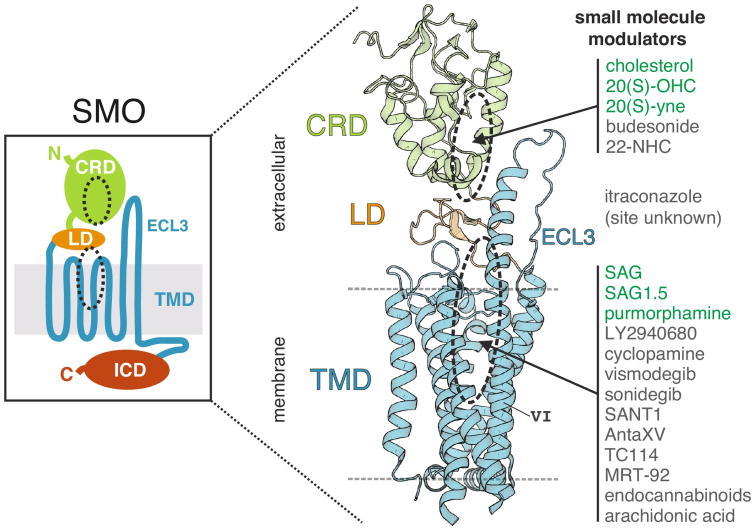
SMO is a Frizzled-class GPCR. Unlike the ‘classical’ Class A GPCRs, SMO contains not just the stereotypical seven-pass α-helical transmembrane bundle (TMD), but also sizeable extracellular and intracellular domains (Figure 2, left panel). The extracellular region of SMO consists primarily of a cysteine-rich domain (CRD), named for its conserved disulphide bonding architecture, which has homologs in the Frizzled receptors (Fzd), Niemann–Pick type-C protein 1 (NPC1), and ribofla-vin-binding protein [11]. The CRD is connected to the TMD through a short linker domain (LD), itself containing a disulphide bond. The intracellular domain of SMO is partly unstructured and has been shown to be important for SMO localisation in primary cilia and subsequent downstream signalling.
Figure 2.
The overall structure of SMO. SMO consists of a large extracellular region, made up of the CRD (green) and LD (orange), and an intracellular domain (ICD, red) in addition to the seven-pass α-helical transmembrane domain (TMD, blue) (left panel). The multi-domain SMO crystal structure revealed a stacked domain arrangement with two physically separable binding sites (right panel). The approximate location of the two binding sites is marked with dashed black ovals in both left and right panels. ECL3 and TMD helix VI are also labeled. Agonist (green) and antagonist (dark grey) small molecule modulators are listed on the right and associated with a particular binding site, if known. This list is not exhaustive.
While structures of the isolated CRD and TMD of SMO have been previously reported and reviewed [12–16], more recent structural studies of a multi-domain SMO protein have revealed the critical interactions between these domains and how they may be modulated by small molecules [17••,18••]. These new structures show a stacked domain arrangement with the CRD perched atop the TMD with an intervening wedged LD. The extracellular extension of TMD helix VI, part of extracellular loop 3 (ECL3), enables the TMD to interact directly with the CRD (Figure 2). This arrangement suggests that signal transmission from the CRD to the TMD is mediated by the LD and ECL3. Contacts between the CRD, LD and TMD seem to be critical for stabilizing the inactive state of vertebrate SMO; complete deletion of the CRD increases the constitutive signalling activity of SMO, as do mutations designed to destabilize the LD or contacts between the LD and the CRD [17••].
Several limitations of these recently reported structures are worth highlighting. First, the SMO structures solved to date do not give any insights into TMD conformational changes associated with activation or insights into how SMO transmits signals to the cytoplasm. Indeed the TMD domains in the SMO structures almost look identical, regardless of whether they are bound to antagonists or agonists [17••,18••,19]. The multi-domain SMO proteins used in these recent structural studies are incapable of coupling to cytoplasmic signalling components for at least three reasons: they lack the C-terminal tail, they contain stabilizing heterologous proteins inserted into the third intracellular loop (critical for the signalling function of many GPCRs) and their TMDs are locked in an inactive state by either mutations or high-potency antagonists.
Small molecule modulators of Smoothened
Unlike many GPCRs, SMO can be activated or inhibited through at least two ligand-binding sites (reviewed in [15]). The first binding site (hereafter the ‘TMD site’), which corresponds to the canonical ‘orthosteric’ ligand-binding site in many GPCRs, is located at the extracellular end of the transmembrane bundle (Figure 2, right panel). The TMD site was first shown to engage the SMO antagonist cyclopamine [20] and subsequently shown to bind to multiple small molecule agonists and antagonists, including the two high-potency inhibitors vismodegib and sonidegib used to treat advanced BCC in the clinic [3,15,21–27]. Pioneering structures of the isolated TMD showed that TMD ligands bind at various depths within a vestibule at the extracellular end of the TMD and revealed how mutations in residues lining this vestibule can lead to resistance to SMO antagonists used in the clinic [12,19]. The second site, formed by a shallow hydrophobic groove on the surface of the CRD, can bind and mediate the effects of oxysterols and cholesterol on SMO activity [13,17••,28,29,30,31••,32••] (Figure 2, right panel).
While these two ligand-binding sites are housed in distinct, physically separable domains, early pharmacological studies suggested that they were allosterically linked [28]. A structural basis for this communication was revealed by multi-domain crystal structures of SMO bound to cholesterol in the CRD site or two high-potency antagonists, vismodegib or TC114, in the TMD site [17••,18••]. Cholesterol was recently shown to be a direct SMO agonist that can bind to the CRD in solution and is sufficient to activate signalling even in the absence of Hh ligands [31••,32••]. In the multi-domain structure, the cholesterol molecule fills the hydrophobic CRD binding groove, forming essential interactions with several residues including Asp95 and Trp109, and is partially shielded from the solvent by ECL3 (Figure 3a) [17••]. The CRD binding site and the cholesterol molecule within it are positioned at the intersection of the CRD, LD and TMD, ideally located to mediate interactions between the three domains that could regulate SMO activity (Figure 3b, left panel). A recent study suggested that the 3b-hydroxyl of cholesterol can form a covalent ester bond with Asp95 [33••], though this was not evident in the high-resolution structure [17••]. While the CRD and TMD binding sites are separated by ∼12 Å, vismodegib and TC114 binding to the TMD alters the orientation of the CRD, LD and ECL3 relative to the TMD, resulting in displacement of cholesterol from the CRD ligand-binding groove [17••,18••](Figure 3b, middle and right panels, Figure 3c). The CRD binding site is occluded by a sugar moiety in the vismodegib-bound structure or by sidechains of several residues from ECL3 in the TC114-bound structure (Figure 3b). Thus, the observed allosteric linkage between the two sites is likely to be facilitated by structural communication mediated by ECL3 and the LD [17••,28] (Figure 3c).
Figure 3.
Multi-domain structures of SMO. (a) Close-up of the cholesterol binding site in the SMO CRD. Residues involved in binding are shown as sticks. Dotted black lines indicate potential hydrogen bonds. Two important residues also discussed in the text are labeled (Asp95 and Trp109). (b) Three multi-domain structures of human SMO, each solved with a different ligand (as indicated beneath each structure), are shown in the same orientation (PDB: 5L7D [17••], 5L7I [17••], 5V57 [18••]). The glycan occluding the CRD-site in the vismodegib complex (middle) is shown in yellow stick representation. Domains in (a) and (b) are coloured as in Figure 2. (c) Conformational changes associated with antagonist binding result in collapse of the CRD binding site, thus precluding cholesterol binding. The three multi-domain structures of SMO were aligned by their TMDs. Each structure is coloured separately with helices shown as solid cylinders and loops omitted for clarity. Red arrows indicate domain movements between structures.
In addition to the TMD and CRD sites noted above, the C-terminal tail of SMO has been proposed to mediate the potentiating effect of phosphatidylinositol 4-phosphate (PI(4)P) on SMO activity, but we lack any structural information on the C-tail or pharmacological information on how this interaction might influence the CRD and TMD ligand binding sites [34,35].
Ligand-binding sites that regulate endogenous SMO signalling
Much of our understanding of SMO function described above comes from studies of signalling in response to exogenously added ligands. But what are the endogenous ligands that regulate SMO activity and do they act through the CRD site, the TMD site or a yet undiscovered third site? Both the CRD and TMD sites have been subjected to mutagenesis to address this question. Mutations in the CRD site can impair signalling, not just in response to oxysterols and cholesterol, but also in response to SHH (which does not act directly on SMO but rather indirectly through PTC1 (Figure 1)) [31••,32••]. The importance of the CRD-site for endogenous signalling was highlighted by the demonstration that a point mutation in Asp95 (which makes a critical hydrogen bond with cholesterol in the SMO structure (Figure 3a)) impairs mouse embryonic development when knocked into the endogenous smo locus [33••], closely phenocopying a null smo allele. In addition, a complete deletion of the CRD (SMO-ΔCRD) or more subtle mutations that disrupt CRD-LD interactions, resulted in SMO molecules with elevated, ligand-inde-pendent constitutive activity [17••,29,30]. SMO-ΔCRD is markedly less sensitive to PTC1 and nearly fully activated, consistent with the model that the CRD site plays an important role in mediating the inhibitory influence of PTC1 on SMO [32••].
In contrast to CRD mutations, several mutations introduced into the TMD site failed to alter either basal or SHH-regulated SMO activity in cultured cells, even though they abrogated the effects of synthetic TMD ligands [36,37]. Thus, the TMD site in SMO that corresponds to the main regulatory site in other GPCRs, may only play a modulatory role in endogenous signalling, despite the fact that it has been critical for therapeutic targeting of SMO. However, there is some evidence that a different site within the TMD may regulate SMO activity in response to Hh ligands. The constitutive signalling activity of SMO-ΔCRD can be suppressed by the transient co-expression of PTC1, suggesting that, at least when over-expressed, PTC1 can inhibit SMO by a mechanism that does not require the CRD [29]. Another hint that a third, undiscovered site exists also comes from the observation that the SMO antagonist itraconazole does not compete for binding with either CRD or TMD ligands [38].
Cholesterol as an endogenous regulator of SMO signalling
Cholesterol, an abundant component of vertebrate cell membranes, is both necessary and sufficient to activate SMO signalling (summarized in [32••]). A permissive function for cholesterol was first suggested by the observation that cellular cholesterol depletion or drugs that impair intracellular cholesterol transport reduce Hh signalling [39–42]). In addition, humans with mutations in genes encoding enzymes of distal cholesterol biosynthesis, such as Smith-Lemli-Opitz syndrome (SLOS), have Hh-related developmental defects [43,44]. There is uncertainty about whether impaired signalling in SLOS is due to a cholesterol deficit or due to accumulation of an inhibitory precursor derived from 7-dehydrocholesterol (7-DHC), the substrate for the enzyme mutated in SLOS, 7-dehydrocholesterol reductase (7-DHCR) [45]. The cholesterol deficiency model is more likely because signalling can be rescued by cholesterol addition in cells carrying 7-DHCR disease mutants [46•] and defects in enzymes earlier in the cholesterol biosynthesis cascade, which do not lead to elevated 7-DHC, also impair Hh signalling in mice [47].
The permissive role of cholesterol has been attributed to the TMD rather than the CRD, because the constitutive signalling activity of SMO-ΔCRD can be reduced by cholesterol depletion [29], and because a SMO protein carrying a mutation in the CRD sterol-binding site remains sensitive to 7-DHCR inhibitors [46•]. Indeed, many transmembrane receptors, including GPCRs (reviewed in [48]), require interactions with membrane cholesterol around their TM domains for proper function.
While cholesterol is required for signalling by many receptors, the role of cholesterol in SMO signalling is distinguished by the fact that it is sufficient to activate the signalling cascade in the absence of native Hh ligands [31••,32••]. This effect, which suggests an instructive rather than a purely permissive role, is mediated by the structurally-defined binding site in the CRD, located nearly ∼12 Å away from the lipid bilayer (Figure 3a and b). While exogenous cholesterol activates SMO through the CRD, definitive evidence that endogenous cellular cholesterol (rather than a different lipidic ligand) engages the SMO CRD site in cells is still incomplete. A plausible alternative is that oxysterols are the physiologically-relevant CRD ligands — exogenous cholesterol activates SMO after it is metabolized to oxysterols. Two observations have pointed to cholesterol as the endogenous CRD ligand. First, fluorinated cholesterol derivatives that cannot be metabolized to oxysterols can still activate signalling [31••]. Second, mutations in the sterol-binding groove that impair activation by oxysterols, but not by cholesterol, have little effect on SHH-driven signalling (while mutations that block cholesterol-induced activation also impair SHH-induced activation) [31••,32••].
Taken together, the above evidence has nominated cholesterol as an endogenous, physiologically-relevant regulator of SMO function and led to the hypothesis that PTC1 regulates SMO by preventing its access to cholesterol [31••,32••]. Indeed, SHH and cholesterol can activate Hh signalling in a synergistic fashion [31••,32••]. This model is also consistent with the observation that PTC1 has homology to the Niemann-–Pick C1 (NPC1) cholesterol transporter in lysosomes [49] and has been shown to be capable of both binding and transporting cholesterol [50]. The additional homology of PTC1 to RND-family transporters suggests that it may be able to use transmembrane ion gradients to actively transport cholesterol, even against a concentration gradient [7].
An unresolved question is whether PTC1 regulates cholesterol access to the CRD, the TMD, or both. Covalent labeling of the CRD ligand-binding site by cholesterol can be reduced by PTC1 in a SHH-regulated fashion, consistent with the idea that PTC1 can regulate cholesterol access to the CRD [33••]. As mentioned above, mutations in the CRD that block cholesterol binding also reduce SMO signalling in both cells and animals. The case that PTC1 may also regulate access of the TMD to cholesterol is based on the observation that PTC1 over-expression can inhibit the high constitutive activity of SMO-ΔCRD [29].
How might PTC1 prevent SMO access to cholesterol, especially given that cholesterol is such an abundant lipid? Biochemically, PTC1 could change the levels of accessible (or chemically active) cholesterol, which is known to regulate its interaction with proteins and is distinct from the tightly-bound pool that plays a structural role in lipid bilayers [51] (Figure 4a and b). Alternatively, PTC1 could function as a lipid flippase that changes the distribution of cholesterol between the two leaflets of the plasma membrane, an activity analogous to the inferred ability of NPC1 to flip cholesterol from the luminal to the cytoplasmic leaflet of the lysosomal membrane (Figure 4c).
Figure 4.
Models for PTC1 function. (a) In the first model, the SMO TMD is constitutively associated with cholesterol but PTC1 prevents the SMO CRD from accessing cholesterol, thereby preventing activation. Upon SHH-binding, PTC1 is inactivated, allowing the CRD to acquire cholesterol and to become activated. (b) In the second model, the SMO CRD contains cholesterol as a necessary co-factor and PTC1 prevents the SMO TMD from accessing cholesterol, thereby preventing activation. Upon SHH-binding, PTC1 is inactivated, allowing the SMO TMD to acquire cholesterol and become activated. (c) In the third model, a variant of the first, PTC1 acts as a cholesterol flippase, shifting cholesterol from the outer to the inner leaflet of the lipid bilayer and thereby preventing the SMO CRD from accessing cholesterol. These models are not mutually exclusive and PTC1 could regulate cholesterol access to both sites. (d) Cholesterol acts as a co-factor for SMO activation, while PTC1 regulates a different lipidic ligand (solid square) which could either function as a SMO antagonist as shown here or as a SMO agonist.
An important caveat to the above discussion is that changes in either local or global cholesterol levels or cholesterol distribution in response to Hh signalling have not yet been demonstrated in an endogenous signalling context. Thus, we cannot rule out the possibility that cholesterol functions as a constitutive co-factor or allosteric regulator of SMO activity, required for SMO to adopt a fully active conformation. In this scenario PTC1 would inhibit SMO through a different small molecule regulator (Figure 4d).
Conclusions and perspectives
The crucial processes of embryonic development and regenerative responses depend upon proper functioning of the Hh signalling pathway. Recent multi-domain structures of SMO have cast a new light on the role of the extracellular domains of SMO and the allosteric interaction between its two defined ligand-binding sites. Functional studies have nominated cholesterol as an endogenous instructive modulator of SMO, mediating the critical regulatory interaction between SMO and the receptor for Hh ligands, PTC1. These insights have advanced our understanding of this key developmental signalling system, suggested strategies to overcome clinically-significant resistance to anti-SMO drugs, and, more generally, suggested the possibility that cholesterol may be used more broadly as a lipid second messenger in signalling systems.
Several important questions remain for future research. Further structural studies will be required to understand how the SMO TMD adopts an active conformation and how it subsequently communicates with candidate downstream regulators, such as GPR161 [52] or hetero-trimeric G-proteins [53,54], to eventually influence GLI activity. Understanding the mechanism by which cholesterol gains access to the CRD site, perched ∼12 Å above the membrane, will also require further studies, since a cholesterol molecule would have to completely desorb from the membrane to gain access to this site. Finally, since the regulation of SMO by PTC1 in vertebrates is thought to be orchestrated at primary cilia or associated membranes, it will be important to test whether changes in sterol lipids or other endogenous SMO regulators are compartmentalized to cilia-associated membrane compartments.
Acknowledgments
This work was supported by Cancer Research UK (C20724/A14414), the European Research Council under the European Union's Horizon 2020 Research and Innovation Programme Grant 647278, the US National Institutes of Health (GM106078, GM105448 and GM118082) and Wellcome Trust (102890/Z/13/Z, 092970/Z/10/Z and 090532/Z/09/Z). Further support by NDM Oxford (E.F.X.B.) and the Ford Foundation (G.L.) is acknowledged.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ingham PW, Nakano Y, Seger C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet. 2011;12:393–406. doi: 10.1038/nrg2984. [DOI] [PubMed] [Google Scholar]
- 2.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 3.Wu F, Zhang Y, Sun B, McMahon AP, Wang Y. Hedgehog signaling: from basic biology to cancer therapy. Cell Chem Biol. 2017;24:252–280. doi: 10.1016/j.chembiol.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24:2001–2012. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen BL, Song JY, Izzi L, Althaus IW, Kang JS, Charron F, Krauss RS, McMahon AP. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell. 2011;20:775–787. doi: 10.1016/j.devcel.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izzi L, Levesque M, Morin S, Laniel D, Wilkes BC, Mille F, Krauss RS, McMahon AP, Allen BL, Charron F. Boc and Gas1 each form distinct Shh receptor complexes with Ptch1 and are required for Shh-mediated cell proliferation. Dev Cell. 2011;20:788–801. doi: 10.1016/j.devcel.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 8.Denef N, Neubuser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and Smoothened. Cell. 2000;102:521–531. doi: 10.1016/s0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 9.Ingham PW, Nystedt S, Nakano Y, Brown W, Stark D, van den Heuvel M, Taylor AM. Patched represses the Hedgehog signalling pathway by promoting modification of the Smoothened protein. Curr Biol. 2000;10:1315–1318. doi: 10.1016/s0960-9822(00)00755-7. [DOI] [PubMed] [Google Scholar]
- 10.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 11.Bazan JF, de Sauvage FJ. Structural ties between cholesterol transport and morphogen signaling. Cell. 2009;138:1055–1056. doi: 10.1016/j.cell.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Wu H, Katritch V, Han GW, Huang XP, Liu W, Siu FY, Roth BL, Cherezov V, Stevens RC. Structure of the human Smoothened receptor bound to an antitumour agent. Nature. 2013;497:338–343. doi: 10.1038/nature12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachtergaele S, Whalen DM, Mydock LK, Zhao Z, Malinauskas T, Krishnan K, Ingham PW, Covey DF, Siebold C, Rohatgi R. Structure and function of the Smoothened extracellular domain invertebrate Hedgehog signaling. Elife. 2013;2:e01340. doi: 10.7554/eLife.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rana R, Carroll CE, Lee HJ, Bao J, Marada S, Grace CR, Guibao CD, Ogden SK, Zheng JJ. Structural insights into the role of the Smoothened cysteine-rich domain in Hedgehog signalling. Nat Commun. 2013;4:2965. doi: 10.1038/ncomms3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharpe HJ, Wang W, Hannoush RN, de Sauvage FJ. Regulation of the oncoprotein Smoothened by small molecules. Nat Chem Biol. 2015;11:246–255. doi: 10.1038/nchembio.1776. [DOI] [PubMed] [Google Scholar]
- 16.Weierstall U, James D, Wang C, White TA, Wang D, Liu W, Spence JC, Bruce Doak R, Nelson G, Fromme P, et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat Commun. 2014;5:3309. doi: 10.1038/ncomms4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Byrne EF, Sircar R, Miller PS, Hedger G, Luchetti G, Nachtergaele S, Tully MD, Mydock-McGrane L, Covey DF, Rambo RP, et al. Structural basis of Smoothened regulation by its extracellular domains. Nature. 2016;535:517–522. doi: 10.1038/nature18934. This study describes multi-domain structures of SMO, which represent the first high-resolution views of a G protein coupled receptor with a large extracellular domain. These structures, with accompanying functional analyses, demonstrated the interaction of cholesterol with the SMO extracellular domain, revealed how structural communication between the extracellular and transmembrane domains can regulate signalling, and provided a structural explanation for SMO mutations that cause resistance to a clinically-used anti-cancer drug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Zhang X, Zhao F, Wu Y, Yang J, Han GW, Zhao S, Ishchenko A, Ye L, Lin X, Ding K, et al. Crystal structure of a multi-domain human Smoothened receptor in complex with a super stabilizing ligand. Nat Commun. 2017;8:15383. doi: 10.1038/ncomms15383. The authors presented the crystal structure of a multi-domain human SMO protein, bound and stabilized by the designed ligand TC114. By combining structural analysis with molecular dynamics simulations and deuterium exchange experiments, they identified key structural elements in SMO — the TMD helix VI and the ECL3 loop — that are important for communication between the extracellular and transmembrane domains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Wu H, Evron T, Vardy E, Han GW, Huang XP, Hufeisen SJ, Mangano TJ, Urban DJ, Katritch V, et al. Structural basis for Smoothened receptor modulation and chemoresistance to anticancer drugs. Nat Commun. 2014;5:4355. doi: 10.1038/ncomms5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, et al. Small-molecule modulators of Hedgehog signaling: identification and characterization of Smoothened agonists and antagonists. J Biol. 2002;1:10. doi: 10.1186/1475-4924-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, Gould SE, Guichert O, Gunzner JL, Halladay J, et al. GDC-0449 — a potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. 2009;19:5576–5581. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 24.Pan S, Wu X, Jiang J, Gao W, Wan Y, Cheng D, Han D, Liu J, Englund NP, Wang Y, et al. Discovery of NVP-LDE225, a potent and selective Smoothened antagonist. ACS Med Chem Lett. 2010;1:130–134. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaliullina H, Bilgin M, Sampaio JL, Shevchenko A, Eaton S. Endocannabinoids are conserved inhibitors of the Hedgehog pathway. Proc Natl Acad Sci USA. 2015;112:3415–3420. doi: 10.1073/pnas.1416463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arensdorf AM, Dillard ME, Menke JM, Frank MW, Rock CO, Ogden SK. Sonic Hedgehog activates phospholipase A2 to enhance Smoothened ciliary translocation. Cell Rep. 2017;19:2074–2087. doi: 10.1016/j.celrep.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Davidow L, Arvanites AC, Blanchard J, Lam K, Xu K, Oza V, Yoo JW, Ng JM, Curran T, et al. Glucocorticoid compounds modify Smoothened localization and hedgehog pathway activity. Chem Biol. 2012;19:972–982. doi: 10.1016/j.chembiol.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myers BR, Sever N, Chong YC, Kim J, Belani JD, Rychnovsky S, Bazan JF, Beachy PA. Hedgehog pathway modulation by multiple lipid binding sites on the Smoothened effector of signal response. Dev Cell. 2013;26:346–357. doi: 10.1016/j.devcel.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nedelcu D, Liu J, Xu Y, Jao C, Salic A. Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat Chem Biol. 2013;9:557–564. doi: 10.1038/nchembio.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Huang P, Nedelcu D, Watanabe M, Jao C, Kim Y, Liu J, Salic A. Cellular cholesterol directly activates Smoothened in hedgehog signaling. Cell. 2016;166:1176–1187.e1114. doi: 10.1016/j.cell.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Luchetti G, Sircar R, Kong JH, Nachtergaele S, Sagner A, Byrne EF, Covey DF, Siebold C, Rohatgi R. Cholesterol activates the G-protein coupled receptor Smoothened to promote Hedgehog signaling. Elife. 2016;5:e20304. doi: 10.7554/eLife.20304. Both studies from Huang et al. and Luchetti et al. showed that cholesterol is sufficient to activate Hh signalling in fibroblasts and neural precursor cells in the absence of native Hh ligands. Mutations in the SMO cysteine-rich domain (CRD) that prevent cholesterol binding also prevent signalling by native Hh ligands, showing that the CRD-cholesterol interaction is important for endogenous signaling. Using mutagenesis, both studies provided evidence that the endogenous CRD ligand is likely to be cholesterol, not oxysterols as previously proposed. Huang et al. also presented high-resolution crystal structures of the isolated Xenopus SMO CRD in complex with an activating oxysterol and with cyclopamine, a SMO inhibitor that binds to both the CRD and TMD sites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Xiao X, Tang JJ, Peng C, Wang Y, Fu L, Qiu ZP, Xiong Y, Yang LF, Cui HW, He XL, et al. Cholesterol modification of Smoothened is required for Hedgehog signaling. Mol Cell. 2017;66:154–162.e110. doi: 10.1016/j.molcel.2017.02.015. The authors presented the unexpected observation that cholesterol can form a covalent ester bond between its 3′-hydroxyl and Asp95 in the sterol-binding groove of the SMO CRD and that PTC1 can inhibit the formation of this SMO-cholesterol adduct. An Asp95N mutation impairs embryonic development in a mouse, pheno-copying a null allele of SMO and highlighting the importance of the CRD sterol-binding groove in the context of an intact animal. [DOI] [PubMed] [Google Scholar]
- 34.Yavari A, Nagaraj R, Owusu-Ansah E, Folick A, Ngo K, Hillman T, Call G, Rohatgi R, Scott MP, Banerjee U. Role of lipid metabolism in Smoothened derepression in hedgehog signaling. Dev Cell. 2010;19:54–65. doi: 10.1016/j.devcel.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang K, Liu Y, Fan J, Zhang J, Li XA, Evers BM, Zhu H, Jia J. PI(4)p promotes phosphorylation and conformational change of Smoothened through interaction with its C-terminal tail. PLoS Biol. 2016;14:e1002375. doi: 10.1371/journal.pbio.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dijkgraaf GJ, Alicke B, Weinmann L, Januario T, West K, Modrusan Z, Burdick D, Goldsmith R, Robarge K, Sutherlin D, et al. Small molecule inhibition of GDC-0449 refractory Smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71:435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 37.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388–399. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- 40.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 41.Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits Sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 42.Incardona JP, Gaffield W, Lange Y, Cooney A, Pentchev PG, Liu S, Watson JA, Kapur RP, Roelink H. Cyclopamine inhibition of Sonic hedgehog signal transduction is not mediated through effects on cholesterol transport. Dev Biol. 2000;224:440–452. doi: 10.1006/dbio.2000.9775. [DOI] [PubMed] [Google Scholar]
- 43.Kelley RL, Roessler E, Hennekam RC, Feldman GL, Kosaki K, Jones MC, Palumbos JC, Muenke M. Holoprosencephaly in RSH/Smith-Lemli-Opitz syndrome: does abnormal cholesterol metabolism affect the function of Sonic Hedgehog? Am J Med Genet. 1996;66:478–484. doi: 10.1002/(SICI)1096-8628(19961230)66:4<478::AID-AJMG22>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 44.Lanoue L, Dehart DB, Hinsdale ME, Maeda N, Tint GS, Sulik KK. Limb, genital, CNS, and facial malformations result from gene/environment-induced cholesterol deficiency: further evidence for a link to sonic hedgehog. Am J Med Genet. 1997;73:24–31. [PubMed] [Google Scholar]
- 45.Sever N, Mann RK, Xu L, Snell WJ, Hernandez-Lara CI, Porter NA, Beachy PA. Endogenous B-ring oxysterols inhibit the Hedgehog component Smoothened in a manner distinct from cyclopamine or side-chain oxysterols. Proc Natl Acad Sci U S A. 2016;113:5904–5909. doi: 10.1073/pnas.1604984113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Blassberg R, Macrae JI, Briscoe J, Jacob J. Reduced cholesterol levels impair Smoothened activation in Smith-Lemli-Opitz syndrome. Hum Mol Genet. 2015 doi: 10.1093/hmg/ddv507. By studying an in vitro model for the autosomal-recessive disorder Smith-Lemli-Opitz syndrome (SLOS), the authors showed that a deficit in cholesterol, rather than the accumulation of an inhibitor precursor sterol, is likely to impair SMO activation and its localization to the primary cilium in this disease. This study suggested that endogenous cholesterol levels or distribution may regulate SMO activation in cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunningham D, DeBarber AE, Bir N, Binkley L, Merkens LS, Steiner RD, Herman GE. Analysis of hedgehog signaling in cerebellar granule cell precursors in a conditional Nsdhl allele demonstrates an essential role for cholesterol in postnatal CNS development. Hum Mol Genet. 2015;24:2808–2825. doi: 10.1093/hmg/ddv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gimpl G. Interaction of G protein coupled receptors and cholesterol. Chem Phys Lipids. 2016;199:61–73. doi: 10.1016/j.chemphyslip.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 50.Bidet M, Joubert O, Lacombe B, Ciantar M, Nehme R, Mollat P, Bretillon L, Faure H, Bittman R, Ruat M, et al. The hedgehog receptor patched is involved in cholesterol transport. PLoS ONE. 2011;6:e23834. doi: 10.1371/journal.pone.0023834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das A, Brown MS, Anderson DD, Goldstein JL, Radhakrishnan A. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. Elife. 2014;3 doi: 10.7554/eLife.02882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 53.DeCamp DL, Thompson TM, de Sauvage FJ, Lerner MR. Smoothened activates Galphai-mediated signaling in frog melanophores. J Biol Chem. 2000;275:26322–26327. doi: 10.1074/jbc.M004055200. [DOI] [PubMed] [Google Scholar]
- 54.Riobo NA, Saucy B, Dilizio C, Manning DR. Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci U SA. 2006;103:12607–12612. doi: 10.1073/pnas.0600880103. [DOI] [PMC free article] [PubMed] [Google Scholar]