Abstract
Objective
This study investigated symptom dimensions and subgroups in the National Institute of Mental Health (NIMH) childhood-onset schizophrenia (COS) cohort and their similarities to adult-onset schizophrenia (AOS) literature.
Method
Scores from the Scales for the Assessment of Positive and Negative Symptoms (SAPS & SANS) from 125 COS patients were assessed for fit with previously established symptom dimensions from AOS literature using confirmatory factor analysis (CFA). K-means cluster analysis of each individual’s scores on the best fitting set of dimensions was used to form patient clusters, which were then compared using demographic and clinical data.
Results
CFA showed the SAPS & SANS data was well suited to a 2-dimension solution, including positive and negative dimensions, out of five well established models. Cluster analysis identified three patient groups characterized by different dimension scores: (1) low scores on both dimensions, (2) high negative, low positive scores, and (3) high scores on both dimensions. These groups had different Full Scale IQ, Children’s Global Assessment Scale (CGAS) scores, ages of onset, and prevalence of some co-morbid behavior disorders (all p<3.57E-03).
Conclusion
Our analysis found distinct symptom-based subgroups within the NIMH COS cohort using an established AOS symptom structure. These findings confirm the heterogeneity of COS and were generally consistent with AOS literature.
Keywords: subgroups, symptomatology, childhood-onset schizophrenia, symptom dimensions
1.1 Introduction
Methods of categorizing the symptoms of schizophrenia and subgrouping schizophrenic patients into homogenous groups have long been studied (Adityanjee et al., 1999; Crow, 1985). More recently, research has shifted from rigid categories towards symptom dimensions and novel subtyping methods to better define and treat this heterogeneous disease (Andreasen and Carpenter, 1993; Bleich-Cohen et al., 2014; Carpenter et al., 1988; Harvey et al., 2016; Reininghaus et al., 2013). Childhood-onset schizophrenia (COS), defined by onset before the 13th birthday, is a rare and more severe version of the adult disorder in which symptom dimensions and subtypes have not been examined (Nicolson and Rapoport, 1999).
There is some consensus in adult-onset schizophrenia (AOS) regarding the broad themes of schizophrenia symptom dimensions, with many studies reporting at least a positive, negative, and disorganized dimension (Dazzi et al., 2016; Peralta and Cuesta, 2001; von Knorring and Lindstrom, 1992; Wallwork et al., 2012). Scale for the Assessment of Positive and Negative Symptoms (SAPS & SANS) studies find between 2 and 4, but commonly three, dimensions (Andreasen, 1995; Crow, 1985; Cuesta et al., 1994; Lewine et al., 1983; Mortimer et al., 1990; Peralta et al., 1994; Peralta and Cuesta, 2001), while studies using the Positive and Negative Symptom Scale (PANSS)(Kay et al., 1987; Wallwork et al., 2012) or the Brief Psychiatric Rating Scale (BPRS)(Overall, 1962) most commonly identify 4 or 5-factor solutions (Dazzi et al., 2016; Mueser et al., 1997; Salokangas et al., 2002; Wallwork et al., 2012). Notably, despite common themes, the items included and variance explained differed between studies, even those using the same scales (Dollfus and Everitt, 1998; Peralta et al., 1994; Peralta and Cuesta, 2001; Potuzak et al., 2012). In studies with additional factors, paranoid, depressive, and hostile/excitement factors were often reported (Peralta and Cuesta, 2001; Wallwork et al., 2012). Studies investigating symptom dimensions are valuable as they provide insight into schizophrenia symptomatology and offer novel framework to explore the relationship between symptoms and clinical, biological, and treatment data (Colasanti et al., 2010; Collin et al., 2012; Docherty et al., 2015; Salokangas et al., 2002; Viher et al., 2016).
Very few studies have examined symptom dimension in early-onset schizophrenia (EOS), defined as onset before 18 (Banaschewski et al., 2000; Bunk et al., 1999; Maziade et al., 1996b, 1996a; McClellan et al., 2002). Generally, these studies reported positive and negative dimensions, with some including two or three additional factors, although a disorganization dimension was not consistently reported. Two studies examined the stability of EOS dimensions over time and found that although EOS results were relatively similar to adult onset schizophrenia (AOS) findings, the dimensions more closely resembled AOS studies when EOS patients were re-examined in adulthood (Bunk et al., 1999; Maziade et al., 1996a). These studies suggest that while the general themes of dimensions are similar between EOS and AOS, there are differences, mainly less clarity regarding a disorganized dimension.
Recent studies in adults have also investigated novel methods of subtyping schizophrenia, including groups based on imaging (Bleich-Cohen et al., 2014), cognitive (Rangel et al., 2015), biological (Chien et al., 2015), genetic (Boks et al., 2008), and symptom data (Voineskos et al., 2013) to better understand the heterogeneous disease. One method for investigating symptom based groups is through cluster analysis of clinical scale scores or subscale scores (Dickinson, 2017; Dollfus et al., 1996; Lastra et al., 2000; Morrison et al., 1990). Studies using SAPS, SANS, or PANSS show some consistency in the broad themes of subgroups, often deriving a “deficit” or severe negative symptom group, a low symptom group, and other groups with mixed negative and positive symptoms (Dollfus et al., 1996; Jackson et al., 1989; Lastra et al., 2000; Morrison et al., 1990; Williams, 1996). Fewer studies have additionally identified groups specifically characterized by positive symptoms (Lastra et al., 2000) or disorganized symptoms (Dollfus et al., 1996). Although diagnostic subtypes are no longer used, new approaches to subtyping offer means of attacking the heterogeneity of schizophrenia and may reveal differences relevant to treatment response, clinical outcomes, and novel targeted treatment (Boks et al., 2008; Carpenter et al., 1988; Chien et al., 2015; Rangel et al., 2015; Villar-Menendez et al., 2014).
Although a few studies have investigated categorical subtypes beyond the classic diagnostic subtypes in EOS(Bellgrove et al., 2006; Eggers et al., 1999; Reddy et al., 1996), none have done so in COS. Notably, one study using cluster analysis of EOS Medicaid claims found two older patient groups, one with more mood dysregulation comorbidities, the other lacking co-morbidities, along with an especially early diagnosis group with higher rates of developmental delays and behavioral co-morbidities (Jerrell et al., 2017). The latter group likely includes COS patients, but these findings provide no further nuance about this population.
To more precisely characterize COS, in the current study, we explore both symptom dimensions and symptom-driven subgroups in the largest known sample of COS patients. Specifically, we examined the fit of SAPS and SANS data with previously established AOS symptom dimensions and then used the best fitting dimensions to form symptom based subgroups. We then compared demographic, clinical, cognitive, and genetic data across the resulting groups. COS has been shown to be mainly continuous with AOS (Jacobsen and Rapoport, 1998; Ordonez et al., 2015) and thus we expected our results to parallel adult research. Nevertheless, given the variation in the adult literature, the slight distinctions noted in EOS literature, and the association between earlier onset and more severe symptoms, cognitive impairment, premorbid disability, and poorer outcomes (Luoma et al., 2008; Ropcke and Eggers, 2005), we also sought to characterize any differences between COS and AOS in symptom dimensions and subtypes.
1.2 Materials and Methods
Sample
Patients were recruited nationally as part of a COS longitudinal study at the National Institute of Mental Health (NIMH). Selection and exclusion criteria have been described previously (Gordon et al., 1994). Briefly, participants were screened by phone, assessed during an outpatient visit, and admitted if history and screening interviews suggested a probable COS diagnosis. Child psychiatrists made a final diagnosis after an inpatient observation of up to three months, including a medication washout, using DSM-III R/DSM-IV criteria. Exclusion criteria were IQ under 70 before COS onset, neurological or medical illness, or substance abuse. Onset age was determined by child psychiatrist as the onset of impairing schizophrenic symptoms based on medical records and parent interview. Data from 125 COS patients were used in this study (Table 1).
Table 1.
Demographic and clinical characteristics of the childhood-onset schizophrenia patient cohort.
| N | n/mean | %/SD | |
|---|---|---|---|
| Female | 125 | 60 | 48.00 |
| Race | 125 | ||
| Caucasian | 68 | 54.40 | |
| African American | 39 | 31.20 | |
| Asian | 6 | 4.80 | |
| Other | 12 | 9.60 | |
| SES | 123 | 59.63 | 28.81 |
| Age Of Onset | 124 | 9.90 | 2.03 |
| Age At Rating | 121 | 13.32 | 2.68 |
| SAPS | 125 | 36.25 | 18.41 |
| SANS | 125 | 49.94 | 25.12 |
| CGAS | 124 | 32.48 | 11.24 |
| Full Scale IQ | 114 | 80.16 | 16.99 |
Neuropsychological and Clinical Measures
At admission, the clinical team conducted structured, including SAPS, SANS, the BPRS, and Children’s Global Assessment Scale (CGAS), and unstructured clinical interviews with patients and their parents. The Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS)(Chambers et al., 1985) was used to determine co-morbid diagnoses, except Pervasive Developmental Disorder (PDD)/Autism Spectrum Disorders (ASD), which involved a psychiatrist evaluation (See Sporn et al., 2004) and the Autism Screening Questionnaire (ASQ)(Berument et al., 1999). Average age at initial rating was 13.3±2.7 years.
During inpatient stay and/or at follow up, trained research staff tested participants using the most recent Wechsler Intelligence Scale for their age. Due to the longitudinal nature of the study and the variety of testing ages, tests included the Wechsler Adult Intelligence Scale-Revised (WAIS-R), Wechsler Abbreviated Scale of Intelligence First Edition (WASI) /second edition (WASI-II), or Wechsler Intelligence Scale for Children-Revised (WISC-R) /third edition (WISC-III) (Wechsler, 1974, 1981, 1991, 1999, 2011). In cases with multiple scores, the highest score was used because symptom severity can impact testing. Average testing age was 15.4±3.1 years.
Genetic data
Genomic DNA from purification of peripheral blood leukocytes was used to identify genetic abnormalities for all patients. Samples were screened using array based single-nucleotide polymorphism genotyping, as extensively described elsewhere (Ahn et al., 2014). Forty-six rare copy number variants (CNVs) associated with risk for development of AOS, intellectual disability, autism, and/or epilepsy were investigated. Previous studies showed the NIMH COS cohort had far higher rates of these disease-related CNVs than controls and AOS patient populations (Ahn et al., 2014). In this study, patients were categorized as carriers or non-carriers of these CNVs.
Statistical analysis
In order to assess the compatibility of the SAPS and SANS data with adult dimension models, confirmatory factor analysis (CFA) was performed using the robust maximum likelihood procedure to test and compare pre-existing symptom dimensions. Data was compared to 5 models, compiled by Dollfus and Everitt, 1998, including two, three, and four factor solutions (originally described by Andreasen, 1995; Peralta et al., 1994). This set of models was selected because it contained solutions reflective of commonly reported themes and allowed us to compare our CFA results directly to AOS findings.
All analyses were performed using R version 3.2.3 (R Core Team, 2014). CFA was performed using the ‘lavaan’ package (Rosseel, 2012). The fit of the model was evaluated using common goodness-of-fit measures (Jackson et al., 2009), including chi-square (χ2) test, comparative fit index (CFI), Tucker Lewis index (TLI), goodness of fit index (GFI) and the Root Mean Square Error of Approximation (RMSEA). Based on previous literature, χ2 statistic with a probability of occurrence > 0.05, CFI, TLI, and GFI values ≥ 0.95, and RMSEA ≤ 0.05 indicated a good fit (Barrett, 2007; Hu and Bentler, 1999; Kline, 2010).
K-means cluster analysis was performed using each patient’s dimension score from the best fitting dimension solution, as assessed by CFA. Cluster number was determined based on elbow criterion, variance explained, average silhouette value, and practical considerations. R packages ‘stats’ and ‘cluster’ were used (Machler et al., 2017).
Demographic and clinical data, which were external to the cluster analysis, were compared between groups. For categorical variables, group differences were assessed using chi-square and Fisher’s exact tests. For continuous variables, Levine’s test (α level=0.05) was used to determine whether group variances were equal. When variances were equal, one-way ANOVA were conducted, otherwise Welch’s ANOVA was used. When appropriate, post hoc tests (Tukey or Games Howell) were performed using ‘userfriendlyscience’ package (Peters, n.d.). After Bonferroni correction for multiple comparisons, p<3.57E-03 (0.05/14) was considered statistically significant.
1.3 Results
Of the five SAPS/SANS models, a two-factor solution (Andreasen, 1995) containing positive and negative dimensions was determined to best fit the COS data (χ2(19)=21.6, p=0.307, CFI:0.989, TLI:0.983, GFI:0.987, RMSE:3.38E-02). A three-factor solution, including positive, negative, and disorganized dimensions, was a less optimal fit (χ2(16)= 27.2, p=0.039, CFI:0.955, TLI:0.921, GFI:0.984, RMSE:7.69E-02). None of the remaining models met any of the criteria for a good model besides GFI (Dollfus, 1998).
K-means cluster analysis was performed using patients’ scores on the positive and negative dimensions from the best fitting model from the CFA. According to elbow criterion, the average silhouette values (2-cluster: 0.42, 3-cluster: 0.41), and variance explained (2-cluster: 47.13%, 3-cluster: 66.42%), both 2 or 3 cluster solutions were possible solutions. We preferred the 3-cluster solution because of the greater variance explained and because it aligned better with our clinical experience of the affected individuals. The three groups had: low scores on both dimensions (n=37), high negative scores with low positive scores (n=33), and high scores on both dimensions (n=55), herein referred to as low mixed, high negative, and high mixed respectively (Figure 1).
Figure 1.
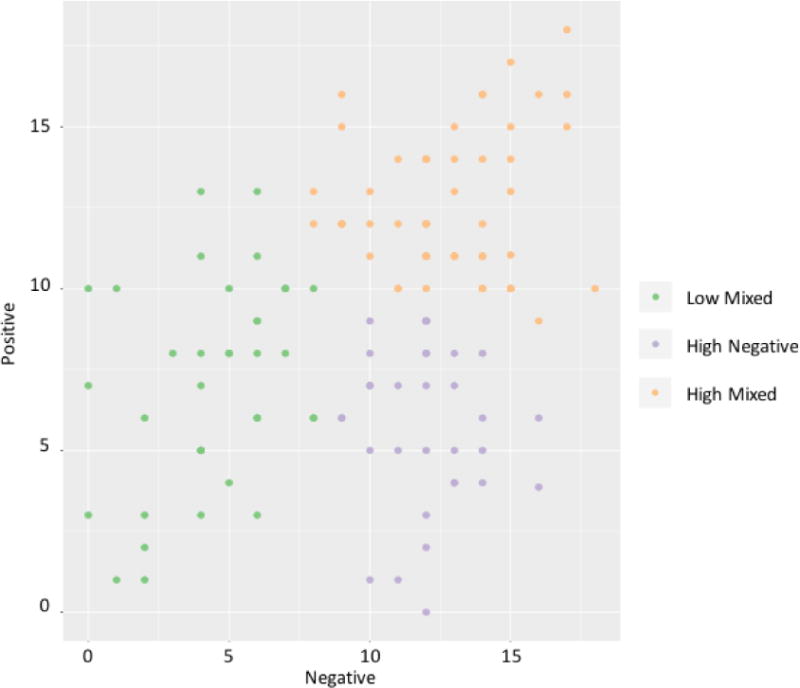
Three patient groups derived from K-means cluster analysis of positive and negative dimension scores.
The Andreasen 2-factor model includes all the SAPS global ratings within the positive dimension, but excluded the alogia, intimacy and closeness, and relationship SANS global ratings from the negative dimension. Thus, SANS scores were compared between groups to compare the two measures of negative symptoms. SANS scores differed (p=3.80E-27), as high negative and high mixed had significantly higher scores than low mixed (p=8.29E-14, p=6.59E-14).
Comparison of the groups’ demographic and clinical information revealed no differences in demographics or CNV status, but showed variance in clinical scores between groups (Table 2). Full scale IQ differed between groups (p=1.97E-03), as low mixed had a significantly higher score (88.34±18.37) than high mixed (75.54±14.19)(p=1.50E-03)(Figure 2). Additionally, although the significance of pairwise comparisons did not survive correction, onset age differed between groups (p=3.09E-03), as high negative had a later onset (10.75±1.57 years) compared to low and high mixed (9.18±2.19, 9.89±1.99 years). The prevalence of behavior disorder comorbidities, including attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and conduct disorder (CD), also differed between groups (p=2.35E-03)(Table 3). Although the significance of pairwise comparison did not survive correction, low mixed had a higher prevalence (41.94%) than high mixed (8.89%) and high negative (18.52%). Finally, groups’ CGAS scores differed (p=4.85E-09), as low mixed had significantly higher scores (40.49±10.31) than high mixed (26.71±9.17))(p=2.13E-09), while high negative had an intermediate score (33.13±9.76)(Table 2).
Table 2.
Comparison of demographics, clinical information, and clinical rating scores between groups. Bold values indicate significant results after Bonferroni correction (p<3.57E-03).
| N | Low Mixed
|
High Negative
|
High Mixed
|
Statistic | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n/Mean | %/SD | n/Mean | %/SD | n/Mean | %/SD | ||||
| Female | 125 | 16 | 43.24 | 16 | 48.49 | 28 | 50.91 | Chi2(2)=0.53 | 7.69E-01 |
| SES | 123 | 62.68 | 25.87 | 57.13 | 28.27 | 59.02 | 31.27 | F(2,120)=0.34 | 7.15E-01 |
| Race | |||||||||
| Caucasian | 125 | 26 | 70.27 | 17 | 51.52 | 25 | 45.45 | Fisher Exact Test | 1.32E-01 |
| African American | 7 | 18.92 | 14 | 42.42 | 18 | 32.73 | |||
| Asian | 1 | 2.70 | 1 | 3.03 | 4 | 7.27 | |||
| Others | 3 | 8.11 | 1 | 3.03 | 8 | 14.55 | |||
| Age of Onset | 124 | 9.18 | 2.19 | 10.75 | 1.57 | 9.89 | 1.99 | F(2,74)=6.25 | 3.09E-03 |
| Age at Rating | 121 | 13.22 | 3.11 | 14.16 | 2.07 | 12.88 | 2.63 | F(2,118)=2.36 | 9.91E-02 |
| CNV | 125 | 5 | 13.51 | 4 | 12.12 | 8 | 14.545 | Chi2(2)=0.10 | 9.50E-01 |
| Negative Dimension | 125 | 4.46 | 2.33 | 12.00 | 1.75 | 12.78 | 2.41 | F(2,122)=170.14 | 5.11E-36 |
| Positive Dimension | 125 | 7.11 | 3.20 | 5.81 | 2.51 | 12.49 | 2.17 | F(2,67)=96.22 | 1.86E-20 |
| SANS | 125 | 19.43 | 11.88 | 59.83 | 15.46 | 64.54 | 17.29 | F(2,122)=104.36 | 3.80E-27 |
| SAPS | 125 | 27.82 | 15.27 | 23.14 | 11.63 | 49.78 | 14.21 | F(2,122)=47.41 | 5.82E-16 |
| CGAS | 124 | 40.49 | 10.31 | 33.13 | 9.76 | 26.71 | 9.17 | F(2,121)=22.52 | 4.85E-09 |
| Full Scale IQ | 114 | 88.34 | 18.37 | 78.55 | 16.90 | 75.54 | 14.19 | F(2,111)=6.59 | 1.97E-03 |
Figure 2.
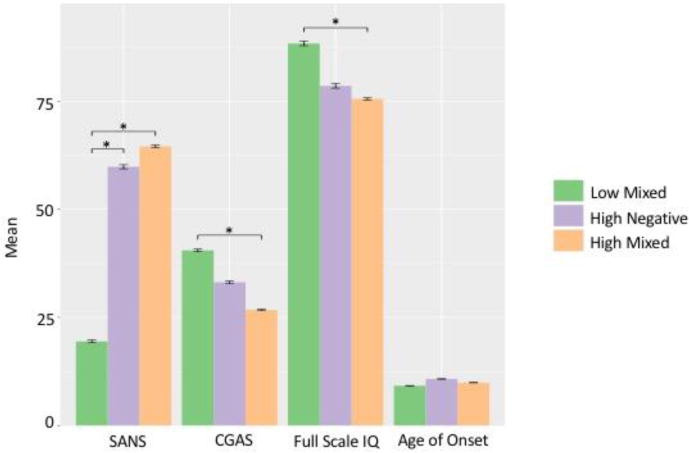
Group comparison of clinical rating scale scores. *Indicates significance in post-hoc test after Bonferroni correction (p<3.57E-03). Bars show standard error. SANS: Scale for the Assessment of Negative Symptoms. CGAS: Children’s Global Assessment Scale.
Table 3.
Comparison of co-morbidities between groups. Affect, anxiety, and behavior disorders were current disorders measured using the Kiddie Schedule for Affective Disorders and Schizophrenia at time of admission. Affect disorders included Depression and Mania. Anxiety disorders included Separation Anxiety Disorder, Generalized Anxiety Disorder, Obsessive Compulsive Disorder, Post-Traumatic Stress Disorder, Panic Disorder, Agoraphobia, Specific Phobia, and Social Phobia. Behavior Disorders included Attention Deficit Hyperactivity Disorder, Oppositional Defiant Disorder, and Conduct Disorder. Bold values indicate significant results after Bonferroni correction (p<3.57E-03).
| N | Low Mixed
|
High Negative
|
High Mixed
|
Statistic | P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n/Mean | %/SD | n/Mean | %/SD | n/Mean | %/SD | ||||
| PDD | 119 | 8 | 22.22 | 6 | 20.00 | 6 | 11.321 | Chi2(2)=2.11 | 3.47E-01 |
| ASQ score | 117 | 9.92 | 9.05 | 10.32 | 9.41 | 7.46 | 6.43 | F(2,114)=1.55 | 2.17E-01 |
| K-SADS Disorders | |||||||||
| Affect disorders | 103 | 7 | 22.58 | 4 | 14.82 | 6 | 13.33 | Chi2(2)=1.21 | 5.45E-01 |
| Anxiety disorder | 104 | 13 | 40.63 | 5 | 18.52 | 18 | 40.00 | Chi2(2)=4.18 | 1.24E-01 |
| Behavior disorders | 103 | 13 | 41.94 | 5 | 18.52 | 4 | 8.89 | Chi2(2)=12.11 | 2.35E-03 |
1.4 Discussion
CFA revealed that a two-factor solution, comprised of a positive and negative dimension, best fit COS clinical data. Although the three-factor solution, which included an additional disorganized dimension, had been found to fit AOS data better in two previous studies (Andreasen, 1995; Dollfus and Everitt, 1998), it provided a less optimal fit for the COS sample. Consistent with our findings, limited studies in EOS generally report positive and negative dimensions, with some reporting additional factors, although notably not disorganized ones (Banaschewski et al., 2000; Bunk et al., 1999; Maziade et al., 1996a; McClellan et al., 2002). This distinction may reflect the scarcity of COS/EOS studies, but might also suggest that disorganized symptoms are less independent and more closely tied to other positive symptoms in earlier onset schizophrenia samples compared to AOS. Interestingly, both studies examining dimension stability in EOS found that the configuration of EOS symptom factors more closely approximated AOS findings when EOS patients were re-tested in adulthood (Bunk et al., 1999; Maziade et al., 1996a), consistent with other reports of an orderly developmental progression between COS/EOS and AOS (Frangou, 2010; Ordonez et al., 2015). Overall, our CFA results were consistent with the limited EOS literature and overlapped with AOS studies in finding positive and negative dimensions, but differed in the lack of a disorganized dimension.
Our cluster analysis identified high negative, high mixed, and low mixed symptom groups. This was highly consistent with the other SAPS/SANS studies, which found the same 3 groups, although the exact dimensions and symptoms included, particularly in the positive dimension, varied (Dollfus et al., 1996; Jackson et al., 1989; Lastra et al., 2000; Morrison et al., 1990; Williams, 1996). Several studies found additional groups including a high positive group and a mid-level mixed symptom group (Dollfus et al., 1996; Lastra et al., 2000; Williams, 1996). Studies using PANSS data commonly identify negative and low symptom groups too, but are otherwise difficult to compare because of the greater representation of non-psychotic psychopathology on that scale (Dickinson, 2017; Dollfus and Petit, 1995). Nevertheless, our groups reflect the general themes found in AOS studies, particularly those using SAPS and SANS.
Group comparisons of demographic and clinical data provided insight into the characteristics of each patient group. There were no differences in demographics or CNV status. The difference in SANS scores paralleled group differences in the negative dimension scores, demonstrating the patients’ factor scores reflected the core negative symptoms included in the negative dimension and that their SANS scores were not greatly influenced by alogia, intimacy and closeness, and relationship global ratings. As high mixed was the largest group (n=55), the most common symptomatology profile in the NIMH cohort consisted of high levels of both positive and negative symptoms. The subgroup proportions may have been affected by selection and referral bias, but the large size of the high mixed group also likely reflects the generally severe nature of COS.
There was also a group difference in full scale IQ, which was driven by the significant difference between the high (88.34±18.37) and low (75.54±14.19) scores of low and high mixed groups respectively. High negative also had a low score (78.55±16.90), although it was not significantly different from either other group. AOS subgrouping studies reported less cognitive deficit in low symptom groups, matching the high score of our low mixed group (Dickinson, 2017; Jackson et al., 1989; Williams, 1996). In AOS literature, severe cognitive deficits are primarily associated with negative symptoms (Gold et al., 1999; Hartmann-Riemer et al., 2015), a trend reflected in the low scores of our high negative and high mixed groups, both of which experience severe negative symptoms.
Additionally, onset age differed between groups. Although the pairwise comparison did not survive correction, high negative was older (10.75±1.57) than low and high mixed at illness onset (9.18±2.19 & 9.89±1.99), perhaps suggesting a longer more insidious path to onset for this subgroup. Conversely to our results, severe symptoms, especially negative ones, are associated with earlier onset age in AOS(Clemmensen et al., 2012; Guerra et al., 2002; Kao and Liu, 2010). However, onset age comparison of AOS and COS is confounded given the early and compressed nature of COS onset.
Although pairwise comparison does not survive correction, the prevalence of externalizing disorders, including ADHD, ODD, and/or CD, also varied by group. Low mixed had the highest prevalence (41.94%), while high mixed had the lowest (8.89%). High negative had an intermediate prevalence (18.52%). There is evidence of overlap between the risk factors, genetics, and symptoms of ADHD and schizophrenia, including high rates of ADHD in the COS general population that is thought to reflect the neurodevelopmental origin of schizophrenia (Barr, 2001; Owen et al., 2011; Ross et al., 2006). Yet, there is little research comparing schizophrenic patients with and without these disorders. Consistent with our findings, one AOS study reported a group of patients with severe attention deficit and lower schizophrenia symptoms than other AOS patients (Bellak, 1985). The connection between symptom severity and these disorders is novel in COS; thus, this finding may provide further insight into additional deficits commonly experienced by COS patients, particularly those with relatively lower levels of positive and negative symptoms. Interestingly, one EOS cluster analysis found a group like our low mixed with highly prevalent ADHD, ODD, and/or CD co-morbidity and a young onset age (Jerrell et al., 2017). All these findings reflect the complex relationship among neurodevelopmental disorders, including schizophrenia, which is not yet fully understood.
Finally, global functioning differed between groups. Low mixed had significantly higher CGAS scores than high mixed. High negative also had a low score, although it was not significant from either of the other groups. These results are consistent with adult literature that typically associates low symptom burden with reduced functional impairment, and severe negative symptoms, found in both our high mixed and high negative groups, with the greater impairment (Diaz-Caneja et al., 2015; Dickinson, 2017; Hawk et al., 1975; Startup et al., 2002). In the COS sample, a mixed positive and negative symptom profile showed the worst functioning. This finding reflects the relative impact of symptom type on ability to function in COS, suggesting psychotic symptomatology and cognitive impairment are more closely related to functioning than the externalizing symptoms or co-morbidities prominent in the low mixed group. Thus, similarly to adults, a combination of low IQ and psychotic symptoms is most toxic for global functioning.
This study has several limitations. The NIMH cohort, although the largest available COS sample, represents a severely ill population and is small due to disease rarity; thus, there are unknown referral and selection biases. Methodologically, some have criticized using cluster analysis for subgroup formation because results vary by method and can be unstable (Kessler, 2002; Marquand et al., 2016). To address the former, methods from AOS studies were replicated as closely as possible and only general thematic comparisons were made. Unfortunately, precise comparison to AOS was still challenging due to illness severity of the COS cohort, variety of items included in dimensions, the rarity of similar analyses, and the prevalence of PANSS use over SAPS/SANS in other studies. Longitudinal analysis to address instability was outside the scope of this paper; however, future studies using latent class growth analysis could address this concern and assess if COS symptomatology, like in EOS, shifts closer to AOS as patients age. Additionally, the significance of several of our results did not survive correction, likely due to the large number of comparisons and contributed to by the small sample size in this study. These findings were included and discussed because they neared significance and aided in investigating distinctions between groups; nevertheless, it is important to note their lack of statistical significance makes these findings less certain and increases the possibility of conflicting results in future COS studies.
In conclusion, we found COS clinical data were well described by positive and negative dimensions, which were used to form three distinct subgroups that differed in clinically meaningful ways. To our knowledge, this is the first study reporting CFA and subgroups in a purely COS population. The dimension findings were consistent with EOS and some AOS studies, although the lack of disorganized dimension was different from most AOS literature. Despite this difference, our subgroups had similar defining characteristics with the few comparable AOS studies. Additionally, the differences between our groups reflected general themes often seen in AOS research, including the influence of symptom severity and type on IQ and global functioning. The main exception was onset age findings that showed opposite trends to AOS. Importantly, our analysis both confirmed the typical severe and impaired characterization of COS, but also revealed the presence of other groups with distinct symptomatology and deficits. Overall, these subgroups and dimensions offer additional nuance in characterizing the NIMH COS population and, for the most part, support the continuity of COS, EOS, and AOS in terms of symptomatology.
Acknowledgments
None
Funding: This research was supported by the NIMH Intramural Research Program (grant number: NCT00049738, annual report number: ZIA MH002581-25).
Abbreviations
- ASD
Autism Spectrum Disorders
- ASQ
the Autism Screening Questionnaire
- AOS
Adult onset schizophrenia
- ADHD
Attention Deficit Hyperactivity Disorder
- BPRS
Brief Psychiatric Rating Scale
- COS
Childhood onset schizophrenia
- CGAS
Children’s Global Assessment Scale
- CD
Conduct Disorder
- CFA
Confirmatory Factor Analysis
- CFI
Comparative Fit Index
- CNVs
Copy Number Variants
- EOS
Early onset schizophrenia
- GFI
Goodness of Fit Index
- K-SADS
Kiddie Schedule for Affective Disorders and Schizophrenia
- NIMH
National Institute of Mental Health
- ODD
Oppositional Defiant Disorder
- PDD
Pervasive Developmental Disorder
- PANSS
Positive and Negative Symptom Scale
- RMSEA
Root Mean Square Error of Approximation
- SAPS
Scale for the Assessment of Positive Symptoms
- SANS
Scale for the Assessment of Negative Symptoms
- TLI
Tucker Lewis index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare they have no conflicts of interest.
Contributors: Dr. Rapoport designed the study and wrote the protocol. Ms. Zhou and Dr. Liu performed the statistical analysis. Dr. Dickinson provided statistical advice and assisted in data interpretation. Mr. Gochman managed the data and contributed to data interpretation. Ms. Craddock performed literature review, interpreted the data, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Adityanjee Aderibigbe YA, Theodoridis D, Vieweg VR. Dementia praecox to schizophrenia: the first 100 years. Psychiatry Clin Neurosci. 1999;53:437–48. doi: 10.1046/j.1440-1819.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- Ahn K, Gotay N, Andersen TM, Anvari AA, Gochman P, Lee Y, Sanders S, Guha S, Darvasi A, Glessner JT, Hakonarson H, Lencz T, State MW, Shugart YY, Rapoport JL. High rate of disease-related copy number variations in childhood onset schizophrenia. Mol Psychiatry. 2014;19:568–72. doi: 10.1038/mp.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. Symptoms of Schizophrenia: Methods, Meanings, and Mechanisms. Arch Gen Psychiatry. 1995;52:341. doi: 10.1001/archpsyc.1995.03950170015003. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Carpenter WT., Jr Diagnosis and classification of schizophrenia. Schizophr Bull. 1993;19:199–214. doi: 10.1093/schbul/19.2.199. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Schulz E, Martin M, Remschmidt H. Cognitive functions and psychopathological symptoms in early-onset schizophrenia. Eur Child Adolesc Psychiatry. 2000;9:11–20. doi: 10.1007/s007870050111. [DOI] [PubMed] [Google Scholar]
- Barr WB. Schizophrenia and Attention Deficit Disorder. Ann N Y Acad Sci. 2001;931:239–250. doi: 10.1111/j.1749-6632.2001.tb05782.x. [DOI] [PubMed] [Google Scholar]
- Barrett P. Structural equation modelling: Adjudging model fit. Personal Individ Differ. 2007;42:815–824. doi: 10.1016/j.paid.2006.09.018. Special issue on Structural Equation Modeling. [DOI] [Google Scholar]
- Bellak L. ADD psychosis as a separate entity. Schizophr Bull. 1985;11:523–527. doi: 10.1093/schbul/11.4.523. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Mattingley JB, Hawi Z, Mullins C, Kirley A, Gill M, Robertson IH. Impaired temporal resolution of visual attention and dopamine beta hydroxylase genotype in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;60:1039–45. doi: 10.1016/j.biopsych.2006.03.062. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–51. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bleich-Cohen M, Jamshy S, Sharon H, Weizman R, Intrator N, Poyurovsky M, Hendler T. Machine learning fMRI classifier delineates subgroups of schizophrenia patients. Schizophr Res. 2014;160:196–200. doi: 10.1016/j.schres.2014.10.033. [DOI] [PubMed] [Google Scholar]
- Boks MP, Hoogendoorn M, Jungerius BJ, Bakker SC, Sommer IE, Sinke RJ, Ophoff RA, Kahn RS. Do mood symptoms subdivide the schizophrenia phenotype? Association of the GMP6A gene with a depression subgroup. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:707–11. doi: 10.1002/ajmg.b.30667. [DOI] [PubMed] [Google Scholar]
- Bunk D, Eggers C, Klapal M. Symptom dimensions in the course of childhood-onset schizophrenia. Eur Child Adolesc Psychiatry. 1999;8(Suppl 1):I29–35. doi: 10.1007/pl00010688. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578–83. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Chien YL, Liu CM, Shan JC, Lee HJ, Hsieh MH, Hwu HG, Chiou LC. Elevated plasma orexin A levels in a subgroup of patients with schizophrenia associated with fewer negative and disorganized symptoms. Psychoneuroendocrinology. 2015;53:1–9. doi: 10.1016/j.psyneuen.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Clemmensen L, Vernal DL, Steinhausen HC. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry. 2012;12:150. doi: 10.1186/1471-244X-12-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti A, Paletta S, Moliterno D, Mazzocchi A, Mauri MC, Altamura AC. Symptom dimensions as predictors of clinical outcome, duration of hospitalization, and aggressive behaviours in acutely hospitalized patients with psychotic exacerbation. Clin Pr Epidemiol Ment Health. 2010;6:72–8. doi: 10.2174/1745017901006010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, Derks EM, van Haren NE, Schnack HG, Hulshoff Pol HE, Kahn RS, Cahn W. Symptom dimensions are associated with progressive brain volume changes in schizophrenia. Schizophr Res. 2012;138:171–6. doi: 10.1016/j.schres.2012.03.036. [DOI] [PubMed] [Google Scholar]
- Crow TJ. The two-syndrome concept: origins and current status. Schizophr Bull. 1985;11:471–86. doi: 10.1093/schbul/11.3.471. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V, de Leon J. Schizophrenic syndromes associated with treatment response. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:87–99. doi: 10.1016/0278-5846(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Dazzi F, Shafer A, Lauriola M. Meta-analysis of the Brief Psychiatric Rating Scale - Expanded (BPRS-E) structure and arguments for a new version. J Psychiatr Res. 2016;81:140–51. doi: 10.1016/j.jpsychires.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Diaz-Caneja CM, Pina-Camacho L, Rodriguez-Quiroga A, Fraguas D, Parellada M, Arango C. Predictors of outcome in early-onset psychosis: a systematic review. NPJ Schizophr. 2015;1:14005. doi: 10.1038/npjschz.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Pratt D, Giangrande E, Grunnagle M, Orel J, Weinberger D, Callicott J, Berman K. Attacking heterogeneity in schizophrenia by deriving clinical subgroups from widely available symptom data. Schizophr Bull. 2017 doi: 10.1093/schbul/sbx039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty AR, Bigdeli TB, Edwards AC, Bacanu S, Lee D, Neale MC, Wormley BK, Walsh D, O’Neill FA, Riley BP, Kendler KS, Fanous AH. Genome-wide gene pathway analysis of psychotic illness symptom dimensions based on a new schizophrenia-specific model of the OPCRIT. Schizophr Res. 2015;164:181–6. doi: 10.1016/j.schres.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollfus S, Everitt B. Symptom structure in schizophrenia: two-, three- or four-factor models? Psychopathology. 1998;31:120–130. doi: 10.1159/000066235. [DOI] [PubMed] [Google Scholar]
- Dollfus S, Everitt B, Ribeyre JM, Assouly-Besse F, Sharp C, Petit M. Identifying subtypes of schizophrenia by cluster analyses. Schizophr Bull. 1996;22:545–555. doi: 10.1093/schbul/22.3.545. [DOI] [PubMed] [Google Scholar]
- Dollfus S, Petit M. Principal-component analyses of PANSS and SANS-SAPS in schizophrenia: their stability in an acute phase. Eur Psychiatry. 1995;10:97–106. doi: 10.1016/0924-9338(96)80320-8. [DOI] [PubMed] [Google Scholar]
- Eggers C, Bunk D, Volberg G, Ropcke B. The ESSEN study of childhood-onset schizophrenia: selected results. Eur Child Adolesc Psychiatry. 1999;8(Suppl 1):I21–8. doi: 10.1007/pl00010687. [DOI] [PubMed] [Google Scholar]
- Frangou S. Cognitive Function in Early Onset Schizophrenia: A Selective Review. Front Hum Neurosci. 2010;3 doi: 10.3389/neuro.09.079.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S, Arndt S, Nopoulos P, O’Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry. 1999;156:1342–8. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- Gordon CT, Frazier JA, McKenna K, Giedd J, Zametkin A, Zahn T, Hommer D, Hong W, Kaysen D, Albus KE, et al. Childhood-onset schizophrenia: an NIMH study in progress. Schizophr Bull. 1994;20:697–712. doi: 10.1093/schbul/20.4.697. [DOI] [PubMed] [Google Scholar]
- Guerra A, Fearon P, Sham P, Jones P, Lewis S, Mata I, Murray R. The relationship between predisposing factors, premorbid function and symptom dimensions in psychosis: an integrated approach. Eur Psychiatry. 2002;17:311–20. doi: 10.1016/s0924-9338(02)00685-5. [DOI] [PubMed] [Google Scholar]
- Hartmann-Riemer MN, Hager OM, Kirschner M, Bischof M, Kluge A, Seifritz E, Kaiser S. The association of neurocognitive impairment with diminished expression and apathy in schizophrenia. Schizophr Res. 2015;169:427–32. doi: 10.1016/j.schres.2015.10.032. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Aslan M, Du M, Zhao H, Siever LJ, Pulver A, Gaziano JM, Concato J. Factor structure of cognition and functional capacity in two studies of schizophrenia and bipolar disorder: Implications for genomic studies. Neuropsychology. 2016;30:28–39. doi: 10.1037/neu0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk AB, Carpenter WT, Strauss JS. Diagnostic Criteria and Five-Year Outcome in Schizophrenia: A Report From the International Pilot Study of Schizophrenia. Arch Gen Psychiatry. 1975;32:343–347. doi: 10.1001/archpsyc.1975.01760210077005. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model Multidiscip J. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Jackson DL, Gillaspy JA, Purc-Stephenson R. Reporting practices in confirmatory factor analysis: an overview and some recommendations. Psychol Methods. 2009;14:6–23. doi: 10.1037/a0014694. [DOI] [PubMed] [Google Scholar]
- Jackson HJ, Minas IH, Burgess PM, Joshua SD, Charisiou J, Campbell IM. Is social skills performance a correlate of schizophrenia subtypes? Schizophr Res. 1989;2:301–309. doi: 10.1016/0920-9964(89)90007-8. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Rapoport JL. Research update: childhood-onset schizophrenia: implications of clinical and neurobiological research. J Child Psychol Psychiatry. 1998;39:101–13. [PubMed] [Google Scholar]
- Jerrell JM, McIntyre RS, Deroche CB. Diagnostic clusters associated with an early onset schizophrenia diagnosis among children and adolescents. Hum Psychopharmacol. 2017;32 doi: 10.1002/hup.2589. [DOI] [PubMed] [Google Scholar]
- Kao YC, Liu YP. Effects of age of onset on clinical characteristics in schizophrenia spectrum disorders. BMC Psychiatry. 2010;10:63. doi: 10.1186/1471-244X-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiological perspectives for the development of future diagnostic systems. Psychopathology. 2002;35:158–61. doi: 10.1159/000065137. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling, Third Edition. 3rd. The Guilford Press; New York: 2010. [Google Scholar]
- Lastra I, Vázquez-Barquero JL, Herrera Castanedo S, Cuesta MJ, Vázquez-Bourgon ME, Dunn G. The classification of first episode schizophrenia: a cluster-analytical approach. Acta Psychiatr Scand. 2000;102:26–31. doi: 10.1034/j.1600-0447.2000.102001026.x. [DOI] [PubMed] [Google Scholar]
- Lewine RR, Fogg L, Meltzer HY. Assessment of negative and positive symptoms in schizophrenia. Schizophr Bull. 1983;9:368–376. doi: 10.1093/schbul/9.3.368. [DOI] [PubMed] [Google Scholar]
- Luoma S, Hakko H, Ollinen T, Jarvelin MR, Lindeman S. Association between age at onset and clinical features of schizophrenia: the Northern Finland 1966 birth cohort study. Eur Psychiatry. 2008;23:331–5. doi: 10.1016/j.eurpsy.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Machler M, Rousseeuw P, Struyf A, Hubert M, Hornik K. cluster: Cluster Analysis Basics and Extensions 2017 [Google Scholar]
- Marquand AF, Wolfers T, Mennes M, Buitelaar J, Beckmann CF. Beyond Lumping and Splitting: A Review of Computational Approaches for Stratifying Psychiatric Disorders. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:433–447. doi: 10.1016/j.bpsc.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziade M, Bouchard S, Gingras N, Charron L, Cardinal A, Roy MA, Gauthier B, Tremblay G, Cote S, Fournier C, Boutin P, Hamel M, Merette C, Martinez M. Long-term stability of diagnosis and symptom dimensions in a systematic sample of patients with onset of schizophrenia in childhood and early adolescence. II: Postnegative distinction and childhood predictors of adult outcome. Br J Psychiatry. 1996a;169:371–8. doi: 10.1192/bjp.169.3.371. [DOI] [PubMed] [Google Scholar]
- Maziade M, Gingras N, Rodrigue C, Bouchard S, Cardinal A, Gauthier B, Tremblay G, Cote S, Fournier C, Boutin P, Hamel M, Roy MA, Martinez M, Merette C. Long-term stability of diagnosis and symptom dimensions in a systematic sample of patients with onset of schizophrenia in childhood and early adolescence. I: nosology, sex and age of onset. Br J Psychiatry. 1996b;169:361–70. doi: 10.1192/bjp.169.3.361. [DOI] [PubMed] [Google Scholar]
- McClellan J, McCurry C, Speltz ML, Jones K. Symptom factors in early-onset psychotic disorders. J Am Acad Child Adolesc Psychiatry. 2002;41:791–8. doi: 10.1097/00004583-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Morrison RL, Bellack AS, Wixted JT, Mueser KT. Positive and negative symptoms in schizophrenia. A cluster-analytic approach. J Nerv Ment Dis. 1990;178:377–84. doi: 10.1097/00005053-199006000-00006. [DOI] [PubMed] [Google Scholar]
- Mortimer AM, Lund CE, McKenna PJ. The positive:negative dichotomy in schizophrenia. Br J Psychiatry. 1990;157:41–49. doi: 10.1192/bjp.157.1.41. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Curren PJ, McHugo GJ. Factor structure of the brief psychiatric rating scale in schizophrenia. Psychol Assess. 1997;9:196–204. [Google Scholar]
- Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46:1418–28. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- Ordonez AE, Luscher ZI, Gogtay N. Neuroimaging findings from childhood onset schizophrenia patients and their non-psychotic siblings. Schizophr Res. 2015 doi: 10.1016/j.schres.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall J, Gorham DR. The Brief Psychiatric Rating Scale Psychol Rep. 1962;10:799–812. [Google Scholar]
- Owen MJ, O’Donovan MC, Thapar A, Craddock N. Neurodevelopmental hypothesis of schizophrenia. Br J Psychiatry. 2011;198:173–175. doi: 10.1192/bjp.bp.110.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. How many and which are the psychopathological dimensions in schizophrenia? Issues influencing their ascertainment. Schizophr Res. 2001;49:269–85. doi: 10.1016/s0920-9964(00)00071-2. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ, de Leon J. An empirical analysis of latent structures underlying schizophrenic symptoms: a four-syndrome model. Biol Psychiatry. 1994;36:726–36. doi: 10.1016/0006-3223(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Peters G. userfriendlyscience: Quantitative analysis made accessible. R package version 0.4-1 2016 [Google Scholar]
- Potuzak M, Ravichandran C, Lewandowski KE, Ongur D, Cohen BM. Categorical vs dimensional classifications of psychotic disorders. Compr Psychiatry. 2012;53:1118–29. doi: 10.1016/j.comppsych.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. [Google Scholar]
- Rangel A, Munoz C, Ocampo MV, Quintero C, Escobar M, Botero S, Marin C, Jaramillo LE, Sanchez R, Rodriguez-Losada J, Ospina-Duque J, Palacio C, J CA, Valencia AV, Aguirre-Acevedo DC, Garcia J. Neurocognitive subtypes of schizophrenia. Actas Esp Psiquiatr. 2015;43:80–90. [PubMed] [Google Scholar]
- Reddy YCJ, Srinath S, Sathyanarayana V, Girimaji S, Seshadri S. Clinical profile of early onset schizophrenia: A review of 43 cases. Nimhans J. 1996;14:93–98. [Google Scholar]
- Reininghaus U, Priebe S, Bentall RP. Testing the psychopathology of psychosis: evidence for a general psychosis dimension. Schizophr Bull. 2013;39:884–95. doi: 10.1093/schbul/sbr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropcke B, Eggers C. Early-onset schizophrenia: a 15-year follow-up. Eur Child Adolesc Psychiatry. 2005;14:341–50. doi: 10.1007/s00787-005-0483-6. [DOI] [PubMed] [Google Scholar]
- Ross RG, Heinlein S, Tregellas H. High rates of comorbidity are found in childhood-onset schizophrenia. Schizophr Res. 2006;88:90–5. doi: 10.1016/j.schres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software, Articles. 2012;48(2) [Google Scholar]
- Salokangas RK, Honkonen T, Stengard E, Koivisto AM. Symptom dimensions and their association with outcome and treatment setting in long-term schizophrenia. Results of the DSP project. Nord J Psychiatry. 2002;56:319–27. doi: 10.1080/080394802760322079. [DOI] [PubMed] [Google Scholar]
- Sporn AL, Addington AM, Gogtay N, Ordonez AE, Gornick M, Clasen L, Greenstein D, Tossell JW, Gochman P, Lenane M, Sharp WS, Straub RE, Rapoport JL. Pervasive developmental disorder and childhood-onset schizophrenia: comorbid disorder or a phenotypic variant of a very early onset illness? Biol Psychiatry. 2004;55:989–94. doi: 10.1016/j.biopsych.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Startup M, Jackson MC, Bendix S. The concurrent validity of the Global Assessment of Functioning (GAF) Br J Clin Psychol. 2002;41:417–422. doi: 10.1348/014466502760387533. [DOI] [PubMed] [Google Scholar]
- Viher PV, Stegmayer K, Giezendanner S, Federspiel A, Bohlhalter S, Vanbellingen T, Wiest R, Strik W, Walther S. Cerebral white matter structure is associated with DSM-5 schizophrenia symptom dimensions. Neuroimage Clin. 2016;12:93–99. doi: 10.1016/j.nicl.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar-Menendez I, Diaz-Sanchez S, Blanch M, Albasanz JL, Pereira-Veiga T, Monje A, Planchat LM, Ferrer I, Martin M, Barrachina M. Reduced striatal adenosine A2A receptor levels define a molecular subgroup in schizophrenia. J Psychiatr Res. 2014;51:49–59. doi: 10.1016/j.jpsychires.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, Lobaugh N, Pollock BG, Mulsant BH. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. 2013;70:472–480. doi: 10.1001/jamapsychiatry.2013.786. [DOI] [PubMed] [Google Scholar]
- von Knorring L, Lindstrom E. The Swedish version of the Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Construct validity and interrater reliability. Acta Psychiatr Scand. 1992;86:463–8. doi: 10.1111/j.1600-0447.1992.tb03298.x. [DOI] [PubMed] [Google Scholar]
- Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137:246–50. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence- Second Edition (WASI-II) Psychological Corporation; New York: 2011. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; New York: 1999. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children- Third Edition. Psychological Corporation; New York: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale- Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children- Revised. Psychological Corporation; New York: 1974. [Google Scholar]
- Williams LM. Cognitive inhibition and schizophrenic symptom subgroups. Schizophr Bull. 1996;22:139–51. doi: 10.1093/schbul/22.1.139. [DOI] [PubMed] [Google Scholar]


