Abstract
While full oral glucose tolerance test (OGTT) helps improve prediction, it requires intravenous access with 6 sample collections for glucose and C-peptide. The objective of this study was to explore less costly and less time-consuming options. All children being prospectively followed by the Diabetes Autoimmunity Study in the Young (DAISY) who had a complete baseline OGTT and at least one confirmed islet autoantibody (Ab+) were included in this study (n=68). Of 68 Ab+ subjects with a baseline OGTT, 25 developed diabetes after a mean follow-up 5.7 yrs, at a mean age of 12.4 yrs. Univariate proportional hazards (PH) models suggested that age at seroconversion, number of Ab+, IA-2A levels, HbA1c and metabolic variables from the OGTT predicted progression to diabetes, while HLA DR3/4, BMI, levels of IAA or GADA did not. Five multivariate PH predictive models were similar (p=0.32). All five models included age at seroconversion, number of Ab+, IA-2A levels and HbA1c, and in addition included: model 1 - 1h glucose and 1h C-peptide; model 2 - 2h glucose and 2h C-peptide; model 3 - glucose sum and C-peptide sum; model 4 - glucose AUC and C-peptide AUC; and model 5: index 60. A model containing age at seroconversion, number of Ab+, IA-2A levels, HbA1c, 1h glucose and 1h C-peptide was as predictive for type 1 diabetes progression as models including all sum or AUC values for glucose and C-peptide from full OGTT. The performance of this model should be confirmed in an independent population of Ab+ children.
Keywords: islet autoimmunity, progression to diabetes, type 1 diabetes, predictors for diabetes, oral glucose tolerance test
1. Introduction
Prospective, longitudinal studies following individuals at high risk for type 1 diabetes, determined by genetic risk markers or family history, have elucidated the typical disease progression prior to the onset of clinical symptoms (1–5). The American Diabetes Association, the Juvenile Diabetes Research Foundation and the Endocrine Society published a joint statement in 2015 that describes distinct stages of type 1 diabetes (6). In Stage 1, a person is euglycemic with no symptoms but positive for multiple islet autoantibodies. Stage 2 occurs when a person with multiple autoantibodies begins to have metabolic abnormalities (dysglycemia) but remains clinically asymptomatic. In Stage 3, a patient has classical diabetes symptoms in the presence of significant dysglycemia and therefore meets standard clinical diagnostic criteria for diagnosis of diabetes.
Dysglycemia precedes clinical diagnosis of diabetes by months or years and has gained interest as a distinct stage of pre-diabetes and a potential window for therapeutic intervention. The ability to reliably identify the dysglycemic period and implement prevention may have important implications for preservation of endogenous insulin secretion. The oral glucose tolerance test (OGTT) has value in predicting progression from islet autoimmunity to type 1 diabetes (7,8), is performed in prospective studies to monitor subjects’ risk of progression, as entry criteria into prevention trials and/or to confirm the diagnosis of diabetes. While full OGTT helps improve prediction, it requires intravenous access with 6 sample collections for glucose and C-peptide, and repeated OGTTs are poorly accepted by children and families.
The objective of this study was to explore whether less costly and less time-consuming options are as accurate as a full OGTT for prediction of type 1 diabetes in children known to be at high genetic risk and positive for islet autoantibodies. We found that a model containing age at seroconversion, number of Ab+, IA-2A levels, HbA1c, 1h glucose and 1h C-peptide was as predictive for type 1 diabetes progression as models including all sum or area under the curve (AUC) values for glucose and C-peptide from a full OGTT.
2. Material and methods
2.1. Study population
Since 1993, DAISY has followed two cohorts of young children at increased risk of type 1 diabetes (total N=2542): a cohort of relatives of type 1 diabetes patients (siblings and offspring), and the general population newborn cohort. The latter consists of children with type 1 diabetes susceptibility HLA-DR/DQ genotypes identified through screening of over 31,000 newborns at St. Joseph Hospital in Denver, Colorado. Recruitment began in 1993 and ended in 2004. The details of screening and follow-up have been previously published(9). DAISY children with at least one confirmed Ab+ on two consecutive visits are offered an OGTT. Only DAISY children who had a complete baseline OGTT were included in this study (N=68). Onset of diabetes was defined according to ADA criteria. Informed consent was obtained from the parents of each study subject. The Colorado Multiple Institutional Review Board approved all study protocols.
2.2. Islet Autoantibodies
Measurement of islet autoantibodies to insulin, GAD65 and IA-2 was performed in the Clinical Immunology Laboratory at the Barbara Davis Center, the core immunology laboratory for TrialNet study antibody testing, using radio-immunoassays as previously described (10–12). In addition, all available samples from children ever positive for any of the above autoantibodies or who developed type 1 diabetes were tested for autoantibodies to ZnT8 as previously described (13). In the 2015 IASP Workshop, sensitivities and specificities were 52% and 100% respectively for mIAA, 82% and 99% respectively for GADA, 72% and 100% respectively for IA-2A, and 70% and 97% respectively for ZnT8A.
2.3. Oral glucose tolerance test (OGTT)
Participants were instructed to fast for 8 hours prior to the study visit. The oral glucose tolerance test (OGTT) was conducted only if glucometer fasting glucose was below 200 mg/dl. A fasting blood sample was drawn for hemoglobin HbA1c, glucose and C-peptide. For the oral glucose tolerance test (OGTT), 1.75g per kilogram glucose dose (maximum 75g of carbohydrate) was ingested within 5 minutes and blood samples for glucose and C-peptide were collected at 6- time points (−10, 0, 30, 60, 90 and 120 minutes).
2.4. Statistical Analysis
Statistical analyses were performed using the SAS software version 9.4. Autoantibody levels were converted to Z scores (SD units away from threshold) and log transformed for analyses. Because of negative values, 1 was added before log transformation and calculation of mean. Progression to diabetes from baseline OGTT visit was analyzed using univariate and multivariate Cox proportional hazard analyses. Follow-up time was defined as time from baseline OGTT to development of type 1 diabetes or last visit for those who did not develop diabetes. AUC was calculated according to the trapezoidal rule. Values from 30, 60, 90 and 120 minutes time points for glucose or C-peptide were combined for the glucose SUM and C-peptide SUM, respectively. Index60 combines log fasting C-peptide, 60-min C-peptide, and 60-min glucose values(14). Receiver operating characteristic (ROC) curves were generated to compare AUC of five different predictive models. As we had incomplete data for ZnT8, ZnT8 was not included in multivariate models. A two-tailed p-value with an alpha level for significance was set at 0.05.
3. Results
The characteristics of study participants at baseline OGTT are shown in Table 1. DAISY Ab+ subjects who progressed to diabetes had a younger age at seroconversion (5.4 ± 2.9 vs 8.1 ± 4.1 yrs respectively, p=0.005). As expected, follow-up time was shorter for those Ab+ subjects who progressed to type 1 diabetes. The percentage of subjects with a first-degree relative (FDR) with type 1 diabetes was high in both groups, and even higher in the Ab+ non-progressors (72% vs 48%, p=0.047).
Table 1.
Characteristics of DAISY Ab+ subjects
| Characteristics | Ab+ non-progressorsa (N=43) | Ab+ subjectsa who progressed to T1D (N=25) | P value |
|---|---|---|---|
| Male Gender, N (%) | 19 (44) | 17 (68) | 0.06 |
| Ethnicity NHW, N (%) | 34 (79) | 23 (92) | 0.19 |
| FDRb with T1D, N (%) | 31 (72) | 12 (48) | 0.047 |
| HLA DR3/3, N (%) | 0 (0) | 1 (4) | 0.20 |
| HLA DR3/4, N (%) | 12 (29) | 9 (36) | |
| HLA DR4/4, N (%) | 6 (14) | 4 (16) | |
| HLA DR3/X, N (%) | 2 (5) | 4 (16) | |
| HLA DR4/X, N (%) | 11 (26) | 5 (20) | |
| HLA DRX/X, N (%) | 11 (26) | 2 (8) | |
| HbA1c (%) c mmol/mol | 5.1 ± 0.3 32 ± 4 |
5.3 ± 0.5 34 ± 6 |
0.08 |
| BMI z-score c | 0.18 ± 0.94 | 0.32 ± 0.84 | 0.51 |
| Multiple Ab+ at seroconversiona, N (%) | 13 (30) | 10 (40) | 0.41 |
| Multiple Ab+ at baseline OGTTa, N (%) | 19 (49) | 19 (76) | 0.09 |
| Age at seroconversion c | 8.1 ± 4.1 | 5.4 ± 2.9 | 0.005 |
| Follow-up from seroconversion c | 9.1 ± 4.4 | 7.1 ± 3.2 | 0.048 |
| Follow-up from baseline OGTT c | 6.9 ± 3.2 | 3.6 ± 2.9 | <0.0001 |
| Age at T1D onset or last visit c | 17.1 ± 4.8 | 12.4 ± 3.5 | <0.0001 |
Ab+: autoantibody positive
FDR: first-degree relative
Mean ± SD
Univariate Cox proportional hazard (PH) models were performed to analyze factors involved in progression to diabetes in Ab+ subjects since baseline OGTT (Table 2). Age at seroconversion, number of Ab+, IA-2A and ZnT8A levels, HbA1c, 1h glucose, 2h glucose, glucose AUC, glucose sum, 1h C-peptide, C-peptide AUC, C-peptide sum and index 60 predicted progression to type 1 diabetes. On the other hand, HLA DR3/4, BMI, FDR with diabetes, levels of IAA or GADA, fasting glucose, fasting C-peptide and 2h C-peptide did not predict progression to diabetes.
Table 2.
Progression to diabetes in Ab+ subjects since baseline OGTT
| Variable | HR and 95%CI | P value |
|---|---|---|
| FDRa with T1D | 0.46 (0.21–1.00) | 0.051 |
| HLA DR3/4*0302 | 1.42 (0.62–3.21) | 0.406 |
| Age at seroconversion | 0.87 (0.76–0.99) | 0.029 |
| Number of positive Abb | 1.89 (1.24–2.88) | 0.003 |
| mIAA | 1.00 (0.99–1.00) | 0.840 |
| GADA | 1.00 (0.99–1.00) | 0.511 |
| IA-2A | 1.00 (1.00–1.01) | 0.002 |
| ZnT8A | 1.03 (1.01–1.05) | 0.004 |
| HbA1c | 3.34 (1.35–8.28) | 0.009 |
| BMI | 0.86 (0.72–1.02) | 0.083 |
| Fasting glucose | 1.01 (0.96–1.06) | 0.766 |
| Fasting C-peptide | 0.76 (0.37–1.56) | 0.451 |
| 1h glucose | 1.03 (1.02–1.04) | <0.0001 |
| 1h C-peptide | 0.79 (0.65–0.97) | 0.023 |
| 2h glucose | 1.03 (1.01–1.04) | <0.0001 |
| 2h C-peptide | 0.88 (0.71–1.09) | 0.241 |
| Glucose SUMc | 1.01 (1.01–1.01) | <0.0001 |
| C-peptide SUMc | 0.94 (0.88–0.99) | 0.031 |
| Index60d | 1.02 (1.01–1.02) | <0.0001 |
| Glucose AUC e | 1.42 (1.24–1.62) | <0.0001 |
| C-peptide AUC e | 1.00 (0.99–1.00) | 0.027 |
Univariate Cox PH analyses
FDR: first-degree relative
Ab+: autoantibody positive
SUM: sum of values from 30, 60, 90 and 120 min
Index60: log fasting C-peptide, 60-min C-peptide, and 60-min glucose values
AUC: calculated according to trapezoidal rule
Five multivariable Cox proportional hazards models predicting progression to diabetes were compared. All models contained the variables that were significant in univariate Cox PH models, i.e. age at seroconversion, number of Ab+, IA-2A levels and HbA1c (ZnT8 levels were not included in multivariate analyses due to incomplete data). In addition to these common variables, the model included significant metabolic variables from the OGTT: model 1: 1h glucose and 1h C-peptide; model 2: 2h glucose and 2h C-peptide; model 3: glucose sum and C-peptide sum; model 4: glucose AUC and C-peptide AUC; model 5: Index60 (Table 3). Factors that remained significantly associated with prediction to diabetes were: model 1: 1h glucose and 1h C-peptide; model 2: IA-2A and 2h glucose; model 3: IA-2A and glucose SUM; model 4: IA-2A and glucose AUC; model 5: Index60.
Table 3.
Progression to diabetes in Ab+ subjects since baseline OGTT
| Model | Variable | HR and 95%CI | P value |
|---|---|---|---|
| Model 1 | Age at seroconversion | 0.97 (0.81–1.16) | 0.715 |
| Number of positive Aba | 1.40 (0.79–2.49) | 0.253 | |
| IA-2A | 1.00 (1.00–1.01) | 0.093 | |
| HbA1c | 0.90 (0.18–4.47) | 0.901 | |
| 1h glucose | 1.03 (1.02–1.05) | <0.0001 | |
| 1h C-peptide | 0.77 (0.62–0.95) | 0.017 | |
| Model 2 | Age at seroconversion | 0.87 (0.73–1.04) | 0.138 |
| Number of positive Aba | 1.36 (0.72–2.57) | 0.342 | |
| IA-2A | 1.01 (1.00–1.01) | 0.037 | |
| HbA1c | 2.24 (0.52–9.68) | 0.281 | |
| 2h glucose | 1.02 (1.01–1.04) | 0.002 | |
| 2h C-peptide | 0.96 (0.76–1.22) | 0.746 | |
| Model 3 | Age at seroconversion | 0.91 (0.75–1.10) | 0.339 |
| Number of positive Aba | 1.50 (0.81–2.78) | 0.195 | |
| IA-2A | 1.01 (1.00–1.01) | 0.047 | |
| HbA1c | 0.97 (0.20–4.68) | 0.974 | |
| Glucose SUMb | 1.01 (1.01–1.02) | <0.0001 | |
| C-peptide SUMb | 0.96 (0.90–1.02) | 0.160 | |
| Model 4 | Age at seroconversion | 0.90 (0.75–1.10) | 0.309 |
| Number of positive Aba | 1.45 (0.80–2.65) | 0.223 | |
| IA-2A | 1.01 (1.00–1.01) | 0.040 | |
| HbA1c | 0.97 (0.21–4.59) | 0.970 | |
| Glucose AUCc | 1.52 (1.27–1.82) | <0.0001 | |
| C-peptide AUCc | 1.00 (0.99–1.00) | 0.093 | |
| Model 5 | Age at seroconversion | 0.97 (0.82–1.16) | 0.750 |
| Number of positive Aba | 1.24 (0.72–2.13) | 0.443 | |
| IA-2A | 1.00 (1.00–1.01) | 0.057 | |
| HbA1c | 1.56 (0.40–6.19) | 0.525 | |
| Index60d | 1.02 (1.01–1.02) | 0.0001 |
Multivariate Cox PH analyses. ZnT8A not included in multivariate Cox PH analyses because of missing values.
Ab: autoantibodies
SUM: sum of values from 30, 60, 90 and 120 min
AUC: calculated according to trapezoidal rule
Index60: log fasting C-peptide, 60-min C-peptide, and 60-min glucose values
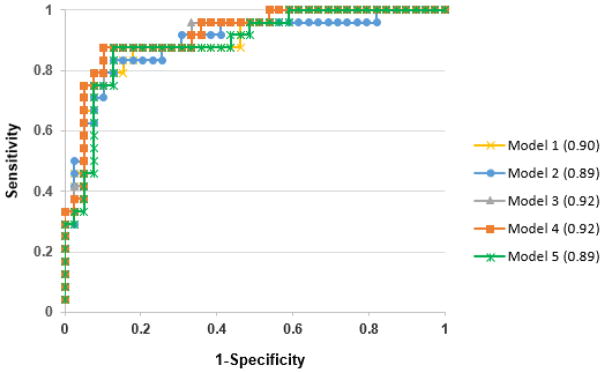
Receiver operating characteristic (ROC) curves were generated to compare area under the curve (AUC) of the five different predictive models. There were no significant differences in the ROC AUC of the five different models (Figure 1, p=0.316), suggesting that a simpler model such as model 1 (1h glucose and 1h C-peptide) was as predictive for type 1 diabetes progression as models including all sum or AUC values for glucose and C-peptide.
Figure 1. ROC curves for 5 predictive models (p=0.32).
All models contained age at seroconversion, number of Ab+, IA-2A levels and HbA1c, and in addition: 1 hour (model 1): 1h glucose and 1h C-peptide; 2 hour (model 2): 2h glucose and 2h C-peptide; SUM (model 3): glucose sum and C-peptide sum; AUC (model 4): glucose AUC and C-peptide AUC; model 5: Index60
4. Discussion
To our knowledge, this is the first study to date to compare whether less costly and less time-consuming options are as accurate as a full OGTT for prediction of type 1 diabetes in children known to be at high genetic risk and positive for islet autoantibodies. Children with islet autoantibodies (Ab+) are at high risk for type 1 diabetes, but the individual risk varies. This study found that a model containing age at seroconversion, number of Ab+, IA-2A levels, HbA1c, 1h glucose and 1h C-peptide was as predictive for type 1 diabetes progression as models including all sum or AUC values for glucose and C-peptide from full OGTT.
Several risk scores for diabetes have been developed, including the DPTRS (15,16) in order to predict risk for diabetes. The DPTRS(15) includes age, log body mass index (BMI), log fasting C-peptide, the glucose sums of 30-, 60-, 90-, and 120-min values and the C-peptide sums of 30-, 60-, 90-, and 120-min values. The DPTRS has been validated and confirmed to be a useful predictor of diabetes risk in TrialNet(16), but requires calculations and values from a full OGTT. In this study, a model containing age at seroconversion, number of Ab+, IA-2A levels, HbA1c, 1h glucose and 1h C-peptide was as predictive for type 1 diabetes progression as more complicated models including all sum or AUC values for glucose and C-peptide from full OGTT. This approach would allow for easier monitoring of children at high risk for type 1 diabetes, involving just one blood draw 60 minutes after ingestion of glucose dose. As studies such as Fr1da and ASK (Autoimmunity Screening for Kids) now begin to screen general population children for islet autoantibodies and risk for type 1 diabetes(17), this modified OGTT would lead to increased acceptance and easier monitoring of these Ab+ high risk children.
While the five multivariate PH predictive models were equivalent, it is interesting to note that the factors that remained significantly associated with prediction to diabetes were either glucose and C-peptide (models 1 and 5) or IA-2A and glucose (models 2,3 and 4). Index60(14) was developed recently from the Diabetes Prevention Trial—Type 1 Diabetes (DPT-1) database using a proportional hazards regression model and includes log fasting C-peptide, 60-min C-peptide, and 60-min glucose values. The ROC curves showed that at baseline, Index60 was a much more accurate predictor for type 1 diabetes than the 2h glucose value after an OGTT(14). In addition, in Ab+ relatives of patients with type 1 diabetes, Index60 also appears superior as a prediagnostic endpoint for diabetes compared to dysglycemia from OGTT(18). Consistent with this study, our study would suggest that a combination of glucose and C-peptide values at 1hour after ingestion of glucose dose is a good predictor for diabetes. In another TrialNet Pathway to Prevention study using recursive partitioning analysis(19), progression from multiple autoantibodies to dysglycemia was associated with IA-2A titers, 2h glucose and fasting C-peptide levels, while progression from dysglycemia to diabetes was associated with number of Ab+, peak C-peptide level, HbA1c and age. Some of the differences in these results are likely due to the different outcomes used in these studies, i.e. progression to dysglycemia versus progression to diabetes. On the other hand, IA-2A levels are confirmed to be important in prediction to diabetes; the presence of autoantibodies that typically develop later such as IA-2A has been described as an increased risk for development of diabetes (20), and IA-2A levels have been associated with rate of progression to diabetes (4).
DAISY is a rare prospective cohort study that has followed a large number of children at increased risk for development of islet autoimmunity and type 1 diabetes for over 20 years. Prospective testing for islet autoantibodies was performed at 9 months, 15 months, 24 months and annually thereafter. If confirmed islet autoantibody positive, subjects were monitored more frequently, every 3–6 months, with islet autoantibodies, HbA1c and OGTT testing. However, as OGTT is offered optionally to these children every 6 months, the numbers of subjects doing an OGTT and included in these analyses was limited (68 subjects). Our findings need validation in an independent group of subjects, which will be possible once other prospective studies with close monitoring such as The Environmental Determinants of Diabetes in the Young (TEDDY) and ASK studies have longer follow-up. The TEDDY study is a multi-site, multi-country prospective cohort that follows children with increased genetic risk of progression to type 1 diabetes since birth (21), while ASK is a program with the goal to screen 50,000 Denver metro-area children for pre-symptomatic type 1 diabetes and celiac disease. In addition, a larger proportion of the DAISY subjects undergoing OGTT were first-degree relatives of a patient with type 1 diabetes, likely due to the higher acceptability of doing OGTT in subjects who have a FDR with diabetes. Another limitation of this study was incomplete data on ZnT8A as autoantibody testing for ZnT8 started after the identification ZnT8A and retrospectively included all subjects who had ever been antibody positive in DAISY. Although ZnT8A was a significant predictor in univariate analyses, it was excluded in multivariate analyses due to incomplete data.
This is the first study to explore less costly and less time-consuming monitoring options in children at high risk for type 1 diabetes. A modified OGTT with a one-time blood draw 60 minutes after ingestion of glucose dose may help with monitoring for progression to type 1 diabetes in children at increased risk and may be more acceptable to families compared to the full OGTT with multiple blood draws. The performance of this model should be confirmed in an independent sample of Ab+ children.
Highlights.
Islet antibody (Ab) positive children need surveillance for diabetes development
HLA DR3/4, BMI, IAA or GADA levels do not seem to predict progression to diabetes
Age, Ab number, IA-2A levels, HbA1c and OGTT variables predict diabetes progression
A 1h glucose/C-peptide model is as predictive as glucose/Cpeptide AUC or sum models
Surveillance with 1h OGTT seems accurate, less costly and less time-consuming
Acknowledgments
This research was supported by NIH grants DK32493, DK32083, DK050979, DK57516, JDRF 17-2013-535 and ADA Grant 1-14-CD-17. AKS takes full responsibility for the contents of the article.
Footnotes
Conflict of Interest
The authors have no relevant conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, Yu L, Palmer JP, Schatz D, Eisenbarth G. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32:2269–2274. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siljander HT, Simell S, Hekkala A, Lahde J, Simell T, Vahasalo P, Veijola R, Ilonen J, Simell O, Knip M. Predictive characteristics of diabetes-associated autoantibodies among children with HLA-conferred disease susceptibility in the general population. Diabetes. 2009;58:2835–2842. doi: 10.2337/db08-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steck AK, Johnson K, Barriga KJ, Miao D, Yu L, Hutton JC, Eisenbarth GS, Rewers MJ. Age of Islet Autoantibody Appearance and Mean Levels of Insulin, but Not GAD or IA-2 Autoantibodies, Predict Age of Diagnosis of Type 1 Diabetes: Diabetes Autoimmunity Study in the Young. Diabetes Care. 2011;34:1397–1399. doi: 10.2337/dc10-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steck AK, Vehik K, Bonifacio E, Lernmark A, Ziegler AG, Hagopian WA, She J, Simell O, Akolkar B, Krischer J, Schatz D, Rewers MJ. Predictors of Progression From the Appearance of Islet Autoantibodies to Early Childhood Diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY) Diabetes Care. 2015;38:808–813. doi: 10.2337/dc14-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Bonifacio E, Eisenbarth GS. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–2479. doi: 10.1001/jama.2013.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark A, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging Presymptomatic Type 1 Diabetes: A Scientific Statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS. Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care. 2007;30:38–42. doi: 10.2337/dc06-1615. [DOI] [PubMed] [Google Scholar]
- 8.Sosenko JM, Skyler JS, Herold KC, Palmer JP Type 1 Diabetes T, Diabetes Prevention Trial-Type 1 Study G. The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial-type 1. Diabetes. 2012;61:1331–1337. doi: 10.2337/db11-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rewers M, Bugawan TL, Norris JM, Blair A, Beaty B, Hoffman M, McDuffie RS, Hamman RF, Klingensmith G, Eisenbarth GS, Erlich HA. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY) Diabetologia. 1996;39:807–812. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge CF, Chase HP, Klingensmith GJ, Erlich H, Norris J, Eisenbarth G. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 1996;81:4264–4267. doi: 10.1210/jcem.81.12.8954025. [DOI] [PubMed] [Google Scholar]
- 11.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Keleman K, Eisenbarth GS. Early expression of anti-insulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci,USA. 2000;97:1701–1706. doi: 10.1073/pnas.040556697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM, Adler K, Ziegler AG, Mueller PW, Schatz DA, Krischer JP, Steffes MW, Akolkar B. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95:3360–3367. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wenzlau JM, Moua O, Sarkar SA, Yu L, Rewers M, Eisenbarth GS, Davidson HW, Hutton JC. SlC30A8 is a major target of humoral autoimmunity in type 1 diabetes and a predictive marker in prediabetes. Ann N Y Acad Sci. 2008;1150:256–259. doi: 10.1196/annals.1447.029. [DOI] [PubMed] [Google Scholar]
- 14.Sosenko JM, Skyler JS, DiMeglio LA, Beam CA, Krischer JP, Greenbaum CJ, Boulware D, Rafkin LE, Matheson D, Herold KC, Mahon J, Palmer JP Type 1 Diabetes TrialNet Study G, Diabetes Prevention Trial-Type 1 Study G. A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care. 2015;38:271–276. doi: 10.2337/dc14-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosenko JM, Skyler JS, Mahon J, Krischer JP, Beam CA, Boulware DC, Greenbaum CJ, Rafkin LE, Cowie C, Cuthbertson D, Palmer JP. The application of the diabetes prevention trial-type 1 risk score for identifying a preclinical state of type 1 diabetes. Diabetes Care. 2012;35:1552–1555. doi: 10.2337/dc12-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sosenko JM, Skyler JS, Palmer JP Diabetes Type T, Diabetes Prevention Trial-Type 1 Study G. The development, validation, and utility of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS) Curr Diab Rep. 2015;15:626. doi: 10.1007/s11892-015-0626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raab J, Haupt F, Scholz M, Matzke C, Warncke K, Lange K, Assfalg R, Weininger K, Wittich S, Lobner S, Beyerlein A, Nennstiel-Ratzel U, Lang M, Laub O, Dunstheimer D, Bonifacio E, Achenbach P, Winkler C, Ziegler AG Fr1da Study G. Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open. 2016;6:e011144. doi: 10.1136/bmjopen-2016-011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan BM, Boulware D, Geyer S, Atkinson MA, Colman P, Goland R, Russell W, Wentworth JM, Wilson DM, Evans-Molina C, Wherrett D, Skyler JS, Moran A, Sosenko JM Type 1 Diabetes T, Diabetes Prevention Trial-Type 1 Study G. Dysglycemia and Index60 as Prediagnostic End Points for Type 1 Diabetes Prevention Trials. Diabetes Care. 2017 doi: 10.2337/dc17-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu P, Krischer JP Type 1 Diabetes TrialNet Study G. Prognostic Classification Factors Associated With Development of Multiple Autoantibodies, Dysglycemia, and Type 1 Diabetes-A Recursive Partitioning Analysis. Diabetes Care. 2016;39:1036–1044. doi: 10.2337/dc15-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decochez K, De Leeuw IH, Keymeulen B, Mathieu C, Rottiers R, Weets I, Vandemeulebroucke E, Truyen I, Kaufman L, Schuit FC, Pipeleers DG, Gorus FK. IA-2 autoantibodies predict impending type I diabetes in siblings of patients. Diabetologia. 2002;45:1658–1666. doi: 10.1007/s00125-002-0949-8. [DOI] [PubMed] [Google Scholar]
- 21.Hagopian WA, Erlich H, Lernmark A, Rewers M, Ziegler AG, Simell O, Akolkar B, Vogt R, Jr, Blair A, Ilonen J, Krischer J, She J. The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes. 2011;12:733–743. doi: 10.1111/j.1399-5448.2011.00774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]